Abstract
Seasonal patterns in pathogen transmission can influence the impact of disease on populations and the speed of spatial spread. Increases in host contact rates or births drive seasonal epidemics in some systems, but other factors may occasionally override these influences. White-nose syndrome, caused by the emerging fungal pathogen Pseudogymnoascus destructans, is spreading across North America and threatens several bat species with extinction. We examined patterns and drivers of seasonal transmission of P. destructans by measuring infection prevalence and pathogen loads in six bat species at 30 sites across the eastern United States. Bats became transiently infected in autumn, and transmission spiked in early winter when bats began hibernating. Nearly all bats in six species became infected by late winter when infection intensity peaked. In summer, despite high contact rates and a birth pulse, most bats cleared infections and prevalence dropped to zero. These data suggest the dominant driver of seasonal transmission dynamics was a change in host physiology, specifically hibernation. Our study is the first, to the best of our knowledge, to describe the seasonality of transmission in this emerging wildlife disease. The timing of infection and fungal growth resulted in maximal population impacts, but only moderate rates of spatial spread.
Keywords: seasonality, emerging infectious disease, fungal pathogen, white-nose syndrome, Myotis lucifugus, hibernation
1. Introduction
Seasonality in pathogen dynamics influences the impact of disease on populations [1], and can enhance pathogen spread [2]. If the timing of peak infectiousness occurs when populations are highly mobile (e.g. during migration or dispersal), spatial spread will be maximized [3–6]. The timing of seasonal mortality can also influence disease impact: impacts will be additive and largest if there is seasonal density-dependent population regulation, and mortality from disease occurs after most density-dependent mortality [7–9]. For example, if birth pulses drive seasonal epidemics, then disease impacts may compensate for naturally occurring density-dependent mortality and dispersing infected young could lead to rapid spatial spread. Understanding the patterns and drivers of seasonality increases our understanding of disease impacts on populations and the rate of spread of invading pathogens.
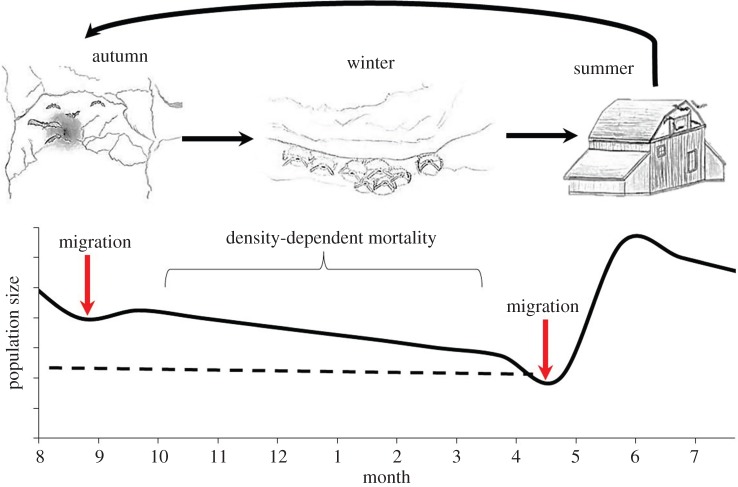
Five mechanisms driving seasonality in transmission have been proposed for directly transmitted pathogens, and these may act independently or in concert with each other to drive transmission. First, sociality varies seasonally for many species and alters transmission by increasing or decreasing contact rates [5,10–12]. Mating frequently increases infectious contacts, whereas territoriality can decrease contact rates among hosts [2,5,13]. Second, seasonal birth pulses can increase transmission by creating an influx of susceptible individuals into an otherwise mostly immune population [14–16]. Third, seasonal changes in host habitat use can influence the transmission and persistence of pathogens by altering contact with infective stages in the environment [17,18]. Fourth, climatic changes may influence the persistence of pathogens outside hosts, and can also change host behaviour, which may work in concert with other factors [19]. Finally, seasonal differences in host immune function also can alter growth of pathogens within hosts [20,21]. Hibernating bat species affected by the novel fungal disease, white-nose syndrome (WNS), exhibit seasonal differences in host physiology, habitat use and sociality, presenting a powerful opportunity to empirically test the influence of these factors on seasonal disease dynamics (figure 1).
Figure 1.
Seasonal life-history patterns of temperate hibernating bats and hypothesized trends in population size in the absence of disease. In the summer, female bats form single-species maternity colonies in human structures, trees or rock crevices, where they give birth to two (Eptesicus fuscus) or one (all other species) pup. During autumn, bats often mate at swarms in and around hibernation sites and use torpor intermittently. In winter, bats of multiple species enter prolonged periods of torpor (hibernation) inside hibernacula. Disease mortality that reduces populations down to the dashed black line may be compensatory mortality, whereas population decreases below the dashed black line will be additive with other sources and will lead to fewer reproducing individuals in summer. (Online version in colour.)
