Abstract
Neophobia—the generalized fear response to novel stimuli—provides the first potential strategy that predator-naive prey may use to survive initial predator encounters. This phenotype appears to be highly plastic and present in individuals experiencing high-risk environments, but rarer in those experiencing low-risk environments. Despite the appeal of this strategy as a ‘solution’ for prey naivety, we lack evidence that this strategy provides any fitness benefit to prey. Here, we compare the relative effect of environmental risk (high versus low) and predator-recognition training (predator-naive versus predator-experienced individuals) on the survival of juvenile fish in the wild. We found that juveniles raised in high-risk conditions survived better than those raised in low-risk conditions, providing the first empirical evidence that environmental risk, in the absence of any predator-specific information, affects the way naive prey survive in a novel environment. Both risk level and experience affected survival; however, the two factors did not interact, indicating that the information provided by both factors did not interfere or enhance each other. From a mechanistic viewpoint, this indicates that the combination of the two factors may increase the intensity, and hence efficacy, of prey evasion strategies, or that both factors provide qualitatively separate benefits that would result in an additive survival success.
Keywords: neophobia, predation risk, predator recognition, experience, naivety, survival
1. Introduction
Owing to the unforgiving nature of predation, prey species have evolved a number of ways to detect and avoid predators. These defensive traits can be linked to life-history adaptations, with individuals altering their age and/or size of maturation [1,2], or transitioning to the next life stage either earlier or later in order to decrease their risk of predation on their present or future life stage, respectively [3,4]. Other defences include altering morphological traits, such as changing body shape or growing defensive spines, to decrease probability of attack (deterrence) or capture by predators [5,6]. Behavioural adaptations are some of the most plastic and well-studied antipredator traits in prey, and encompass, for example, very short-term (whether or not to look up for vigilance or retreat for shelter) to longer-term decisions (which habitat to forage in or where to set up a territory). Regardless of the types of adaptations used, all prey face the same trade-off. Antipredator defences are costly, in terms of either investment or loss of opportunities, and their expressions should be weighed against their benefits [7]. A number of studies have shown that prey invest in defences in a threat-sensitive manner, i.e. increase their investment when the risk of predation is greater [8], but theory predicts that prey should always overestimate risk in situations of uncertainty in order to increase chances of survival [9].
The antipredator performance of individual prey is often linked to their age or experience. Young prey may suffer mortality due to either their inherent vulnerability (e.g. altricial young) or simply their lack of knowledge regarding predators (naivety). Although some predation-related cues may be recognized ‘innately’ by prey, such as those from injured conspecifics [10], for most species the basic recognition of whom their predators are, where they live and when they attack requires experience, i.e. learning [11,12]. In social species or those providing parental care, such information can be transmitted by more knowledgeable conspecifics [12,13]. In species lacking such social support, like most aquatic species, information must be acquired from personal experience or via publicly available information [14]. While acquiring predator-related information is a very efficient process where one exposure is often enough to retain the information [11], the question remains as to whether prey have adaptive ways to deal with their first, and often most dangerous, predator encounter.
Recent studies [15–17] demonstrated that inexperienced fish and larval amphibians raised in high-risk environments display neophobic responses to novel cues, while those raised in low-risk conditions do not. Similar generalized fear responses to novel stimuli have been documented in the context of foraging [18,19], but relatively little is known on the occurrence of this phenomenon in the context of predation. For example, Schleidt [20] showed that young turkeys display a fearful response to large silhouettes passing above them, a response pattern attributed to unfamiliarity. In the above-mentioned experiments, the background level of risk was provided by non-predator-specific cues such as those from injured conspecifics [15]. This allowed the prey to be informed about the risky nature of their environment without knowing the density, diversity of predators or types of predators causing this risk. These studies provided new insight as to how completely naive prey could survive first-time predator encounters, without knowing who the threat was. This generalized fear response to novel stimuli is likely very costly due the lost opportunities endured while responding to non-threatening stimuli, and should be favoured in environments where the risk of encountering a real threat is high. Despite the appealing nature of this strategy, no empirical evidence yet exists demonstrating a survival benefit associated with this trait.
