Abstract
Studies are increasingly demonstrating that individuals differ in their rate of ageing, and this is postulated to emerge from a trade-off between current and future reproduction. Recent theory predicts a correlation between individual personality and life-history strategy, and from this comes the prediction that personality may predict the intensity of senescence. Here we show that boldness correlates with reproductive success and foraging behaviour in wandering albatrosses, with strong sex-specific differences. Shy males show a strong decline in reproductive performance with age, and bold females have lower reproductive success in later adulthood. In both sexes, bolder birds have longer foraging trips and gain more mass per trip as they get older. However, the benefit of this behaviour appears to differ between the sexes, such that it is only matched by high reproductive success in males. Together our results suggest that personality linked foraging adaptations with age are strongly sex-specific in their fitness benefits and that the impact of boldness on senescence is linked to ecological parameters.
Keywords: individual behavioural differences, boldness, ageing, foraging, life-history trade-offs, biologging
1. Introduction
In the last decade, there has been a surge in studies highlighting senescence in wild populations [1,2]. These reductions in reproductive performance or survival probability at old ages have deleterious effects on individual fitness [3]. It is widely thought that senescence may occur as a result of an accumulation of cellular damage caused during the lifetime of an individual, such that there is conflict between investment in current reproduction and investment in repair of organismal damage when resources are limited [4,5]. There is increasing empirical evidence that individuals differ in their rate of senescence, and this suggests individual level trade-offs between current and future reproduction [1,6–8], but what leads individuals to engage in different life-history tactics is poorly understood.
Concurrently, research on consistent individual differences in behaviour, or personalities, has increased exponentially in the last decade [9]. In recent years, evolutionary ecologists have postulated that life-history trade-offs may lead to the evolution and maintenance of such variation [10–13], resulting in the prediction that different personality types should engage in different life-history strategies. There is empirical support to suggest that personality correlates with single life-history traits, such as survival or fecundity [12,14–16], and some evidence that there is an interaction between age and personality on fitness, with bolder individuals having higher reproductive success in old age [17]. However, while life-history predicts that an individual's personality will correlate with the level of energy allocation in current versus future reproduction, and that high allocation corresponds to an investment involving increased senescence at old age [10–12], to our knowledge, no study to date has examined the relationship between personality and reproductive senescence in the wild.
Wandering albatrosses (Diomedea exulans) are one of the longest lived wild bird species, and their personality and life-history traits are already well understood [18–22]. Boldness has previously been shown to be repeatable and heritable in this species [22], and albatrosses show strong age-specific variation in life-history traits such as senescence in reproductive success [19–21,23] and changes in foraging behaviour with age [24], all of which are found predominantly in males. Furthermore, in a closely related species, the black browed albatross (Thalassarche melanophrys), personality has been shown to have sexually antagonistic effects on fitness, mediated by environmental conditions [25], but age effects were unexplored. In this study, we address the potential link between life-history traits and personalities by using one of the longest demographic and foraging datasets available in a wild species, in combination with personality scores for over 1300 birds. We ask whether an individual's boldness to a human approacher, a proxy for personality type, correlates with age at first breeding and changes in reproductive success with age, and thus senescence in reproductive success. We combine these data with foraging measures from 205 individuals, across 307 trips, to examine whether boldness is linked to changes in foraging behaviour and efficiency with age. As previous work has found stronger senescence in reproductive success in males of this species [21,24], we extend these analyses to consider sexually antagonistic effects. If males demonstrate greater changes in foraging behaviour and reproductive success with age, we suggest that the interaction with personality may be stronger in this sex.
2. Material and methods
(a). Data collection
(i). Study system
Wandering albatrosses are one of the largest flying birds, weighing between 8 and 12 kg, showing sexual size dimorphism, with males on average 20% larger. They are among the longest lived wild species, reaching over 50 years of age [26], with a very high annual survival and delayed breeding until at least 7 years old [27]. Pairs breed biennially, and share incubation and chick provisioning over an 11 month period, until the offspring fledges. Divorce is low (ca 0.3% of pairs; [28]) and pairs remain mainly monogamous, separating normally owing to failed breeding attempts or partner death. We examine how boldness, a proxy for personality, influences several reproductive parameters: age at first reproduction; reproductive success with age; and foraging behaviour.
The wandering albatross population of Possession Island, Crozet Islands, Southwestern Indian Ocean (46° S, 51° E), has been monitored since 1966 [27]. Each year, the pair identity, presence of an egg, presence of a chick and fledging of a chick are recorded for the whole island population. Birds are sexed from size and plumage dimorphism within each pair. All adults and chicks are fitted with a metal ring with a unique number to allow their survival and reproductive success to be followed throughout their lifetime. Reproductive success was defined as the successful fledging of a chick, for a nest with an egg laid. Birds normally breed annually if unsuccessful in their last attempt but biannually if they succeed in rearing a chick. Given this complexity, here we examine only the success of reproductive attempts and not breeding frequency. As a result, our study focuses on how boldness correlates with annual reproductive success not total fitness. In total, 1317 birds of known personality were known to have commenced breeding and of these 1142 (n reproductive success) were followed for between 1 and 42 years, for a total of 7534 breeding attempts. This creates a dataset that is partially cross sectional and partially longitudinal (see the electronic supplementary material, appendices S1 and S5 for full discussion). The mean population reproductive success was 77 ± 0.48%, driven mainly by hatching success (85 ± 0.42%; electronic supplementary material, appendix S1) and for birds which hatched a chick, the fledging success was very high (94 ± 0.30%; electronic supplementary material, appendix S1).
