Abstract
Mating has profound effects on animal physiology and behaviour, not only in females but also in males, which we show here for olfactory responses. In cotton leafworm moths, Spodoptera littoralis, odour-mediated attraction to sex pheromone and plant volatiles are modulated after mating, producing a behavioural response that matches the physiological condition of the male insect. Unmated males are attracted by upwind flight to sex pheromone released by calling females, as well as to volatiles of lilac flowers and green leaves of the host plant cotton, signalling adult food and mating sites, respectively. Mating temporarily abolishes male attraction to females and host plant odour, but does not diminish attraction to flowers. This behavioural modulation is correlated with a response modulation in the olfactory system, as shown by electro-physiological recordings from antennae and by functional imaging of the antennal lobe, using natural odours and synthetic compounds. An effect of mating on the olfactory responses to pheromone and cotton plant volatiles but not to lilac flowers indicates the presence of functionally independent neural circuits within the olfactory system. Our results indicate that these circuits interconnect and weigh perception of social and habitat odour signals to generate appropriate behavioural responses according to mating state.
Keywords: olfactory modulation, behaviour, mating, Spodoptera littoralis, electrophysiology, host plant attraction
1. Introduction
Animals express stereotypic behaviours that are controlled by sensorimotor circuits and modulated by external and internal factors [1]. Reproductive state, for example, modulates sensory processing and thus influences behavioural choices. In females of the cotton leafworm, Spodoptera littoralis (Lepidoptera, Noctuidae), mating modulates olfactory sensitivity and switches odour-mediated attraction from floral food to green host plant cues [2]. A similar behavioural switch in preference, from pheromone to host cues, has been observed in female fruit flies [3] and parasitoid wasps [4,5].
By contrast, little is known about how mating affects behavioural responses to olfactory signals in male insects. Mating-induced downregulation of male attraction to sex pheromone has been investigated in the parasitoid wasp, Spalangia endius, and the noctuid moth, Agrotis ipsilon [6,7]. In A. ipsilon males, this behavioural inhibition is correlated with a decreased sensitivity of antennal lobe (AL) neurons to the conspecific sex pheromone [7–9] and an increased response to a plant odour [9,10]. Available data also suggest that mating-induced changes in the olfactory response are distinct for pheromone and non-pheromone coding pathways [9]. In moths and other animals, males often show a post-ejaculatory refractory period of sexual inactivity but the role of the olfactory system in this behavioural modulation is not understood [6,7,11].
Cotton leafworm, S. littoralis, is a suitable species to study peripheral and central olfactory modulation. Its behaviour in response to pheromone and plant odours, and the physiology and morphology of the olfactory apparatus, have been extensively studied (e.g. [2,12–17]). We have investigated whether mating-induced olfactory and behavioural modulation to floral and green cues observed in female moths [2] is sex specific, and whether the response to female sex pheromone is modulated in males after mating.
We show here that males show a mating-induced olfactory and behavioural modulation to green host plant cues, albeit the response is distinct from the female switch of preference from floral to green [2]. After mating, S. littoralis males show a reversible downregulation in their attraction to green mating site plant odours, and additionally to conspecific female sex pheromone. This behavioural modulation is reflected by changes in sensitivity of the peripheral olfactory system and the responses elicited in the AL. By contrast, the behavioural and olfactory response to floral odour is not significantly affected by mating. Our findings indicate the presence of separate olfactory circuits, each responding differentially to mating-induced modulation.
2. Material and methods
(a). Insects
Spodoptera littoralis were reared under controlled conditions on semi-artificial diet under a 16 L : 8 D photoperiod [2]. Two-to-three day-old unmated males, and males 3 h or 24 h post mating, were used in the analyses. All analyses of virgin and mated males were started after 4 h of the scotophase. For timing the start of experiments with mated males, about 30 males and females were placed together, either at the beginning of the scotophase (for testing them 3 h post mating) or 3 h after beginning of the scotophase (for testing them 24 h post mating). Most moths started mating within a few minutes, which resulted in a sufficiently high number of males that finished mating within the given mating period of 1 h.
(b). Plant material
Cotton plants (Gossypium hirsutum) were grown from seeds (produced in-house from a cultivar of DPL 90, Delta and Pine Land Company, Scott, MS, USA) in a greenhouse, and lilac (Syringa vulgaris) flowers were field-collected in Alnarp, Sweden [2].
(c). Plant headspace collection and chemical analyses
Headspace collections from both non-flowering cotton plants and lilac flowers were performed using a dynamic headspace apparatus, drawing charcoal-filtered air over the plant materials onto an air filter with Super Q adsorbent (80/100 mesh, Altech, Deerfield, IL, USA) [2]. Trapped plant volatiles were subsequently eluted from the filter and analysed by gas chromatography-mass spectrometry (GC–MS; 6890 GC and 5975 MS; Agilent Technologies, Palo Alto, CA, USA), as described earlier [2].
(d). Female pheromone gland extraction and chemical analysis
Pheromone glands of calling, two-to-three day-old, unmated S. littoralis females were dissected from the extruded ovipositors using a pair of fine forceps. Pheromone glands were extracted in redistilled heptane (Labscan, 20 µl/10 glands) as previously described [18]. A subsample of extracts was used for chemical analysis. For bioassays, extracts were pooled and divided into volumes equivalent to 10 female glands. Extracts were stored in sealed glass capillaries at –20°C until analysis.
Chemical analysis of pheromone extracts was performed by GC–MS, equipped with a fused silica DB-Wax capillary column (30 m × 0.25 mm, d.f. = 0.25 µm; J&W Scientific, Folsom, CA, USA). Helium was used as carrier gas at an average linear flow of 35 cm s−1. The initial temperature of the GC was set to 80°C (5 min hold) and then increased 10°C min−1 to a final temperature of 225°C (10 min hold). Pheromone components were identified by co-injection of authentic synthetic samples and comparison with a NIST mass spectra library (Agilent) and our own library [18].
