Abstract
Size-structured predator–prey interactions can be altered by the history of exploitation, if that exploitation is itself size-selective. For example, selective harvesting of larger sized predators can release prey populations in cases where only large individuals are capable of consuming a particular prey species. In this study, we examined how the history of exploitation and recovery (inside marine reserves and due to fisheries management) of California sheephead (Semicossyphus pulcher) has affected size-structured interactions with sea urchin prey in southern California. We show that fishing changes size structure by reducing sizes and alters life histories of sheephead, while management measures that lessen or remove fishing impacts (e.g. marine reserves, effort restrictions) reverse these effects and result in increases in density, size and biomass. We show that predation on sea urchins is size-dependent, such that the diet of larger sheephead is composed of more and larger sized urchins than the diet of smaller fish. These results have implications for kelp forest resilience, because urchins can overgraze kelp in the absence of top-down control. From surveys in a network of marine reserves, we report negative relationships between the abundance of sheephead and urchins and the abundance of urchins and fleshy macroalgae (including giant kelp), indicating the potential for cascading indirect positive effects of top predators on the abundance of primary producers. Management measures such as increased minimum size limits and marine reserves may serve to restore historical trophic roles of key predators and thereby enhance the resilience of marine ecosystems.
Keywords: California sheephead, kelp forest resilience, marine protected areas, recovery from fishing, Semicossyphus pulcher, size-structured trophic interactions
1. Introduction
The development of ecological theory about size-structured interactions has greatly advanced our understanding of population and community dynamics [1], in addition to providing a framework to address wildlife conservation problems. Size-structured interactions are a common element of many ecological systems as the feeding preferences and capabilities of predators with broad diets often change with size and age [i.e. ontogenetic diet shifts]. In aquatic systems, predation by fishes is generally size-dependent [1–4] and vulnerability of a prey to a predator often depends on the gape size or other consumption limits of the predator, along with patterns of prey selection [5,6]. In addition, predator size structure may vary across space and through time in response to variability in recruitment, environmental conditions, resource availability, predation and the history of exploitation [7,8]. Therefore, the ecological role of key predators and the strength of predator–prey interactions may change as a function of predator size structure. A failure to account for size-dependent shifts in trophic interactions could lead to erroneous predictions about the response of species assemblages to management intervention.
The history of exploitation of top-predators has been shown to alter food webs and ecosystem function through direct and indirect pathways in terrestrial and aquatic systems [9]. Fishing, like many types of harvest, but unlike natural mortality, is often size-selective for the largest individuals. In addition to reducing abundance, fishing can reduce average and maximum body sizes [7], alter life-history and population demographic traits such as age and size at maturity [8,10,11] and change predator–prey interactions [12–14]. With recovery from overfishing via protection inside no-take marine reserves, both abundance and size of individuals of fished species predictably increase (reviewed by Lester et al. [15]), and this may directly or indirectly affect associated communities [16]. Typically, researchers have examined how increasing predator abundance or total biomass inside marine protected areas can result in increased levels of prey mortality, with cascading effects on lower trophic levels [17–19]. What is often overlooked is that management actions resulting in changes in size structure, independent of changes in abundance, may have profound impacts on prey populations if predator–prey interactions are size-dependent [19,20]. For example, reductions in predator body size in response to fishing, even in the absence of changes in aggregate predator biomass, can lead to shifts in the trophic structure of whole ecosystems [21]. Given that changes in size structure of fished species are a common response to protection from fishing [15], indirect effects on prey species through size-structured interactions may be more important than previously thought.
California sheephead (Semicossyphus pulcher) are generalist carnivores that occur on rocky reefs and in kelp beds from southern California to Baja California, Mexico. They can be important consumers in regulating populations of sea urchins and other invertebrate prey in some locations [22,23], leading to the suggestion that these fish (along with spiny lobsters) are critical for maintaining kelp forest ecosystem health by suppressing urchin grazing [24]. Commercial and recreational fisheries target sheephead and exploitation increased dramatically from the 1980s through the early 2000s [25]. Previously, we showed that intense size-selective fishing reduced fish size, timing of maturation and timing of sex change of sheephead [8]. In addition, predator–prey interactions are size-dependent as sheephead diets change ontogenetically [26].
Here, we expand on these findings by presenting patterns of life-history and diet variation from locations differing in their history of sheephead exploitation. We then assess changes in the abundance of kelp, urchins and sheephead inside and outside of a network of marine reserves and estimate direct and indirect effects in a community context. We ask whether the history of exploitation leads to location-dependent and ontogenetic shifts in the ecological role of sheephead in response to size-dependent changes in predator–prey interactions, and whether recovery of the size structure of these urchin predators inside marine reserves is associated with changes in kelp and urchin abundance after a decade of protection.
2. Material and methods
(a). Study sites and collections to assess temporal changes in response to fishing
To examine the response of California sheephead populations to fishing pressure, we compared changes in size structure and life-history traits for populations on San Nicolas and Catalina Islands over multiple decades (electronic supplementary material, figure S1). Historic samples from relatively unfished populations were reported from Catalina [27] and San Nicolas [28]. More recent samples were collected in 1998 [8] and again in 2007 by the authors. Individual sheephead were collected by spear on SCUBA [as in 27,28]. We recorded morphometrics (e.g. total length (TL), weight) and determined sex macroscopically by observing the colour, texture and appearance of the gonads [as in 28]. We prepared thin sections of dorsal spines for age analysis following methods described in [8].
To examine differences in the predicted maximum size of individuals in each population in response to fishing, we fit von Bertalanffy growth functions to the size at age data using least-squares techniques according to Hamilton et al. [29]. The size or age at maturity was defined as the size or age at which females began to predominate over immatures in the population (i.e. length or age at 50% mature female). Comparably, the size or age at sex change was defined as the size or age at which males began to predominate over mature females in the population (i.e. length or age at 50% male). Survivorship of mature fish from each population was calculated as reported in [8].
Fisheries landings data were provided by the California Department of Fish and Wildlife (CDFW) for the commercial and recreational fishing sectors. Total landings were available from 1915 for the commercial fishery and 1947 for the recreational fishery ([25]; electronic supplementary material, figure S2). Spatial and temporal variation in landings by CDFW fishing block (10 × 10 nautical miles) were available from 1993 to 2007 [30]. For our study locations, the commercial live-fish fishery predominates on San Nicolas Island, while the recreational fishery is responsible for the bulk of landings on Catalina Island. Therefore, to investigate how fishing pressure may explain changes in size structure and life-history traits, we examined temporal trends in landings for the commercial fishing blocks on San Nicolas Island and the recreational fishing blocks on Catalina Island.