Age was known for all birds born in the population banded as chicks (n = 1149) and was estimated for all birds banded as adults (n = 168), assuming the minimum age at first reproduction was 7 years [27]. As in recently published studies [20], extreme values of age (more than 42 years) were collapsed into a single group (42 years) for analyses to avoid extreme values, with very low sample sizes, driving relationships.
(ii). Boldness
Individual boldness was measured as the response to a human approacher from 5 m, between 2009 and 2013, for incubating birds. The response was classified along an ordinal scale from 0, no response; 1, bird lifts head; 2, bird rises onto tarsus; 3, bird vocalizes; 4, bird stands up. As boldness has only been recorded during the last 5 years, all birds of known boldness are potentially still active breeders. As wandering albatrosses get older they breed less frequently, and to ensure accurate survival estimates, for a simple survival analysis birds must be absent from the colony for more than 4 years to be considered ‘dead’. While it is possible to use more complex multi-state models to estimate survival, this was beyond the scope of this paper. We were therefore unable to calculate survival estimates in this study. While we have several repeat measures for some individuals, to accurately measure within individual plasticity in boldness, at least three observations per individual are required, and as such we have insufficient data to adequately assess individual plasticity in boldness over time for all individuals in this study (for details see the electronic supplementary material, appendix S2). Although boldness has only been measured over the last 5 years, there is little evidence of a change in boldness with age nor evidence that is an interaction between personality and plasticity (electronic supplementary material, appendix S2). These results clearly show that any plasticity in boldness is not correlated with age supporting the use of these estimates as proxies of boldness across the lifetime of birds.
(iii). Foraging behaviour
Between 1989 and 2013, foraging movements were recorded using observations, satellite tags and GPS loggers [29–31]. All birds in this analysis were monitored during incubation, during which foraging behaviour is linked to self-maintenance [30]. Between 1989 and 1992, study nests were checked daily to record the changeover of partners and estimate trip duration (n trips = 40). From 1994 to 2013, trip duration was estimated using biologging devices. Devices were attached using Tesa tape [31] and recorded accurate positions every hour (Argos system: n trips = 76) or highly accurate locations at a resolution of 15 min (GPS: n trips = 191). For a subsection of trips, birds were weighed at departure and return, allowing the change in mass during the trips to be calculated (n trips = 90).
(b). Analyses
(i). Boldness
The maximum recorded response to a human approacher was used as an individual's score and this was scaled controlling for observation number and observer [22]. Briefly, models including all observations were fitted using a Bayesian generalized linear mixed model with an ordinal error structure fitted in MCMCglmm [32]. Observation number and observer were fitted as fixed effects and individual bird identification (ID) as a random effect. Using a parameter expanded prior, with V = 1, ν = 1000, α.µ = 0 and α.V = 1 for random effects and the residual variance was fixed at 1. We extracted 500 000 values for each individual bird, from a model with 600 000 iterations after a burn-in of 100 000. Visual, autocorrelation and convergence checks were performed. We extracted the posterior mode as the boldness score for each individual, and a matrix of 1000 boldness estimates per bird, drawn from the 95% creditability intervals, to estimate the impact of uncertainty in boldness on final parameter estimates.
(ii). Age at first reproduction
Age at first reproduction may be important for reproductive success as birds show an increase in success with experience in very early adulthood and age at first reproduction has previously been shown to differ among the sexes [27]. Furthermore, commencing breeding early may have a cost in later adulthood. Here we examined the effect of boldness on age at first reproduction. The interaction between boldness and sex was fitted as the age at first reproduction is independent among pairs. Models were fitted with a Poisson error distribution with log-link function, using only birds of exact known age (n = 1149).
(iii). Reproductive success
Given that reproductive success changes as quadratically with age (electronic supplementary material, appendix S3) we conducted two main analyses.
(1) Full data analysis. We fitted the interaction between boldness × age2 (see the electronic supplementary material, appendix S3 for consideration of other model structures; n = 7534), to examine boldness mediated senescence and fitted age at first reproduction to examine the impact of early breeding on reproductive success across a bird's lifetime. Reproductive outcome: 0, egg laid but no chick surviving to fledging; 1, egg laid produced a fledgling. Reproductive success was fitted with a binary error structure, using a logit-link function. Individual ID and year were fitted as random effects and subcolony (nine levels) as a fixed effect. Given that males and females from the same pair share a reproductive success, we ran all models separately for each sex. This analysis included cross-sectional and longitudinal data and an analysis using exclusively cross-sectional values for boldness and reproductive success, which were collected in the same year, are presented in the electronic supplementary material, appendix S5, showing strong congruence with the main models.
-
(2) Early and late adulthood analyses. For the full dataset, because not all individuals were studied across their entire lifetime, there may be an interaction between individual level changes in reproductive success with age and sampling regime, which has previously been reported in studies examining wandering albatross senescence [20]. Given the complexity of a model accounting for such an interaction, we are unable to fit this model, prohibiting the estimation of within individual senescence effects. Instead, following the methods of Froy et al. [20], we divide the data into ‘early’ and ‘late’ adulthood, allowing us to estimate within individual and personality effects in the same models. We divided the life cycle into ‘early adulthood’, defined as before 22 years of age (see turning point of quadratic curve in figure 1) and ‘late adulthood’, defined as 22 years or more. For each stage, individuals were selected if reproductive data was available across at least 90% of the stage duration.