The goal of our study was twofold. First, we aimed to test the hypothesis that predator-naive prey raised in a high-risk environment would benefit from a survival advantage driven by neophobic responses, when compared with predator-naive prey raised in a low-risk environment—hereby testing the effect of ‘environmental risk’. Second, we wanted to investigate the type of benefits (if any) provided by this neophobic response. If neophobia allowed predator-naive prey to respond to novel predator cues, then one could predict that it would provide the same survival benefits as those provided by experience—that is, the ability to display antipredator responses to predator cues. It has been shown that inexperienced prey trained in the laboratory to recognize a few common predators had much higher survival in the field, compared with prey that did not have this training [21]. If neophobia was qualitatively equivalent to predator-recognition training, at least during first encounters, we should predict that prey possessing neophobic responses only, training only or both would show similar survival rates. In fact, neophobic individuals may survive better than trained ones, as their antipredator response might not be limited to those few, previously learned predators. However, if having both a neophobic phenotype and training leads to a higher survival rate than either one of those options alone, then we could conclude that the type of benefits provided by the two strategies differ.
To test those predictions, we used a predator/prey system that would allow us to conduct survival trials in the most natural setting possible. The whitetail damselfish, Pomacentrus chrysurus, is a common coral reef fish in the Indo-Pacific region, typically associated with coral rubble in shallow (less than 10 m depth) reef waters. It has a bipartite life history typical of many reef fishes, with a planktonic larval stage maintained for approximately 20–25 days, before young fish recruit to coral reefs and transition to benthic juveniles that are highly territorial. This transition involves a severe population bottleneck, with more than 60% of individuals succumbing to predation within 1–2 days of settlement to the reef [22]. These figures illustrate the importance of predation in structuring these communities. The lack of parental care in this species and predator inexperience associated with this life-history transition to the reef makes these juvenile damselfish a perfect model species for our experiment.
2. Material and methods
(a). Test species
Settlement-stage juveniles of the whitetail damselfish were collected overnight using light traps [23] moored in open water seaward of the reef crest around Lizard Island (14°40′ S, 145°28′ E), in the northern Great Barrier Reef, Australia in November 2013. Fish were captured approximately 100 m away from the reef and hence were naive to the specific predators that awaited them upon settlement. This species naturally settles on rubble reef environments where juveniles are exposed to a diverse range of predators that use a variety of feeding modes including ambush (lizardfish, Synodus dermatogenys, and small cod, Cephalopholis microprion) and pursuit (dottybacks, Pseudochromis fuscus, and moonwrasse, Thalassoma lunare). These predatory fishes can be observed consuming juveniles that venture too far from shelter. We collected three individuals of each of three species of predators (lizardfish (83–89 mm standard length, SL), dottybacks (90–94 mm SL) and moonwrasse (102–119 mm SL)) by SCUBA using nets and/or clove oil. These individuals provided both predatory visual and chemical cues for our experiment.
(b). Risk background and predator training
Our experiment consisted of two phases. In the first phase, we manipulated the background level of risk for individual prey fish using previously established protocols [17]. Following capture, juvenile damselfish were taken to the laboratory and placed in groups of 10 in a series of 24 3 l flow-through plastic aquaria with a flow rate of approximately 3 l h−1. The fish were fed ad libitum with newly hatched brine shrimp three times per day and were allowed to acclimate for 24 h before starting the experimental treatment. Fish were then exposed to high- or low-risk conditions by introducing a solution of injured conspecific cues (hereafter, alarm cue—high risk) or a seawater control (low risk) into the tanks three times per day for 4 days. Half the fish (12 randomly selected tanks) received the high-risk treatment and the fish in the remaining half (12 tanks) received the low-risk treatment. The alarm cue solution was prepared minutes prior to being used by making six vertical cuts on each side of six freshly euthanized (cold-shocked and pithed) donor conspecifics (13–14 mm SL) using a scalpel, and then rinsing these fish in 60 ml of seawater. We injected 5 ml of this alarm cue solution into the conditioning tanks using a hose attached to a 5 ml syringe, giving a concentration of two cuts per litre when injected into the tanks. The timing of the three injections occurred randomly between 08.00 and 18.00 h with a minimum of 1.5 h between successive injections.