(b). Marine reserve responses and kelp forest community surveys
We have conducted community surveys in nearshore kelp beds and rocky reefs (depths < 20 m) in the northern Channel Islands since 1999 (see fig. 1 in [31]). On each of five islands, surveys were conducted annually inside and outside of marine reserves established in 2003 [31]. At each site, we survey 8–12 fish transects that are 30 × 2 × 2 m at multiple levels in the water column: benthic, midwater and kelp canopy (when present). Transects are laid out in a stratified random design, with multiple non-permanent transects located in fixed strata (i.e. outer, middle and inner edges of the reef). At each level in the water column, one diver counts and sizes all fish to the nearest centimetre (TL). In addition, we survey four to six benthic transects (also 30 × 2 m swaths) at each site to characterize community structure of invertebrates and macroalgae. For giant kelp (Macrocystis pyrifera), we enumerate the number of individual kelp plants and the number of stipes per plant to estimate stipe density. For sea urchins (red sea urchin, Mesocentrotus franciscanus and purple sea urchin, Stronglyocentrotus purpuratus), we count adult urchins greater than 2.5 cm in test diameter. Red sea urchins are typically larger than purple sea urchins and are targeted by a commercial fishery, predominately in the western islands. The per cent cover of the benthos was estimated using uniform point contact methodology at 30 points along each transect, by identifying the organism (e.g. macroalgae, sessile invertebrates) or non-living category underneath each point. We focused our analyses on responses of taxa categorized as fleshy understory macroalgae or crustose coralline algae (CCA).
To examine differences in abundance, size and biomass of sheephead inside and outside reserves, we calculated annual site means from 2003 to 2012. We used length–weight relationships (W = 0.0144 × TL3.04, TL in centimetres, n = 499, r2 = 0.98, p < 0.0001) to estimate observed weights and used those values and the density information to estimate biomass (metric tons per hectare). We also calculated densities of legal-sized sheephead as the number of individuals that were larger than the minimum size limit of 30 cm TL. We examined evidence for differences in the abundance of giant kelp and sea urchins inside and outside of reserves by using the density data from benthic transects. We compared density, size and/or biomass between reserve and non-reserve sites on each island separately using the site-level means as replicates. Reserve responses were tested with ANOVA using the factors of Reserve status, Island and their interaction.
To examine the role of sheephead in regulating sea urchin populations and indirectly influencing giant kelp, fleshy understory algae and CCA, we examined relationships between grazer (sea urchin) and primary producer abundance, as well as relationships between predator biomass (California sheephead) and prey abundance (sea urchin). Best-fit relationships were determined using linear and nonlinear least-squares regression. We compared linear and exponential models using model selection techniques with corrected Aikake's information criteria, following [32]. Statistical significance was determined in cases where the difference in AICc values (ΔAICc) was greater than or equal to 2. We used path analysis to evaluate the strength of direct and indirect interactions between sheephead, sea urchins and benthic algal functional groups. We calculated standardized correlation coefficients for the hypothesized direct and indirect interactions following methods in [33].
(c). Ontogenetic changes in trophic role
In 2007–2008, we collected sheephead from nine distinct populations in southern California ([26]; electronic supplementary material, figure S1). Stomach contents were weighed to the nearest 0.1 g and stored in 10% buffered formalin. To assess the proportional contribution of different prey items to the diet of sheephead, we sorted the gut contents into 26 prey classes, following [23]. Where sea urchin spines were intact, we measured the length and diameter of the largest spine as a proxy for sea urchin test diameter [34]. We assessed ontogenetic variation in the importance of sea urchins in the diet by testing for changes in the per cent volume of the gut contents comprised urchins as a function of sheephead size. A regression of sea urchin spine length as a function of sheephead size was also performed on the subsample of individuals that had intact spines in their guts.
3. Results
(a). Effects of fishing on California sheephead populations
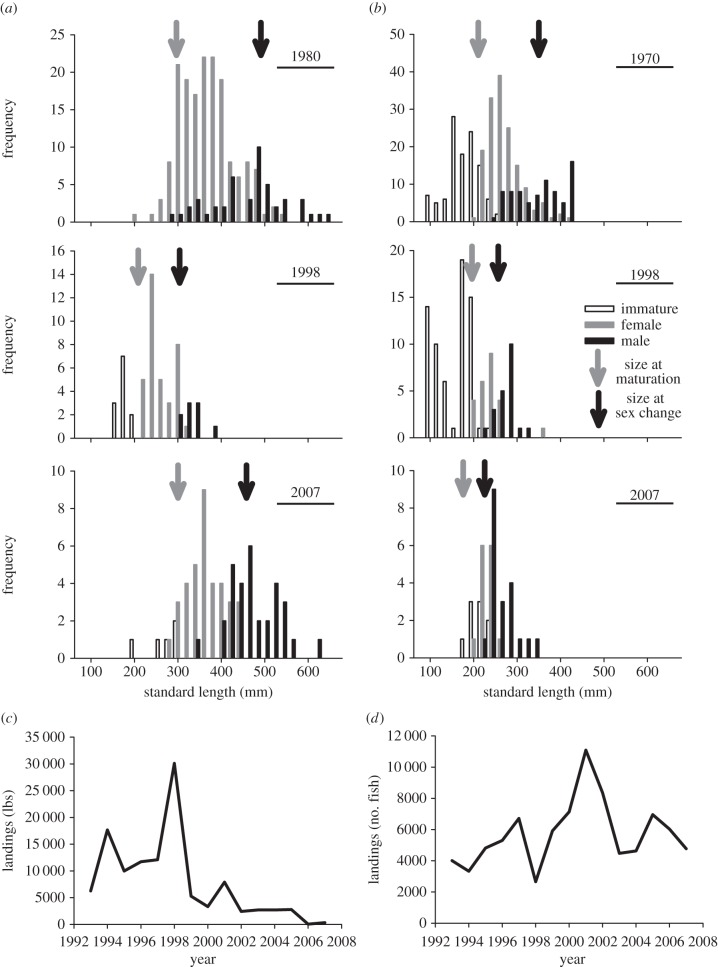
San Nicolas Island is remote (greater than 100 km offshore of Los Angeles), is exposed and had remained relatively unfished by commercial and recreational fishing until the expansion of the commercial live-fish fishery in the 1990s (electronic supplementary material, figure S2; figure 1c). Correspondingly, size frequency distributions of male and female sheephead were severely truncated between the early 1980s and the peak year of commercial fishing in 1998 (figure 1a). With increased harvest and a subsequent decline in annual survivorship, this population experienced a significant decrease in size, maturation and sex change, while sex ratios became more female biased (electronic supplementary material, table S1). However, following the implementation of fishing regulations in the late 1990s (e.g. size limits, limited entry, total allowable catch) and other economic factors, commercial landings decreased sharply (figure 1c). In response to a reduction in fishing pressure over a 10 year period, mean size and survivorship of sheephead increased and sex ratios became more even (electronic supplementary material, table S1; figure 1a). In addition, the size at maturation and the size and age at sex change increased (though not quite to pre-fishing levels), as well as the predicted maximum size (electronic supplementary material, table S1; figure 1a).
