Abstract
Mixed-species assemblages are often unintentionally introduced into new ecosystems. Analysing how assemblage structure varies during transport may provide insights into how introduction risk changes before propagules are released. Characterization of introduction risk is typically based on assessments of colonization pressure (CP, the number of species transported) and total propagule pressure (total PP, the total abundance of propagules released) associated with an invasion vector. Generally, invasion potential following introduction increases with greater CP or total PP. Here, we extend these assessments using rank-abundance distributions to examine how CP : total PP relationships change temporally in ballast water of ocean-going ships. Rank-abundance distributions and CP : total PP patterns varied widely between trans-Atlantic and trans-Pacific voyages, with the latter appearing to pose a much lower risk than the former. Responses also differed by taxonomic group, with invertebrates experiencing losses mainly in total PP, while diatoms and dinoflagellates sustained losses mainly in CP. In certain cases, open-ocean ballast water exchange appeared to increase introduction risk by uptake of new species or supplementation of existing ones. Our study demonstrates that rank-abundance distributions provide new insights into the utility of CP and PP in characterizing introduction risk.
Keywords: ballast water, colonization pressure, community structure, invasion vectors, invasive species, propagule pressure
1. Introduction
Ecologists have long been interested in the structure of biological communities, namely the species abundance distribution or relative abundance of all species recorded in an area [1]. Species abundance distributions have been studied extensively in natural systems to test models of community assembly including the unified neutral theory of biodiversity and biogeography, to compare multiple communities along environmental gradients, to monitor communities across time and to identify rare species for conservation [1]. Species abundance distributions are visualized by plotting log-abundance versus species rank [1], and are widely used in various sub-disciplines of ecology but rarely in invasion ecology [2,3].
Species assemblages are often unintentionally introduced into areas beyond their natural range by invasion vectors such as ships' ballast water or fouled hulls, bait worm packaging, straw and hay and wood dunnage [4], resulting in introduction of non-indigenous species. An introduction event begins with a vector that entrains individuals, often randomly, from a source community. If a sufficiently large number of individuals are entrained, many or all species in the source community may be represented within the transport vector [5]. Generally, only a fraction of entrained individuals and species survive transportation and are released into a new environment [2,6]. Vector management efforts such as open-ocean ballast water exchange (BWE) may further reduce the number of entrained individuals and species [6]. Therefore, the structure of the entrained assemblage may vary from that in the source community as it transitions through the transportation phase prior to release. Analysing how characteristics of an entrained assemblage vary over time during transport may provide insights into how introduction risk changes before propagules are released.
Invasion ecologists often focus on the number of transported species (i.e. colonization pressure (CP)) and their respective abundances (i.e. propagule pressure (PP) of individual species or of the entire taxonomic group (total PP)) when characterizing species assemblages transported by vectors [6,7]. Theoretical and empirical studies have demonstrated a positive relationship between PP of a species and its probability of establishment [8,9]. For a single introduction event, large PP reduces the likelihood of demographic stochasticity and Allee effects [8]. Similarly, greater CP also increases invasion risk by increasing the probability that at least one species will tolerate the new environment and form a reproducing population, as matching environmental conditions between source and recipient regions enhances probability of establishment [5]. Recently, researchers have begun to explore relationships between CP and PP for species assemblages entrained by vectors [5,10,11]. If species introduction is a random sampling process, CP should be positively related to PP because larger inocula will include more species than smaller ones [5].
While CP and PP are important parameters for characterizing introduction risk, they provide no information regarding the structure of the entrained assemblage. Given the stochastic nature of the entrainment process, many assemblage structures are plausible, ranging from uneven assemblages with steep rank-abundance gradients to those with shallow rank-abundance gradients [3]. The former case of uneven assemblage structure is of special concern because both mean abundance per species (mean PP) and total PP fail to describe the variation in abundances across species [3]. These measures may underestimate population size of dominant species and overestimate that of rare ones [3]. Given the strong ecological consequence of biased estimates, Drake et al. [3] argued that the rank-abundance distribution should be considered in addition to CP and PP when characterizing risk associated with transported species assemblages.