(e). Synthetic chemicals
Synthetic compounds used for electroantennogram (EAG) and optical imaging (OI) analyses included plant-related components (benzaldehyde, benzyl methyl ether, (E)-β-ocimene, phenylacetaldehyde, acetophenone, benzyl alcohol, (+)-(S)-linalool, 1,4-dimethoxy benzene, estragole, β-myrcene, Z3-hexenyl acetate, p-cymene, (+)-(R)-limonene and nonanal), female-emitted sex pheromone components (Z9,E11-14Ac, Z9,E12-14Ac, Z9-14Ac, E11-14Ac, Z11-14Ac and 14Ac) and the synthetic mimic of the sex pheromone blend (ratio according to [18], 10(Z9,E11-14Ac) : 0.4(Z9,E12-14Ac) : 1(Z11-14Ac) : 2(E11-14Ac) : 3(Z9-14Ac) : 0.3(14Ac); see electronic supplementary material, table S5, for further details on the synthetic chemicals). For single sensillum recordings (SSRs), Z9,E11-14Ac, Z9,E12-14Ac and Z9-14Ac were tested individually. Synthetic compounds were diluted in decadic steps in redistilled n-hexane (Labscan) except for benzyl alcohol, which was diluted in diethyl ether (Fluka).
(f). Wind tunnel bioassay
Wind tunnel experiments were performed according to the protocol described earlier with cotton plant, lilac flowers [2] or calling females [18] (approximately five calling females at a time) as attractants. Take-off, upwind-directed flight and landing at the source were recorded. Behavioural inhibition as described in the Results section for males 3 h post mating (§3(a) Wind tunnel) was observed within the first hour after mating. However, the time required for preparation of mated males for OI (see below) set the starting time for experiments to 3 h post mating at the earliest (corresponding to 4 h after onset of the scotophase for virgin and mated moths).
(g). Electro-physiological and optical imaging recordings
Gas chromatography–electroantennodetection (GC–EAD) analysis with simultaneous (1 : 1 split) flame ionization detection (GC–FID) of the plant headspace or pheromone extracts, as well as stationary EAG recordings, were done as previously described [2]. Single sensillum recordings were performed from the antennal long trichoid sensilla known to respond to pheromone components [16].
Optical imaging was performed as described before [2], with the exception of using Calcium Green-1 AM (Invitrogen) instead of Calcium Green-2 AM. In short, the head of a fixed moth was dissected to expose the brain, bathed for 1 h in Calcium Green-1 AM and then rinsed with Ringer solution and transferred to a microscope (Olympus, Tokyo, Japan) for imaging of odour-evoked responses. Glomerular numbering was based on the number assignment of Couton et al. [19].
(h). Odour stimulation for electroantennogram, sensillum recording and optical imaging
Odour stimulation for EAG and OI recordings was performed according to earlier described practice [2], with loadings on filter paper (1 × 1 cm) ranging from 1 ng to 100 µg or 1 mg for individual plant-related and pheromone components, 0.001–100 min equivalents for cotton plant and lilac flower headspace collections, and 0.001–100 female equivalents (FE) for the synthetic pheromone blend. Odour delivery from filter paper and physiological stimulation are dependent on compound-specific evaporation rates. Stimulus loadings of individual plant and pheromone compounds (1 ng–100 µg or 1 mg) are therefore not equivalent to absolute amounts inducing stimulation. The applied loadings, however, cover a suitable range for physiological stimulation and offer the possibility for replication of the described procedure.
Because of identical OI responses to pheromone gland extract and the synthetic mimic of the pheromone blend (based on GC–MS qualification and quantification, see §2e), EAG and OI experiments were carried out using the synthetic mimic. Odour stimulation for SSR experiments was done as described before [16] with doses ranging from 1 ng to 100 µg.
(i). Statistical analysis
Contrast analysis was used to analyse the effect of mating status (all possible comparisons between virgin, 3 h and 24 h post mating) on the behavioural response of males to lilac flowers, cotton plant or calling females, using a generalized linear model (GzLM) with quasi-binomial distribution [20]. Repeated-measurements (RM) ANOVA, followed by Bonferroni post hoc test, were used to compare the EAG and OI responses, respectively, between unmated and mated males to sequential dilutions of the headspace extracts, the sex pheromone blend and the individual synthetic components of both. The non-parametric Kruskal–Wallis test followed by Dunn's post hoc test were used to compare SSR spike frequencies induced by sequential dilutions of the pheromone components in unmated and mated males.
To estimate the congruence (magnitude of biological effects by mating among assays) between behavioural (wind tunnel) and physiological (EAG and OI) data, we calculated the effect size, a unitless measure, which scales the differences between a pair of means by their pooled standard deviation (as the conservative, bias corrected Hedges' g) [21]. For OI, we added the values of responding glomeruli (#17, 18 and 37 for pheromone; #5, 6, 7, 10, 11 and 15 for cotton, and #8, 10 and 16 for lilac) to get only one data point per assay and stimuli, similar to EAG measurements. Similarly, take-off, as the primary step of odour-mediated upwind flight behaviour, was used to calculate effect size for the wind tunnel assay. The stimuli and dose used for each such effect size corresponded to a 10 min collection of volatiles of plants or 10 FE, respectively.
Analyses were done with R-Studio software v. 0.96.316 (R Development Core Team, Free Software Foundation Boston, MA, USA), Graphpad Prism v. 5.0a, and IBM SPSS.19.
3. Results
(a). Wind tunnel
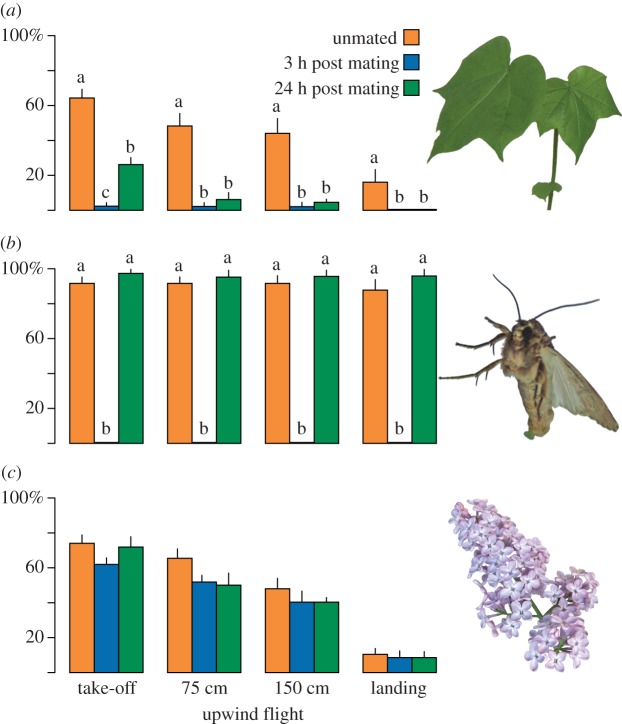
Changes in odour-mediated upwind flight attraction illustrated a mating-induced olfactory modulation in male S. littoralis. Male moths were attracted to social and plant cues depending on odour stimulus, mating status and time post mating (figure 1). Unmated males were attracted to calling females, cotton plants and lilac flowers (figure 1). Pheromone-mediated upwind flight was followed by landing at the calling female (figure 1b), and several unmated males landed also on cotton or lilac flowers (figure 1a,c). Shortly after mating, the response to cotton plants and calling females was entirely abolished. However, 24 h after mating, the response to calling females was fully restored and the response to cotton plant was partially restored (figure 1a,b). In comparison, males were highly attracted to lilac flowers, independent of mating status (figure 1c).