Figure 1.
Changes in California sheephead size structure and fishing pressure at San Nicolas and Catalina Islands. Top panels show shifts in size frequency distributions (SL, mm) of immature, female and male individuals at (a) San Nicolas and (b) Catalina from 1970 or 1980, 1998 and 2007. Arrows depict the size at maturation (50% probability that fish are mature female; grey arrows) and size at sex change (50% probability that fish are male; black arrows) calculated based on logistic regression. No data on immature size frequencies were available from San Nicolas in 1980. Bottom panels show (c) commercial and (d) recreational landings of California sheephead from 1993 to 2007 from fishing blocks around San Nicolas and Catalina, respectively. Note that commercial take is reported in pounds landed, whereas recreational take is reported as the number of fish landed on commercial passenger fishing vessels.
Santa Catalina Island is one of the few populated Channel Islands and its close proximity to Los Angeles results in this island experiencing the greatest take of sheephead by the recreational fishing sector, including spearfishing and charter boats [30]. Recreational landings increased in southern California, since the 1970s (electronic supplementary material, figure S2) and have remained high at Catalina (figure 1d). In response to fishing, this population experienced a decrease in annual survivorship and a truncation of the size frequency distribution from 1970 and 1998 (electronic supplementary material, table S1; figure 1b). Size-selective take of larger individuals resulted in a significant decline in the timing of sex change and a reduction in the predicted asymptotic size (electronic supplementary material, table S1). Sex ratios became even as annual survivorship declined. From 1998 to 2007 recreational take of sheephead on Catalina remained high (figure 1d), despite additional fishing regulations including a minimum size limit (30 cm TL) and a daily bag limit (five fish) [25]. As a result, the population continued to be dominated by small individuals (figure 1b). In 2007, the size at sex change and predicted maximum size continued to decline and the sex ratio became biased in favour of males (electronic supplementary material, table S1). By 2007, only male fish were above the minimum size limit for the fishery.
(b). Response of California sheephead to marine reserves in the northern Channel Islands
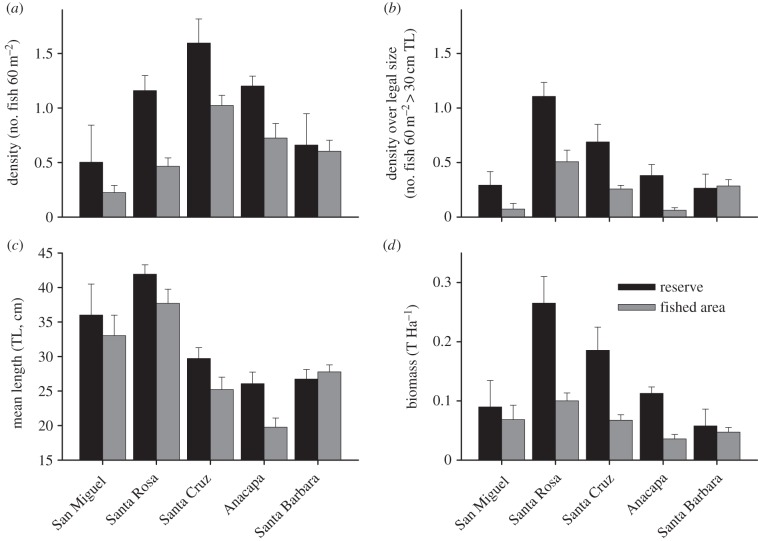
A network of marine reserves was implemented in the northern Channel Islands in 2003. Ten years after implementation, densities of sheephead were significantly greater inside reserves on all five islands (figure 2a), despite spatial differences in absolute abundance among islands (ANOVA: Reserve, F1,82 = 14.2, p = 0.0003; Island, F4,82 = 9.4, p < 0.0001; Reserve × Island, F4,82 = 1.2, p = 0.32). Density differences between reserve and non-reserve areas were more pronounced when considering only legal-sized individuals (figure 2b), as would be expected since fishing removes fish greater than legal size (ANOVA: Reserve, F1,82 = 13.47, p = 0.0005; Island, F4,82 = 8.66, p < 0.0001; Reserve × Island, F4,82 = 2.03, p = 0.10). On average, sheephead are significantly larger inside marine reserves and fish are larger on average at the western Islands of San Miguel and Santa Rosa, which are bathed by cooler, more productive waters (ANOVA: Reserve, F1,82 = 4.75, p = 0.033; Island, F4,82 = 18.74, p < 0.0001; Reserve × Island, F4,82 = 0.93, p = 0.45). As a result of the consistently higher density and larger size of sheephead inside reserves, fish biomass was also significantly greater in reserve than non-reserve areas and exhibited even greater differences, based on reserve status, than the density effects alone (ANOVA: Reserve, F1,82 = 19.6, p < 0.0001; Island, F4,82 = 9.1, p < 0.0001; Reserve × Island, F4,82 = 3.0, p = 0.023). We did find a significant but non-crossing interaction term, indicating that sheephead biomass increased more inside reserves on some islands.
Figure 2.
Responses of California sheephead to marine reserves in the northern Channel Islands. Shown are differences in (a) density, (b) density of legal-sized individuals (c) average size and (d) biomass of sheephead in no-take marine reserves and areas open to fishing on five islands. Values represent means ± 1 s.e. from underwater visual surveys.
