Abstract
Resurveys of historical collecting localities have revealed range shifts, primarily leading edge expansions, which have been attributed to global warming. However, there have been few spatially replicated community-scale resurveys testing whether species' responses are spatially consistent. Here we repeated early twentieth century surveys of small mammals along elevational gradients in northern, central and southern regions of montane California. Of the 34 species we analysed, 25 shifted their ranges upslope or downslope in at least one region. However, two-thirds of ranges in the three regions remained stable at one or both elevational limits and none of the 22 species found in all three regions shifted both their upper and lower limits in the same direction in all regions. When shifts occurred, high-elevation species typically contracted their lower limits upslope, whereas low-elevation species had heterogeneous responses. For high-elevation species, site-specific change in temperature better predicted the direction of shifts than change in precipitation, whereas the direction of shifts by low-elevation species was unpredictable by temperature or precipitation. While our results support previous findings of primarily upslope shifts in montane species, they also highlight the degree to which the responses of individual species vary across geographically replicated landscapes.
Keywords: global change, geographical range, elevation gradient, occupancy, museum specimens, vertebrates
1. Introduction
Evidence for the biotic responses to recent climate change has continued to accumulate [1–5] and is central to the prediction of vulnerability to future change [6]. There is a general trend toward upward and poleward shifts of elevational and latitudinal boundaries of species' ranges [1–5,7,8], with ‘leading edge’ expansions detected more often than ‘lagging edge’ contractions [9–11]. However, there is considerable heterogeneity in the direction and magnitude of species' responses, and ranges of many species have not changed at all [12–14]. Some of this heterogeneity may be attributable to local variation in climate change, but few studies have accounted for spatial heterogeneity in climate change across the landscape [1,14,15]. Moreover, there is potential for considerable sampling error because local colonization and extinction cannot be demonstrated convincingly unless detectability (i.e. the probability of ‘false absence’) is explicitly incorporated into models of occurrence change [16]. Insights into the dynamics of species' responses to recent climate change are likely to be gained from spatially replicated resurveys [17,18] combined with analytical methods that have statistical power to detect both range contractions and expansions.
An unusually detailed historic dataset, combined with contemporary resurveys, allows us to evaluate robustly a century of range responses of mammals to climate change in montane California (figure 1a). Joseph Grinnell and colleagues studied the elevational distributions of vertebrates of California in the early 1900s [19–21]. These data laid the foundation for Grinnell's concept of the ecological niche and for understanding the climatic limits of species' distributions [22]. They also provided a benchmark for documenting changes in the elevational ranges of species in California over the past century [11,13,14,23], during which time the mean annual temperature in California has increased by approximately 0.6°C [24–26] (electronic supplementary material, figure S1). Precipitation changes were more spatially heterogeneous, with spatial covariation increasing across the northern part of the state and decreasing across the southern part [27,28]. Elevational ranges of species in California over this period have shifted heterogeneously, including species moving upslope, downslope or not at all [13,14,29]. Heterogeneity in movements of species has been partly explained by incorporating local-scale measures of climatic change for both temperature and precipitation [14,30]; increases in the former usually favour upslope shifts, while increases in the latter typically favour downslope movements. Local changes in habitat structure owing to fire and grazing are also factors in some areas [31,32].
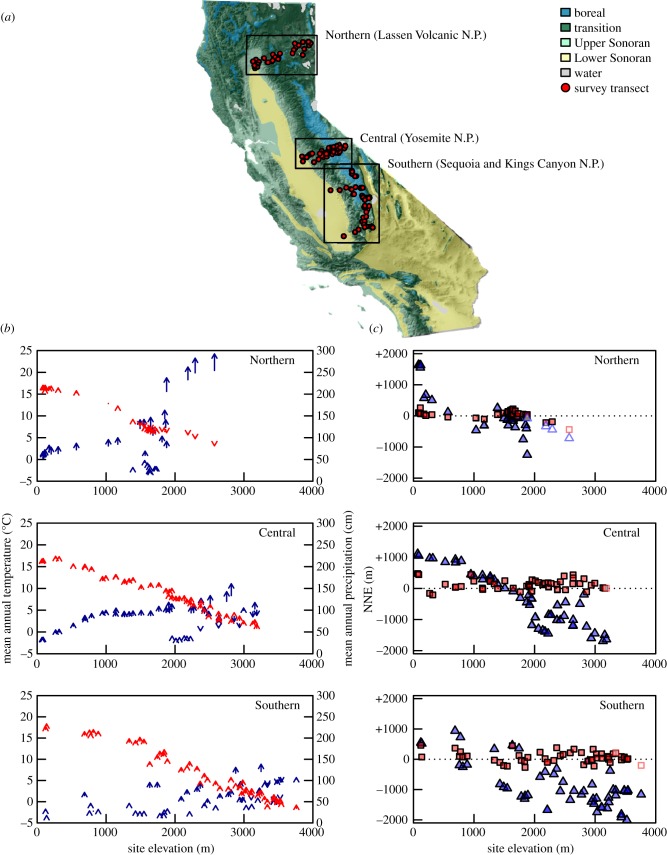
Figure 1.
Climate change and expected elevation shifts across sampling sites. (a) Map of historical survey localities in relation to survey region and life zone (N.P., National Park); (b) change in mean annual temperature (red) and mean annual precipitation (blue) between the historical (base of arrow) and modern (tip of arrow) eras across elevation; (c) average expectation of elevation shift in the modern era to achieve the most similar value of mean annual temperature (red squares) or mean annual precipitation (blue triangles) as historical localities across elevation, based on our climatic nearest neighbour analysis (see §2); open squares and triangles indicate historical sites where similar climate is underrepresented regionally within the historical era (i.e. rare) or in the modern era (i.e. disappearing). NNE, nearest neighbour elevation (m).
Here we characterized spatial variation in elevational range responses of small mammals in protected areas of montane California by expanding our analysis centred on a single region in central California (Yosemite National Park, central Sierra Nevada) [13] to other regions in the north (Lassen Volcanic National Park, southern Cascade Range) and south (Sequoia/Kings Canyon National Parks, southern Sierra Nevada). We controlled for variation in detectability among species and survey eras to compare elevational limits from the early twentieth century (1911–1934) to the present (2003–2010). With data from multiple, geographically separated regions of montane California that have experienced limited land-use change, we tested four predictions of the influence of climate warming on elevational ranges that emerged from patterns observed in the Yosemite region [13] and in birds across montane California [14]. If an average warming trend across California is the predominant driver of elevational range change, then (i) upslope shifts should be the most common change across all regions and (ii) range contractions at lower limits should be more frequent in high-elevation species, and range expansions at upper limits more common in low-elevation species. If, however, species have responded to the heterogeneous climate change across the landscape and to both temperature and precipitation change, then (iii) elevational ranges of species should shift inconsistently across regions; and (iv) upslope and downslope shifts should both occur and be associated with local changes in temperature and precipitation, respectively [14].