Figure 1.
Upwind attraction of unmated and mated (3 or 24 h post mating) male S. littoralis to (a) cotton plant G. hirsutum, (b) calling S. littoralis females and (c) lilac flowers Sy. vulgaris in a wind tunnel (mean ± s.e.m., n = 50). Different letters indicate significant differences (p < 0.05) according to GzLM with quasi-binomial distribution. (Online version in colour.)
(b). Gas chromatography–electroantennodetection and gas chromatography–mass spectrometry
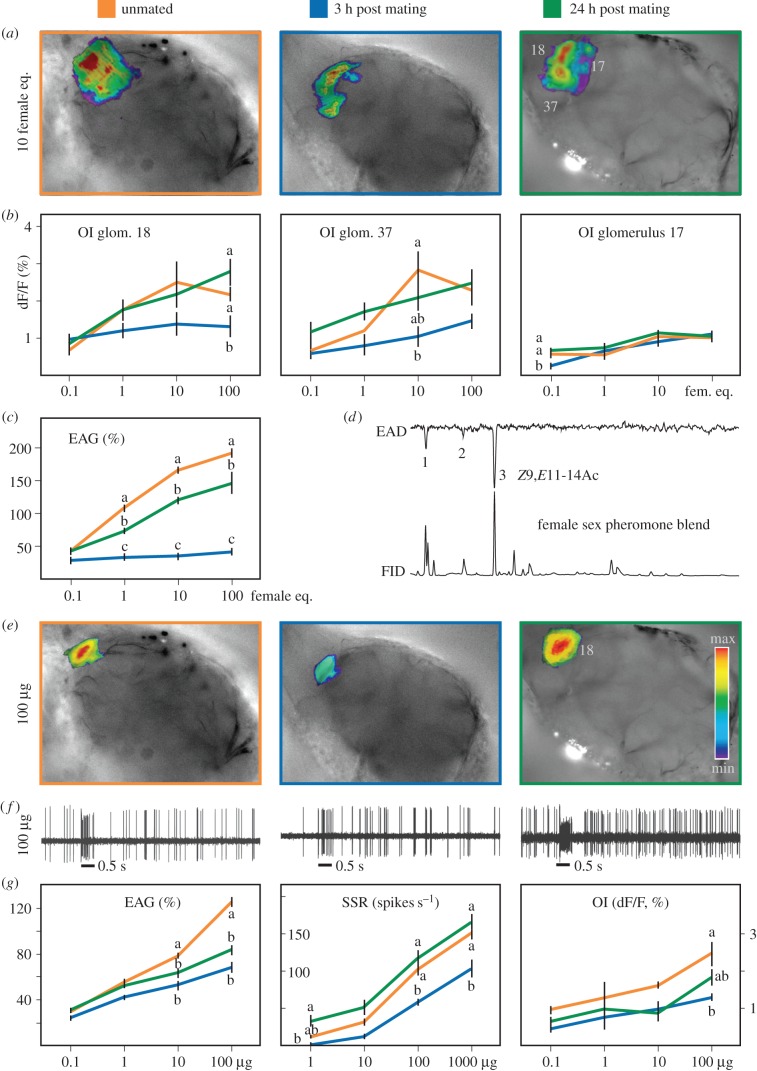
GC–EAD and GC–MS analyses were performed to identify antennally active compounds for subsequent studies on olfactory modulation by EAG, SSR and OI. Three active components in the female pheromone gland extract elicited a response in male antennae, Z9,E11-14Ac, Z9,E12-14Ac and Z9-14Ac (figure 2d).
Figure 2.
Optical imaging and electro-physiological recordings of unmated and mated (3 or 24 h post mating) male S. littoralis olfactory responses to different loadings of (a–c) the synthetic pheromone blend and (e–g) the main component of the pheromone blend (Z9,E11-14Ac) by OI, EAG and SSR. (d) Antennal active components in the pheromone gland extract are (1) Z9-14Ac, (2) Z9,E12-14Ac and (3) Z9,E11-14Ac as identified by synchronous signals from GC–FID and the coupled electroantennodetection (EAD). (f) SSR recordings from unmated (left) and mated males, 3 h (middle) or 24 h (right) post mating. (c,g left) EAG (%), (f,g middle) SSR (spikes s−1) responses and (a,b,e,g right) OI intensities (ΔF/F; %) obtained on antennal lobe glomeruli. Stimulus loadings from 0.1 to 100 FE for the synthetic pheromone blend and (0.1 – 100 µg) for Z9,E11-14Ac were applied on filter paper. Insignificant baseline responses at lower loadings are not shown. Different letters within the same dose indicate significant difference (p < 0.05, Bonferroni post hoc tests following two-way RM ANOVA for EAG and OI; Dunn's multiple comparison following Kruskal–Wallis test for SSR). SSR: horizontal bar represents 0.5 s. (Online version in colour.)