(c). The role of California sheephead as sea urchin predators in kelp forest ecosystems
Sheephead are generalist invertebrate predators and the importance of urchins in their diet varies geographically and with size. The mean per cent volume of urchins in the diet varied from 8 to 35% across nine sampling locations (electronic supplementary material, figure S1). The importance of sea urchins in the diet of sheephead also varied ontogenetically. The average proportion of the gut contents composed of urchins increased significantly with sheephead size (r2 = 0.35, p < 0.0001; figure 3a), from a cross-site average of 2.8% of the gut volume for fish less than 200 mm standard length (SL) up to 38.4% for fish greater than 450 mm SL. The pattern of increasing sea urchin dominance in the gut contents of larger sheephead was consistent (non-significant interaction term) across the nine sampling locations (ANCOVA: model r2 = 0.60; Size, F1,45 = 14.5, p = 0.0007; Location, F8,45 = 1.9, p = 0.09; Size × Location, F8,45 = 0.4, p = 0.89). Larger sheephead also consume larger sea urchins (r2 = 0.39, p < 0.0001; figure 3b), when using urchin spine length as a proxy for test diameter. On Anacapa and Santa Cruz Islands, we collected a subsample of sheephead from inside marine reserves. On average the fish inside reserves were larger and the per cent volume of sea urchins in the guts was also higher than that from fish from outside reserves on the same island (Anacapa: reserve, 38.9 ± 15.6%, n = 7; non-reserve, 28.9 ± 3.5%, n = 37; Santa Cruz: reserve, 27.9 ± 7.16%, n = 19; non-reserve, 17.8 ± 3.4%, n = 51).
Figure 3.
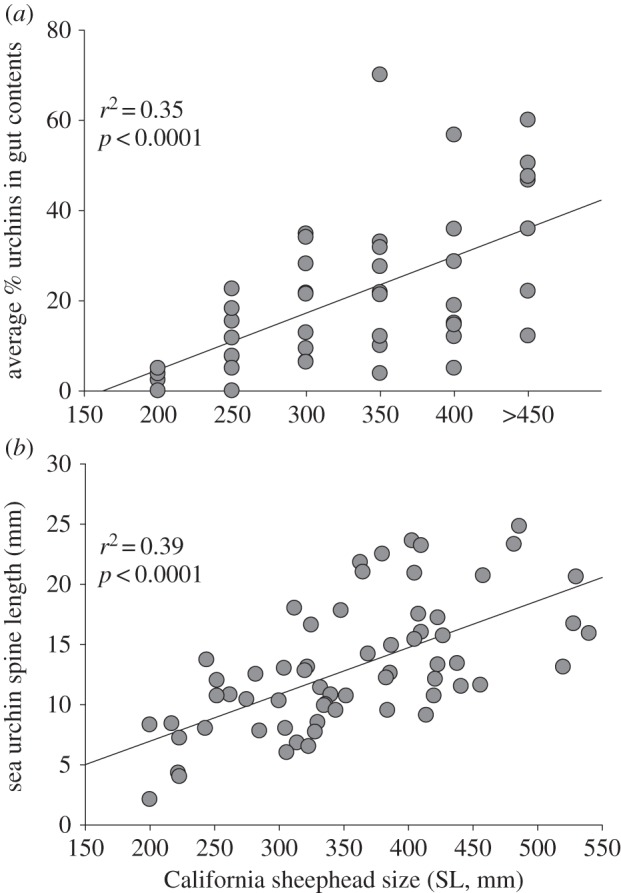
Predation impacts on sea urchins as a function of California sheephead size. (a) Plot depicts a positive relationship between fish length and the average per cent volume of gut contents containing sea urchins. Points represent the average values in 50 cm size classes (SL, mm) across nine populations. (b) Relationship between California sheephead length and the length of intact sea urchin spines found in the gut contents (i.e. as a proxy for sea urchin size). Larger California sheephead are capable of consuming larger sea urchins.
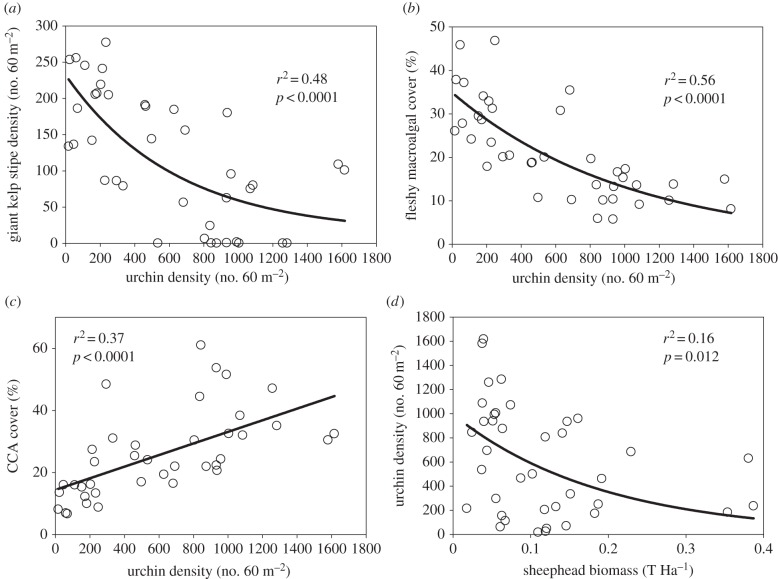
Sheephead and sea urchins are associated with changes in the community composition of the benthos (figure 4; electronic supplementary material, table S2). SCUBA surveys from the northern Channel Islands show a nonlinear negative relationship between the abundance of urchins and the abundance of giant kelp (r2 = 0.48, p < 0.0001; figure 4a). Where sea urchins are relatively rare, giant kelp thrives, however where sea urchins are overabundant, giant kelp is less common or non-existent (with some sites characterized as urchin barrens). Sea urchin densities are also negatively associated with the per cent cover of fleshy macroalgae (r2 = 0.56, p < 0.0001; figure 4b), such that locations with more urchins are characterized by having less red and brown understory algae. By contrast, sea urchin densities are positively associated with the per cent cover of CCA (r2 = 0.37, p < 0.0001; figure 4c). Sheephead biomass and sea urchin densities follow a negative nonlinear relationship (r2 = 0.16, p = 0.012; figure 4d). Sea urchins can occur at both high and low densities where sheephead are rare, but only occur at low densities in locations where sheephead biomass is high. Path analysis evaluating the strength of direct and indirect interactions between benthic macroalgae, urchins and sheephead illustrate the potential importance of both predation/grazing and space competition in influencing community composition in the Channel Islands (figure 5). The model indicates that urchins have strong direct negative effects on kelp densities and the per cent cover of fleshy macroalgae, resulting in an indirect positive effect on CCA cover. The indirect effect may be realized through space competition between kelp and fleshy algae and CCA. By contrast, sheephead have an indirect positive effect on kelp densities and fleshy algal cover, probably mediated through consumptive effects on urchins (figure 5).
Figure 4.
Relationships between sheephead (predators), sea urchins (grazers) and benthic macroalgal taxa (primary producers). Shown are associations between the abundance of sea urchins and (a) giant kelp, (b) fleshy understory macroalgae and (c) crustose coralline algae (CCA) and (d) sheephead biomass. Points represent the mean density, per cent cover or biomass per site.
Figure 5.