2. Material and methods
(a). Survey regions and twentieth century climate change
Historical surveys were made between 1911 and 1934 [16,17,20,21] along elevation transects across three regions of montane California (figure 1a; electronic supplementary material, table S1): a northern region, in the southern Cascade Range, around Lassen Volcanic National Park (‘Northern’), a central region, in the central Sierra Nevada, around Yosemite National Park (‘Central’) and a southern region, in the southern Sierra Nevada, around Kings Canyon and Sequoia National Parks (‘Southern’). The three regions of montane California differed considerably in climate and physiognomy. The Northern region was the coolest and wettest, and had the smallest elevation range and the least topographic complexity. The Southern region was the driest, and had the largest elevation range and greatest topographic complexity. Climate change over the past century differed among the three regions (figure 1b; electronic supplementary material, figure S1). The Central region experienced the greatest and the Northern region the least increase in mean annual temperature, whereas precipitation increased in both of these regions but not in the Southern region. Across all three regions, the maximum temperature of the warmest month was constant, whereas the minimum temperature of the coldest month increased (electronic supplementary material, figure S1). Given that small mammals of California may respond differently to changes in minimum, maximum or mean annual temperature based on differences in life history (e.g. hibernators versus non-hibernators), we examined multiple measures of climate change in our climatic nearest neighbour analyses below. See Tingley et al. [14] for additional details of the sampling regions.
(b). Survey and resurvey data
We used historical maps, written descriptions in field notes and modern ground-truthing with historical photographs and hand-held GPS units to georeference historical localities. Modern trapline coordinates were obtained from hand-held GPS units, with coordinates recorded at the beginning, middle and end of each trapline. We defined localities or sampling sites as an aggregate of concurrent surveys (i.e. traplines) conducted within a 2 km distance and 100 m elevation [13]. Each site was georeferenced, and elevation was determined using a Digital Elevation Model derived from the Shuttle Radar Topography Mission (v4) with a resolution of 1 arc second. We verified these values by manual comparison to elevations determined on the ground or on topographic maps (electronic supplementary material, table S1).
From 1911 to 1934, Grinnell and staff of the Museum of Vertebrate Zoology at the University of California, Berkeley (MVZ) surveyed 134 historical localities included in this study. Of these sites, 34 were in the Northern, 47 were in the Central and 53 were in the Southern region of montane California (figure 1a). Between 2003 and 2010, we surveyed a total of 166 sites with 38 in the Northern, 81 in the Central and 47 in the Southern region of montane California ([13,14]; figure 1a). Our combined dataset included 85 sites surveyed in both historical and modern eras, 49 sites surveyed in the historical era and 81 sites surveyed in the modern era. Additional modern sites were selected to maximize elevation coverage and to serve as proxies for otherwise inaccessible historical sites. We obtained details of historical survey efforts including number of traps set, number of each species recorded daily, location maps and habitats from more than 2500 pages of field notebooks held in the MVZ Archives (available online at http://bscit.berkeley.edu/mvz/volumes.html). Each historical site was surveyed for 1–16 nights (median = 5) for a total of 681 survey nights. In the modern era, we surveyed each site for 1–11 nights (median = 6) for a total of 916 survey nights. For most sites, surveys were conducted over consecutive nights. For each historical site, the average number of traps per night ranged from 6 to 335 (median = 96). For each modern site, the average number of traps per night ranged from 3 to 339 (median = 65). Historical trapping efforts used snap traps, Macabee gopher traps, mole traps and steel traps that were set in suitable locations in various habitats around a central camp. In the historical era, shooting resulted in additional opportunistic records of diurnal mammals, primarily squirrels and pikas. In the modern era, we used a combination of live traps (Sherman and Tomahawk), with standard traplines containing 40 Sherman traps and 10 Tomahawk traps run for four consecutive nights in suitable spots. Pitfall traps, consisting of 32 oz plastic cups placed in the ground, were used to collect shrews and were set at the same time as the Sherman lines. Pocket gophers were trapped using Macabee gopher traps where gopher mounds were observed. Additional observational records were made daily for both historical and modern eras. For this study, we documented a total of 15 277 historical mammal occurrence records. Of these, 8688 were backed by voucher specimens in the MVZ and the remaining 6589 were recorded in field notes (http://arctos.database.museum/project/historic-grinnell-survey-lassen-transect, http://arctos.database.museum/project/historic-grinnell-survey-yosemite-transect, http://arctos.database.museum/project/historic-grinnell-survey-southern-sierra-nevada-transect). With our modern surveys, we documented 14 316 mammal occurrence records. Of these, 6144 were backed by voucher specimens in the MVZ and the remaining 8172 were recorded in field notes (http://arctos.database.museum/project/grinnell-resurvey-project-lassen-transect, http://arctos.database.museum/project/grinnell-resurvey-project-yosemite-transect, http://arctos.database.museum/project/grinnell-resurvey-project-southern-sierra-nevada-transect). Combined with the historical survey data, this resulted in a total of 29 593 records of 67 species from 215 sites.
(c). Species set
We present the elevation distribution for 67 species of rodents, shrews and lagomorphs recorded in the historical and modern surveys (electronic supplementary material, table S2). Our resurvey protocols were not designed to detect carnivores, ungulates or bats, so these were not included. We also considered only those west slope species that are characteristic of the Sierra Nevada and Cascade Range. For example, we did not include Mojave Desert species such as Neotoma lepida or Perognathus longimembris. Statistical analyses of range shifts were restricted to 34 species that were detected at more than 10% of sites for at least one region in both eras. Of these, we were able to model detectability and occupancy for 28 species because they were detected through repeated nights of trapping at sites where the number of traps set was reported (hereafter ‘quantitative trapping’). We include an additional six species in our range shift analyses as we made systematic efforts to detect and record these easily observed species. Elevational profiles of species are presented separately for the east and west slope sites with the exception of sites at elevations above the Yellow Pine (Pinus ponderosa) belt (approx. 2500 m in elevation) on the east slope that are included with west slope analyses. The habitats at these elevations are effectively connected between east and west slopes. Following Moritz et al. [13], we adjusted the slope cut-off for Peromyscus truei to reflect known boundaries between Sierra Nevada and Great Basin subspecies [33]. To test if species' responses differed based on their elevational distributions, we categorized each species as low-elevation (historical elevation ranges within Lower Sonoran—transition life zones), high-elevation (Transition—alpine) or widespread (Lower Sonoran—alpine) [13,17,20,21] (electronic supplementary material, table S3). Thomomys bottae was classified as low-elevation based on its range in the Northern and Central regions, consistent with Moritz et al. [13].