The second phase consisted of training the fish to recognize, both visually and chemically, three common predators on the reef, namely moonwrasse, dottyback and lizardfish, using a methodology similar to that of Lönnstedt et al. [21]. The day following the end of the first phase, fish were placed in 20 l flow-through tanks in groups of four fish, each fish of matching risk treatment (high or low risk) but coming from separate conditioning tanks. The tank contained a sandy substrate, an airstone and a small coral object at one end of the tank to provide shelter. The end of a 2 m long plastic tube was attached to the airstone. This tube was used to inject the chemical stimuli into the tank, close to the airstone to facilitate the distribution of the cues throughout the tank. Each injection of cue (alarm cues, predator odour or water controls—see below for cue preparation) was followed by a 60 ml injection of tank water (previously withdrawn from the tank), to ensure the stimulus was completely flushed into the tank and did not remain in the hose. The fish were left to acclimate for 2 h. The tanks from each risk treatment were randomly allocated to one of two training groups: the fish would learn to recognize the three predators (predator-recognition training group), or alternatively, they would undergo the same experimental manipulation, but would not learn any information about the predators (naive, untrained controls).
The training protocol consisted of three exposure blocks (one for each predator), each separated by 2 min. At the start of each block, 5 ml of alarm cues were injected into the tank. Seconds later, a clear, sealed, 1 l plastic bag filled with seawater and containing a small amount of sand (to ensure it would sink) and a live predator was gently lowered into the tank opposite to the coral object, and 20 ml of odour from that same predator was injected into the tank. This provided the fish with a reliable indicator of risk (alarm cues) paired with both visual and chemical cues from the predator. This pairing of alarm and predator cues has been shown many times to mediate learned predator recognition [10]. The predator was removed from the tank after 2 min, and we waited another 2 min before starting the next block. The order of presentation of the three predators was randomized across trials. The untrained control fish underwent the three-block procedure, with the exception that the bags contained sand with no predator (empty bags), and that the injections of predator odour were replaced by blank water controls; they still received the alarm cue solution, but were not provided with any information about predators. The alarm cue solution was prepared fresh, by euthanizing 10 conspecifics (13–14 mm SL), and making six cuts on each flank of each fish using a scalpel. The 10 fish were then rinsed in 120 ml of seawater. We injected 5 ml of this solution into each tank at the beginning of each block. The odour of the predators was collected fresh. We maintained three individuals from each of the three predator species into three 30 l flow-through tanks and fed them a heterospecific diet of apogonids, once every 2 days. Two hours prior to the use of the predator odour, the flow-through system was stopped, and the water volume in the tank dropped to 10 l to let the odour accumulate. We injected 20 ml of this solution during the training blocks. The juveniles were left undisturbed for 1 h, prior to being released onto the reef.
(c). Survival assay
We used a well-documented assay to assess survival [21,24,25]. Following their conditioning, fish were tagged in a random order with an elastomer, photographed and placed into individually labelled 1 l plastic bags filled with seawater. This tagging procedure has been shown not to affect fish survival [25]. The bags were kept in a water bath of flowing seawater until deployment in the field. To reduce transport and handling stress, fish in bags were transported to the field site in a 60 l bin of seawater (to reduce temperature fluctuations) under subdued light conditions.