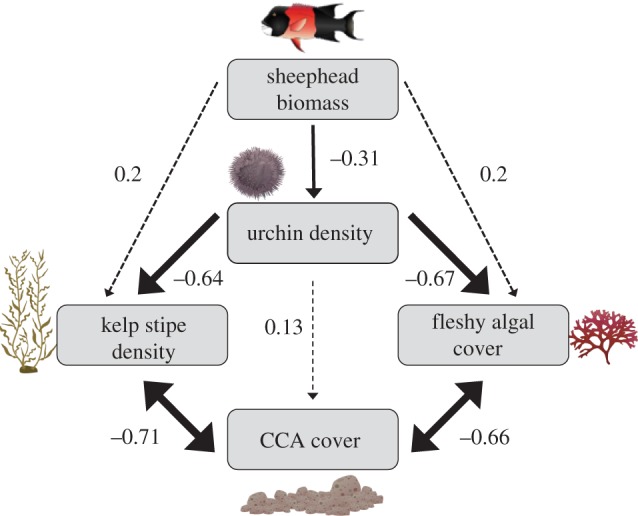
Path diagram showing standardized correlation coefficients of the direct (solid arrows) and indirect (dashed arrows) effects of sheephead and urchins on benthic algal community composition in the Channel Islands. Arrow thickness represents the strength of the correlation. (Online version in colour.)
Despite significant increases in sheephead density and biomass inside reserves, differences in the abundance of kelp and urchins between reserve and non-reserve sites on each island were mixed, as might be expected after only one decade of protection in this highly dynamic system. Giant kelp stipe densities did not differ based on reserve status, but did differ from island to island (ANOVA: Reserve, F1,82 = 0.08, p = 0.78; Island, F4,82 = 9.6, p < 0.0001; Reserve × Island, F4,82 = 1.2, p = 0.31). Stipe densities were higher inside reserves on Santa Rosa, Anacapa and Santa Barbara Islands, but showed the opposite trend on San Miguel and Santa Cruz (electronic supplementary material, figure S3). We found no difference in urchin densities based on reserve status but did find a significant Reserve × Island interaction (ANOVA: Reserve, F1,82 = 0.6, p = 0.45; Island, F4,82 = 17.5, p < 0.0001; Reserve × Island, F4,82 = 4.9, p = 0.0015). Urchins, primarily S. purpuratus, were more abundant outside reserves in the eastern channel islands of Santa Barbara and Anacapa (electronic supplementary material, figure S3). By contrast, the fished M. franciscanus were more abundant inside reserves on San Miguel, Santa Rosa and Santa Cruz Islands (where the fishery concentrates). Across the Channel Islands, geographical gradients in urchin abundance were strong and followed the opposite pattern of kelp, with more urchins and less kelp in the east (where sheephead and lobster fishing concentrate) and fewer urchins and more kelp in the west (colder and more productive), indicating that environmental conditions and fishing play a role in community interactions in this system.
4. Discussion
(a). Exploitation and recovery of California sheephead in southern California
California sheephead were heavily exploited by commercial and recreational fishing sectors in the 1920s and 1930s and again from 1980–2000. Management intervention in 1999–2000, in the form of size and catch limits [25], served to reduce take of sheephead in locations where the commercial fishery occurred. By 1998, size-selective harvest over multiple decades resulted in predictable shifts in life-history traits of sheephead at San Nicolas and Catalina Islands (electronic supplementary material, table S1; figure 1), such that with increased harvest average and maximum fish sizes were reduced and individuals matured and changed sex at smaller sizes. With a dramatic reduction in fishing pressure at San Nicolas by 2007, sheephead exhibited a rapid recovery of size structure and return of life-history traits to values measured during periods of low exploitation [14]. By contrast, size structure and life-history traits did not change at Catalina from 1998 to 2007, where recreational fishing pressure on sheephead remained high. Similar shifts in life histories and size structure of have been reported for other temperate fishery species [10,11].
In the northern Channel Islands, we observed increases in fish sizes, density, biomass and the density of fish above the legal size limit inside marine reserves that were closed to recreational and commercial fishing activities for almost a decade. These positive responses of sheephead to protection were consistent inside and outside of reserves across the five islands where surveys occurred, despite strong geographical gradients in environmental conditions, including sea surface temperature, wave exposure and productivity [31,35]. The responses of sheephead on San Nicolas Island mirrored the changes observed inside reserves, however, those changes were not due to reserve protection but rather different fisheries management measures (i.e. size limit and total allowable catch limits) that effectively reduced fishing pressure. Similar increases in density, size and biomass of sheephead and other targeted fish species have been observed inside other marine reserves in southern California [31,36]. On a global scale, marine reserves have consistently reported to increase these metrics for species that are targeted by fishing activities [15]. However, evidence for indirect effects of reserves on communities via changes in abundance and size structure of predators and other protected species is just now accumulating [17–20,37]. One important outstanding question for management of rocky reefs in southern California is the extent to which increased densities and sizes of sheephead inside reserves, or in response to other management measures, will affect kelp forest community structure. The issue is particularly timely in the light of the recent implementation of a very large network of marine protected areas, protecting approximately 16% of state waters in California [38].
(b). Do California sheephead enhance kelp forest ecosystem resilience?
Sheephead are common inhabitants in kelp forests and are reported to be strong interactors in these systems [22–24]. Because sheephead consume different prey in different places, their trophic impacts on prey communities may be location-dependent. Hamilton et al. [26] showed that sheephead diets differ throughout the Channel Islands and along the mainland coast in southern California and their consumption of urchins varies geographically and as function of prey abundance. At San Nicolas Island prior to increasing fishery harvest, sheephead consumed 20–33% of the red urchin population annually [23]. Experimental removal of sheephead from an isolated reef in the same study resulted in a 26% annual increase in red urchin densities, indicating strong trophic interactions, despite the fact that urchins were only the fourth most important prey taxa in the diet. In addition, Cowen [23] reported that the proportion of exposed urchins increased with decreasing sheephead densities across five sites spanning the species' range, suggesting strong indirect trophic effects on grazer behaviour. Similarly, Tegner & Dayton [22] demonstrated that in the absence of California sheephead and spiny lobsters in a Pt. Loma kelp bed, size frequency distributions of purple and red urchins changed, urchins occurred at higher densities and a greater proportion of individuals were found exposed and outside of shelter. Shifts in urchin size structure have also been reported between a 30-year-old reserve and fished areas in the Channel Islands [18]. Bimodal size distributions of M. franciscanus and S. purpuratus characterize the reserve sites; intermediate urchin sizes (those targeted by predators) are rare in reserves but common where urchin predators are fished. The predatory impacts of sheephead may vary geographically and their presumed role as urchin predators may only be realized in certain locations. Geographical shifts in predator–prey interactions may be related to differences in prey availability, but our results suggest that size-specific predation abilities or preferences (which in turn are likely to be affected by fishing) must also be considered.