(d). Modelling changes in elevational ranges
We used the program MARK v. 6.0 to simultaneously estimate the probability of detection (p) and the probability of occupancy (Ψ) of each species at each site in each era [34,35]. To fit these models, we included the 28 species and 215 sites (85 historical and modern, 49 historical and 81 modern) for which quantitative trapping data were available. Because not all sites were paired in this study, we implemented an ‘unpaired-site’ framework [16] and used the single-season occupancy-modelling framework to test for temporal changes in occupancy by fitting time period (‘era’) as a covariate effect. We used the package ‘RMark’ v. 2.0.1 in the R v. 2.12.2 framework to build design matrices, combine models and to compare AIC weights among models [36].
To develop detection-adjusted elevation range profiles for each species in each era and region, we parametrized 25 occupancy models (Ψ) building on the model set of Moritz et al. [13] and Tingley et al. [14]. The 25 models included all two- and three-way interactions among the following variables: era (categorical: historical or modern), elevation (linear), elevation (quadratic) and region (categorical: Northern, Central or Southern), as well as a constant model (.). The full Ψ model set is listed in electronic supplementary material, table S4. Following Moritz et al. [13], we estimated the probability of detection per survey night (p) based on 34 competing models with the following variables: era (historical or modern), trend (linear change in detections over sequential nights due to the collection of trapped individuals, trap habituation or trap-shyness), trap effort (number of traps/100 and the log10 of the number of traps), the interaction between era and trend, and the interactions between era and trap effort variables. We built detection models with all additive combinations of these independent variables, as well as a constant model (.). We ran this full candidate p model set with two parametrizations of Ψ: a constant model and a fully parametrized model. From these analyses, we selected the set of p models that incorporated the best (lowest AIC) model and all models with ΔAIC < 2 for each species (electronic supplementary material, table S5). This subset of p models (n = 16; electronic supplementary material, table S4) were then combined with the full set of 25 Ψ models for a total of 400 competing models that were run for each species and compared using AIC [13].
We estimated temporal shifts in the lower and upper range limits for each species in each region following Moritz et al. [13]. For elevation distributions, we used all detection data including quantitatively trapped specimens, incidentally collected (shot or salvaged) specimens and observational records (electronic supplementary material, figure S2). We plotted all localities in each transect for each era against elevation, and coded each species at a locality as present or undetected. We then calculated the change in elevation of each range limit from the historical to the modern era. We determined the statistical significance of shifts by calculating the probability of false absence (Pfa [16]) of a species across the sites spanning the difference in elevation limit between the two eras. First, we estimated site-specific detection probabilities (p*) by model averaging model-specific p estimated using AIC weights from our 400 occupancy models [13,37] (electronic supplementary material, figure S3). From these, we calculated Pfa by multiplying 1 − p* for all sites where a species was undetected in one era and that were located between the lower or upper range limits of the two eras. Range limit shifts with Pfa ≤ 0.05 were considered statistically significant and ‘ecologically relevant’ if the movement was both more than 10% of the species' historical elevation range and more than 100 m in elevation [13].
(e). Testing predictors of range shifts
To understand the heterogeneity in species' range shifts based on regional (Northern, Central and Southern) and elevational (low and high) differences, we used a series of generalized linear mixed models (GLMM) and one-sided binomial tests. We included elevation limit (upper or lower) as a categorical variable to account for the possibility that upper and lower limits of species would respond differently. All GLMM models used the logit link function run in R with the ‘lme4’ package and model performance was assessed by AIC [38]. To focus on general patterns of region, limit and elevation effects, we included species identity as a random scalar effect (intercept only; see [14]). We first used GLMMs to evaluate what factors were associated with occurrence of a range shift (as a binary variable). The two species that are widespread across elevations (Peromyscus maniculatus and Otospermophilus beecheyi) were excluded from this analysis because they could neither be categorized into low- or high-elevation species nor could they be analysed as a separate category. We defined 11 models comprising a null model (intercept only) and all additive combinations and one-way interactions between three categorical explanatory variables: (i) limit, (ii) region and (iii) elevational zone. Second, to resolve interaction effects associated with zone, we analysed low-elevation and high-elevation species separately, retaining limit and region variables.
We used one-sided binomial tests to evaluate whether upslope shifts were the most common across regions (prediction i), whether range contractions were more likely in high-elevation species and range expansions were more common in low-elevation species (prediction ii) and to evaluate whether the patterns of range shifts were consistent across regions (prediction iii). For each of these analyses, we included only those species that exhibited significant shifts determined from the Pfa analysis above.
(f). Climatic nearest neighbour
Predicting a priori how individual species responded over the past century to the complex spatial heterogeneity in both temperature and precipitation change observed for the three regions of this study is not feasible. To address this uncertainty, we developed a method for identifying if climates observed at each historical site would predict upslope or downslope shifts given the geographical distribution of climates in the modern era (prediction iv). First, we identified the nearest climatic neighbours of historical localities under modern climate conditions, following the approach described in Tingley et al. [14]. Using four standard BIOCLIM variables (mean annual temperature, B1; maximum temperature of the warmest month, B5; minimum temperature of the coldest month, B6; and mean annual precipitation, B12) from the Parameter-elevation Regressions on Independent Slope Model (PRISM [39]) at a resolution of 30 arc second (1 km2), we calculated 20-year averages for the historical (1910–1930) and modern (1989–2009) survey periods. Climatic distances for each of the BIOCLIM variables were calculated between each historical locality and modern era PRISM grid cells within the same region, which was defined by a 20-km buffer around the minimum convex polygon that encompassed all survey sites within each region. For each historical site, we identified the 5% of modern cells that were most similar climatically. To test for availability of similar environments within the historical era, we also identified the 5% of historical cells that were most similar climatically. A critical assumption of our nearest climate neighbour analysis is that similar environments are in fact available on the landscape (both within the historical era and across eras). Thus, for each climatic variable at each site, we also identified rare and disappearing climates using climatic thresholds of 1°C temperature or 10 cm precipitation. We defined rare climates as those that occurred within climatic thresholds at less than 2.5% of historical cells. We defined disappearing climates as those that occurred within climatic thresholds at 5% or more of historical cells and less than 2.5% of modern cells. We excluded this subset of site-specific climate change from nearest neighbour comparisons. We calculated nearest climatic neighbour and rare and disappearing climates for each climatic variable separately using the Euclidean distance. To calculate the predicted shift in elevation based on nearest climatic neighbour, we subtracted the elevation of the historical site from the average elevation of the modern nearest climate neighbour cells; positive values indicated upslope movement in climate space. We recorded these values (positive or negative) for the two historical localities defining the upper and lower limits of each species on each transect. These values provided a climate-based prediction for movement of species at their range limits for each region (i.e. upslope or downslope). To test if incorporating local-scale climate data improved predictions of range shifts, we compared these climate data-derived models to an ‘overall warming model’ that assumes an increased temperature at all grid cells over the same time period. Under this latter model, cooler temperatures are always found upslope and species are always predicted to move upslope. We used a one-sided binomial to test if the upslope movement predicted from the overall warming model and predictions from each of the BIOCLIM variables were consistent with the direction of observed shifts (prediction iv). All models, whether based on nearest climatic neighbour predictions or an overall warming model, assume the same process of species responding to climate change by tracking similar climates. Thus, our comparison is intended to assess the methodological implications of using climate change averages (overall warming model) or derived estimates of local climate change (nearest neighbour).