Briski et al.'s [2] conceptual model characterizing community dynamics in ballast water has not been tested empirically using repeated measurements during transportation, nor does it consider the effect of biogeographic source region or BWE. In this study, we examined changes in assemblage structure of different taxa in control and exchanged ballast water during trans-Atlantic and trans-Pacific voyages to Canada. We use rank-abundance distributions in transported assemblages to test three hypotheses: changes in assemblage structure and CP : total PP during transportation are the same for (i) different voyage routes or (ii) different taxonomic groups or (iii) in response to BWE.
2. Material and methods
(a). Plankton datasets
To analyse temporal variation in CP and total PP, we examine three datasets representing 16 invertebrate, 12 diatom and 8 dinoflagellate assemblages collected from ballast water during two trans-Atlantic and two trans-Pacific voyages, hereafter referred to as ‘Atlantic’ and ‘Pacific’, respectively (table 1). Datasets were selected on the basis of common methodologies and taxonomic resolution to the species level. Methods for sample collection, enumeration and identification are detailed elsewhere [12–15]. For Atlantic voyages, eight tanks were sampled for each taxonomic group at different time points (i.e. eight assemblages for each group). Only invertebrate and diatom data were available for Pacific voyages. For Pacific voyage 1, invertebrate and diatom samples were collected by repeatedly sampling four tanks at different time points. During Pacific voyage 2, four tanks were sampled for invertebrates only. As a result, eight invertebrate and four diatom assemblages were surveyed during Pacific voyages. For both Atlantic and Pacific voyages, half of tanks underwent BWE, hereafter referred to as ‘exchanged tanks'. Remaining tanks served as controls, hereafter ‘control tanks'. We compiled a database recording the number and identity of all species, total abundance of each species, sampling time point and date of BWE, if conducted, for each plankton assemblage. We excluded individuals that were not identified to species level because they could obscure the relationship between CP and total PP; approximately 70–95% of the data in each dataset was retained for analyses.
Table 1.
Plankton datasets used in this study including data source, voyage route (Atlantic versus Pacific), origin of ballast water, destination port, duration, taxonomic group (invertebrates, diatoms and dinoflagellates), BWE status (Y, yes; N, no) and number of sampled tanks.
| voyage route | origin of ballast water | destination port | duration (days) | taxonomic group | BWE (Y/N) | no. sampled tanks | data source |
|---|---|---|---|---|---|---|---|
| Atlantic 1 | Rotterdam, The Netherlands | Sept-Îles, Canada | 7 | invertebrates | Y N |
2 2 |
[12] |
| diatoms | Y N |
2 2 |
[12] | ||||
| dinoflagellates | Y N |
2 2 |
[12] | ||||
| Atlantic 2 | Rotterdam, The Netherlands | Sept-Îles, Canada | 9 | invertebrates | Y N |
2 2 |
[12] |
| diatoms | Y N |
2 2 |
[12] | ||||
| dinoflagellates | Y N |
2 2 |
[12] | ||||
| Pacific 1 | Hakata, Japan | Vancouver, Canada | 24 | invertebrates | Y N |
2 2 |
[13] |
| Pacific 2 | Osaka, Japan | Vancouver, Canada | 23 | invertebrates | Y N |
2 2 |
[13] |
| Pacific 1 | Hakata, Japan | Vancouver, Canada | 24 | diatoms | Y N |
2 2 |
[14] |
(b). Rank-abundance distributions and colonization pressure: total propagule pressure curves
For each plankton assemblage, we constructed a series of rank-abundance distributions at different time points during the transoceanic voyage. We then generated corresponding CP : total PP curves for each rank-abundance distribution using Monte Carlo simulations in R v. 3.0.2 [16]. Individuals were randomly selected from the empirical distribution, without replacement, with a sample size ranging from 1 to 2500 (total n). One thousand iterations were conducted at each sample size, with the resulting mean number of species (i.e. mean CP) determined for each total PP. In addition, we estimated asymptotic species richness for each assemblage at different time points using the Chao-1 estimator in SPADE v. 23 October 2012 to account for under-sampling [17]. While rank-abundance distributions and CP : total PP curves were estimated for each sampling day, we present results for only 5 days, for brevity, corresponding with the beginning, middle (particularly the last and first available time point immediately before and after BWE, respectively, in exchanged tanks) and end of voyages. We also compared results obtained from our rank-abundance distributions with those from more traditional analyses of total and mean PP, in order to examine differences in the methodologies.