California sheephead change their diet ontogenetically. As sheephead get larger in size, their diets shift from small sessile filter feeders to larger mobile invertebrates, as indicated by gut contents and stable isotopes. In this study (figure 3) and in [26], we showed that in southern California the per cent volume of the gut contents containing urchins increased consistently with fish size across nine study locations. In addition to consuming more urchins, larger fish also consumed larger urchins. Thus, larger sheephead will have a different predatory impact on benthic communities than smaller sheephead. Wainwright [6] showed that the ability of the Caribbean hogfish (Lachnolaimus maximus) to crush molluscs was more limited by the crushing force exerted than gape size per se, and this mechanism could apply to sheephead preying on urchins. Sheephead grow fast and attain some of the largest sizes at San Nicolas Island [8,29], which is bathed by the productive California Current. At this island, we found that the history of exploitation and recovery can lead to shifts in dietary niche breadth as larger sheephead expand their diet to include larger mobile invertebrates, such as urchins, in addition to smaller bivalves and brachyuran crabs typically consumed by smaller fish [14]. Our results indicate that only in places where large individuals are present, will sheephead have strong trophic impacts on urchin populations. In the light of the size-based changes in the consumption of urchins that we observed, these results suggest that fishing could indirectly reduce kelp forest resilience by removing the large fish that are capable of handling and successfully preying on urchins. If urchin grazing potential is a function of urchin size, then the effects of large sheephead on kelp forest resilience may occur by both changing urchin abundance and urchin size structure [as in 18]. A recent study in Tasmania showed a similar effect, such that kelp forest resilience was enhanced inside marine reserves because lobsters attained large enough sizes to effectively prey upon an invasive urchin species, which was responsible for widespread kelp loss outside reserves where large lobsters were overfished [20].
Kelp forest surveys in the Channel Islands revealed a negative relationship between sheephead and urchin densities (figure 4), which could reflect the consumptive effects of sheephead (and other important urchin predators; e.g. California spiny lobsters (Panulirus interuptus) and sunflower sea stars (Pycnopodia helianthoides)) on urchin populations. Alternatively, this negative correlation could occur if sheephead actively avoid urchin barrens. At this point, we are not able to distinguish between these two alternative hypotheses. We also observed a negative association between urchin densities and kelp density and fleshy algal cover, illustrating the potential for strong grazing effects of urchins in this system (figures 4 and 5; electronic supplementary material, figure S3). Arkema et al. [39] reported a similar negative relationship between kelp and urchin densities at mainland sites in southern California, in addition to the effects of space and light competition on benthic community composition. Sheephead biomass is indirectly positively associated with kelp density and macroalgal cover, presumably mediated through their consumption of sea urchins (figure 5). Further evidence for the predatory role of sheephead is reflected in the data showing that they are more abundant and larger inside reserves and that urchins are typically less abundant inside reserves, especially in the eastern islands (electronic supplementary material, figure S3) where predators such as sheephead and lobsters are more common, and more commonly targeted by fishers outside reserves [31,40]. As mentioned previously, sheephead are presumed to be important predators of urchins and it has been suggested that along with lobsters they can regulate urchin populations and help prevent the phase transition from kelp forests to urchin barrens in southern California [18,22–24,37]. Spiny lobsters are also more abundant and larger inside reserves in the Channel Islands [40] and likely exert similar or stronger (R. Jenkinson, 2014) top-down control on urchins.
In New Zealand, predatory snappers and lobsters serve similar roles as urchin predators, and protection of these species inside marine protected areas has resulted in the recovery of kelp beds following a reduction of urchin populations [17]. The effects of predator recovery on kelp abundance in New Zealand and other temperate systems, consistently occurred after decades of reserve protection [37] despite rapid initial changes in the abundance of targeted predators, illustrating that the indirect effects of predators on primary producers may not be rapid. In the Mediterranean, Guidetti [19] reported that recovery of the abundance and size structure of urchin predators (two species of sea bream, genus Diplodus) inside marine reserves after 10 years of protection resulted in large reductions in urchin abundance and concomitant shifts in benthic community composition from barrens to an algal-dominated state. Stomach content analysis showed that predation on urchins by these Diplodus species was also size-dependent. After a decade of protection, we witnessed island-specific responses of sheephead and urchins to reserves across the Channel Islands; differences in density between reserves and fished areas were in the expected direction based on harvest, predation pressure and environmental gradients. Sheephead were consistently more abundant and larger inside reserves, while urchins were less abundant, except in the western, colder islands (i.e. San Miguel, Santa Rosa, Santa Cruz) where the commercial sea urchin fishery (targeting red urchins) predominates. Red urchins were more abundant inside reserves on the western islands, indicating that reserves are benefitting populations of this fished species. By contrast, we did not detect significant differences in kelp abundance between reserve and fished areas after only 10 years of reserve protection. Synthetic analyses by Babcock et al. [37] indicate that these indirect effects typically take 10–15+ years to occur in marine reserves, so our study may have simply been too early to document strong indirect ecosystem-level changes. In addition, the northern Channel Islands are located in a very dynamic and complex oceanographic transition zone with strong gradients of temperature, nutrients and wave exposure [31,35] that increase the difficulty of teasing apart the processes controlling kelp dynamics in the area. Recent studies [41] have highlighted the importance of disturbance in the form of large storms in controlling kelp productivity in California and a debate over the relative importance and roles of top-down and bottom-up factors, and disturbance, continues [42]. Recent work shows that globally, urchins are capable of controlling kelp abundance in any system where they occur in high densities, have high consumptive potential, and occur against a backdrop of strong environmental variability [43]. These conditions are likely to be met in strongly fished locations in southern California.
Inside marine reserves in southern California, populations of sheephead have increased in density, size and biomass [31,36]. Increases in predator sizes afforded by protection may have the potential to indirectly increase predation rates on urchins and alter urchin size structure, which may enhance kelp forest resilience inside marine reserves. The persistent kelp forest state of the Anacapa Island Ecological Reserve (protected since 1978) may be due in part to predation on urchins by large sheephead and spiny lobsters [18,37]. Along with the implementation of marine reserves, additional management actions, such as raising size limits, may increase predation on sea urchins and could enhance ecosystem resilience and biodiversity by promoting the maintenance of productive kelp beds over less productive urchin barrens. Our study demonstrates the importance of moving beyond simple measures of abundance and biomass as metrics of success when assessing management actions such as marine reserves. Size-structured predator–prey dependencies, such as we demonstrate here, probably occur broadly in nature, especially in aquatic systems with fish predators because many species are indeterminate growers with ontogenetic dietary shifts. Through an improved understanding of geographical and ontogenetic changes in the trophic ecology of functionally important predators, such as sheephead, we may be able to improve the management of coastal ecosystems in the future.