3. Results
(a). Mammalian elevational range shifts over the past century
Of the 67 small mammal species we detected in either the historical or modern surveys (electronic supplementary material, table S2 and figure S2), we were able to use robust statistical methods to evaluate range shifts of 34 species. Across the three regions, we detected 52 significant range limit shifts, representing 31.3% of the 166 region-specific historical range limits across the 34 species analysed (figure 2; electronic supplementary material, figure S3). We observed no significant range limit shifts in nine species (26.4%), including two gophers (Thomomys bottae and T. monticola), three chipmunks (Tamias merriami, T. quadrimaculatus and T. amoenus), two shrews (Sorex trowbridgii and S. vagrans), a widespread deer mouse (P. maniculatus) and the pika (Ochotona princeps). The remaining 25 species (74.6%) shifted at least one range limit in one or more regions (figure 2; electronic supplementary material, table S2).
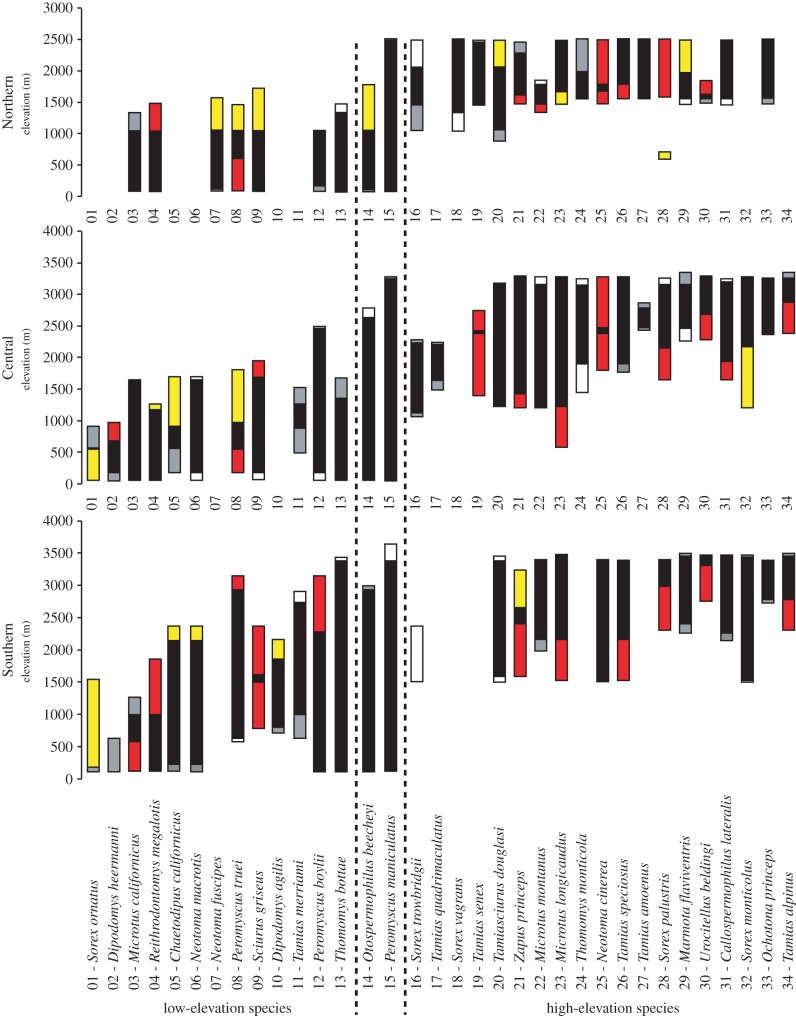
Figure 2.
Elevation range limit shifts by region of the 34 modelled species, arranged by increasing average elevation range. Species were classified as low-elevation, high-elevation or widespread based on their range limits in relation to life zone, following [13]: low-elevation (historical elevation ranges within Lower Sonoran—transition life zones; 01–13), high-elevation (Transition—alpine; 16–34) and widespread species (14 and 15). For each species, statistically significant elevation range contractions (red) and expansions (yellow) between the historical and modern eras are shown, along with non-significant contractions (grey) and expansions (white). Lack of a bar indicates that species is not found in that region.
We observed little consistency in directionality of range shifts of individual species among regions providing the primary evidence that species' responses were not spatially consistent. None of the 22 species found in all three regions shifted both their upper and lower limits in the same direction in all three regions (figure 2). For example, both the bushy tailed woodrat (Neotoma cinerea) and the pinyon mouse (P. truei) showed substantial changes in elevational ranges in the Northern and Central regions but not in the Southern region, while the western gray squirrel (Sciurus griseus) contracted strongly in the Southern and marginally in the Central, but expanded its range in the Northern.