WNS is caused by the fungus, Pseudogymnoascus destructans and emerged in North America in the winter of 2006 [22–24]. This disease currently threatens several hibernating bat species with extinction [25], has killed millions of individuals and has resulted in the collapse of little brown bat populations across eastern North America [25,26]. Morbidity and mortality appear to be linked to fungal invasion of tissues that disrupt bat physiology, and lead to dehydration and increased arousals [22] that deplete fat reserves [27]. Pseudogymnoascus destructans grows best at the cool temperatures at which many bats hibernate, with optimal fungal growth between 7°C and 16°C, and no growth above 20°C [28]. Hibernacula are known reservoirs for the fungus [29,30], and P. destructans can survive for long periods in the absence of bats [31].
We quantified seasonal patterns of P. destructans infection and pathogen loads (infection intensity) to examine the relative influences of colony size, birth pulses, habitat use and physiology on transmission (the change in prevalence over time) and pathogen amplification (the increase in infection intensity) on hosts. If colony size is the primary mechanism driving transmission, we predict that prevalence would increase faster in seasons when colonies are larger. A yearly birth pulse may be important in driving transmission if either colony size or acquired immunity is important, and would result in sharp increases in prevalence in mid-summer after females have given birth [32]. If contact with an environmental reservoir drives transmission, then prevalence would be predicted to increase significantly as bats contact infected environments. Hibernacula, where bats swarm and spend the winter are known reservoirs for the fungus [30], and it is unknown whether differences in seasonal exposure in different habitats may drive seasonal patterns of WNS. Finally, changes in host physiology, in particular, hibernation (when bats' lower their body temperatures for sustained periods to conditions where P. destructans can grow [32,33]) may drive seasonal transmission. Increases in infectiousness as the fungus grows on hibernating bats may drive winter transmission, with loads increasing throughout the winter. We examined the influence of these mechanisms on the seasonality of WNS by quantifying patterns of infection prevalence and pathogen loads on six bat species over the year at 30 hibernacula and maternity sites spanning much of the current distribution of the fungus.
2. Material and methods
(a). Study sites
We sampled bats at 30 hibernacula and maternity colonies in New York, Vermont, Massachusetts, Virginia, New Hampshire and Illinois where P. destructans had been present for at least 1 year. We sampled bats in one or more periods of their life cycle which roughly correspond with seasons, including early autumn swarm (late August to mid-September), late autumn swarm (late September to late October), early winter hibernation (November and December), late winter hibernation (March and early April), early summer maternity (May) and late summer maternity (late June to July; figure 2). Phenology varies by latitude; bats in southern sites have shorter hibernation seasons, swarm later in the autumn and return to maternity colonies earlier [34]. Winter colonies included one to six bat species: little brown myotis (Myotis lucifugus), northern long-eared myotis (Myotis septentrionalis), eastern small-footed myotis (Myotis leibii), Indiana myotis (Myotis sodalis), tri-colored bats (Perimyotis subflavus) and big brown bats (Eptesicus fuscus). We sampled two species in the summer that roost in human dwellings (e.g. barns), little brown myotis and big brown bats. Other species' maternity sites are difficult to locate and therefore were not sampled. Hibernacula counts were conducted primarily during late winter visits. At maternity colonies, we conducted emergence counts twice to determine total colony size: once before, and once after the young of the year had become volant. We followed field hygiene protocols in accordance with United States Fish & Wildlife Service WNS Decontamination Guidelines, and individual state recommendations [35]. All research was conducted under protocol number 11-022 approved by the IACUC of Boston University.
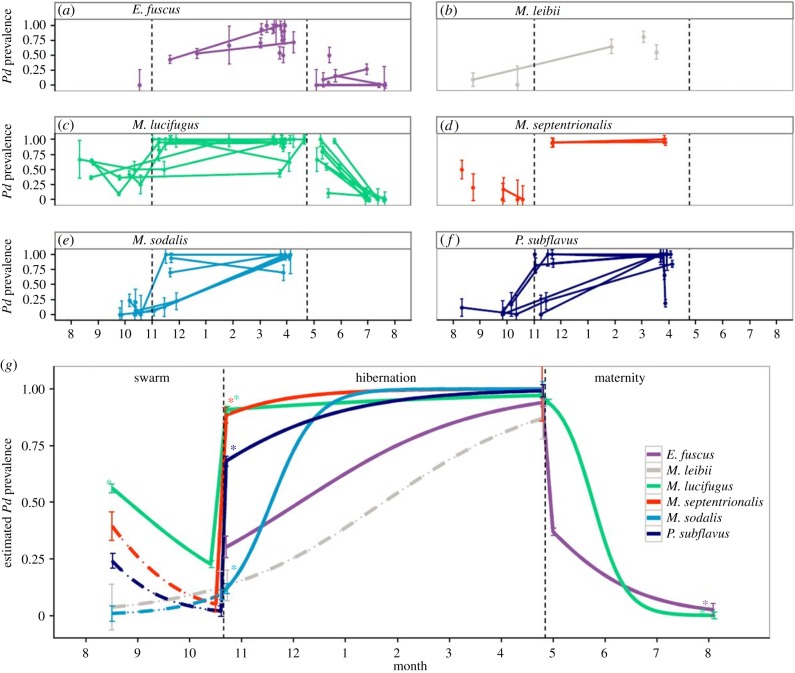
Figure 2.