Supplementary Material
Acknowledgements
We thank K. Loke-Smith, C. Lowe, M. Love, D. Schroeder, J. Rosales-Casian, O. Sosa-Nishizaki and numerous assistants for help in the field and laboratory. We wish to thank one anonymous reviewer and K. Demes for constructive comments.
Ethics statement
Recent samples, collected in 2007 by the authors, were approved on IACUC Protocol no. 4–07–729 at UCSB and CDFW permit SC-6477.
Data accessibility
Kelp forest survey data are publically available from PISCO (www.piscoweb.org) and through the Knowledge Network for Biocomplexity (https://knb.ecoinformatics.org/). Specific datasets used were: fish surveys (doi:10.6085/AA/pisco_subtidal.150.2); benthic surveys (doi:10.6085/AA/pisco_subtidal.152.3) and (doi:10.6085/AA/pisco_subtidal.151.2). Data on sheephead size structure and diet from 2007 to 2008 collections (doi:10.5061/dryad.79qd9) are available through Dryad (www.datadryad.org).
Funding statement
Funding was provided by NOAA (NA04OAR4170038), California Sea Grant and the Ocean Protection Council (R/OPC-FISH05), the National Science Foundation (CAMEO #1041489) and the Partnership for Interdisciplinary Studies of Coastal Oceans (PISCO), which is funded by the David and Lucille Packard Foundation and the Gordon and Betty Moore Foundation. This is PISCO publication no. 449.
References
- 1.Werner EE, Gilliam JF. 1984. The ontogenetic niche and species interactions in size-structured populations. Ann. Rev. Ecol. Syst. 15, 393–425. ( 10.1146/annurev.es.15.110184.002141) [DOI] [Google Scholar]
- 2.Brooks JL, Dodson SI. 1965. Predation, body size, and the composition of plankton. Science 150, 28–35. ( 10.1126/science.150.3692.28) [DOI] [PubMed] [Google Scholar]
- 3.Rice JA, Crowder LB, Rose KA. 1993. Interactions between size-structured predator and prey populations: experimental test and model comparison. T. Am. Fish. Soc. 122, 481–491. () [DOI] [Google Scholar]
- 4.Sogard SM. 1997. Size-selective mortality in the juvenile stage of teleost fishes: a review. Bull. Mar. Sci. 60, 1129–1157. [Google Scholar]
- 5.Schmitt RJ, Holbrook SJ. 1984. Gape-limitation, foraging tactics and prey size selective of two microcarnivorous species of fish. Oecologia 63, 6–12. ( 10.1007/BF00379778) [DOI] [PubMed] [Google Scholar]
- 6.Wainwright PC. 1987. Biomechanical limits to ecological performance: mollusk-crushing by the Caribbean hogfish, Lachnolaimus maximus (Labridae). J. Zool. 213, 283–297. ( 10.1111/j.1469-7998.1987.tb03704.x) [DOI] [Google Scholar]
- 7.Ricker WE. 1981. Changes in the average size and average age of Pacific salmon. Can. J. Fish. Aquat. Sci. 38, 1636–1656. ( 10.1139/f81-213) [DOI] [Google Scholar]
- 8.Hamilton SL, Caselle JE, Standish JD, Schroeder DM, Love MS, Rosales-Casian JA, Sosa-Nishizaki O. 2007. Size-selective harvesting alters life histories of a temperate sex-changing fish. Ecol. Appl. 17, 2268–2280. ( 10.1890/06-1930.1) [DOI] [PubMed] [Google Scholar]
- 9.Estes JA, et al. 2011. Trophic downgrading of planet earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 10.Rijnsdorp AD. 1993. Fisheries as a large-scale experiment on life-history evolution: disentangling phenotypic and genetic effects in changes in maturation and reproduction of North sea plaice, Pleuronectes platessa. Oecologia 96, 391–401. ( 10.1007/BF00317510) [DOI] [PubMed] [Google Scholar]
- 11.Hutchings JA. 2005. Life history consequences of overexploitation to population recovery in Northwest Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 62, 824–832. ( 10.1139/f05-081) [DOI] [Google Scholar]
- 12.Hunsicker ME, et al. 2011. Functional responses and scaling in predator–prey interactions of marine fishes: contemporary issues and emerging concepts. Ecol. Lett. 14, 1288–1299. ( 10.1111/j.1461-0248.2011.01696.x) [DOI] [PubMed] [Google Scholar]
- 13.Kaplan IC, Gray IA, Levin PS. 2013. Cumulative impacts of fisheries in the California Current. Fish. Fish. 14, 515–527. ( 10.1111/j.1467-2979.2012.00484.x) [DOI] [Google Scholar]
- 14.Hamilton SL, Newsome SD, Caselle JE. 2014. Dietary niche expansion of a kelp forest predator recovering from intense commercial exploitation. Ecology 95, 164–172. ( 10.1890/13-0014.1) [DOI] [PubMed] [Google Scholar]
- 15.Lester SE, et al. 2009. Biological effects within no-take marine reserves: a global synthesis . Mar. Ecol. Prog. Ser. 384, 33–46. ( 10.3354/meps08029) [DOI] [Google Scholar]
- 16.Micheli F, Halpern BS, Botsford LW, Warner RR. 2004. Trajectories and correlates of community change in no-take marine reserves. Ecol. Appl. 14, 1709–1723. ( 10.1890/03-5260) [DOI] [Google Scholar]
- 17.Shears NT, Babcock RC. 2002. Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 132, 131–142. ( 10.1007/s00442-002-0920-x) [DOI] [PubMed] [Google Scholar]
- 18.Behrens MD, Lafferty KD. 2004. Effects of marine reserves and urchin disease on southern California rocky reef communities. Mar. Ecol. Prog. Ser. 279, 129–139. ( 10.3354/meps279129) [DOI] [Google Scholar]
- 19.Guidetti P. 2006. Marine reserves reestablish lost predatory interactions and cause community changes in rocky reefs. Ecol. Appl. 16, 963–976. ( 10.1890/1051-0761(2006)016[0963:MRRLPI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 20.Ling SD, Johnson CR, Frusher SD, Ridgway KR. 2009. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc. Natl Acad. Sci. USA 106, 22 341–22 345. ( 10.1073/pnas.0907529106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shackell NL, Frank KT, Fisher JAD, Petrie B, Leggett WC. 2010. Decline in top predator body size and changing climate alter trophic structure in an oceanic ecosystem. Proc. R. Soc. B 277, 1353–1360. ( 10.1098/rspb.2009.1020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tegner MJ, Dayton PK. 1981. Population structure, recruitment and mortality of two sea urchins (Stronglyocentrotus franciscanus and S. purpuratus) in a kelp forest. Mar. Ecol. Prog. Ser. 5, 255–268. ( 10.3354/meps005255) [DOI] [Google Scholar]