Nevertheless, some consistent patterns emerged across regions when testing the frequency and directionality of shifts irrespective of species identity. Overall, the GLMM for ‘All Species' revealed an overwhelming zone × limit interaction (zone : limit, AIC wt = 1.00; electronic supplementary material, table S5), and none of the models that included region was well supported (AIC wt = 0.00; electronic supplementary material, table S5). For both high- and low-elevation species, the limit-only GLMM model was the top model (‘high-elevation species', AIC wt = 0.63; ‘low-elevation species', AIC wt = 0.73) with high-elevation species more likely to shift at the lower limits and low-elevation species more likely to shift at the upper limits. Both analyses of high- and low-elevation species recovered only limited support for models that included region. Across all three regions, species' elevational limits were more than twice as likely to move upslope (69.2%) as downslope (31.8%; one-sided binomial test, n = 52 species' limits, p = 0.004; figure 3). Furthermore, significant differences among high- and low-elevation species emerged when examining the directionality of shifts when species shifted their ranges. High-elevation species were significantly more likely to contract their ranges than to expand them (79% contract, n = 29 species' limits, p = 0.001; high-elevation pie chart, figure 3), whereas, contrary to our expectations based on an average warming trend, low-elevation species contracted their limits as often as they expanded them (50% contract, n = 22 species' limits, p = 0.584; low-elevation pie chart, figure 3). These patterns emerged because there were significantly more upslope than downslope shifts of the lower limit of high-elevation species (n = 21 lower limits, p < 0.001; arrows, figure 3), whereas shifts in the upper limit of low-elevation species were heterogeneous, with nearly as many downslope shifts as upslope shifts (n = 17 upper limits, p = 0.315).
Figure 3.
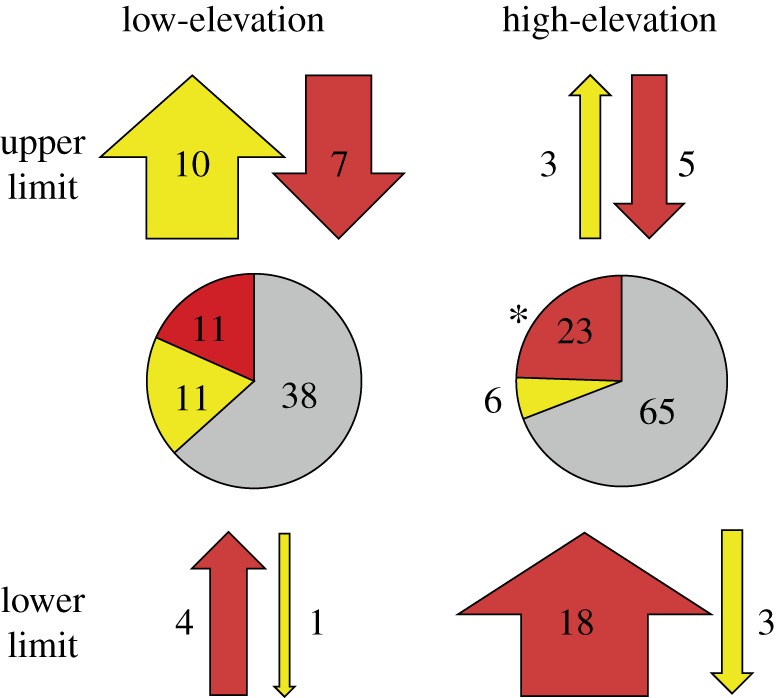
Number and proportion of upper and lower range shifts of high- and low-elevation species across all regions. Pie charts display the proportions of range limits that exhibited significant expansions (yellow), contractions (red), or no significant change (grey). Numbers represent the number of individual shifts observed in each category. An asterisk next to a pie indicates that significantly more contractions were observed than expansions. For each elevation and limit category, the arrows above and below each pie indicate the direction (up or down) and the number of shifts observed in each direction at each range limit, with the width of the arrow indicating the relative proportion observed within each category. The colours of arrows indicate whether the shift resulted in an expansion (yellow) or contraction (red).
(b). Range shifts in relation to local climate change
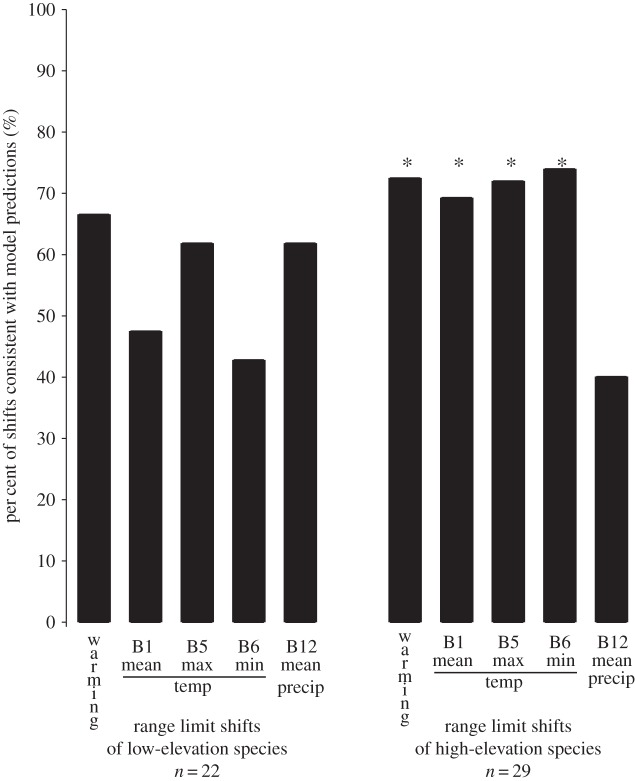
Nearest climatic neighbour analyses revealed that local-scale temperature change predicted shifts either upslope or downslope depending on location (figure 1c). For low-elevation species no model was significantly better than random (figure 4). By contrast, for high-elevation species, predictions from each of the four temperature variables were significantly better than random (figure 4, p < 0.05). Mean annual precipitation (40% of shifts) did not explain more shifts than a random model (p = 0.21). Change in minimum annual temperature was the best predictor of the direction of range limit shifts and explained 74.1% of range limit shifts observed. However, change in minimum temperature was only a slight improvement over an overall warming model (72.4% of shifts), local mean annual temperature change (69.2% of shifts) and local maximum annual temperature (72.0% of shifts). Although temperature variables were highly correlated within eras (historical and modern: B1 versus B5, rS = 0.96; B1 versus B6, rS = 0.96; B5 versus B6, rS = 0.89), they did not always predict shifts in the same direction. Minimum temperature showed the most dramatic change between eras and varied across regions, whereas mean and maximum temperature did not (electronic supplementary material, figure S1e–h).
Figure 4.
Significant range limit shifts of high- and low-elevation species in relation to climate predictions. Bars represent the per cent of observed limit shifts that are consistent with the predictions from an overall warming model (i.e. all upwards) and with nearest neighbour analyses for each of the four BIOCLIM variables. Sample sizes indicate the number of range limit shifts within high- and low-elevation species that were significant from the Pfa analysis. Symbols above bars denote predictions that were significantly better than random (*p < 0.05).