(a–f) Seasonal prevalence (±1 s.e.) of Pseudogymnoascus destructans for six species across all sites. Lines connect estimates from the same site. (g) Model predicted prevalence (± standard error of predicted mean) of P. destructans for six species from autumn to summer. Dashed lines indicate species that were poorly sampled across that season. Asterisks denote time points in which prevalence was significantly different (p < 0.0001 for all comparisons) than the previous season. Vertical dashed lines in all panels divide seasons (autumn swarm, winter hibernation, summer maternity.). (Online version in colour.)
(b). Sample collection and analysis
We sampled a mean of 12 individuals (±0.26, range: 5–80) of each species present at each site to determine infection prevalence and P. destructans infection intensity. We sampled bats by dipping a sterile polyester swab in sterile water to moisten it and then rubbing the swab five times across both the forearm and muzzle of a bat. Swabs were stored in RNAlater for preservation until extraction. Samples were tested for presence and quantity of P. destructans DNA using real-time PCR [36]. We quantified the amount of P. destructans based on the cycle threshold (Ct) value to estimate the fungal load on each bat, with a Ct cut-off of 40 cycles. The standard curve for quantification was generated using genomic DNA from P. destructans ATCC MYA-4855 quantified with the Quant-IT PicoGreen double-stranded DNA assay kit (Life Technologies, Carlsbad, CA) in conjunction with a DynaQuant 300 fluorometer (Harvard Bioscience, Inc., Holliston, MA). Serial dilutions of the DNA from 10 ng to 1000 fg were prepared and analysed with IGS qPCR, resulting in a significant curve from 17.33 to 30.74 Ct (ng of P. destructans = −3.348*Ct + 22.049, r2 = 0.986).
(c). Statistical analysis
We used generalized linear-mixed models (function glmer in package lme4 [37] in R v. 3.02 [38]) to compare changes in P. destructans prevalence and intensity for each species over time. To measure the change in prevalence or load over time, we used a modified time axis where 0 represented the first day of autumn swarm sampling and expressed time (in units of partial months). We examined differences in seasonal transmission (the change in prevalence over time) and changes in infection intensity as the fixed effect of time interacting with season (autumn, winter, spring) to estimate a slope (representing the transmission rate or fungal growth rate) and intercept for each season. We included time nested within site as a random effect to allow for variation among sites in transmission rate or fungal growth rate on bats.
3. Results
We sampled a total of 1512 bats of six species at 20 hibernacula and 717 bats of two species at 10 maternity sites where P. destructans had been present for at least 1 year. Infection prevalence in early autumn when bats returned to infected hibernacula to swarm was between 5% and 50% for the six species (figure 2). Surprisingly, prevalence decreased during the autumn for M. lucifugus at all three sites where this species was sampled multiple times, and more limited data for other species also suggested a decline in prevalence during this season (figure 2 and the electronic supplementary material, table S1), despite high contact rate during promiscuous mating. By contrast, prevalence spiked when bats entered hibernation and was significantly higher than late autumn prevalence for all sampled species (figure 2g). During winter when bats were in hibernation, prevalence increased significantly for three species, M. sodalis (figure 2e), P. subflavus (figure 2f) and E. fuscus (figure 2a). For two other species, M. lucifugus and M. septentrionalis, prevalence was already nearly 100% in early hibernation and showed no change over time (figure 2c,d,g and the electronic supplementary material, table S1). In the sixth species, M. leibii, prevalence also increased significantly between autumn and late winter, but a lack of early winter samples from this relatively rare species prevented finer characterization of winter trends (figure 2b and the electronic supplementary material, table S1). During the summer, when bats in maternity colonies use torpor much less frequently and their body temperature is typically 15–20°C higher than the upper growth limit of P. destructans, prevalence of both species sampled, M. lucifugus and E. fuscus, decreased rapidly (figure 2a,c and the electronic supplementary material, table S1), and were not significantly different from zero by late summer (figure 2).