- 23.Cowen RK. 1983. The effect of sheephead (Semicossyphus pulcher) predation on red sea urchin (Stronglyocentrotus franciscanus) populations: an experimental analysis. Oecologia 58, 249–255. ( 10.1007/BF00399225) [DOI] [PubMed] [Google Scholar]
- 24.Tegner MJ, Dayton PK. 2000. Ecosystem effects of fishing in kelp forest ecosystems. ICES J. Mar. Sci. 57, 579–589. ( 10.1006/jmsc.2000.0715) [DOI] [Google Scholar]
- 25.Alonzo SH, Key M, Ish T, MacCall A. 2004. Status of the California sheephead (Semicossyphus pulcher) stock, p. 146 Sacramento, CA: California Department of Fish and Game. [Google Scholar]
- 26.Hamilton SL, et al. 2011. Extensive geographic and ontogenetic variation characterizes the trophic ecology of a temperate reef fish on southern California (USA) rocky reefs. Mar. Ecol. Prog. Ser. 429, 227–244. ( 10.3354/meps09086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warner RR. 1975. The reproductive biology of the protogynous hermaphrodite Pimelometopon pulchrum (Pisces: Labridae). Fish. Bull. 73, 262–283. [Google Scholar]
- 28.Cowen RK. 1990. Sex change and life history patterns of the labrid Semicossyphus pulcher, across an environmental gradient. Copeia 3, 787–795. ( 10.2307/1446444) [DOI] [Google Scholar]
- 29.Hamilton SL, Wilson JR, Ben-Horin T, Caselle JE. 2011. Utilizing spatial demographic and life history variation to optimize sustainable yield of a temperate sex-changing reef fish. PLoS ONE 6, e24580 ( 10.1371/journal.pone.0024580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright N, Kum J, King C, Knaggs E, Leos B, Perez C. 2000. California sheephead, Semicossyphus pulcher 1993–1999 Commercial catch by ports and blocks. Marine Fishery Profiles, Volume 1: Nearshore. Sacramento, CA: California Department of Fish and Game Marine Region. [Google Scholar]
- 31.Hamilton SL, Caselle JE, Malone DP, Carr MH. 2010. Incorporating biogeography into evaluations of the Channel Islands marine reserve network. Proc. Natl Acad. Sci. USA 107, 18 272–18 277. ( 10.1073/pnas.0908091107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnhan KP, Anderson DR. 1998. Model selection and inference: a practical information theoretic approach, p. 353 New York, NY: Springer. [Google Scholar]
- 33.Mitchell RJ. 2001. Path analysis. In Design and analysis of ecological experiments, 2nd edn (eds Scheiner SM, Gurevitch J.), pp. 217–234. Oxford, UK: Oxford University Press. [Google Scholar]
- 34.Ebert TA. 1968. Growth rates of the sea urchin Strongylocentrotus purpuratus related to food availability and spine abrasion. Ecology 49, 1075–1091. ( 10.2307/1934491) [DOI] [Google Scholar]
- 35.Blanchette CA, Broitman BR, Gaines SD. 2006. Intertidal community structure and oceanographic patterns around Santa Cruz Island, CA, USA. Mar. Biol. 149, 689–701. ( 10.1007/s00227-005-0239-3) [DOI] [Google Scholar]
- 36.Tetreault I, Ambrose RF. 2007. Temperate marine reserves enhance targeted but not untargeted fishes in multiple no-take MPAs. Ecol. Appl. 17, 2251–2267. ( 10.1890/06-0161.1) [DOI] [PubMed] [Google Scholar]
- 37.Babcock RC, et al. 2010. Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects. Proc. Natl Acad. Sci. USA 107, 18 256–18 261. ( 10.1073/pnas.0908012107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botsford LW, White JW, Carr MH, Caselle JE. 2014. Marine protected area networks in California, USA. Adv. Mar. Biol. 69, 205–251. ( 10.1016/B978-0-12-800214-8.00006-2) [DOI] [PubMed] [Google Scholar]
- 39.Arkema KK, Reed DC, Schroeter SC. 2009. Direct and Indirect effects of giant kelp determine benthic community structure and dynamics. Ecology 90, 3126–3137. ( 10.1890/08-1213.1) [DOI] [PubMed] [Google Scholar]
- 40.Kay MC, Lenihan HS, Guenther CM, Wilson JR, Miller CJ, Shrout SW. 2012. Collaborative assessment of California spiny lobster population and fishery responses to a marine reserve network. Ecol. Appl. 22, 322–335. ( 10.1890/11-0155.1) [DOI] [PubMed] [Google Scholar]
- 41.Reed DC, Rassweiler A, Carr MH, Cavanaugh KC, Malone DP, Siegel DA. 2011. Wave disturbance overwhelms top-down and bottom-up control of primary production in California kelp forests. Ecology 92, 2108–2116. ( 10.1890/11-0377.1) [DOI] [PubMed] [Google Scholar]
- 42.Foster MS, Schiel DR. 2010. Loss of predators and the collapse of southern California kelp forests (?): alternatives, explanations, and generalizations. J. Exp. Mar. Biol. Ecol. 393, 59–70. ( 10.1016/j.jembe.2010.07.002) [DOI] [Google Scholar]
- 43.Byrnes JE, et al. 2013. The sea urchin—the ultimate herbivore and biogeographic variability in its ability to deforest kelp ecosystems. PeerJ 1, e174v1.24109558 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Kelp forest survey data are publically available from PISCO (www.piscoweb.org) and through the Knowledge Network for Biocomplexity (https://knb.ecoinformatics.org/). Specific datasets used were: fish surveys (doi:10.6085/AA/pisco_subtidal.150.2); benthic surveys (doi:10.6085/AA/pisco_subtidal.152.3) and (doi:10.6085/AA/pisco_subtidal.151.2). Data on sheephead size structure and diet from 2007 to 2008 collections (doi:10.5061/dryad.79qd9) are available through Dryad (www.datadryad.org).