Using existing models [2,3,5] as foundations, we developed a conceptual model describing four relative introduction risk scenarios: high risk: high CP and high total PP; moderate risk: high CP and low total PP or low CP and high total PP; and low risk: low CP and low total PP (figure 1a). We overlaid final CP and total PP values for the last sampling point on our model to estimate relative introduction risk among transported assemblages. All plankton abundance data were standardized to individuals m–3. Our comparisons assume uniform inherent invasibility, establishment probabilities and environmental suitability among transported species. We characterized total PP rather than mean PP because partitioning of total abundance evenly across all species makes it impossible to obtain high both CP and total PP. Our analysis estimates introduction risk per ballast sample. To estimate introduction risk for an individual vessel, one must multiply total PP and CP by total ballast discharge volume.
Figure 1.
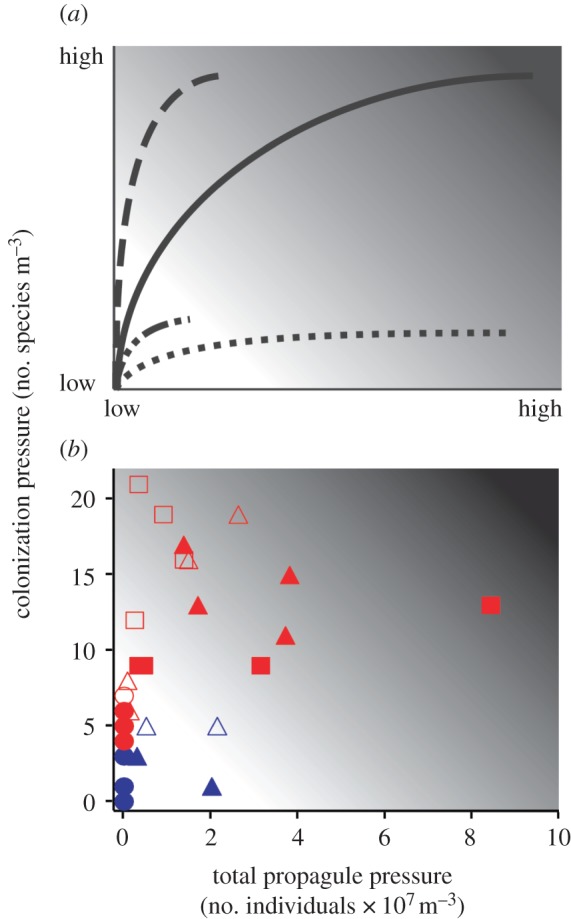
(a) Conceptual model of the relationship between CP and total PP for species assemblages translocated by a transport vector under four different scenarios: high CP and total PP for species-rich and high-abundance assemblages (solid line); high CP and low total PP for species-rich assemblages with low abundance of most or all species (dashed line); low CP and high total PP for species assemblages with at least one abundant species (dotted line); and low CP and total PP for assemblages represented by a few species in low numbers (dotted-dashed line). (b) Final CP and total PP for invertebrate (circles), diatom (triangles) and dinoflagellate (squares) assemblages at the end of two trans-Atlantic (red) and two trans-Pacific (blue) voyages. Solid and open markers denote assemblages collected from control and exchanged tanks, respectively. Background shading in both panels indicates relative introduction risk with light and dark shadings representing relatively low and high risk, respectively.