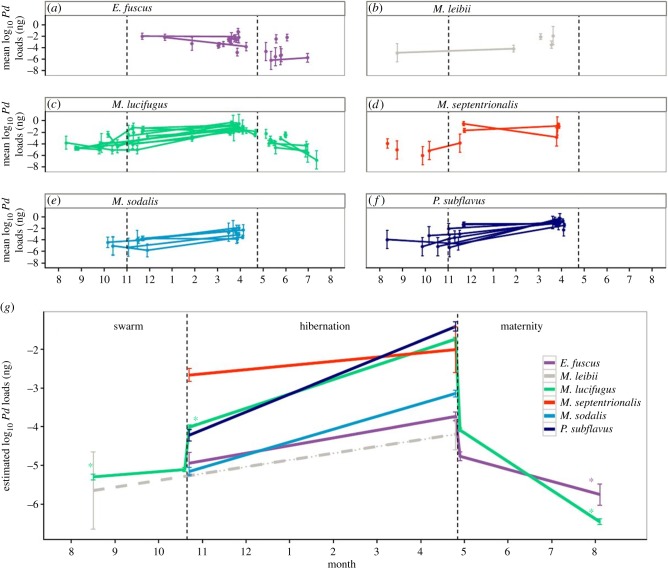
Loads of P. destructans on bats showed trends similar to prevalence patterns (figure 3). During the autumn, loads remained very low on all species. Loads increased significantly in most species during hibernation (figure 3c,e,f,g and the electronic supplementary material, table S2) and peaked on all species at the end of hibernation (figure 3). However, as soon as bats became active and migrated to maternity colonies, loads decreased substantially and fell to zero for most individuals by the end of summer (figure 3). Decreases in loads and prevalence over the summer did not parallel changes in colony size for M. lucifugus, which broadly overlapped and were not significantly different between summer and winter (figure 4; t19.21 = 0.46, p = 0.65). Colony sizes at maternity sites grew to be approximately 2.2 times larger over the summer, suggesting that most active bats were clearing the pathogen rather than dying of latent infection, and some immigration occurred between the counts.
Figure 3.
(a–f) Seasonal loads (±1 s.e.) of Pseudogymnoascus destructans for six species across all sites. Lines connect estimates from the same site. (g) Model predicted log10 P. destructans loads (± standard error of predicted mean) among species of P. destructans for six species from autumn to summer. Dashed lines indicate species that were poorly sampled across that season. Asterisks denote time points in which loads were significantly different (p < 0.0001 for all comparisons) than the previous season. Vertical dashed lines in all panels divide seasons (autumn swarm, winter hibernation, summer maternity). (Online version in colour.)
Figure 4.
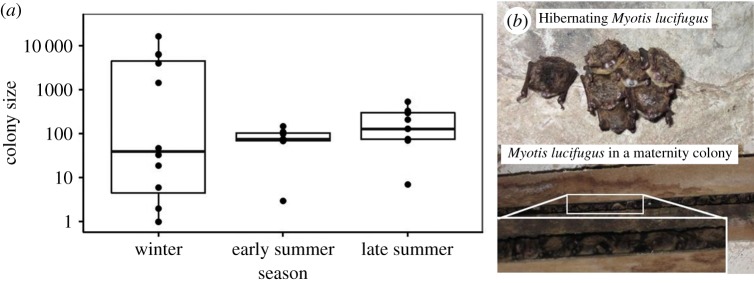
(a) Boxplot of colony sizes, on a log scale, of M. lucifugus at winter hibernacula and summer maternity colonies. (b) Photos of M. lucifugus roosting in groups in winter (top) and summer (bottom). (Online version in colour.)
4. Discussion
The seasonal patterns of prevalence and loads of P. destructans were remarkably consistent for all six bat species, with a sharp increase in prevalence between autumn and early winter when bats began to hibernate and a peak in fungal load in late winter at the end of hibernation. In contrast to many other systems [2,15,16,39], we found no support for the hypothesis that birth pulses drive seasonality. Prevalence and intensity actually decreased precipitously in summer when all species gave birth and previously uninfected young of the year join the adult population. This suggests that an influx of susceptible individuals is not driving transmission dynamics. Furthermore, for M. lucifugus, transmission was unrelated to seasonal changes in colony size. Colony sizes among seasons broadly overlapped despite directionally opposite patterns of infection prevalence, suggesting that larger winter colony sizes are not the primary driver of differences in transmission among seasons. Although winter colonies affected by WNS decline over the winter, colony sizes would have to be 100 times higher in early winter to alter the qualitative relationship between seasonal colony size and transmission. Finally, although contact with contaminated hibernacula [29,30,40] in autumn initiated infection in bats, transmission and infection intensity remained low until bats increased prolonged torpor use [33], suggesting that habitat selection is not the primary factor driving disease dynamics. Furthermore, prevalence and loads decreased during the summer, suggesting that if summer maternity colony sites are infected, the routine use of body temperatures above the growth range of P. destructans probably prevented infection or growth.
Hibernation appeared to be the dominant factor determining transmission dynamics and pathogen growth. It was only after bats began to fully hibernate during the winter that transmission increased, and shortly thereafter nearly 100% of individuals became infected at many sites. Fungal loads also increased substantially with the onset of hibernation. The rapid increase in prevalence between late autumn and early hibernation could have been caused by the large increase in loads which increased infectiousness. Contact rates during hibernation are unknown, but males are known to mate with torpid females [33], and a combination of high infectiousness and moderately high contact rates could facilitate rapid transmission. Temperatures of bats during hibernation are approximately the same as ambient temperatures of hibernacula [41], and are within the range of temperatures that the pathogen can grow [28], resulting in explosive amplification of P. destructans on hibernating bats. In summary, the seasonality of P. destructans transmission appears to be driven by host physiology, specifically a sustained decrease in body temperature.