Patch reefs used in the field experiment were composed of pieces of healthy and dead bushy hard coral, Pocillopora damicornis (approx. 18 × 15 × 18 cm), and placed on a sandflat, arranged 3 m apart, approximately 3 m away from the reef edge of the Lizard Island fringing reef. Biotic and abiotic conditions were similar throughout the range of the location of our patch reefs. Patches were cleared of any fishes or invertebrates using a hand net prior to release and one fish was randomly allocated to each patch reef. The divers were blind to the treatment when releasing the fish. A small wire cage (approx. 30 × 30 × 30 cm, 12 mm mesh size) was placed over the patch to allow the fish to acclimate to their new surroundings while being protected from predators. Cages were removed 40–60 min after release of the fish between 11.45 and 12.45 h. Fish presence was monitored twice daily (i.e. after the initial acclimation period, the evening after release and the following morning, etc.) for approximately 77 h. Again, divers were blind with respect to experimental treatments. Owing to their highly territorial nature, and the high mortality rate related to migrating to other reefs, juveniles released on the coral stay on the coral (100% survival observed with caged reefs), unless consumed by predators [24,25]. A total of 102 fish (n = 24–27 per treatment) were released for the survival experiment (SL: 13–14 mm)
(d). Statistical analyses
Survival (up to 77 h) of P. chrysurus among the four treatments (2 risk levels × 2 training treatments) was compared using multiple-sample survival analysis, which uses a Cox's proportional hazard model (Statistica 12.0). Survival curves for fish within each treatment were calculated and plotted using the Kaplan–Meier product-limit method. The Kaplan–Meier method is a non-parametric estimator of survival that incorporates incomplete (censored) observations, such as those cases where fish had not died by the end of the census period. Differences in fish survival between particular pairs of treatments were compared using the Cox–Mantel test with a Cox's F statistic. Unfortunately, this method does not allow us to test for any interactions between our two factors (risk and training). Hence, we used a logistic regression testing the effect of risk (high versus low), training (untrained versus trained) and their interaction on the state of the fish at the last census (dead versus alive).
3. Results
The survival analysis indicated a significant difference in survival among treatment groups (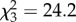 , p < 0.001), with high-risk fish surviving more than low-risk fish (Cox's F4,26 = 7.8, p < 0.001), and trained fish surviving more than untrained ones (Cox's F4,20 = 5.7, p = 0.003). Post-hoc comparisons revealed three outcomes: untrained fish in the high-risk group survived as well as trained fish in the low-risk group; trained fish in the high-risk group survived significantly more than fish in any other treatment (p < 0.001), while untrained fish in the low-risk group survived significantly less than fish in any other treatments (p < 0.001, figure 1).
, p < 0.001), with high-risk fish surviving more than low-risk fish (Cox's F4,26 = 7.8, p < 0.001), and trained fish surviving more than untrained ones (Cox's F4,20 = 5.7, p = 0.003). Post-hoc comparisons revealed three outcomes: untrained fish in the high-risk group survived as well as trained fish in the low-risk group; trained fish in the high-risk group survived significantly more than fish in any other treatment (p < 0.001), while untrained fish in the low-risk group survived significantly less than fish in any other treatments (p < 0.001, figure 1).
Figure 1.
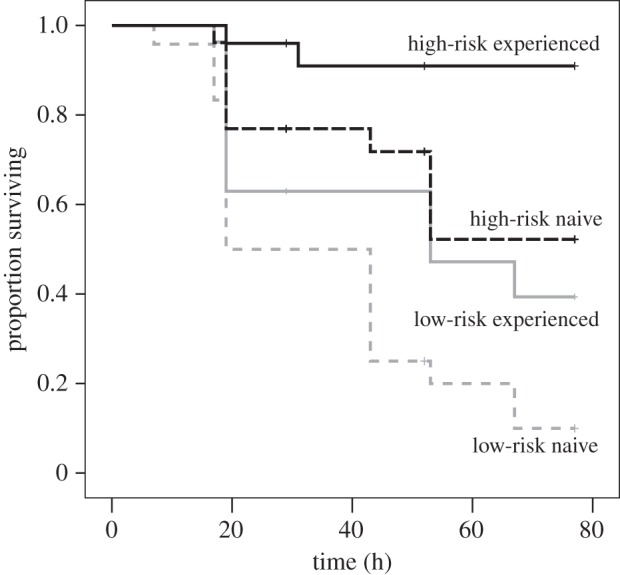
In situ survival curves (up to 77 h) of juvenile Pomacentrus chrysurus exposed to high- (black) or low- (grey) risk conditions for 4 days, and either experienced (solid lines) or naive (dashed lines) to three common predators.