For ‘early’ adulthood, data was subset using birds whose reproductive success was measured until at least 20 years of age (n attempts = 3344; n individuals = 545). For ‘late’ adulthood, data was subset to include only birds whose reproduction was monitored until they were at least 38 years old (n attempts = 409; n individuals = 38). These cut-offs were chosen as they maximized sample size, while ensuring that birds had been sampled over at least 90% of the range used. Logistic regressions were fitted in keeping with those used for the full dataset with the following changes. Boldness × age was fitted as a linear interaction (as the data were split at the turning point of the quadratic), again assessing boldness mediated changes in reproduction with age. Colony was grouped into a two-level factor (based on geographical location), as the datasets were considerably reduced and there was insufficient power to fit a nine-level effect. Age at first reproduction was fitted as fixed effect to examine the impact of early commencement on reproductive success in both early and late adulthood. An additional individual level random slope was fitted and age was mean centred at the population level for fixed effects and at the individual level for the random slopes.
Figure 1.
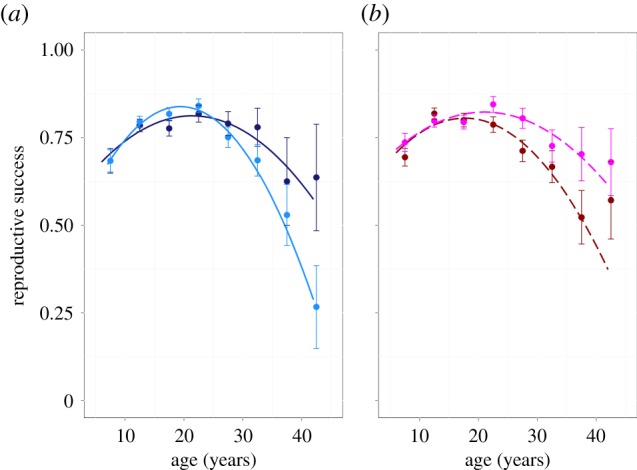
The change in reproductive success with age and boldness. (a) Bolder males senesce less quickly than shyer males, with solid lines showing the interaction between boldness and age. (b) Females do not show detectable differences in senescence with boldness. For illustrative purposes dashed lines show non-statistically significant differences in reproductive success with age between the personality types. While age and boldness are continuous measures in all analyses, for illustrative purposes only, they are grouped here. Age was grouped into 5-year bins from 5 to 45 years and the mean reproductive success (±s.e.) plotted against the mid- point of the bin. Females are plotted in shades of red and males in shades of blue. Boldness was grouped into two categories: bold (upper 1/2 of boldness scores; dark shades); shy (lower 1/2 of boldness scores; pale shades). (Online version in colour.)
(iv). Foraging behaviour
Trip duration (days) was calculated for 307 birds of known age and personality. Mass change (mass at return to the nest (g) − mass at departure (g)), was estimated controlling for the mass loss on the nest of 0.9% per day [30]. There was little evidence to support a quadratic change in foraging behaviour with age, so we fitted these models examining the interaction between boldness and age (see the electronic supplementary material, appendix S3 for model selection). Analyses were run on the sexes together fitting the interaction between boldness × age and year and month were fitted as random effects.
Age at first reproduction models were fitted using lm and all other analyses using lmer [33] in R [34]. First, all models were run using the posterior mode estimate for boldness per individual to extract parameter estimate and standard errors. ANOVA model comparisons were run with and without the term of interest to obtain p-values. Non-statistically significant fixed effect interaction terms and non-statistically significant random slopes (where the variance = 0) were dropped from final models. Then each analysis described was run 1000 times, using the 1000 estimates of boldness extracted per individual bird. This was carried out by fitting the models below, using a loop which varied the boldness scores input per iteration of the model. For every parameter in the model, we present the 95% CIs for every model parameter, based on the 1000 iterations [35]. We present the full tables of results in the electronic supplementary material, appendix S4, with all parameter estimates on the link scale.
3. Results
(a). Age at first reproduction
There was no interaction between boldness and sex on age at first reproduction (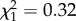 ; p = 0.57; electronic supplementary material, appendix S4, table S3) nor an effect of boldness (
; p = 0.57; electronic supplementary material, appendix S4, table S3) nor an effect of boldness (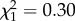 ; p = 0.59 electronic supplementary material, appendix S4, table S3). However, as shown in previous studies, age at first reproduction was strongly influenced by sex itself, with females starting breeding at a younger age to males (females: 8.75 years ± 1.01; males: 9.69 ± 1.02;
; p = 0.59 electronic supplementary material, appendix S4, table S3). However, as shown in previous studies, age at first reproduction was strongly influenced by sex itself, with females starting breeding at a younger age to males (females: 8.75 years ± 1.01; males: 9.69 ± 1.02; 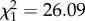 ; p < 0.001; electronic supplementary material, appendix S4, table S3).
; p < 0.001; electronic supplementary material, appendix S4, table S3).