(c). Statistical analyses
We applied three broad analytical approaches using linear mixed-effects models to investigate patterns observed in rank-abundance distributions and CP : total PP curves for each taxonomic group. These models are appropriate because the datasets have hierarchical structure, in which ballast tanks are nested within ships and repeated measurements are collected from individual tanks [18]. We used the observed CP rather than estimated species richness in these analyses because the number of singletons and doubletons increased with time as the abundance of most species decreased, inflating the number of rare species and thus overestimating complete species richness. First, we constructed separate models to test the effect of time on CP and total PP using data collected from control tanks. We treated time (i.e. days since uptake of ballast water) and quadratic variant of time (time2) as fixed explanatory variables. Twelve models (two response variables (CP and total PP) × three taxonomic groups × two voyage routes) were constructed for this. Second, to test the effect of voyage route (Atlantic versus Pacific) on CP and total PP, we treated time, time2, voyage route and the interaction between time and voyage (time × voyage) as fixed explanatory variables in four separate models (two each for invertebrates and diatoms). To distinguish the effects of biogeographic region and voyage length, we constructed four follow-up models using the same fixed explanatory variables and included only the first 9 days so that both routes encompassed the same voyage length, allowing examination of the importance of route.
Finally, we constructed models to test the effect of BWE on CP and total PP for each taxonomic group using data from both control and exchanged tanks. As a first step, we built six separate models (two response variables × three taxonomic groups) to determine if there were inherent differences in CP and total PP between control and exchanged tanks prior to BWE. We included time, time2, voyage route, BWE status, time × voyage route, as well as the interactions between time and BWE status (time × BWE status), and between BWE status and voyage route (BWE status × voyage route), as fixed explanatory variables. Here, time was defined as the number of days between ballast water uptake and BWE. Measurements collected after BWE were excluded in these analyses. Results of the models suggested that BWE status was not related to CP and total PP in all models (results not presented). Therefore, we concluded there were no inherent differences in CP and total PP between treatment tanks prior to BWE. Next, we fitted six separate models (two response variables × three taxonomic groups) to test the effect of BWE on CP and total PP. We included time, time2, voyage route, BWE status, time × voyage route, time × BWE status and voyage route × BWE status as fixed explanatory variables. Time was defined as the number of days after BWE. To investigate the effects of time × voyage route and voyage route × BWE status on CP and total PP, we constructed four follow-up models (two each for invertebrates and diatoms) with time, time2, BWE status and time × BWE status as fixed explanatory variables.
We included tank and ship as hierarchical grouping variables in all models to accommodate the nested nature of the datasets. In addition, we specified a simple autoregressive covariance structure for the models to account for temporal autocorrelation between repeated measurements [19]. We applied the top-down strategy to identify optimal models having both fixed and random effects beginning with three beyond-optimal models containing all explanatory variables and their interactions but different random components: (i) no random terms except for ordinary residuals; (ii) a random intercept model; and (iii) a random intercept and slope model [18]. We assessed random components by comparing Akaike information criterion values estimated using restricted maximum-likelihood estimation. Once the optimal random structure was found, we identified the optimal fixed structure by conducting sequential t-tests using restricted maximum-likelihood estimation [18]. Non-significant fixed effects were removed in a stepwise manner until minimum adequate models containing only significant factors remained. We used visual inspection of model residuals to check for a normal distribution and homogeneity of variance. All analyses were conducted using the lme and gls functions in R. The latter function was used on models lacking random effects [18].
We used multivariate analysis of variance (MANOVA) to test the effects of voyage route, taxonomic group, BWE status and their interactions on final CP and total PP. If significant, a follow-up univariate analysis of variance and Bonferroni post hoc tests were performed. CP and total PP were log(x + 1)-transformed to meet the assumptions of parametric tests.