Our nearest climatic neighbour analysis also identified climatic conditions that are disappearing from the landscape (i.e. a reduction of their historical geographical representation to less than half in the modern era). Twenty-one of the 134 historical sites in our study (15.7%) had climatic conditions that fit this definition of disappearing climates for at least one climate variable and all of these sites were above 1500 m elevation. Minimum annual temperature showed the most substantial effect, with values at 15% of historical sites disappearing from the modern landscape. Mean annual temperature (1.5% of historical sites), maximum annual temperature (3.7% of historical sites) and mean annual precipitation (0 historical sites) did not decline as dramatically across the landscape.
4. Discussion
Our results from a multi-region, community-wide analysis of elevational transects across montane California suggested strong but locally heterogeneous impacts of recent climate change on the range limits of small mammals. None of the species shifted both their upper and lower limits in the same direction in all three regions (figure 2). There were, however, consistent and climatically predictable upslope range contractions in high-elevation species, while low-elevation species exhibited heterogeneous and climatically unpredictable directionality of range limit shifts. High-elevation species that showed consistent range reduction included Belding's ground squirrel (Urocitellus beldingi), the alpine chipmunk (Tamias alpinus) that is endemic to central-southern montane California, the Pacific jumping mouse (Zapus princeps) and the water shrew (Sorex palutris). These observations have been confirmed in more extensive analyses of U. beldingi and T. alpinus [11,40]. Elevational ranges of the pika (O. princeps), which attracted considerable attention because of extirpations and upslope retractions in the Great Basin [41,42], were stable across all three regions. This result is consistent with a more extensive study across montane California that found pika thriving across wider geographical and elevation ranges than reported historically [43].
While a coherent pattern of upslope movement was found for high-elevation species, there was substantial heterogeneity in the response of low-elevation species. The vast majority of sites in our study, especially at mid- to high elevations, were located in protected reserves or public lands with minimal land-use conversion, although grazing, fire regimes and forestry practices may have altered habitat structure [44]. One might expect that low-elevation species should be more likely to experience impacts from land-use change at their lower limits in the Central Valley and foothills [45,46]. However, we detected few contractions at lower limits of low-elevation mammals (figure 3), and shifts were significantly more common at their upper limits where potential land-use impacts were less evident. Greater heterogeneity in responses of low-elevation species may reflect stronger biotic influences [25,31], such as interspecific competition [40], seral dynamics of habitats [31] and the spread of invasive species [32]. Indeed, for the Central region, low-elevation species tracked changes in the extent of preferred habitats more closely than high-elevation taxa [46].
Heterogeneous range shifts have been demonstrated in a range of taxa [5,25], suggesting that species' responses to twentieth century climate change were both influenced by local factors and were context dependent. Tingley et al. [14] found even greater heterogeneity in Californian birds sampled over the past century in the same regions; only half the observed range limit shifts of birds were upslope. Thus, while our findings confirmed overall results from our initial study of small mammals in central montane California [13] and reflect heterogeneity observed in resurveys of birds [14], butterflies [47] and plants [28] over similar spatial and temporal scales, they amplify the complex and variable ways that species have changed over the past century in California [25]. Moreover, intra-species heterogeneity in range shifts appears widespread from our data but is probably under-reported in the literature owing to the infrequency of studies replicating range shift studies across spatially and ecophysically distinct survey regions.
Intra-species heterogeneity in range shifts among regions may be attributed to region-specific changes or local changes in temperature and precipitation [48]. In previous resurvey studies of birds and plants across the same regions, local changes in precipitation as well as temperature were related to range changes [14,28]. For small mammals, however, temperature was the only reliable predictor of the direction of shifts. Furthermore, local-scale minimum temperature change models provided only a slight improvement over an overall warming model, suggesting that local-scale climate models, at least as implemented in this study, cannot explain all the spatial heterogeneity in species' responses. Nevertheless, increased minimum temperatures are particularly troubling for mammals. While mammals can avoid heat stress by behavioural means (e.g. shifting daily activity), warming winters lead to increased energy expenditures for hibernators [49] and reduce the snow layer, which acts as insulation for non-hibernators [50].
Global climate projections suggest that disappearing climates will be an increasing challenge for predicting future species' responses [51,52]. Across the Sierra Nevada, minimum temperature values (but not precipitation) recorded at several historical sites are disappearing from the landscape (i.e. declining from >5% of grid cells in the historical era to <2.5% of grid cells in the modern era of our study). These conditions leave species with fewer options for shifting their ranges to compensate for changing temperature. Consistent with the contractions observed for several high-elevation mammal species, all sites with disappearing climates occur above 1500 m elevation [53,54].
Our rigorous study of elevational range shifts of mammals across montane California revealed heterogeneous responses of species within and among regions that were consistent with studies of other taxa [25] but that were most influenced by temperature change consistent with twentieth century warming [2,5]. A suite of high-elevation mammals appears to be undergoing range contraction amid disappearing high-elevation climates. The challenge ahead is to understand the proximate causes of the diverse species' responses to improve predictions of vulnerability [6]. We need a better understanding of whether and how species track climatic niches in response to local variation in climate change [30], as our tests of species' responses to local climate failed to demonstrate a strong improvement in range limit shift predictions. We also need a better understanding of whether range changes are mediated by other non-climatic processes such as ecosystem dynamics or species interactions. Moreover, identifying the life-history traits (e.g. dispersal ability, reproductive rate and degree of ecological specialization) that best predict persistence or vulnerability [9,55,56] may provide key insights into the mechanisms of species- and region-specific responses to climate change. The diverse responses among closely related taxa that we find here (e.g. among different species of mice, chipmunks, ground squirrels and woodrats) provide the basis for the detailed comparative studies that are necessary to improve our knowledge of vulnerability.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the numerous field resurvey team participants, data miners and land agencies (US National Park Service, US Forest Service, Bureau of Land Management, and California Fish and Game) for their contribution and support.
Ethics statement
All animals were trapped and collected in the modern era following procedures approved by the UC Berkeley Animal Care and Use Committee (Permit number R278–0315).
Data accessibility
Primary data for this study are available online via the MVZ website (http://arctos.database.museum/project/historic-grinnell-survey-lassen-transect, http://arctos.database.museum/project/historic-grinnell-survey-yosemite-transect, http://arctos.database.museum/project/historic-grinnell-survey-southern-sierra-nevada-transect, http://arctos.database.museum/project/grinnell-resurvey-project-lassen-transect, http://arctos.database.museum/project/grinnell-resurvey-project-yosemite-transect, http://arctos.database.museum/project/grinnell-resurvey-project-southern-sierra-nevada-transect). Our occupancy analysis input file for MARK is available on Dryad Digital Repository at http://doi:10.5061/dryad.pp37p.