(b). Reproductive success
(i). Males
There were strong differences in the change in reproductive success with age in males, such that shyer birds senesced more quickly (boldness × age2: 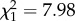 ; p = 0.005; electronic supplementary material, appendix S4, table S4; figure 1). These differences were driven by variation in hatching success, as is population reproductive success (electronic supplementary material, appendix S1). These results were supported by analyses examining early and late adulthood behaviour individually. These showed no significant interaction between boldness and age on early adulthood reproductive success (boldness × age:
; p = 0.005; electronic supplementary material, appendix S4, table S4; figure 1). These differences were driven by variation in hatching success, as is population reproductive success (electronic supplementary material, appendix S1). These results were supported by analyses examining early and late adulthood behaviour individually. These showed no significant interaction between boldness and age on early adulthood reproductive success (boldness × age: 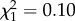 ; p = 0.76; electronic supplementary material, appendix S4, table S5), nor an effect of boldness (boldness:
; p = 0.76; electronic supplementary material, appendix S4, table S5), nor an effect of boldness (boldness: 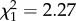 ; p = 0.13; electronic supplementary material, appendix S4, table S5) but a positive increase with age (
; p = 0.13; electronic supplementary material, appendix S4, table S5) but a positive increase with age (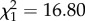 ; p < 0.001; electronic supplementary material, appendix S4, table S5). However, in late adulthood shy males senesced more quickly (boldness × age:
; p < 0.001; electronic supplementary material, appendix S4, table S5). However, in late adulthood shy males senesced more quickly (boldness × age: 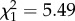 ; p = 0.02; electronic supplementary material, appendix S4, table S6), supporting the patterns seen using the full dataset.
; p = 0.02; electronic supplementary material, appendix S4, table S6), supporting the patterns seen using the full dataset.
Males that started breeding later had a higher reproductive success throughout their lifetime (age at first reproduction: 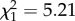 ; p = 0.02; electronic supplementary material, appendix S4, table S4) but this effect was not seen when examining the two life stages individually (age at first reproduction—early adulthood:
; p = 0.02; electronic supplementary material, appendix S4, table S4) but this effect was not seen when examining the two life stages individually (age at first reproduction—early adulthood: 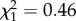 ; p = 0.50; electronic supplementary material, appendix S4, table S5; late adulthood:
; p = 0.50; electronic supplementary material, appendix S4, table S5; late adulthood: 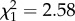 ; p = 0.11; electronic supplementary material, appendix S4, table S6). We found no support for the inclusion of within individual differences in senescence (individual slopes—early adulthood: 0.00 ± 0.07; electronic supplementary material, appendix S4, table S5; late adulthood: 0.00 ± 0.08; electronic supplementary material, appendix S4, table S6). Studies which have previously demonstrated strong within individual senescence rates had not considered that such individual variation may be explained by boldness. By fitting boldness as a fixed effect, we suggest that this accounts for a large amount of variation in individual senescence rates.
; p = 0.11; electronic supplementary material, appendix S4, table S6). We found no support for the inclusion of within individual differences in senescence (individual slopes—early adulthood: 0.00 ± 0.07; electronic supplementary material, appendix S4, table S5; late adulthood: 0.00 ± 0.08; electronic supplementary material, appendix S4, table S6). Studies which have previously demonstrated strong within individual senescence rates had not considered that such individual variation may be explained by boldness. By fitting boldness as a fixed effect, we suggest that this accounts for a large amount of variation in individual senescence rates.
(ii). Females
Females showed no evidence of an interaction between boldness and age on reproductive success, indicative of personality mediated rates of senescence across birds' lifetimes (boldness × age2: 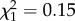 ; p = 0.70; electronic supplementary material, appendix S4, table S4; figure 1; boldness × age:
; p = 0.70; electronic supplementary material, appendix S4, table S4; figure 1; boldness × age: 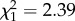 ; p = 0.12; electronic supplementary material, appendix S4, table S4) or within stages (early adulthood: boldness × age:
; p = 0.12; electronic supplementary material, appendix S4, table S4) or within stages (early adulthood: boldness × age: 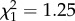 ; p = 0.26; electronic supplementary material, appendix S4, table S5; late adulthood: boldness × age:
; p = 0.26; electronic supplementary material, appendix S4, table S5; late adulthood: boldness × age: 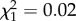 ; p = 0.87; electronic supplementary material, appendix S4, table S6). However, while boldness did not influence reproductive success in early adulthood (boldness:
; p = 0.87; electronic supplementary material, appendix S4, table S6). However, while boldness did not influence reproductive success in early adulthood (boldness: 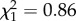 ; p = 0.35; electronic supplementary material, appendix S4, table S5), bolder females had a lower reproductive success in late adulthood (boldness:
; p = 0.35; electronic supplementary material, appendix S4, table S5), bolder females had a lower reproductive success in late adulthood (boldness: 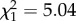 ; p = 0.02; electronic supplementary material, appendix S4, table S6).
; p = 0.02; electronic supplementary material, appendix S4, table S6).
We found no evidence that the age at first reproduction was linked to reproductive success in females (age at first reproduction—all data: 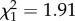 ; p = 0.17; electronic supplementary material, appendix S4, table S4; early adulthood:
; p = 0.17; electronic supplementary material, appendix S4, table S4; early adulthood: 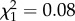 ; p = 0.77; electronic supplementary material, appendix S4, table S5; late adulthood:
; p = 0.77; electronic supplementary material, appendix S4, table S5; late adulthood: 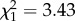 ; p = 0.06; electronic supplementary material, table S6) nor support for within individual differences in senescence (individual slopes—early adulthood: 0.00 ± 0.06; electronic supplementary material, appendix S4, table S5; late adulthood: 0.00±0.05; electronic supplementary material, appendix S4, table S6).
; p = 0.06; electronic supplementary material, table S6) nor support for within individual differences in senescence (individual slopes—early adulthood: 0.00 ± 0.06; electronic supplementary material, appendix S4, table S5; late adulthood: 0.00±0.05; electronic supplementary material, appendix S4, table S6).