Changes in body temperature are also important for other diseases. Hibernation has been shown to be important for another pathogen of bats (rabies; [14]). However, in rabies, hibernation allows the virus to persist in a quiescent phase, whereas for WNS, hibernation increased both transmission among bats and pathogen replication on hosts. Host body temperature is also important in driving host impacts in the fungal pathogen of amphibians [42], Batrachochytrium dendrobatidis, highlighting a similarity between these important pathogens.
The timing of P. destructans transmission and increases in infection intensity probably maximize the impact of WNS on bat populations. Infection peaks when bat populations are near their annual minima, just prior to when females give birth, thereby reducing the reproductive population (figure 1). In addition, bats rely on colonial roosts for thermoregulatory benefits to both raise young in the summer and survive winter hibernation [43,44]. As a result, mortality is occurring at a time when bats may experience positive density dependence (i.e. Allee effects), meaning survival and reproduction would decrease with decreasing colony size [45]. Finally, transmission and disease-caused mortality are absent in late summer and early autumn, when density-dependent food limitation may be strongest because bats forage intensely to obtain enough food and fat to survive over winter [46]. Thus, the seasonal timing of transmission and pathogen growth probably results in nearly maximal disease impacts of this pathogen on bat populations and contributes to exceedingly high population declines across a wide region (more than 90% in several species; [25,26]). By contrast, the timing of peak disease mortality in many other systems often coincides with reproduction. For example, transmission and avian mortality from West Nile virus peaks in late summer and autumn, just after the seasonal birth pulse [47]. In this case, disease mortality reduces density-dependent regulation before populations reach minima overwinter.
The seasonality in P. destructans infection patterns, while leading to maximum disease impacts, probably reduces the rate of spatial spread of P. destructans [48] because of a mismatch between periods of high mobility and high pathogen prevalence and load. Bats are highly mobile during the autumn when they travel among hibernacula to mate, and at the end of summer when they migrate from maternity sites to hibernacula [34,49]. However, pathogen loads and prevalence were relatively low during these periods. If infection loads and prevalence in autumn or late summer were at levels observed in winter, bats would be much more infectious, and spatial spread would probably be much faster. The high prevalence and infection load during winter make occasional movements among hibernacula during winter (either in winter or early spring) [49] potentially important in pathogen spread.
Although bats travel substantial distances from hibernacula to summer maternity sites [49,50], this is unlikely to facilitate spatial spread among hibernacula. While the high fungal loads and nearly 100% prevalence on bats at the end of winter facilitates rapid spread to their summer maternity sites, the high body temperatures and hot maternity roosts bats use during summer are too high for pathogen growth [28] and lead to bats clearing P. destructans infection from their skin. The combination of the seasonality of infection and the hot environments used by bats during the summer has probably slowed the geographical spread of P. destructans compared with pathogens where transmission peaks at the same time when animals are dispersing or migrating, such as West Nile virus [51] and avian influenza [4].
The seasonal patterns of transmission we have documented can be used to more effectively guide management interventions. When bats first become infected with P. destructans, loads, and therefore tissue invasion and damage, are relatively low. Therefore, applying treatments that reduce or clear infection during the autumn and early winter would be most effective for reducing transmission, impacts and spread to new sites. However, if treatments offer only short-term protection, our data suggest that treated bats will probably be rapidly re-infected upon return to natural environments owing to exceedingly high infection prevalence in other hosts. Our results also suggest that another management strategy, culling bats to remove infected individuals, would be ineffective during the winter, because nearly 100% of individuals are already infected by early hibernation. Finally, while rearing temperate bats in captivity is exceedingly challenging [52], if this strategy were attempted, capturing bats during late summer would maximize the fraction of uninfected bats that could be brought into captivity.
In the 7 years since the detection of WNS, bat populations have crashed to a small fraction of their former size [26], with several species at risk of extinction [25]. Our findings illustrate how the seasonality of transmission and infection intensity drives the impact of this deadly disease. Commonality across host physiology, specifically, hibernation for extended periods at temperatures that allow growth of the pathogen, has created a perfect storm, and led to the deaths of millions of individuals of multiple species. More broadly, our results demonstrate the importance of seasonal timing of infection in driving impacts and spread of emerging pathogens, and show how understanding seasonal patterns of transmission can provide critical information for mitigating the devastating impacts of wildlife disease.
Acknowledgements
We thank the numerous personnel that have contributed to collecting data for this work, in particular Wil Orndorff, Karen Powers, Ryan von Linden, Ashley Banks, Elizabeth Braun de Torrez, Joy Collins and Amanda Hunt. We also thank the numerous land owners who permitted access to these sites, and the state agencies that assisted in coordination (NY, VA, IL, NH, VT and MA.) We thank Maya McCrea for contributed artwork. We thank Bruce Lyon, Stephan Munch and two semi-anonymous reviewers for helpful comments on the manuscript.