The results of the logistic regression provided statistical support to the survival analysis, revealing a significant effect of risk (Wald: 8.1, d.f. = 1, p = 0.004) and training (Wald: 5.5, d.f. = 1, p = 0.019), but no interaction between the two factors (Wald: 0.002, d.f. = 1, p = 0.97).
4. Discussion
Fish from the high-risk environment survived significantly better than those from the low-risk environment, providing, we believe, the first empirical evidence that being raised in a high-risk environment—but in the absence of any predator-specific information—conferred a survival advantage. As expected, trained fish survived better than untrained fish, in line with previous studies [21]. Interestingly, untrained fish raised in a high-risk environment survived as well as trained fish coming from a low-risk environment. This could indicate that receiving predator-recognition training or coming from a high-risk environment provides prey with similar or redundant information, which would lead to similar survival rates. If it was the case that the information was redundant, however, we should expect that fish having both strategies (being trained and coming from a high-risk environment) would not survive any better than those being trained alone, or those coming from a high-risk environment alone. Statistically speaking, this would translate into an antagonistic interaction between the two factors. Yet, we found a lack of interaction between risk level and experience, indicating that, in fact, the benefits provided are additive. One of two scenarios could explain this pattern. First, the two strategies could lead to qualitatively similar response patterns (for instance, vigilance), but the addition of the second factor simply increases the intensity of the response, rendering it more effective. Alternatively, the benefits provided by each of two factors are different and, put together, provide additive benefits. For instance, the detection of known predator cues (training effect) may force the fish to spend more time being vigilant and less time foraging. Being raised in a high-risk environment (risk effect) may lead the juveniles to stay closer to shelter. Trained fish raised in a high-risk environment may stay close to shelter and also spend a greater time being vigilant, hence enjoying higher survival rates than those benefiting from only one of those strategies. However, we do not have underwater behavioural data to be able to tease apart those two alternatives.
While the survival benefits associated with neophobia are clear, one can wonder about the costs. Being able to survive predator encounters may come at a cost of missed foraging opportunities. But how severe are those losses and how long can an individual sustain them? Just like any antipredator strategy, one way to balance costs and benefits would be to invest in the response with an intensity that matches the perceived level of risk [8]. Recent work with cichlids, Amatitlania nigrofasciata, has indicated that the neophobic response was modulated, not by the intensity of the cue detected, but rather by the intensity of the background risk [16]. Indeed, fish exposed to high-risk conditions responded with the same intensity to increasing concentration of the odour of a rainbow trout, Oncorhynchus mykiss. This result seems sensible, given that naive prey may not have an innate sense of what is ‘concentrated’ or what is ‘dilute’, since different species vary in their ‘smelliness’ [26]. However, when the concentration of alarm cues used to create the background level of risk increased, the intensity of antipredator response displayed towards the predator cues increased. This threat-sensitive neophobia may modulate the relative costs of neophobia.
An unknown variable is the duration for which this neophobia is present. Presumably, repeated exposure to the same cue in the absence of negative reinforcement will lead to the extinction of the response, as the animal learns to categorize these cues as ‘non-threatening’. A similar phenomenon known as latent inhibition is present in non-neophobic individuals: the repeated exposure to an innocuous stimulus will prevent the one-time association usually documented between alarm cues and novel stimulus [27,28]. However, repeated pairing with risk does eventually result in an association between the previously ‘safe’ stimulus and risk [29], indicating a change in the ‘riskiness’ of the cue. A similar updating process would occur with risky stimuli, where initial uninformed neophobic responses would be replaced by well-informed learned responses, after an individual's experience allows it to categorize the cue as a known threat. Consequently, both benefits and costs provided by neophobia may be substantial during the initial exploring phase of a naive individual in a novel environment, but may wane quickly as individuals rely on experience to make decisions. More work is needed to fully comprehend the extent to which neophobia exists as a strategy in naive prey species, and the environmental factors that may affect the expression and duration of this phenotype.