(c). Foraging behaviour
Bolder birds made longer foraging trips with increasing age (boldness × age: 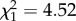 ; p = 0.03; electronic supplementary material, appendix S4, table S7; figure 2) and was driven mainly by changes in late life (boldness × age: early adulthood:
; p = 0.03; electronic supplementary material, appendix S4, table S7; figure 2) and was driven mainly by changes in late life (boldness × age: early adulthood: 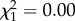 ; p = 0.97; late adulthood:
; p = 0.97; late adulthood: 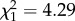 ; p = 0.04; electronic supplementary material, appendix S4, table S7). These results are coupled with a trend that bolder birds gain more mass per foraging trip (boldness:
; p = 0.04; electronic supplementary material, appendix S4, table S7). These results are coupled with a trend that bolder birds gain more mass per foraging trip (boldness: 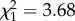 ; p = 0.06; electronic supplementary material, appendix S4, table S8) and this relationship was significant in late adulthood (
; p = 0.06; electronic supplementary material, appendix S4, table S8) and this relationship was significant in late adulthood (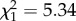 ; p = 0.02; electronic supplementary material, appendix S4, table S8; figure 3) but not early adulthood (
; p = 0.02; electronic supplementary material, appendix S4, table S8; figure 3) but not early adulthood (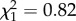 ; p = 0.37; electronic supplementary material, appendix S4, table S8). There was no interaction between boldness and age on mass gain (boldness × age: all data:
; p = 0.37; electronic supplementary material, appendix S4, table S8). There was no interaction between boldness and age on mass gain (boldness × age: all data: 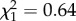 ; p = 0.42; early adulthood:
; p = 0.42; early adulthood: 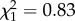 ; p = 0.36; late adulthood:
; p = 0.36; late adulthood: 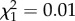 ; p = 0.92; electronic supplementary material, appendix S4, table S8).
; p = 0.92; electronic supplementary material, appendix S4, table S8).
Figure 2.
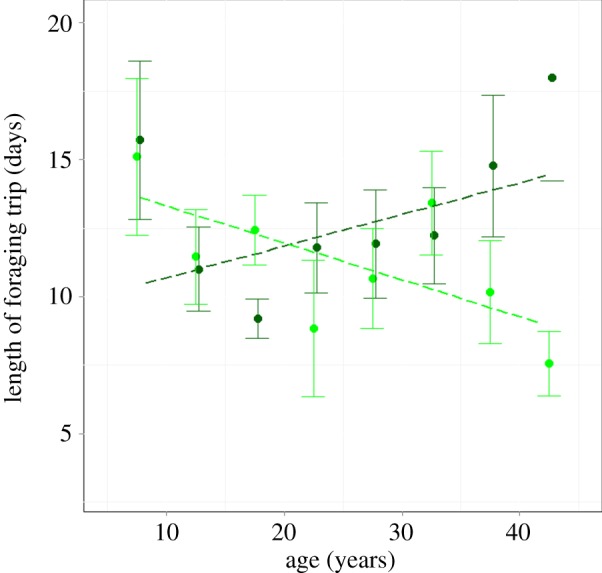
Changes in foraging behaviour with age and personality. Bolder birds make longer foraging trips as they get older. While age and boldness are continuous measures in all analyses, for illustrative purposes only, they are grouped here. Age was grouped into 5 year bins from 5 to 45 years and the mean foraging behaviour (±s.e.) plotted against the mid- point of the bin. Boldness was grouped into two categories: bold (upper 1/2 of boldness scores; dark colour); shy (lower 1/2 of boldness scores; pale colour). (Online version in colour.)
Figure 3.
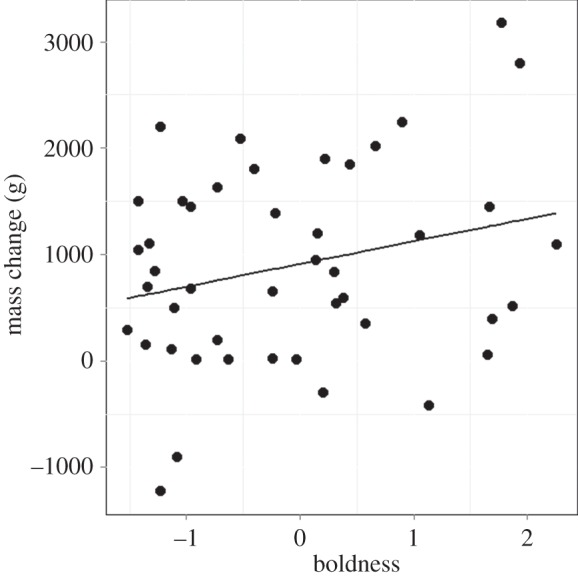
Independent of age and sex, bolder birds gain more mass per foraging trip in late adulthood.
4. Discussion
Our study reveals senescence in reproductive success in wandering albatrosses and links this to a measure of individual personality. These patterns appear to be sex specific, with shyer males demonstrating stronger senescence and bolder females having lower reproductive success in later life. Furthermore, we find that boldness is correlated with foraging trip duration in both sexes, with bolder birds making longer trips in later adulthood, gaining more mass per foraging trip. These results highlight the potential importance of sexually antagonistic effects of personality and foraging behaviour on reproductive success and senescence in a wide-ranging long-lived species.
(a). Foraging behaviour
Here we show evidence that boldness is linked to foraging behaviour in wandering albatrosses. Male wandering albatrosses are known to forage further south with increasing age [24,29], and here we can suggest that these changes in foraging behaviour are driven predominantly by bolder individuals making longer foraging trips in later adulthood. Boldness is often correlated with explorative behaviour [9] and superficial exploration [11,36] and so bolder individuals may be more likely to explore further from the colony when ageing. When males increase the duration of their foraging trips, they can reach more southerly waters, which are highly productive, and may confer an advantage to the individual in old age, when they are potentially less efficient or less competitive closer to the colonies.