Ethics statement
All research was conducted under protocol number 11-022 approved by the IACUC of Boston University.
Data accessibility
Data are deposited in Dryad Digital Repository [53]. Exact site locations are not disclosed to protect endangered species and landowners. Those interested in collaborations using these data should email corresponding author (klangwig@gmail.com) for additional information.
Funding statement
This work was supported by the National Science Foundation (DGE-0741448 to K.E.L., DEB-1115895 to T.H.K., W.F.F., J.T.F. and A.M.K.), Bat Conservation International and the National Geographic Society.
References
- 1.Altizer S, Bartel R, Han BA. 2011. Animal migration and infectious disease risk. Science 331, 296–302. ( 10.1126/science.1194694) [DOI] [PubMed] [Google Scholar]
- 2.Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. 2006. Seasonality and the dynamics of infectious diseases. Ecol. Lett. 9, 467–484. ( 10.1111/j.1461-0248.2005.00879.x) [DOI] [PubMed] [Google Scholar]
- 3.Hochachka WM, Dhondt AA. 2000. Density-dependent decline of host abundance resulting from a new infectious disease. Proc. Natl Acad. Sci. USA 97, 5303–5306. ( 10.1073/pnas.080551197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilpatrick AM, Chmura AA, Gibbons DW, Fleischer RC, Marra PP, Daszak P. 2006. Predicting the global spread of H5N1 avian influenza. Proc. Natl Acad. Sci. USA 103, 19 368–19 373. ( 10.1073/pnas.0609227103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altizer S, Hochachka WM, Dhondt AA. 2004. Seasonal dynamics of mycoplasmal conjunctivitis in eastern North American house finches. J. Anim. Ecol. 73, 309–322. ( 10.1111/j.0021-8790.2004.00807.x) [DOI] [Google Scholar]
- 6.Rappole JH, Derrickson SR, Hubalek Z. 2000. Migratory birds and spread of West Nile virus in the Western Hemisphere. Emerg. Infect. Dis. 6, 319–328. ( 10.3201/eid0604.000401) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooley HS, Wielgus RB, Koehler GM, Robinson HS, Maletzke BT. 2009. Does hunting regulate cougar populations? A test of the compensatory mortality hypothesis. Ecology 90, 2913–2921. ( 10.1890/08-1805.1) [DOI] [PubMed] [Google Scholar]
- 8.Singer FJ, Harting A, Symonds KK, Coughenour MB. 1997. Density dependence, compensation, and environmental effects on elk calf mortality in Yellowstone National Park. J. Wildl. Manage. 61, 12–25. ( 10.2307/3802410) [DOI] [Google Scholar]
- 9.Kistner EJ, Belovsky GE. 2014. Host dynamics determine responses to disease: additive vs. compensatory mortality in a grasshopper–pathogen system. Ecology 95, 2579–2588. ( 10.1890/13-0969.1) [DOI] [Google Scholar]
- 10.Bjornstad ON, Finkenstadt BF, Grenfell BT. 2002. Dynamics of measles epidemics: estimating scaling of transmission rates using a time series SIR model. Ecol. Monogr. 72, 169–184. ( 10.2307/3100023) [DOI] [Google Scholar]
- 11.Dhondt AA, States SL, Dhondt KV, Schat KA. 2012. Understanding the origin of seasonal epidemics of mycoplasmal conjunctivitis. J. Anim. Ecol. 81, 996–1003. ( 10.1111/j.1365-2656.2012.01986.x) [DOI] [PubMed] [Google Scholar]
- 12.Nunn CL, Altizer S. 2006. Infectious diseases in primates: behavior, ecology, and evolution. New York, NY: Oxford University Press. [Google Scholar]
- 13.Hamede RK, Bashford J, McCallum H, Jones M. 2009. Contact networks in a wild Tasmanian devil (Sarcophilus harrisii) population: using social network analysis to reveal seasonal variability in social behaviour and its implications for transmission of devil facial tumour disease. Ecol. Lett. 12, 1147–1157. ( 10.1111/j.1461-0248.2009.01370.x) [DOI] [PubMed] [Google Scholar]
- 14.George DB, Webb CT, Farnsworth ML, O'Shea TJ, Bowen RA, Smith DL, Stanley TR, Ellison LE, Rupprecht CE. 2011. Host and viral ecology determine bat rabies seasonality and maintenance. Proc. Natl Acad. Sci. USA 108, 10 208–10 213. ( 10.1073/pnas.1010875108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosseini PR, Dhondt AA, Dobson A. 2004. Seasonality and wildlife disease: how seasonal birth, aggregation and variation in immunity affect the dynamics of Mycoplasma gallisepticum in house finches. Proc. R. Soc. Lond. B 271, 2569–2577. ( 10.1098/rspb.2004.2938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Bakker M, Bakker KM, King AA, Rohani P. 2014. Human birth seasonality: latitudinal gradient and interplay with childhood disease dynamics. Proc. R. Soc. B 281, 20132438 ( 10.1098/rspb.2013.2438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breban R, Drake JM, Stallknecht DE, Rohani P. 2009. The role of environmental transmission in recurrent avian influenza epidemics. PLoS Comput. Biol. 5, e1000346 ( 10.1371/journal.pcbi.1000346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohani P, Breban R, Stallknecht DE, Drake JM. 2009. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc. Natl Acad. Sci. USA 106, 10 365–10 369. ( 10.1073/pnas.0809026106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaman J, Pitzer VE, Viboud C, Grenfell BT, Lipsitch M. 2010. Absolute humidity and the seasonal onset of influenza in the continental United States. PLoS Biol. 8, e1000316 ( 10.1371/journal.pbio.1000316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillgarth N, Ramenofsky M, Wingfield J. 1997. Testosterone and sexual selection. Behav. Ecol. 8, 108–109. ( 10.1093/beheco/8.1.108) [DOI] [Google Scholar]