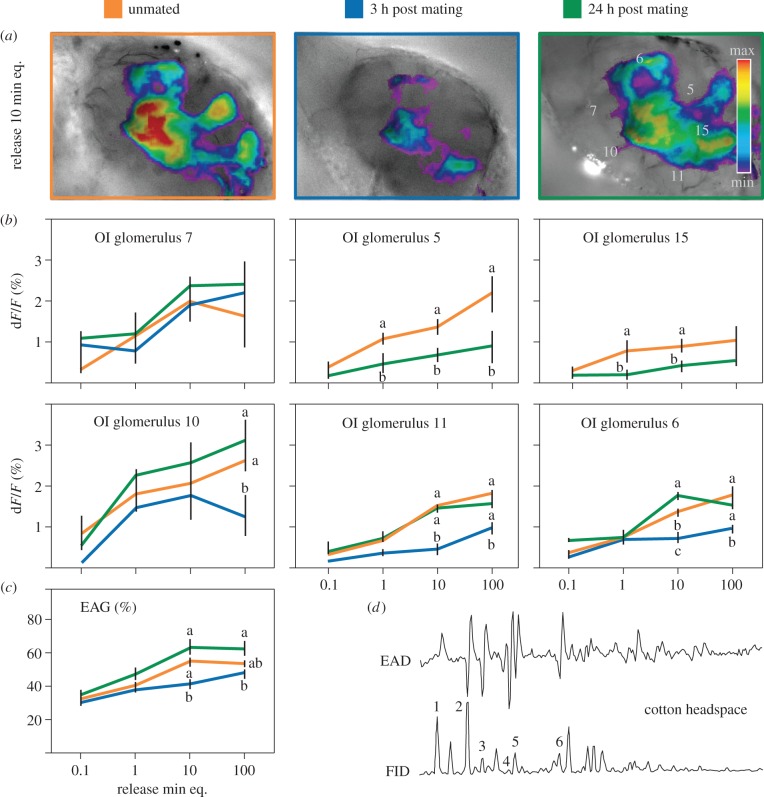
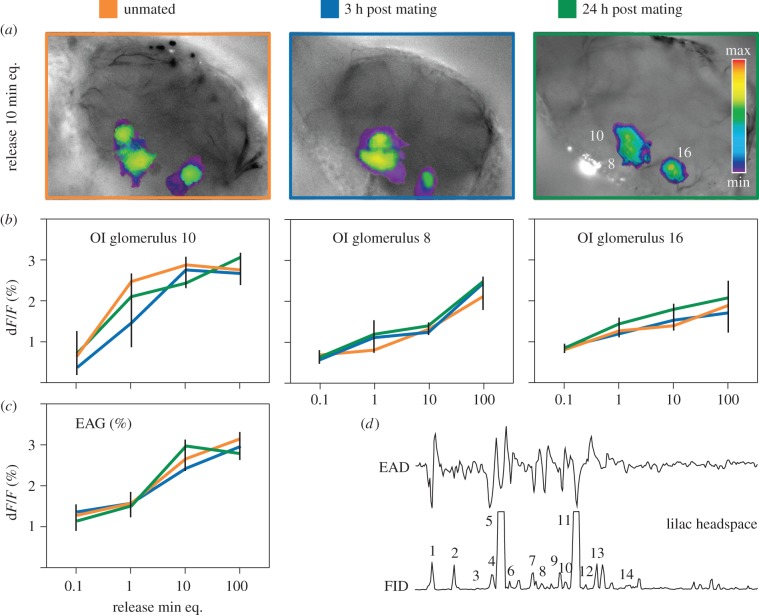
In headspace collections from cotton plants and lilac flowers, 6 and 14 compounds, respectively, elicited an antennal response (figures 3d and 4d). Benzaldehyde was the only compound co-occurring in leaf and flower headspace.
Figure 3.
(a,b) The OI of antennal lobe glomeruli and (c) EAG responses of unmated and mated (3 or 24 h post mating) male S. littoralis to authentic cotton head space. (d) GC–EAD active components are (1) benzaldehyde, (2) β-myrcene, (3) (Z)-3-hexenyl acetate, (4) p-cymene, (5) (+)-(R)-limonene and (6) nonanal. EAG responses (%) and optical imaging intensities (ΔF/F; %) obtained with increasing loadings (applied on filter paper) of cotton plant headspace samples equivalent to 0.1–100 min of sampling. Insignificant baseline responses at lower loadings are not shown. Different letters within the same dose indicate significant differences (p < 0.05, Bonferroni post hoc tests following two-way RM ANOVA for EAG and OI). (Online version in colour.)
Figure 4.
(a,b) The OI of antennal lobe glomeruli and (c) EAG responses of unmated and mated (3 or 24 h post mating) male S. littoralis to authentic lilac head-space. (d) GC–EAD active components are (1) benzaldehyde, (2) benzyl methyl ether, (3) 4-methylanisol, (4) (Z)-β-ocimene, (5) (E)-β-ocimene, (6) acetophenone, (7) unknown, (8) (+)-(S)-linalool, (9) lilac aldehyde A, (10) lilac aldehyde B, (11) 1,4-dimethoxy benzene, (12) unknown, (13) estragol and (14) lilac alcohols. EAG responses (%) and optical imaging intensities (ΔF/F; %) obtained with increasing loadings (applied on filter paper) of lilac flower headspace samples equivalent to 0.1–100 min of sampling. Insignificant baseline responses at lower loadings are not shown. There were no significant differences between mating status. (Online version in colour.)
(c). Electroantennogram
Olfactory modulation at the antennal level was then studied by EAG, applying a synthetic pheromone blend, plant headspace samples as well as single pheromone or plant components. Three hours after mating, the antennal response of mated males to a synthetic pheromone blend was significantly lower than in unmated males (figure 2c). Tested singly, three of the six synthetic components induced a significantly different response in males 3 h post mating compared with unmated ones: Z9,E11-14Ac (figure 2g), Z9,E12-14Ac and Z9-14Ac (electronic supplementary material, figure S1). Twenty-four hours after mating, the antennal responses to these components had partially recovered (figure 2c,g; electronic supplementary material, figure S1).
In agreement with attraction to cotton (figure 1a), EAG responses to cotton headspace were lowest in males 3 h after mating (figure 3c). Two of the six cotton volatiles producing an antennal response, β-myrcene and p-cymene, elicited significantly different responses in unmated males and males 3 h post mating (electronic supplementary material, figure S2).
Stimulation with lilac flower headspace revealed no significant difference in response of unmated and mated males (figure 4c), in accordance with a consistent behavioural response (figure 1c). None of the nine lilac flower components tested with EAG showed differences in response amplitudes between unmated males and males 3 h after mating (electronic supplementary material, figure S3).
(d). Sensillum recording
Next, modulation at the sensory neuron level was studied for the three pheromone components that showed modulation in EAG recordings. Single sensillum recordings from long trichoid sensilla confirmed the transient downregulation observed in EAG recordings. Spike frequencies elicited by Z9,E11-14Ac (figure 2g) and Z9-14Ac (electronic supplementary material, figure S4a) were decreased in males 3 h after mating, compared with unmated males. Responses did not differ between unmated males and males tested 24 h post mating (figure 2g; electronic supplementary material, figure S4a).