Interestingly, while bold females also make longer foraging trips in later adulthood, and gain more mass per trip, this is associated with a lower reproductive success during this time. Females are foraging in different water masses than males, as they travel north from the colony to warmer waters [29,37]. Increasing trip length is associated with the possibility of exploring nutrient poor tropical zones [30] and therefore poorer quality resources. Longer trips in females may be energetically costly, and while birds are able to gain more mass, this may come at a much greater cost than to bold males. As such, these results suggest that changes in foraging behaviour may not confer an advantage to females. Sexually antagonistic selection can maintain a trait in the population if it confers an advantage in one sex, even if it is detrimental to the other sex (reviewed by [38]) and has been proposed as an explanation for the persistence of personality variation within populations [39]. As such, the correlation between boldness and foraging trip length may be adaptive in males, but persist as a carryover effect of selection in females.
In the closely related black browed albatross, boldness has been shown to link to foraging range, such that bolder birds feed nearer the colony [25]. In this species, shyer males and bolder females generally had higher fitness, although this was mediated by environmental conditions and foraging location, and age effects were not examined. Competition has a strong influence on foraging tactics in this species as birds forage as part of a group, close to the colony. This study suggested that boldness may have varying benefits depending on an individual's overlap with other birds and competitive ability. In wandering albatross, birds are mainly solitary feeders and during incubation, when this study was carried out, feed well beyond the local waters [29,37]. As such, we suggest that the influences of boldness on competitive ability may not be under strong selection in this species and may be of decreasing importance with age, as birds extend foraging ranges. Range expansion into new waters may increase the importance of exploration behaviour, altering the impact of boldness on fitness. These results together suggest that the fitness consequences of personality may be highly dependent on the ecological niche of species and the extent of segregation between the sexes. In wandering albatrosses, increased trip duration yields a fitness benefit to males as a result of their foraging locations but it is easy to imagine that under different circumstances boldness may result in very different effects on fitness.
(b). Reproductive failure
As reproductive failures occur mainly during incubation [21], these results infer that pairs with a bold male or shy female are less likely to abandon the egg in old age. Abandonment is associated with a decrease in mass below a critical threshold [19]. As bolder males are able to gain more mass during their foraging trips, this may place them further from the abandonment threshold and enable longer incubation stints. In addition, around this threshold, individuals may differ in their propensity to abandon [19] and more risk taking birds may remain on the nest longer, requiring a higher threshold to abandon the egg. As bolder birds have been shown to be more risk taking in the past [9], these individuals may be more likely to endure increase starvation risks, which in turn may reduce their incubation failure rate. If bold females suffer a greater cost to long foraging trips, or have differing nutritional demands to males, they may be less able to remain on the egg for long periods, and this behaviour in itself could lead to abandonment and nest failure. In addition, the long-term consequences of increased incubation shifts for survival are unknown, but increased energy allocation in a single year season affects survival probabilities in future years.
(c). Trade-offs
Life-history predictions infer that personality may be linked to senescence through the trade-off between current and future reproduction [10,11]. We find no effect of boldness on early adulthood reproduction in either sex, providing little support that boldness drives these differences. An alternative hypothesis which we suggest for this species, given its very high reproductive success during most of its early adulthood, is that it may be only during late adulthood that the trade-off between current and future reproduction is strong, and differences with boldness may begin to emerge. The results showing that bolder individuals are spending longer foraging, indicate increased allocation to reproduction. In males this increased foraging time, and the subsequent change in foraging grounds, may enable then to maintain their reproductive success in late adulthood. However, our study focuses on the link between boldness, foraging and reproductive success. We do not examine the reproductive output of individuals or lifetime fitness. Bold males and shy females may have higher reproductive success in later life, but may attempt to breed less frequently. Hence, the allocation to current versus future reproduction may differ, based on the risks and foraging ecology of the sexes.
Life-history models predict that bolder birds will have lower survival and should therefore allocate more resources to the current reproductive attempt (fast pace of life; [40]). In wandering albatross, reproductive success and survival (post recruitment) during early adulthood is very high and as such, shows little variation between individuals [21]. It is not until later in adulthood (ca 22 years of age) that reproductive success becomes more variable, and survival begins to decrease [21]. While we are unable to test for survival differences between the personality types, the differences we report here may be coupled with varying survival probabilities. Individuals may face a direct trade-off between reproduction in late adulthood and survival, such that senescence in reproductive success correlates with increased survival. It is possible that the distribution of phenotypes in older birds is representative of birds which have survived to old age. As such, we may only see high quality bold males, who are able to maintain their reproductive success and survive. Furthermore, as boldness was measured only in late life for old birds, our results report the reproductive success differences for birds that are bold and shy when old. It is possible that there are individual differences in the stability in personality and fitness consequences to this plasticity in behaviour. Future work should focus on the lifetime fitness of personality types and continue to measure boldness over long time periods, to examine whether such trade-offs exist and whether plasticity in behaviour may be under selection.
Evidence suggesting bold females have a low fitness in old age may arise if bolder females allocate more resources to survival, making data on longevity essential to be able to assess the lifetime fitness consequences of differences in boldness. Furthermore, the estimates of personality were collected only in late adulthood for old birds, and as such, we are unable to examine how plasticity in boldness may correlate with senescence. Individuals that are able to change their behaviour, and potentially their personality, may be better able to resist senescence in later life. While this offers an interesting extension to this work, exploring the idea that life-history trade-offs emerge only in later adulthood, our current study provides evidence linking a personality trait to both senescence in the wild, and changes in foraging behaviour with age. The evidence suggesting that bold birds invest more heavily in current reproduction, paves the way for future studies examining the consequences for future survival and reproduction.