- 21.Mougeot F, Irvine JR, Seivwright L, Redpath SM, Piertney S. 2004. Testosterone, immunocompetence, and honest sexual signaling in male red grouse. Behav. Ecol. 15, 930–937. ( 10.1093/beheco/arh087) [DOI] [Google Scholar]
- 22.Warnecke L, Turner JM, Bollinger TK, Lorch JM, Misra V, Cryan PM, Wibbelt G, Blehert DS, Willis CKR. 2012. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Natl Acad. Sci. USA 109, 6999–7003. ( 10.1073/pnas.1200374109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorch JM, et al. 2011. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature 480, 376–378. ( 10.1038/nature10590) [DOI] [PubMed] [Google Scholar]
- 24.Blehert DS, et al. 2008. Bat white-nose syndrome: an emerging fungal pathogen? Science 323, 227 ( 10.1126/science.1163874) [DOI] [PubMed] [Google Scholar]
- 25.Langwig KE, Frick WF, Bried JT, Hicks AC, Kunz TH, Kilpatrick AM. 2012. Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white-nose syndrome. Ecol. Lett. 15, 1050–1057. ( 10.1111/j.1461-0248.2012.01829.x) [DOI] [PubMed] [Google Scholar]
- 26.Frick WF, Pollock JF, Hicks AC, Langwig KE, Reynolds DS, Turner GG, Butchkoski CM, Kunz TH. 2010. An emerging disease causes regional population collapse of a common North American bat species. Science 329, 679–682. ( 10.1126/science.1188594) [DOI] [PubMed] [Google Scholar]
- 27.Warnecke L, Turner JM, Bollinger TK, Misra V, Cryan PM, Blehert DS, Wibbelt G, Willis CKR. 2013. Pathophysiology of white-nose syndrome in bats: a mechanistic model linking wing damage to mortality. Biol. Lett. 9, 20130177 ( 10.1098/rsbl.2013.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verant ML, Boyles JG, Waldrep W, Wibbelt G, Blehert DS. 2012. Temperature-dependent growth of Geomyces destructans, the fungus that causes bat white-nose syndrome. PLoS ONE 7, e46280 ( 10.1371/journal.pone.0046280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorch JM, Lindner DL, Gargas A, Muller LK, Minnis AM, Blehert DS. 2013. A culture-based survey of fungi in soil from bat hibernacula in the eastern United States and its implications for detection of Geomyces destructans, the causal agent of bat white-nose syndrome. Mycologia 105, 237–252. ( 10.3852/12-207) [DOI] [PubMed] [Google Scholar]
- 30.Lorch JM, Muller LK, Russell RE, O'Connor M, Lindner DL, Blehert DS. 2013. Distribution and environmental persistence of the causative agent of white-nose syndrome, Geomyces destructans, in bat hibernacula of the eastern United States. Appl. Environ. Microbiol. 79, 1293–1301. ( 10.1128/aem.02939-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoyt J, Langwig K, Okoniewski J, Frick W, Stone W, Kilpatrick AM. In press. Long-term persistence of Pseudogymnoascus destructans, the causative agent of white-nose syndrome, in the absence of bats. EcoHealth ( 10.1007/s10393-014-0981-4) [DOI] [PubMed] [Google Scholar]
- 32.Barbour R, Davis W. 1969. Bats of America. Lexington, KY: The University Press of Kentucky. [Google Scholar]
- 33.Thomas DW, Fenton MB, Barclay RMR. 1979. Social behavior of the little brown bat, Myotis lucifugus. I. Mating behavior. Behav. Ecol. Sociobiol. 6, 129–136. ( 10.1007/BF00292559) [DOI] [Google Scholar]
- 34.Kunz TH, Lumsden L. 2003. Colony size. In Bat ecology (eds TH Kunz, MB Fenton), pp. 49–56. Chicago, IL: University of Chicago Press. [Google Scholar]
- 35.Shelley V, Kaiser S, Shelley E, Williams T, Kramer M, Haman K, Keel K, Barton HA. 2013. Evaluation of strategies for the decontamination of equipment for Geomyces destructans, the causative agent of white-nose snydrome (WNS). J. Cave Karst Stud. 75, 1–10. ( 10.4311/2011lsc0249) [DOI] [Google Scholar]
- 36.Muller LK, Lorch JM, Lindner DL, O'Connor M, Gargas A, Blehert DS. 2013. Bat white-nose syndrome: a real-time TaqMan polymerase chain reaction test targeting the intergenic spacer region of Geomyces destructans. Mycologia 105, 253–259. ( 10.3852/12-242) [DOI] [PubMed] [Google Scholar]
- 37.Bates D, Maechler M, Bolker B.2011. lme4: linear mixed-effects models using S4 classes. R package version 0999375-42 http://CRAN.R-project.org/package=lme4 .