3. Results
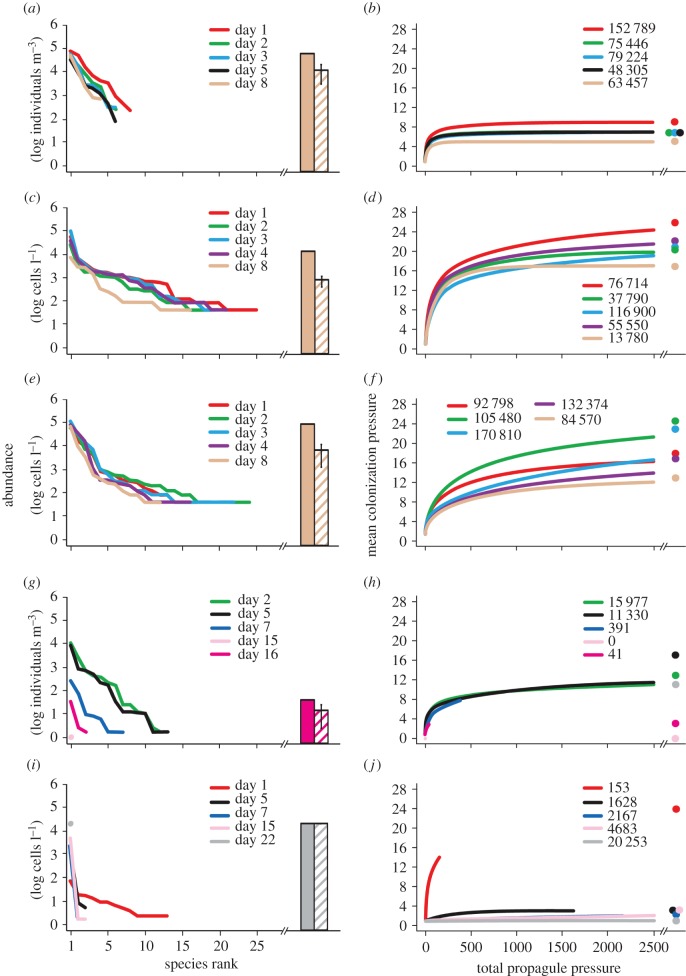
Rank-abundance distributions and CP : total PP curves varied temporally across voyage routes and taxonomic groups (figure 2 and table 2; electronic supplementary material, figures S1–S4). For Atlantic voyages, no changes in CP but attenuation of total PP was observed for invertebrates (figure 2 and table 2; electronic supplementary material, figure S2); the opposite pattern was noted for diatoms (figure 2 and table 2; electronic supplementary material, figure S3). CP for dinoflagellates decreased at a greater than linear rate over time, whereas total PP remained unchanged (figure 2 and table 2; electronic supplementary material figure S4). Reductions in total PP for invertebrates and dinoflagellates were attributed to decreases in abundance of mainly moderately common species and a few rare species, whereas losses in total PP for diatoms resulted from uniform decreases across all species (figure 2; electronic supplementary material figures S1, S3 and S4). As a result, rank-abundance gradients were steeper over time for invertebrates and dinoflagellates than for diatoms (figure 2; electronic supplementary material, figures S1, S3 and S4).
Figure 2.
(a,c,e,g,i) Rank-abundance distributions and (b,d,f,h,j) corresponding CP : total PP curves (CP : total PP) illustrating changes in the structure of plankton assemblages in unexchanged ballast water of ships during (a–f) trans-Atlantic and (g–j) trans-Pacific voyages, for (a,b,g,h) invertebrates, (c,d,i,j) diatoms and (e,f) dinoflagellates. Samples for different taxonomic groups were collected from the same tank and ship for each voyage. The five lines on each graph depict the rank-abundance distributions or CP : total PP relationships on five different days. Colour scheme for different days of the voyages applies to the entire figure. Also shown in each left panel are total PP (solid bar) and mean PP (±s.e.m.; hatched bar) recorded at the last time point. Numeric values and circles in each right panel indicate observed total PP and estimated asymptotic species richness (Chao-1). Circles are offset when values overlap.
Table 2.
Results of linear mixed-effects models testing the fixed effects of (a) time and quadratic term of time (time2) and (b) time, time2, voyage route and the interaction term between time and voyage route (time × voyage route) for CP and total PP associated with invertebrate and diatom assemblages in control tanks during trans-Atlantic and trans-Pacific voyages separately and pooled together, respectively. (c) Follow-up models were conducted to distinguish the effects of biogeographic region and voyage length associated with voyage route. These additional models standardized voyage length for Pacific and Atlantic route to the first 9 days only. Time and time2 were included as fixed effects in (a) models for dinoflagellates. Dashes indicate non-significant variables that were removed from the final optimal models.