Supplementary Material
Acknowledgements
We thank Deborah Pardo, Niels Dingemanse, Jean-Michel Gaillard and two anonymous reviewers whose comments greatly improved the manuscript, Christophe Barbraud, Tobias Uller, Alastair Wilson, Hannah Froy, Dan Nussey and Denis Réale for helpful discussion, and Karine Delord and Dominique Besson for database support. Thanks to all fieldworkers who collected data at Possession Island over the past four decades, particularly Julien Collet, Susan Waugh, Timothée Poupart and Franck Théron for invaluable help and support.
Ethics statement
Licences and permissions were granted by the Ethic Committee of Institut Polaire Francais (IPEV) and by the Préfet of Terres australes et antarctiques francaises (TAAF) after advice from the Comité de l'Environnement Polaire (CEP).
Data accessibility
The data sets used in this article will be available from dryad at 10.5061/dryad.sn711 after an embargo period.
Funding statement
The Institut Polaire Français Paul Emile Victor (IPEV, programme 109) and the Terres Australes and Antarctique Françaises (TAAF) provided logistical and financial support, and S.C.P. was funded by a Marie Curie Intra-European fellowship (ALBASPECIALISATION).
References
- 1.Nussey DH, Coulson T, Festa-Bianchet M, Gaillard JM. 2008. Measuring senescence in wild animal populations: towards a longitudinal approach. Funct. Ecol. 22, 393–406. ( 10.1111/j.1365-2435.2008.01408.x) [DOI] [Google Scholar]
- 2.Nussey DH, Froy H, Lemaitre JF, Gaillard JM, Austad SN. 2013. Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res. Rev. 12, 214–225. ( 10.1016/j.arr.2012.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monaghan P, Charmantier A, Nussey DH, Ricklefs RE. 2008. The evolutionary ecology of senescence. Funct. Ecol. 22, 371–378. ( 10.1111/j.1365-2435.2008.01418.x) [DOI] [Google Scholar]
- 4.Kirkwood TBL, Rose MR. 1991. Evolution of senescence: late survival sacrificed for reproduction. Phil. Trans. R. Soc. Lond. B 332, 15–24. ( 10.1098/rstb.1991.0028) [DOI] [PubMed] [Google Scholar]
- 5.Kirkwood TBL, Holliday R. 1979. Evolution of aging and longevity. Proc. R. Soc. Lond. B 205, 531–546. ( 10.1098/rspb.1979.0083) [DOI] [PubMed] [Google Scholar]
- 6.Aubry LM, Koons DN, Monnat JY, Cam E. 2009. Consequences of recruitment decisions and heterogeneity on age-specific breeding success in a long-lived seabird. Ecology 90, 2491–2502. ( 10.1890/08-1475.1) [DOI] [PubMed] [Google Scholar]
- 7.Aubry LM, Cam E, Koons DN, Monnat JY, Pavard S. 2011. Drivers of age-specific survival in a long-lived seabird: contributions of observed and hidden sources of heterogeneity. J. Anim. Ecol. 80, 375–383. ( 10.1111/j.1365-2656.2010.01784.x) [DOI] [PubMed] [Google Scholar]
- 8.Bouwhuis S, Charmantier A, Verhulst S, Sheldon BC. 2010. Individual variation in rates of senescence: natal origin effects and disposable soma in a wild bird population. J. Anim. Ecol. 79, 1251–1261. ( 10.1111/j.1365-2656.2010.01730.x) [DOI] [PubMed] [Google Scholar]
- 9.Sih A, Bell AM, Johnson JC, Ziemba RE. 2004. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 79, 241–277. ( 10.1086/422893) [DOI] [PubMed] [Google Scholar]
- 10.Stamps JA. 2007. Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol. Lett. 10, 355–363. ( 10.1111/j.1461-0248.2007.01034.x) [DOI] [PubMed] [Google Scholar]
- 11.Wolf M, van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 12.Biro PA, Stamps JA. 2008. Are animal personality traits linked to life-history productivity? Trends Ecol. Evol. 23, 361–368. ( 10.1016/j.tree.2008.04.003) [DOI] [PubMed] [Google Scholar]
- 13.Luttbeg B, Sih A. 2010. Risk, resources and state-dependent adaptive behavioural syndromes. Phil. Trans. R. Soc. B 365, 3977–3990. ( 10.1098/rstb.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boon AK, Reale D, Boutin S. 2007. The interaction between personality, offspring fitness and food abundance in North American red squirrels. Ecol. Lett. 10, 1094–1104. ( 10.1111/j.1461-0248.2007.01106.x) [DOI] [PubMed] [Google Scholar]
- 15.Aarts G, MacKenzie M, McConnell B, Fedak M, Matthiopoulos J. 2008. Estimating space-use and habitat preference from wildlife telemetry data. Ecography 31, 140–160. ( 10.1111/j.2007.0906-7590.05236.x) [DOI] [Google Scholar]
- 16.Smith BR, Blumstein DT. 2008. Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455. ( 10.1093/beheco/arm144) [DOI] [Google Scholar]
- 17.Reale D, Martin J, Coltman DW, Poissant J, Festa-Bianchet M. 2009. Male personality, life-history strategies and reproductive success in a promiscuous mammal. J. Evol. Biol. 22, 1599–1607. ( 10.1111/j.1420-9101.2009.01781.x) [DOI] [PubMed] [Google Scholar]