Supplementary Material
Acknowledgements
We should like to thank all the staff at the Lizard Island Research Station, and all the students and volunteers that helped with the light traps and fish sorting. M.C.O.F. and D.P.C. designed the experiment; M.C.O.F. and D.P.C. conducted the laboratory portion of the study, M.I.M. and M.G.M. collected survival data, M.I.M. and M.C.O.F. analysed the data, M.C.O.F. wrote the first draft of the paper; all authors contributed to the final version of the manuscript.
Ethics statement
All work carried herein was in accordance with the James Cook University Animal Ethics guidelines (JCU Animal Ethics approval no. A2005, collection permit G12/35117.1).
Data accessibility
Data have been submitted as electronic supplementary material.
Funding statement
Funding was provided by an Australian Research Council Discovery Grant to M.I.M., M.C.O.F., D.P.C. and M.G.M.
Conflict of interests
We have no competing interests.
References
- 1.Abrams PA, Rowe L. 1996. The effects of predation on the age and size of maturity of prey. Evolution 50, 1052–1061. ( 10.2307/2410646) [DOI] [PubMed] [Google Scholar]
- 2.Appleton RD, Palmer AR. 1988. Water-borne stimuli released by predatory crabs and damaged prey induced more predator-resistant shells in a marine gastropod. Proc. Natl Acad. Sci. USA 85, 4387–4391. ( 10.1073/pnas.85.12.4387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benard MF. 2004. Predator-induced phenotypic plasticity in organisms with complex life histories. Annu. Rev. Ecol. Evol. Syst. 35, 651–673. ( 10.1146/annurev.ecolsys.35.021004.112426) [DOI] [Google Scholar]
- 4.Warkentin KM. 1995. Adaptive plasticity in hatching age: a response to predation risk trade-offs. Proc. Natl Acad. Sci. USA 92, 3507–3510. ( 10.1073/pnas.92.8.3507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. 2005. Ecological consequences of phenotypic plasticity. Trends Ecol. Evol. 20, 685–692. ( 10.1016/j.tree.2005.08.002) [DOI] [PubMed] [Google Scholar]
- 6.Arnqvist G, Johansson F. 1998. Ontogenetic reaction norms of predator-induced defensive morphology in dragonfly larvae. Ecology 79, 1847–1858. ( 10.1890/0012-9658(1998)079[1847:ornopi]2.0.co;2) [DOI] [Google Scholar]
- 7.Stankowich T, Blumstein DT. 2005. Fear in animals: a meta-analysis and review of risk assessment. Proc. R. Soc. B 272, 2627–2634. ( 10.1098/rspb.2005.3251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helfman GS. 1989. Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behav. Ecol. Sociobiol. 24, 47–58. ( 10.1007/BF00300117) [DOI] [Google Scholar]
- 9.Bouskila A, Blumstein DT. 1992. Rules of thumb for predation hazard assessment—predations from a dynamic model. Am. Nat. 139, 161–176. ( 10.1086/285318) [DOI] [Google Scholar]
- 10.Ferrari MCO, Wisenden BD, Chivers DP. 2010. Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can. J. Zool. 88, 698–724. ( 10.1139/Z10-029) [DOI] [Google Scholar]
- 11.Brown GE, Chivers DP. 2005. Learning as an adaptive response to predation. In Ecology of predator–prey interactions (eds Barbosa P, Castellanos I.), pp. 34–54. Oxford, UK: Oxford University Press. [Google Scholar]
- 12.Griffin AS. 2004. Social learning about predators: a review and prospectus. Learn. Behav. 32, 131–140. ( 10.3758/BF03196014) [DOI] [PubMed] [Google Scholar]
- 13.Crane AL, Ferrari MCO. 2013. Social learning of predation risk: a review and prospectus. In Social learning theory: phylogenetic considerations across animal, plant, and microbial taxa (ed. Clark KB.), pp. 53–82. New York, NY: Nova Science Publisher. [Google Scholar]