In addition to a change in action potential frequency, temporal patterns of the sensory neuron responses differed according to mating state. Neurons of unmated males and males 24 h after mating showed phasic responses to Z9,E11-14Ac, whereas responses in males 3 h post mating were phasic-tonic (figure 2f; electronic supplementary material, figure S4b). Similar temporal characteristics were observed for neurons responding to Z9-14Ac and Z9,E12-14Ac (data not shown).
(e). Optical imaging
Mating-dependent modulation of the antennal responses, as measured by EAG and SSR, was corroborated by functional imaging of the AL. The synthetic pheromone blend evoked visible calcium activity in three AL glomeruli (figure 2a,b). At the highest dose, the activity of glomerulus 18 was significantly decreased in males 3 h post mating compared with unmated males, and males 24 h post mating. Three pheromone components, Z9,E11-14Ac, Z9,E12-14Ac and Z9-14Ac elicited visible responses in the AL (figure 2e; electronic supplementary material, figure S1). The main compound Z9,E11-14Ac (figure 2g) and Z9-14Ac (electronic supplementary material, figure S1) elicited a significantly lower response 3 h after mating.
Mating-dependent modulation was also visible in response to cotton volatiles. Cotton plant headspace evoked activity in six glomeruli in unmated males. Calcium activity in these glomeruli was either completely or partially downregulated 3 h after mating; activity was either partially or fully restored after 24 h (figure 3a,b). Of the six cotton volatiles eliciting antennal response, four elicited AL activity: benzaldehyde, Z3-hexenyl acetate, p-cymene and nonanal (electronic supplementary material, figure S2). Responses to p-cymene and nonanal were significantly lower 3 h after mating; there was no difference in males before and 24 h after mating for any of the cotton volatiles (electronic supplementary material, figure S2).
In contrast to stimulation with pheromone or cotton headspace, no AL modulation was observed in response to lilac odour in three responding glomeruli (figure 4a,b). Six out of nine antennally active lilac volatiles (benzaldehyde, benzyl methyl ether, phenyl acetaldehyde, acetophenone, benzyl alcohol and (S)-(+)-linalool) evoked glomerular activity, but no significant difference in unmated and mated males was observed (electronic supplementary material, figure S3).
(f). Neuronal and behavioural responses reflect the concurrent modulation to sex and host plant cues
We finally analysed the congruence of effects induced by mating among assays. Analyses of effect size showed that the behavioural modulation, measured by wind tunnel responses, to three different stimulus types was reflected in neuronal responses measured by EAG and OI. Declines 3 h post mating and the reversed effects 24 h after mating were similar in effect size and confirmed olfactory modulation as a result of mating.
The changes measured with different techniques, for each stimulus, mirror each other in the sense that magnitudes of decline and recovery effects are numerically similar. Hence, there is a strong correlation (r2 = 0.83) between electro-physiological, imaging and behavioural experiments, with an intercept of zero and a slope close to unity (figure 5). The magnitude of decline (3 h) and recovery (24 h after mating) differs among stimuli, where pheromone and cotton stimuli induce strong effect sizes (|g| > 1 and several were 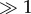 ; figure 5). In response to lilac, none of the techniques revealed a strong modulation (|g| > 1). The confidence interval for the effect size shows that six out of six effects (mating and recovery for each of the three techniques) were significant for both pheromone and cotton. In comparison, only one (recovery EAG) of six possible effects was significant for lilac (electronic supplementary material, table S6). Hence, a strong concurrent modulation of neuronal and behavioural responses was manifest in response to sex and host plant cues only.
; figure 5). In response to lilac, none of the techniques revealed a strong modulation (|g| > 1). The confidence interval for the effect size shows that six out of six effects (mating and recovery for each of the three techniques) were significant for both pheromone and cotton. In comparison, only one (recovery EAG) of six possible effects was significant for lilac (electronic supplementary material, table S6). Hence, a strong concurrent modulation of neuronal and behavioural responses was manifest in response to sex and host plant cues only.
Figure 5.
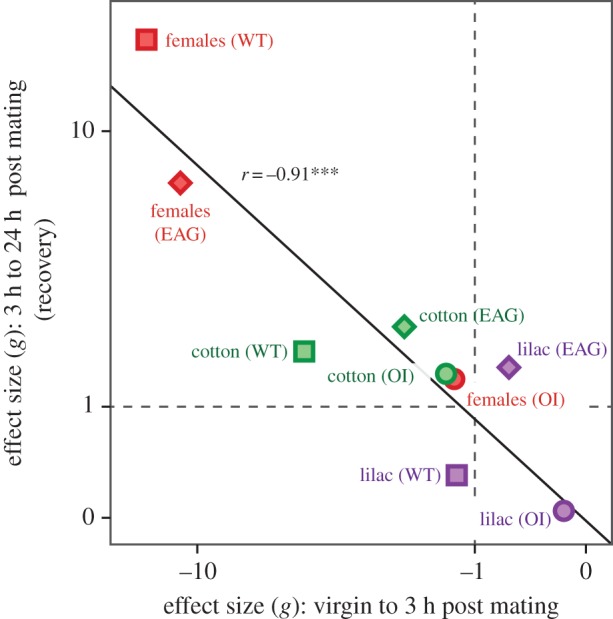
Behavioural and neuronal responses (biological effects determined in behavioural or physiological assays) reflect the concurrent modulation to sex and host plant cues. The observed modulation is demonstrated by a decline in responses 3 h after mating (appear as negative effect sizes on x-axis, ‘mating’) and a recovery after 24 h (positive effect sizes on y-axis, ‘recovery’). Effect sizes are estimates of differences between bioassay means scaled by their pooled standard deviations [21]. The effects that we estimated, for each combination of technique and stimuli, nicely mirror each other as their magnitudes of decline and recovery are numerically similar, giving a regression with an intercept of zero, a slope close to unity and hence a strong correlation (p = 0.0006; confirmed also by a non-parametric correlation rS = −0.88**). Squares, behavioural wind tunnel tests (WT); diamonds, physiology, EAG; and circles, physiology, OI. Colour codes used for stimuli: red, female sex pheromone; green, cotton leaf; and lilac, lilac flower adult food source. (Online version in colour.)
4. Discussion
Host-, food- and mate-finding in insects are distinct, experimentally tractable types of behaviour, which are largely guided by olfactory cues. We show that modulation of these odour-mediated behaviours enables S. littoralis male moths to respond to sensory stimuli in accordance with their physiological condition.