- 18.Weimerskirch H, Barbraud C, Lys P. 2000. Sex differences in parental investment and chick growth in wandering albatrosses: fitness consequences. Ecology 81, 309–318. ( 10.1890/0012-9658(2000)081[0309:SDIPIA]2.0.CO;2) [DOI] [Google Scholar]
- 19.Weimerskirch H. 1992. Reproductive effort in long-lived birds: age specific patterns of condition, reproduction and survival in the wandering albatross. Oikos 64, 464–473. ( 10.2307/3545162) [DOI] [Google Scholar]
- 20.Froy H, Phillips RA, Wood AG, Nussey DH, Lewis S. 2013. Age-related variation in reproductive traits in the wandering albatross: evidence for terminal improvement following senescence. Ecol. Lett. 16, 642–649. ( 10.1111/ele.12092) [DOI] [PubMed] [Google Scholar]
- 21.Pardo D, Barbraud C, Weimerskirch H. 2013. Females better face senescence in the wandering albatross. Oecologia 173, 1283–1294. ( 10.1007/s00442-013-2704-x) [DOI] [PubMed] [Google Scholar]
- 22.Patrick SC, Charmantier A, Weimerskirch H. 2013. Differences in boldness are repeatable and heritable in a long-lived marine predator. Ecol. Evol. 3, 4291–4299. ( 10.1002/ece3.748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weimerskirch H, Gault A, Cherel Y. 2005. Prey distribution and patchiness: factors in foraging success and efficiency of wandering albatrosses. Ecology 86, 2611–2622. ( 10.1890/04-1866) [DOI] [Google Scholar]
- 24.Lecomte VJ, et al. 2010. Patterns of aging in the long-lived wandering albatross. Proc. Natl Acad. Sci. USA 107, 6370–6375. ( 10.1073/pnas.0911181107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patrick SC, Weimerskirch H. 2014. Personality, foraging and fitness consequences in a long lived seabird. PLoS ONE 9, e87269 ( 10.1371/journal.pone.0087269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tickell WLN. 2000. Albatrosses. New Haven, CT: Yale University Press. [Google Scholar]
- 27.Weimerskirch H, Brothers N, Jouventin P. 1997. Population dynamics of wandering albatross Diomedea exulans and Amsterdam albatross D. amsterdamensis in the Indian Ocean and their relationships with long-line fisheries: conservation implications. Biol Conserv. 79, 257–270. ( 10.1016/S0006-3207(96)00084-5) [DOI] [Google Scholar]
- 28.Jouventin P, Lequette B, Dobson FS. 1999. Age-related mate choice in the wandering albatross. Anim. Behav. 57, 1099–1106. ( 10.1006/anbe.1999.1083) [DOI] [PubMed] [Google Scholar]
- 29.Weimerskirch H, Cherel Y, Delord K, Jaeger A, Patrick SC, Riotte-Lambert L. 2014. Lifetime foraging patterns of the wandering albatross: life on the move! J. Exp. Mar. Biol. Ecol. 450, 68–78. ( 10.1016/j.jembe.2013.10.021) [DOI] [Google Scholar]
- 30.Weimerskirch H. 1995. Regulation of foraging trips and incubation routine in male and female wandering albatross. Oecologia 102, 37–43. [DOI] [PubMed] [Google Scholar]
- 31.Weimerskirch H, Doncaster CP, Cuenotchaillet F. 1994. Pelagic seabirds and the marine-environment: foraging patterns of wandering albatross in relation to prey availability and distribution. Proc. R. Soc. Lond. B 255, 91–97. ( 10.1098/rspb.1994.0013) [DOI] [Google Scholar]
- 32.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–22.20808728 [Google Scholar]
- 33.Bates D, Maechler M. 2010. lme4: linear mixed-effects models using S4 classes. R package version 0.999375-35. See http://CRAN.R-project.org/package=lme4.
- 34.R DeveLopment Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 35.Nicolaus M, Tinbergen JM, Bouwman KM, Michler SPM, Ubels R, Both C, Kempenaers B, Dingemanse NJ. 2012. Experimental evidence for adaptive personalities in a wild passerine bird. Proc. R. Soc. B 279, 4885–4892. ( 10.1098/rspb.2012.1936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Careau V, Bininda-Emonds ORP, Thomas DW, Réale D, Humphries MM. 2009. Exploration strategies map along fast–slow metabolic and life-history continua in muroid rodents. Funct. Ecol. 23, 150–156. ( 10.1111/j.1365-2435.2008.01468.x) [DOI] [Google Scholar]
- 37.Weimerskirch H, Salamolard M, Sarrazin F, Jouventin P. 1993. Foraging strategy of wandering albatrosses through the breeding season: a study using satellite telemetry. Auk 110, 325–342. [Google Scholar]
- 38.Cox RM, Calsbeek R. 2009. Sexually antagonistic selection, sexual dimorphism, and the resolution of intralocus sexual conflict. Am. Nat. 173, 176–187. ( 10.1086/595841) [DOI] [PubMed] [Google Scholar]
- 39.Schuett W, Tregenza T, Dall SR. 2010. Sexual selection and animal personality. Biol. Rev. 85, 217–246. ( 10.1111/j.1469-185X.2009.00101.x) [DOI] [PubMed] [Google Scholar]
- 40.Reale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used in this article will be available from dryad at 10.5061/dryad.sn711 after an embargo period.