- 14.Wisenden BD, Chivers DP. 2006. The role of public chemical information in antipredator behaviour. In Communication in fishes (eds Ladich F, Collin SD, Moller P, Kapoor PG.), pp. 259–278. NJ: Science Publishers. [Google Scholar]
- 15.Brown GE, Ferrari MCO, Elvidge CK, Ramnarine I, Chivers DP. 2013. Phenotypically plastic neophobia: a response to variable predation risk. Proc. R. Soc. B 280, 20122712 ( 10.1098/rspb.2012.2712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown GE, Chivers DP, Elvidge CK, Jackson CD, Ferrari MCO. 2014. Background level of risk determines the intensity of predator neophobia in juvenile convict cichlids. Behav. Ecol. Sociobiol. 68, 127–133. ( 10.1007/s00265-013-1629-z) [DOI] [Google Scholar]
- 17.Chivers DP, McCormick MI, Mitchell MD, Ramasamy RA, Ferrari MC. 2014. Background level of risk determines how prey categorize predators and non-predators. Proc. R. Soc. B 281, 20140355 ( 10.1098/rspb.2014.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg R. 1990. Feeding neophobia and ecological plasticity: a test of the hypothesis with captive sparrows. Anim. Behav. 39, 375–379. ( 10.1016/S0003-3472(05)80884-X) [DOI] [Google Scholar]
- 19.Greenberg R, Mettke-Hofmann C. 2001. Ecological aspects of neophobia and neophilia in birds. Curr. Ornith. 16, 119–178. [Google Scholar]
- 20.Schleidt WM. 1961. Reaktionen von Truthühnern auf fliegende Raubvögel und Versuche zur Analyse ihrer AAM's. Zeitsch. Tierpsychol. 18, 534–560. ( 10.1111/j.1439-0310.1961.tb00241.x) [DOI] [Google Scholar]
- 21.Lönnstedt OM, McCormick MI, Meekan MG, Ferrari MCO, Chivers DP. 2012. Learn and live: predator experience and feeding history determines prey behaviour and survival. Proc. R. Soc. B 279, 2091–2098. ( 10.1098/rspb.2011.2516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almany GR, Webster MS. 2006. The predation gauntlet: early post-settlement mortality in reef fishes. Coral Reefs 25, 19–22. ( 10.1007/s00338-005-0044-y) [DOI] [Google Scholar]
- 23.Meekan MG, Wilson SG, Halford A, Retzel A. 2001. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar. Biol. 139, 373–381. ( 10.1007/s002270100577) [DOI] [Google Scholar]
- 24.Munday PL, Dixson DL, McCormick MI, Meekan M, Ferrari MCO, Chivers DP. 2010. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl Acad. Sci. USA 107, 12 930–12 934. ( 10.1073/pnas.1004519107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick MI, Holmes TH. 2006. Prey experience of predation influences mortality rates at settlement in a coral reef fish, Pomacentrus amboinensis. J. Fish Biol. 68, 969–974. ( 10.1111/j.1095-8649.2006.00982.x) [DOI] [Google Scholar]
- 26.Ferrari MCO, Capitania-Kwok T, Chivers DP. 2006. The role of learning in the acquisition of threat-sensitive responses to predator odours. Behav. Ecol. Sociobiol. 60, 522–527. ( 10.1007/s00265-006-0195-z) [DOI] [Google Scholar]
- 27.Acquistapace P, Hazlett BA, Gherardi F. 2003. Unsuccessful predation and learning of predator cues by crayfish. J. Crustacean Biol. 23, 364–370. ( 10.1163/20021975-99990346) [DOI] [Google Scholar]
- 28.Ferrari MCO, Chivers DP. 2006. The role of latent inhibition in acquired predator recognition by fathead minnows. Can. J. Zool. 84, 505–509. ( 10.1139/z06-027) [DOI] [Google Scholar]
- 29.Mitchell MD, McCormick MI, Ferrari MCO, Chivers DP. 2011. Friend or foe? The role of latent inhibition in predator and non-predator labelling by coral reef fishes. Anim. Cogn. 14, 707–714. ( 10.1007/s10071-011-0405-6) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data have been submitted as electronic supplementary material.