Funding statement
This project was supported financially by the Yosemite Foundation, the National Parks Service and National Science Foundation (DEB 064859).
References
- 1.Walther G, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J, Hoegh-Guldberg O, Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395. ( 10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 2.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 3.Root T, Price JT, Hall KR, Schneider SH, Pounds JA. 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60. ( 10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 4.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 5.Chen I, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 6.Moritz CM, Agudo R. 2013. The future of species under climate change: resilience or decline? Science 341, 504–508. ( 10.1126/science.1237190) [DOI] [PubMed] [Google Scholar]
- 7.Thomas CD, Lennon JJ. 1999. Birds extend their ranges northwards. Nature 399, 213 ( 10.1038/20335) [DOI] [Google Scholar]
- 8.Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H. 2008. A significant upward shift in plant species optimum elevation during the 20th century. Science 320, 1768–1771. ( 10.1126/science.1156831) [DOI] [PubMed] [Google Scholar]
- 9.Angert AL, Crozier LG, Rissler LJ, Gilman SE, Tewksbury JJ, Chunco AJ. 2011. Do species’ traits predict recent shifts at expanding range edges? Ecol. Lett. 14, 677–689. ( 10.1111/j.1461-0248.2011.01620.x) [DOI] [PubMed] [Google Scholar]
- 10.Hill JK, Griffiths HM, Thomas CD. 2011. Climate change and evolutionary adaptations at species’ range margins. Annu. Rev. Entomol. 56, 143–159. ( 10.1146/annurev-ento-120709-144746) [DOI] [PubMed] [Google Scholar]
- 11.Morelli T, Smith AB, Kastley CR, Mastroserio I, Moritz C, Beissinger SR. 2012. Anthropogenic refugia ameliorate the severe climate-related decline of a montane mammal along its trailing edge. Proc. R. Soc. B 279, 4279–4286. ( 10.1098/rspb.2012.1301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parmesan C, et al. 1999. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399, 579–583. ( 10.1038/21181) [DOI] [Google Scholar]
- 13.Moritz C, Patton JL, Conroy CJ, Parra JL, White GC, Beissinger SR. 2008. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 322, 261–264. ( 10.1126/science.1163428) [DOI] [PubMed] [Google Scholar]
- 14.Tingley MW, Koo MS, Moritz C, Rush AC, Beissinger SR. 2012. The push and pull of climate change causes heterogeneous shifts in avian elevational ranges. Glob. Change Biol. 18, 3279–3290. ( 10.1111/j.1365-2486.2012.02784.x) [DOI] [Google Scholar]
- 15.Pinsky ML, Worm B, Fogarty MJ, Sarmiento JL, Levin SA. 2013. Marine taxa track local climate velocities. Science 341, 1239–1242. ( 10.1126/science.1239352) [DOI] [PubMed] [Google Scholar]
- 16.Tingley MW, Beissinger SR. 2009. Detecting range shifts from historical species occurrences: new perspectives on old data. Trends Ecol. Evol. 24, 625–633. ( 10.1016/j.tree.2009.05.009) [DOI] [PubMed] [Google Scholar]
- 17.Gottfried M, et al. 2012. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2, 111–115. ( 10.1038/nclimate1329) [DOI] [Google Scholar]
- 18.Menéndez R, González-Megías A, Jay-Robert P, Marquéz-Ferrando R. 2014. Climate change and elevational range shifts: evidence from dung beetles in two European mountain ranges. Glob. Ecol. Biogeogr. 23, 646–657. ( 10.1111/geb.12142) [DOI] [Google Scholar]
- 19.Grinnell J, Storer TI. 1924. Animal life in the Yosemite: an account of the mammals, birds, reptiles, and amphibians in a cross-section of the Sierra Nevada. Berkeley, IL: University of California Press. [Google Scholar]
- 20.Grinnell J, Dixon JS, Linsdale JM. 1930. Vertebrate natural nistory of a section of northern California through the Lassen Peak Region. Berkeley, IL: University of California Press. [Google Scholar]
- 21.Sumner L, Dixon JS. 1953. Birds and mammals of the Sierra Nevada: with records from Sequoia and Kings Canyon National Parks. Berkeley, IL: University of California Press. [Google Scholar]
- 22.Grinnell J. 1917. The niche-relationships of the California Thrasher. Auk 34, 427–433. ( 10.2307/4072271) [DOI] [Google Scholar]
- 23.Grinnell J. 1910. The methods and uses of a research museum. Pop. Sci. Mon. 77, 163–169. [Google Scholar]
- 24.Bonfils C, Duffy PB, Santer BD, Wigley TML, Lobell DB, Phillips TJ, Doutriaux C. 2008. Identification of external influences on temperatures in California. Clim. Change 87(Suppl. 1), S43–S55. ( 10.1007/s10584-007-9374-9) [DOI] [Google Scholar]
- 25.Rapacciuolo G, et al. 2014. Beyond a warming fingerprint : individualistic biogeographic responses to heterogeneous climate change in California. Glob. Change Biol. 29, 2841–2855. ( 10.1111/gcb.12638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobrowski SZ, Abatzoglou J, Swanson AK, Greenberg JA, Mynsberge AR, Holden ZA, Schwartz MK. 2013. The climate velocity of the contiguous United States during the 20th century. Glob. Change Biol. 19, 241–251. ( 10.1111/gcb.12026) [DOI] [PubMed] [Google Scholar]
- 27.Kelly AE, Goulden ML. 2008. Rapid shifts in plant distribution with recent climate change. Proc. Natl Acad. Sci. USA 105, 11 823–11 826. ( 10.1073/pnas.0802891105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crimmins SM, Dobrowski SZ, Greenberg JA, Abatzoglou JT, Mynsberge AR. 2011. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 331, 324–327. ( 10.1126/science.1199040) [DOI] [PubMed] [Google Scholar]
- 29.Hargrove L, Rotenberry JT. 2011. Breeding success at the range margin of a desert species: implications for a climate-induced elevational shift. Oikos 120, 1568–1576. ( 10.1111/j.1600-0706.2011.19284.x) [DOI] [Google Scholar]
- 30.Tingley MW, Monahan WB, Beissinger SR, Moritz C. 2009. Birds track their Grinnellian niche through a century of climate change. Proc. Natl Acad. Sci. USA 106(Suppl. 2), 19 637–19 643. ( 10.1073/pnas.0901562106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe RJ, Finarelli JA, Rickart EA. 2010. Range dynamics of small mammals along an elevational gradient over an 80-year interval. Glob. Change Biol. 16, 2930–2943. ( 10.1111/j.1365-2486.2009.02150.x) [DOI] [Google Scholar]
- 32.Rowe RJ, Terry RC, Rickart EA. 2011. Environmental change and declining resource availability for small-mammal communities in the Great Basin. Ecology 92, 1366–1375. ( 10.1890/10-1634.1) [DOI] [PubMed] [Google Scholar]
- 33.Yang D, Conroy CJ, Moritz C. 2011. Contrasting responses of Peromyscus mice of Yosemite National Park to recent climate change. Glob. Change Biol. 17, 2559–2566. ( 10.1111/j.1365-2486.2011.02394.x) [DOI] [Google Scholar]
- 34.White GC, Burnham KP. 1999. Program MARK: survival estimation from populations of marked animals. Bird Study 46(Suppl. 001), S120–S139. ( 10.1080/00063659909477239) [DOI] [Google Scholar]
- 35.MacKenzie DI, Nichols JD, Lachman GB, Droege S, Royle JA, Langtimm CA. 2002. Estimating site occupancy rates when detection probabilities are less than one. Ecology 83, 2248–2255. ( 10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2) [DOI] [Google Scholar]
- 36.Laake JL. 2013. RMark: an R interface for analysis of capture–recapture data with MARK. Alaska Fish. Sci. Cent. Processed Rep. 2013–01, p. 25.