- 38.R Development Core Team 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Pathak AK, Boag B, Poss M, Harvill ET, Cattadori IM. 2011. Seasonal breeding drives the incidence of a chronic bacterial infection in a free-living herbivore population. Epidemiol. Infect. 139, 1210–1219. ( 10.1017/s0950268810002311) [DOI] [PubMed] [Google Scholar]
- 40.Lindner DL, Gargas A, Lorch JM, Banik MT, Glaeser J, Kunz TH, Blehert DS. 2011. DNA-based detection of the fungal pathogen Geomyces destructans in soils from bat hibernacula. Mycologia 103, 241–246. ( 10.3852/10-262) [DOI] [PubMed] [Google Scholar]
- 41.Thomas D. 1995. The physiological ecology of hibernation in vespertilionid bats. Symp. Zool. Soc. Lond. 67, 233–244. [Google Scholar]
- 42.Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, Rohr JR. 2013. Disease and thermal acclimation in a more variable and unpredictable climate. Nat. Clim. Change 3, 146–151. ( 10.1038/nclimate1659) [DOI] [Google Scholar]
- 43.Altringham JD. 2011. Bats: from evolution to conservation, 2nd edn New York, NY: Oxford University Press. [Google Scholar]
- 44.Boyles J, Storm J, Brack V. 2008. Thermal benefits of clustering during hibernation: a field test of competing hypotheses on Myotis sodalis. Funct. Ecol. 22, 632–636. ( 10.1111/j.1365-2435.2008.01423.x) [DOI] [Google Scholar]
- 45.Courchamp F, Clutton-Brock T, Grenfell B. 1999. Inverse density dependence and the Allee effect. Trends Ecol. Evol. 14, 405–410. ( 10.1016/S0169-5347(99)01683-3) [DOI] [PubMed] [Google Scholar]
- 46.Kunz TH, Wrazen JA, Burnett CD. 1998. Changes in body mass and fat reserves in pre-hibernating little brown bats (Myotis lucifugus). Ecoscience 5, 8–17. [Google Scholar]
- 47.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. 2006. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 4, 606–610. ( 10.1371/journal.pbio.0040082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maher SP, Kramer AM, Pulliam JT, Zokan MA, Bowden SE, Barton HD, Magori K, Drake JM. 2012. Spread of white-nose syndrome on a network regulated by geography and climate. Nat. Commun. 3, 1306 ( 10.1038/ncomms2301) [DOI] [PubMed] [Google Scholar]
- 49.Norquay KJO, Martinez-Nunez F, Dubois JE, Monson KM, Willis CKR. 2013. Long-distance movements of little brown bats (Myotis lucifugus). J. Mammal 94, 506–515. ( 10.1644/12-mamm-a-065.1) [DOI] [Google Scholar]
- 50.Matheson AL, Campbell KL, Willis CKR. 2010. Feasting, fasting and freezing: energetic effects of meal size and temperature on torpor expression by little brown bats Myotis lucifugus. J. Exp. Biol. 213, 2165–2173. ( 10.1242/jeb.040188) [DOI] [PubMed] [Google Scholar]
- 51.Kilpatrick AM, LaDeau SL, Marra PP. 2007. Ecology of West Nile virus transmission and its impact on birds in the Western Hemisphere. Auk 124, 1121–1136. ( 10.1642/0004-8038(2007)124[1121:eownvt]2.0.co;2) [DOI] [Google Scholar]
- 52.Racey PA. 1970. The breeding, care and management of vespertilionid bats in the laboratory. Lab Anim. 4, 171–183. ( 10.1258/002367770781071635) [DOI] [PubMed] [Google Scholar]
- 53.Langwig KE, et al. 2015. Hibernation drives seasonal dynamics of a fungal disease. Data from: Dryad Digital Repository. ( 10.5061/dryad.k4h77) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are deposited in Dryad Digital Repository [53]. Exact site locations are not disclosed to protect endangered species and landowners. Those interested in collaborations using these data should email corresponding author (klangwig@gmail.com) for additional information.