| invertebrates |
diatoms |
dinoflagellates |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | β | t | p | d.f. | β | t | p | d.f. | β | t | p | |
| (a) CP (Atlantic) | ||||||||||||
| time | — | — | — | — | 23 | −0.7 | −2.9 | <0.01 | — | — | — | — |
| time2 | — | — | — | — | — | — | — | — | 20 | −0.2 | −3.8 | <0.01 |
| total PP (Atlantic) | ||||||||||||
| time | 27 | −5778.6 | −3.3 | <0.01 | — | — | — | — | — | — | — | — |
| time2 | — | — | — | — | — | — | — | — | — | — | — | — |
| CP (Pacific) | ||||||||||||
| time | 23 | −3.4 | −3.4 | <0.01 | 24 | −1.3 | −4.7 | <0.01 | n.a. | n.a. | n.a. | n.a. |
| time2 | 23 | 0.1 | 2.3 | 0.03 | 24 | 0.1 | 3.7 | <0.01 | n.a. | n.a. | n.a. | n.a. |
| total PP (Pacific) | ||||||||||||
| time | 23 | −23 825.3 | −4.7 | <0.01 | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| time2 | 23 | 1119.0 | 3.9 | <0.01 | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| (b) CP (full voyages) | ||||||||||||
| time | 50 | −3.1 | −4.6 | <0.01 | 44 | −1.1 | −4.8 | <0.01 | n.a. | n.a. | n.a. | n.a. |
| time2 | 50 | 0.11 | 3.0 | <0.01 | 44 | 0.03 | 3.5 | <0.01 | n.a. | n.a. | n.a. | n.a. |
| voyage route | 50 | −13.8 | −5.9 | <0.01 | — | — | — | n.a. | n.a. | n.a. | n.a. | |
| time × voyage route | 50 | 1.9 | 4.9 | <0.01 | — | — | — | n.a. | n.a. | n.a. | n.a. | |
| total PP (full voyages) | ||||||||||||
| time | 50 | −23 901.3 | −5.0 | <0.01 | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| time2 | 50 | 1107.23 | 4.2 | <0.01 | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| voyage route | — | — | — | — | 50 | 37 752.9 | 5.0 | <0.01 | n.a. | n.a. | n.a. | n.a. |
| time × voyage route | 50 | 6489.2 | 2.4 | 0.02 | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| (c) CP (first 9 days) | ||||||||||||
| time | — | — | — | 31 | −0.8 | −4.5 | <0.01 | n.a. | n.a. | n.a. | n.a. | |
| time2 | — | — | — | — | — | — | n.a. | n.a. | n.a. | n.a. | ||
| voyage route | 2 | −7.1 | −5.8 | 0.03 | — | — | — | n.a. | n.a. | n.a. | n.a. | |
| time × voyage route | — | — | — | — | — | — | n.a. | n.a. | n.a. | n.a. | ||
| total PP (first 9 days) | ||||||||||||
| time | 41 | −32 839.7 | −4.6 | <0.01 | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| time2 | 41 | 2295.5 | 2.8 | <0.01 | — | — | — | n.a. | n.a. | n.a. | n.a. | |
| voyage route | — | — | — | — | 35 | 29 853.6 | 2.8 | <0.01 | n.a. | n.a. | n.a. | n.a. |
| time × voyage route | — | — | — | — | — | — | — | n.a. | n.a. | n.a. | n.a. | |
For Pacific voyages, both CP and total PP decreased for invertebrates as a quadratic function of time (table 2), the latter due to decreases in abundance of moderately common and rare species, though dominant species also suffered high mortality (figure 2; electronic supplementary material, figure S2). By contrast, diatoms exhibited greater than linear reduction in CP but no changes in total PP (figure 2 and table 2; electronic supplementary material, figure S3). Rank-abundance distributions for both groups had increasingly steep gradients over time (figure 2; electronic supplementary material, figures S2 and S3).
Changes in rank-abundance distributions and CP : total PP were generally less apparent for Atlantic than for Pacific voyages (figure 2; electronic supplementary material, figures S1–S4). CP for invertebrates was affected by voyage route, with lower CP observed for Atlantic than for Pacific routes (table 2b). However, CP declined at a slower rate for the Atlantic than the Pacific as indicated by the interaction between time and voyage route (i.e. different regression slopes; table 2b). There were no differences in total PP for invertebrates between voyage routes, but it decreased at a slower rate for Atlantic than Pacific voyages (time × voyage route interaction; table 2b). CP for diatoms decreased over time but it did not vary by voyage route, whereas total PP did not vary over time but was higher for Atlantic than Pacific voyages (table 2b). We could not compare the effect of voyage route for dinoflagellates, as only Atlantic data were available.