Furthermore, we show that odour-mediated responses to host plants and to females, which are elicited by different olfactory signals, are linked by a concurrent mating-induced modulation. The responses of S. littoralis males to sex pheromone and host plant volatiles are strongly inhibited or downregulated shortly after mating, but are partly or fully restored to pre-mating levels 24 h after mating. The concurrency of this response modulation is supported by the magnitude of behavioural and physiological effects. The response to floral odours, on the other hand, is not significantly affected by mating, which clearly demonstrates that male moths distinguish between floral odour and host plant odour, signalling adult food and mating sites, respectively. In addition, our comparative physiological analysis of antennae, single sensory neurons and the AL suggests that this modulation occurs already at the peripheral olfactory level.
For male moths, attraction to host plant volatiles has been reported in a few species (e.g. [22,23]), but modulation of flight attraction to host plants has not been described. In S. littoralis females, we have previously shown a mating-induced modulation of attraction behaviour to host plant and adult food odour [2].
Attraction to the floral scent of lilac and the green scent of non-flowering cotton in female and male S. littoralis suggests that the neural circuitry underpinning the behaviours related to foraging and host plant attraction is shared between the sexes. However, differential modulation of these circuits in males and females is sex specific and suggests differences in behavioural prioritization [24]. Females engage either in foraging or host-seeking behaviour depending on mating status [2], whereas males display plasticity in their response to host plant volatiles, but not to foraging cues. In addition, males display a drastic mating status-dependent plasticity in their response to sex pheromone.
The concurrent modulation of olfactory response to sex pheromone and host plant volatiles hints at a link between the two distinct, morphologically segregated, olfactory subsystems dedicated to environmental or social signals [25], and accordingly a common motif of neuromodulation. The fact that sex pheromones and volatiles of cotton, as a host plant and mating site, apparently are compounded into a context of social cues reflects a close ecological relevance of these signals. A similar sensory pathway has been suggested for Drosophila melanogaster, in which feeding sites often equate to mating sites and where food odours promote male courtship behaviour [26]. Plant volatiles are also known to synergize attraction to sex pheromone in moths [27–32], reflecting a profound integration of these signals in the AL [33,34]. This emphasizes the idea that sex pheromone is naturally embedded in a host plant volatile background for male moths [27,34,35].
In moths as in other sexually reproducing animals, mating is costly and sperm production in males that mate with several females is generally limited [36–38]. Reproductive investments of male butterflies deplete with multiple mating and male mating-history effects female fitness [39–41]. Accordingly, lepidopteran females generally have a higher reproductive output after mating with a virgin male than after mating with a previously mated male [42]. A refractory period will likely increase male reproductive capacity as it allows replenishment of sperm and accessory gland secretions, and balancing of metabolic energy by food intake and avoiding fruitless arousal and sexual activity. In D. melanogaster, offspring production decreases with successive male matings and females discriminate against recently mated males but not against males that had time to replenish their sperm reserves [38]. Similar mechanisms may exist in S. littoralis. Our data suggest that a strong downregulation of response to both females and host plant rendezvous sites in freshly mated males abolishes subsequent investments in unproductive matings. By contrast, continuous responsiveness to food odour (flowers) likely manifests the requirement of energy for prolonged flight, refilling sex accessory glands and the production of new spermatophores [7,11,36].
Mechanisms of olfactory and behavioural modulation in insects have been studied in D. melanogaster. In female flies, modulation of a number of physiological and behavioural responses is induced by the male sex peptide and other seminal fluid components [43–49]. Neuromodulators mediate behavioural flexibility in response to internal physiological state [1,24] and Drosophila tachykinin or short neuropeptide F were shown to modulate presynaptic activities in olfactory sensory neurons affecting odour-guided behaviour [50,51]. It is plausible that similar mechanisms exist in S. littoralis males, in which mating may regulate the release of neuroactive substances leading to the observed changes in olfactory sensitivity and olfactory-guided behaviour. One such neuroactive substance could be the biogenic amine octopamine, which has been shown to selectively modulate the activity of pheromone sensitive sensilla, but not the sensilla responding to generic plant odours [52]. Likewise, juvenile hormone has been shown to selectively modulate pheromone responses in the AL [53,54].
This study contributes to our understanding of the odour-mediated regulation of value-based decisions as a function of internal physiological condition. Our findings provide an important insight into how behavioural hierarchies can be altered through changes in the olfactory system. Ongoing studies aim at elucidating the neuromodulatory mechanism underlying the observed concurrent modulation of olfactory responses in male S. littoralis.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Funding statement
This study was supported by the Linnaeus initiative ‘Insect Chemical Ecology, Ethology and Evolution’ IC-E3 (The Swedish Research Council Formas, SLU).