- 37.Burnham KP, Anderson DR. 2002. Model selection and multi-model inference: a practical information-theoretic approach. New York, NY: Springer. [Google Scholar]
- 38.Bates D, Maechler M. 2009. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375–32.
- 39.Daly C, Gibson WP, Taylor GH, Johnson GL, Pasteris P. 2002. A knowledge-based approach to the statistical mapping of climate. Clim. Res. 22, 99–113. ( 10.3354/cr022099) [DOI] [Google Scholar]
- 40.Rubidge EM, Monahan WB, Parra JL, Cameron SE, Brashares JS. 2011. The role of climate, habitat, and species co-occurrence as drivers of change in small mammal distributions over the past century. Glob. Change Biol. 17, 696–708. ( 10.1111/j.1365-2486.2010.02297.x) [DOI] [Google Scholar]
- 41.Beever EA, Brussard PF, Berger J. 2003. Patterns of apparent extirpation among isolated populations of pikas (Ochotona princeps) in the Great Basin. J. Mammal. 84, 37–54. () [DOI] [Google Scholar]
- 42.Beever EA, Ray C, Wilkening JL, Brussard PF, Mote PW. 2011. Contemporary climate change alters the pace and drivers of extinction. Glob. Change Biol. 17, 2054–2070. ( 10.1111/j.1365-2486.2010.02389.x) [DOI] [Google Scholar]
- 43.Millar CI, Westfall RD. 2010. Distribution and climatic relationships of the American Pika (Ochotona princeps) in the Sierra Nevada and Western Great Basin, U.S.A.; periglacial landforms as refugia in warming climates. Arct. Antarct. Alp. Res. 42, 76–88. ( 10.1657/1938-4246-42.1.76) [DOI] [Google Scholar]
- 44.Collins BM, Everett RG, Stephens SL. 2011. Impacts of fire exclusion and recent managed fire on forest structure in old growth Sierra Nevada mixed-conifer forests. Ecosphere 2, 51 ( 10.1890/ES11-00026.1) [DOI] [Google Scholar]
- 45.Nogués-Bravo D, Araújo MB, Romdal T, Rahbek C. 2008. Scale effects and human impact on the elevational species richness gradients. Nature 453, 216–219. ( 10.1038/nature06812) [DOI] [PubMed] [Google Scholar]
- 46.Santos MJ, Thorne JH, Moritz C. 2014. Synchronicity in elevation range shifts among small mammal and vegetation over the last century is stronger for omnivores. Ecography 37, 1–13. ( 10.1111/ecog.00931) [DOI] [Google Scholar]
- 47.Forister ML, McCall AC, Sanders NJ, Fordyce JA, Thorne JH, O'Brien J, Waetjen DP, Shapiro AM. 2010. Compounded effects of climate change and habitat alteration shift patterns of butterfly diversity. Proc. Natl Acad. Sci. USA 107, 2088–2092. ( 10.1073/pnas.0909686107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrison S, Damschen EI, Grace JB. 2010. Ecological contingency in the effects of climatic warming on forest herb communities. Proc. Natl Acad. Sci. USA 107, 19 362–19 367. ( 10.1073/pnas.1006823107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Humphries M, Thomas D, Speakman J. 2002. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature 418, 313–316. ( 10.1038/nature00903.1) [DOI] [PubMed] [Google Scholar]
- 50.Hayhoe K, et al. 2004. Emissions pathways, climate change, and impacts on California. Proc. Natl Acad. Sci. USA 101, 12 422–12 427. ( 10.1073/pnas.0404500101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams JW, Jackson ST, Kutzbach JE. 2007. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738–5742. ( 10.1073/pnas.0606292104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burrows MT, et al. 2014. Geographical limits to species-range shifts are suggested by climate velocity. Nature 507, 492–495. ( 10.1038/nature12976) [DOI] [PubMed] [Google Scholar]
- 53.Kullman L. 2010. A richer, greener, and smaller alpine world: review and projection of warming-induced plant cover change in the Swedish Scandes. Ambio 39, 159–169. ( 10.1007/s13280-010-0021-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dirnböck T, Essl F, Rabitsch W. 2011. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Glob. Change Biol. 17, 990–996. ( 10.1111/j.1365-2486.2010.02266.x) [DOI] [Google Scholar]
- 55.Schloss CA, Nuñez TA, Lawler JJ. 2012. Dispersal will limit ability of mammals to track climate change in the Western Hemisphere. Proc. Natl Acad. Sci. USA 109, 8606–8611. ( 10.1073/pnas.1116791109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearson RG, et al. 2014. Life history and spatial traits predict extinction risk due to climate change. Nature Clim. Change 4, 217–221. ( 10.1038/nclimate2113) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Primary data for this study are available online via the MVZ website (http://arctos.database.museum/project/historic-grinnell-survey-lassen-transect, http://arctos.database.museum/project/historic-grinnell-survey-yosemite-transect, http://arctos.database.museum/project/historic-grinnell-survey-southern-sierra-nevada-transect, http://arctos.database.museum/project/grinnell-resurvey-project-lassen-transect, http://arctos.database.museum/project/grinnell-resurvey-project-yosemite-transect, http://arctos.database.museum/project/grinnell-resurvey-project-southern-sierra-nevada-transect). Our occupancy analysis input file for MARK is available on Dryad Digital Repository at http://doi:10.5061/dryad.pp37p.