When analysis was limited to the first 9 days of voyages, CP for invertebrates did not change over time, but was lower for Atlantic than Pacific voyages (table 2c). Conversely, total PP for invertebrates decreased over time but was not associated with voyage route (table 2c). Both CP and total PP of diatoms were predicted by the same explanatory variables as the full models, except that the former changed in a linear rather than quadratic fashion (table 2c).
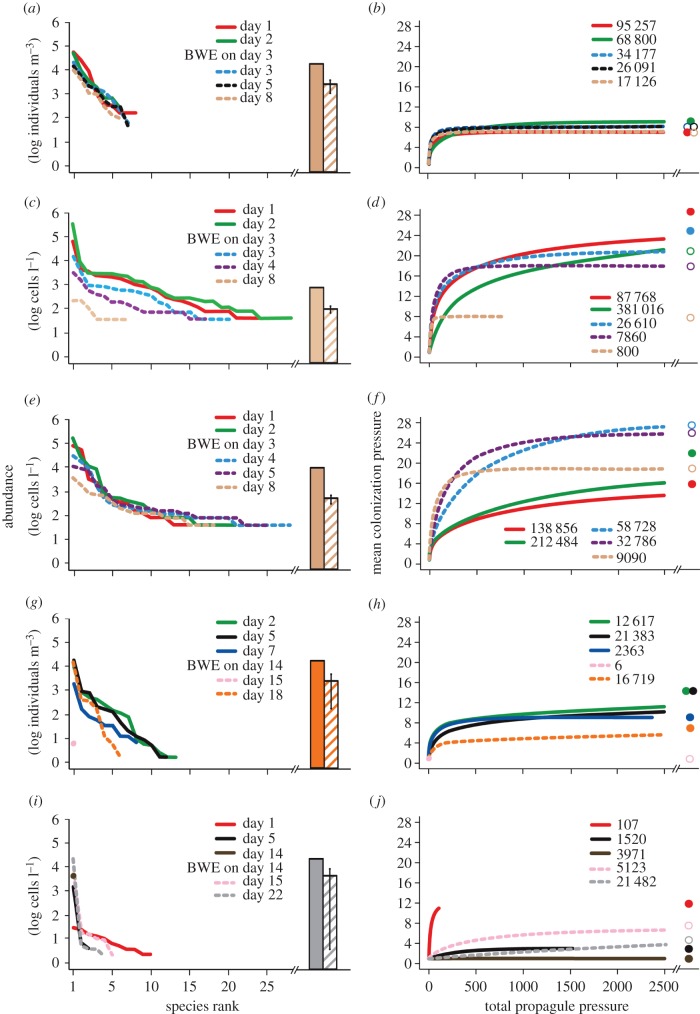
Visual comparisons of rank-abundance distributions and CP : total PP at the last sampling point prior to BWE versus those at the last sampling point near the end of the voyage in exchanged tanks suggest that the effect of BWE varied across voyage routes and taxonomic groups (figure 3; electronic supplementary material, figures S5–S8). For Atlantic routes, there were few changes in CP for invertebrates and moderate attenuation of total PP after BWE owing to reductions in population abundance of moderately common and rare species; rank-abundance gradients were similar before and after BWE (figure 3; electronic supplementary material, figure S5). Changes in CP after BWE varied from relatively strong reductions to slight increases in CP, accompanied by relatively moderate to strong attenuation of total PP for diatoms; rank-abundance gradients remained relatively shallow owing to uniform reductions in population abundance of all species or additions of new species at low abundance (figure 3; electronic supplementary material, figure S7). Similarly, we noted strong increases in CP for dinoflagellates owing to addition of new species at low abundances and marked reductions in total PP due to decreases in abundance of dominant species after BWE; rank-abundance gradients were generally more even post-BWE (figure 3; electronic supplementary material, figure S8).
Figure 3.