References
- 1.Palmer CR, Kristan WB., Jr 2011. Contextual modulation of behavioral choice. Curr. Opin. Neurobiol. 21, 520–526. ( 10.1016/j.conb.2011.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saveer AM, et al. 2012. Floral to green: mating switches moth olfactory coding and preference. Proc. R. Soc. B 279, 2314–2322. ( 10.1098/rspb.2011.2710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang EB. 1995. Effects of mating and accessory gland injections on olfactory-mediated behavior in the female Mediterranean fruit fly, Cerratitis capitata. J. Insect Physiol. 41, 705–710. ( 10.1016/0022-1910(95)00015-M) [DOI] [Google Scholar]
- 4.Steiner S, Ruther J. 2009. Mechanism and behavioral context of male sex pheromone release in Nasonia vitripennis. J. Chem. Ecol. 35, 416–421. ( 10.1007/s10886-009-9624-6) [DOI] [PubMed] [Google Scholar]
- 5.Ruther J, Thal K, Blaul B, Steiner S. 2010. Behavioural switch in the sex pheromone response of Nasonia vitripennis females is linked to receptivity signalling. Anim. Behav. 80, 1035–1040. ( 10.1016/j.anbehav.2010.09.008) [DOI] [Google Scholar]
- 6.Fischer CR, King BH. 2008. Sexual inhibition in Spalangia endius males after mating and time for ejaculate replenishment. J. Insect Behav. 21, 1–8. ( 10.1007/s10905-007-9099-7) [DOI] [Google Scholar]
- 7.Gadenne C, Dufour MC, Anton S. 2001. Transient post-mating inhibition of behavioural and central nervous responses to sex pheromone in an insect. Proc. R. Soc. Lond. B 268, 1631–1635. ( 10.1098/rspb.2001.1710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrozo RB, Gadenne C, Anton S. 2010. Switching attraction to inhibition: mating-induced reversed role of sex pheromone in an insect. J. Exp. Biol. 213, 2933–2939. ( 10.1242/jeb.043430) [DOI] [PubMed] [Google Scholar]
- 9.Barrozo RB, Jarriault D, Deisig N, Gemeno C, Monsempes C, Lucas P, Gadenne C, Anton S. 2011. Mating-induced differential coding of plant odour and sex pheromone in a male moth. Eur. J. Neurosci. 33, 1841–1850. ( 10.1111/j.1460-9568.2011.07678.x) [DOI] [PubMed] [Google Scholar]
- 10.Deisig N, et al. 2012. Differential interactions of sex pheromone and plant odour in the olfactory pathway of a male moth. PLoS ONE 7, e33159 ( 10.1371/journal.pone.0033159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitecek S, Maria A, Blais C, Duportets L, Gaetner C, Dufour M-C, Siaussat D, Debernard S, Gadenne C. 2013. Is the rapid post-mating inhibition of pheromone response triggered by ecdysteroids or other factors from the sex accessory glands in the male moth Agrotis ipsilon? Hormones Behav. 63, 700–708. ( 10.1016/j.yhbeh.2013.03.010) [DOI] [PubMed] [Google Scholar]
- 12.Anderson P, Hansson BS, Löfqvist J. 1995. Plant-odour-specific receptor neurones on the antennae of female and male Spodoptera littoralis . Physiol. Entomol. 20, 189–198. ( 10.1111/j.1365-3032.1995.tb00001.x) [DOI] [Google Scholar]
- 13.Sadek MM, Hansson BS, Rospars JP, Anton S. 2002. Glomerular representation of plant volatiles and sex pheromone components in the antennal lobe of the female Spodoptera littoralis . J. Exp. Biol. 205, 1363–1376. [DOI] [PubMed] [Google Scholar]
- 14.Anderson P, Hansson BS, Nilsson U, Han Q, Sjöholm M, Skals N, Anton S. 2007. Increased behavioral and neuronal sensitivity to sex pheromone after brief odor experience in a moth. Chem. Senses 32, 483–491. ( 10.1093/chemse/bjm017) [DOI] [PubMed] [Google Scholar]
- 15.Martel V, Anderson P, Hansson BS, Schlyter F. 2009. Peripheral modulation of olfaction by physiological state in the Egyptian leaf worm Spodoptera littoralis (Lepidoptera: Noctuidae). J. Insect Physiol. 55, 793–797. ( 10.1016/j.jinsphys.2009.04.012) [DOI] [PubMed] [Google Scholar]
- 16.Binyameen M, Anderson P, Ignell R, Seada MA, Hansson BS, Schlyter F. 2012. Spatial organization of antennal olfactory sensory neurons in the female Spodoptera littoralis moth: differences in sensitivity and temporal characteristics. Chem. Senses 37, 613–629. ( 10.1093/chemse/bjs043) [DOI] [PubMed] [Google Scholar]
- 17.Anderson P, Sadek MM, Larsson MC, Hansson BS, Thöming G. 2013. Larval host plant experience modulates both mate finding and oviposition choice in a moth. Anim. Behav. 85, 1169–1175. ( 10.1016/j.anbehav.2013.03.00) [DOI] [Google Scholar]
- 18.Saveer AM, Becher PG, Birgersson G, Hansson BS, Witzgall P, Bengtsson M. 2014. Mate recognition and reproductive isolation in the sibling species Spodoptera littoralis and Spodoptera litura. Front. Ecol. Evol. 2, 18 ( 10.3389/fevo.2014.00018) [DOI] [Google Scholar]
- 19.Couton L, Minoli S, Kieu K, Anton S, Rospars J-P. 2009. Constancy and variability of identified glomeruli in antennal lobes: computational approach in Spodoptera littoralis . Cell Tissue Res. 337, 491–511. ( 10.1007/s00441-009-0831-9) [DOI] [PubMed] [Google Scholar]
- 20.McCullagh P, Nelder JA. 1989. Generalized linear models, monographs on statistics and applied probability. London, UK: Chapman & Hall. [Google Scholar]
- 21.Nakagawa S, Cuthill IC. 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. 82, 591–605. ( 10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- 22.Landolt PJ. 1989. Attraction of the cabbage looper to host plants and host plant odor in the laboratory. Entomol. Exp. Appl. 53, 117–124. ( 10.1111/j.1570-7458.1989.tb01295.x) [DOI] [Google Scholar]
- 23.Coracini M, Bengtsson M, Liblikas I, Witzgall P. 2004. Attraction of codling moth males to apple volatiles. Entomol. Exp. Appl. 110, 1–10. ( 10.1111/j.0013-8703.2004.00124.x) [DOI] [Google Scholar]
- 24.Mowrey WR, Portman DS. 2012. Sex differences in behavioral decision-making and the modulation of shared neural circuits. Biol. Sex Differ. 3, 8 ( 10.1186/2042-6410-3-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen TA, Hildebrand JG. 2002. Pheromonal and host-odor processing in the insect antennal lobe: how different? Curr. Opin. Neurobiol. 12, 393–399. ( 10.1016/S0959-4388(02)00336-7) [DOI] [PubMed] [Google Scholar]
- 26.Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GSXE, Benton R. 2011. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature 478, 236–240. ( 10.1038/nature10428) [DOI] [PubMed] [Google Scholar]
- 27.Landolt PJ, Phillips TW. 1997. Host plant influences on sex pheromone behavior of phytophagous insects. Annu. Rev. Entomol. 42, 371–391. ( 10.1146/annurev.ento.42.1.371) [DOI] [PubMed] [Google Scholar]
- 28.Ochieng SA, Park KC, Baker TC. 2002. Host plant volatiles synergize responses of sex pheromone-specific olfactory receptor neurons in male Helicoverpa zea. J. Comp. Physiol. A 188, 325–333. ( 10.1007/s00359-002-0308-8) [DOI] [PubMed] [Google Scholar]
- 29.Yang ZH, Bengtsson M, Witzgall P. 2004. Host plant volatiles synergize response to sex pheromone in codling moth, Cydia pomonella. J. Chem. Ecol. 30, 619–629. ( 10.1023/B:JOEC.0000018633.94002.af) [DOI] [PubMed] [Google Scholar]
- 30.Namiki S, Iwabuchi S, Kanzaki R. 2008. Representation of a mixture of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori. J. Comp. Physiol. A 194, 501–515. ( 10.1007/s00359-008-0325-3) [DOI] [PubMed] [Google Scholar]
- 31.Schmidt-Busser D, von Arx M, Guerin PM. 2009. Host plant volatiles serve to increase the response of male European grape berry moths, Eupoecilia ambiguella, to their sex pheromone. J. Comp. Physiol. A 195, 853–864. ( 10.1007/s00359-009-0464-1) [DOI] [PubMed] [Google Scholar]
- 32.von Arx M, Schmidt-Busser D, Guerin PM. 2012. Plant volatiles enhance behavioral responses of grapevine moth males, Lobesia botrana to sex pheromone. J. Chem. Ecol. 38, 222–225. ( 10.1007/s10886-012-0068-z) [DOI] [PubMed] [Google Scholar]
- 33.Trona F, Anfora G, Bengtsson M, Witzgall P, Ignell R. 2010. Coding and interaction of sex pheromone and plant volatile signals in the antennal lobe of the codling moth Cydia pomonella . J. Exp. Biol. 213, 4291–4303. ( 10.1242/jeb.047365) [DOI] [PubMed] [Google Scholar]
- 34.Trona F, Anfora G, Balkenius A, Bengtsson M, Tasin M, Knight A, Janz N, Witzgall P, Ignell R. 2013. Neural coding merges sex and habitat chemosensory signals in an insect herbivore. Proc. R. Soc. B 280, 20130267 ( 10.1098/rspb.2013.0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thöming G, Larsson MC, Hansson BS, Anderson P. 2013. Comparison of plant preference hierarchies of male and female moths and the impact of larval rearing hosts. Ecology 94, 1744–1752. ( 10.1890/12-0907.1) [DOI] [PubMed] [Google Scholar]
- 36.Dewsbury DA. 1982. Ejaculate cost and male choice. Am. Nat. 119, 601–610. ( 10.1086/283938) [DOI] [Google Scholar]
- 37.Svensson MGE, Marling E, Löfqvist J. 1998. Mating behavior and reproductive potential in the turnip moth Agrotis segetum (Lepidoptera: Noctuidae). J. Insect Behav. 11, 343–359. ( 10.1023/A:1020998513316) [DOI] [Google Scholar]
- 38.Loyau A, Blanchet S, Van Laere P, Clobert J, Danchin E. 2012. When not to copy: female fruit flies use sophisticated public information to avoid mated males. Sci. Rep. 2, 768 ( 10.1038/srep00768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carroll AL. 1994. Interactions between body size and mating history influence the reproductive success of males of a tortricid moth, Zeiraphera canadensis. Can. J. Zool. 72, 2124–2132. ( 10.1139/z94-284) [DOI] [Google Scholar]
- 40.Cook PA, Gage MJG. 1995. Effects of risks of sperm competition on the numbers of eupyrene and apyrene sperm ejaculated by the moth Plodia interpunctella (Lepidoptera: Pyralidae). Behav. Ecol. Sociobiol. 36, 261–268. ( 10.1007/BF00165835) [DOI] [Google Scholar]
- 41.Iyengar VK. 2009. Experience counts: females favor multiply mated males over chemically endowed virgins in a moth (Utetheisa ornatrix). Behav. Evol. Sociobiol. 63, 847–855. ( 10.1007/s00265-009-0724-7) [DOI] [Google Scholar]
- 42.Torres-Vila LM, Jennions MD. 2005. Male mating history and female fecundity in the Lepidoptera: do male virgins make better partners? Behav. Ecol. Sociobiol. 57, 318–326. ( 10.1007/s00265-004-0857-7) [DOI] [Google Scholar]
- 43.Peng J, Chen S, Busser S, Liu HF, Honegger T, Kubli E. 2005. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 15, 207–213. ( 10.1016/j.cub.2005.01.034) [DOI] [PubMed] [Google Scholar]
- 44.Kubli E. 2008. Sexual behaviour: a receptor for sex control in Drosophila females. Curr. Biol. 18, R210–R212. ( 10.1016/j.cub.2007.12.047) [DOI] [PubMed] [Google Scholar]
- 45.Yapici N, Kim YJ, Ribeiro C, Dickson BJ. 2008. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 451, 33–37. ( 10.1038/nature06483) [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro C, Dickson BJ. 2010. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila . Curr. Biol. 20, 1000–1005. ( 10.1016/j.cub.2010.03.061) [DOI] [PubMed] [Google Scholar]
- 47.Haussman IU, Hemai Y, Wijesekera T, Dauwalder B, Soller M. 2013. Multiple pathways mediate the sex-peptide-regulated switch in female Drosophila reproductive behaviours. Proc. R. Soc. B 280, 20131938 ( 10.1098/rspb.2013.1938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rezával C, Nojima T, Neville MC, Lin AC, Goodwin SF. 2014. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 24, 725–730. ( 10.1016/j.cub.2013.12.051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heifetz M, Lindner M, Garini Y, Wolfner MF. 2014. Mating regulates neuromodulator ensembles at nerve termini innervating the Drosophila reproductive tract. Curr. Biol. 24, 731–737. ( 10.1016/j.cub.2014.02.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ignell R, Root CM, Birse RT, Wang JW, Nässel DR, Winther ÅME. 2009. Presynaptic peptidergic modulation of olfactory receptor neurons in Drosophila. Proc. Natl Acad. Ssci. USA 106, 13 070–13 075. ( 10.1073/pnas.0813004106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Root CM, Ko KI, Jafari A, Wang JW. 2011. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell 145, 133–144. ( 10.1016/j.cell.2011.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pophof B. 2002. Octopamine enhances moth olfactory responses to pheromones, but not those to general odorants. J. Comp. Physiol. A 188, 659–662. ( 10.1007/s00359-002-0343-5) [DOI] [PubMed] [Google Scholar]
- 53.Gadenne C, Renou M, Sreng L. 1993. Hormonal control of pheromone responsiveness in the male black cutworm Agrotis ipsilon . Experientia 49, 721–724. ( 10.1007/BF01923960) [DOI] [Google Scholar]
- 54.Greiner B, Gadenne C, Anton S. 2002. Central processing of plant volatiles in Agrotis ipsilon males is age-independent in contrast to sex pheromone processing. Chem. Senses 27, 45–48. ( 10.1093/chemse/27.1.45) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.