(a,c,e,g,i) Rank-abundance distributions and (b,d,f,h,j) corresponding CP : total PP curves highlighting changes in the structure of plankton assemblages in ships' ballast water before and after BWE during (a–f) trans-Atlantic and (g–j) trans-Pacific voyages, for (a,b,g,h) invertebrates, (c,d,i,j) diatoms and (e,f) dinoflagellates. Samples for different taxonomic groups were collected from the same tank and ship (paired-tank of the same ship as figure 2) for each voyage. Solid and dotted lines indicate results before and after BWE, respectively. Descriptions of symbols used are given in figure 2.
For Pacific routes, responses in CP for invertebrates ranged from relatively moderate reductions to mild increases owing to either losses or gains of rare species, whereas total PP consistently increased due to higher population abundances of dominant species after BWE; rank-abundance gradients were typically steeper after BWE (figure 3; electronic supplementary material, figure S6). While we observed relatively mild increases in CP due to addition of rare diatom species, changes in total PP varied from relatively moderate reductions to strong increases in total PP owing to increased abundance of dominant species; rank-abundance gradients were steeper after BWE in some but not all cases (figure 3; electronic supplementary material, figure S7).
Comparisons of CP and total PP between exchanged and control tanks further indicate that the effect of BWE varied across voyage routes and taxonomic groups (table 3). CP for invertebrates was associated with time, voyage route, their interaction and BWE status (table 3; electronic supplementary material, table S1). CP for invertebrates was higher in both exchanged and control tanks for Atlantic than Pacific routes; however, CP decreased over time for the Atlantic, whereas it increased over time for the Pacific (table 3; electronic supplementary material, table S1). When comparing between exchanged and control tanks, CP for invertebrates was higher in exchanged than in control tanks for both voyage routes (table 3; electronic supplementary material, table S1). Total PP for invertebrates was associated with voyage route as well as the interactions between time and voyage route, and BWE status and voyage route (table 3; electronic supplementary material, table S1). Total PP for invertebrates was higher in all tanks for Atlantic than Pacific voyages (table 3). While total PP for invertebrates decreased over time for Atlantic voyages, it did not change for Pacific ones (table 3; electronic supplementary material, table S1). There was lower total PP for invertebrates in exchanged than control tanks for Atlantic voyages, whereas no differences were observed for Pacific routes (table 3; electronic supplementary material, table S1). For diatoms, there were no predictors of CP, probably due to high variation observed across assemblages and small sample size (table 3; see also figure 3; electronic supplementary material, figure S7). Total PP for diatoms was associated with the interaction between BWE status and voyage route (table 3). Total PP for diatoms was lower in exchanged than control tanks for Atlantic voyages; however, there were no differences in total PP between treatments for Pacific ones (table 3; electronic supplementary material, table S1). Finally, for dinoflagellates, BWE status was a predictor of CP, which surprisingly was higher in exchanged than control tanks (table 3). By contrast, there were no differences in total PP for dinoflagellates between treatments (table 3).
Table 3.
Results of linear mixed-effects models testing the fixed effects of time, time2, voyage route, BWE status, time × voyage route, the interaction terms between time and BWE status (time × BWE status) and between BWE status and voyage route (BWE status × voyage route) for CP and total PP associated with invertebrate and diatom assemblages in control and BWE tanks during trans-Atlantic and trans-Pacific voyages after BWE. Time, time2 and BWE status were included as fixed effects in models for dinoflagellates. Time was defined as the number of days since BWE. Time2 and time × BWE status were not retained in any model. Dashes indicate non-significant variables that were excluded in the final optimal models.
| invertebrates |
diatoms |
dinoflagellates |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | β | t | p | d.f. | β | t | p | d.f. | β | t | p | |
| CP | ||||||||||||
| time | 57 | 1.3 | 6.1 | <0.01 | — | — | — | — | — | — | — | — |
| voyage route | 57 | 6.3 | 12.1 | <0.01 | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| BWE status | 57 | 1.9 | 6.5 | <0.01 | — | — | — | 5 | 7.7 | 4.2 | <0.01 | |
| time × voyage route | 57 | −1.5 | −6.5 | <0.01 | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| BWE status × voyage route | — | — | — | — | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| total PP | ||||||||||||
| time | — | — | — | — | — | — | — | 31 | −3255.7 | −3.1 | <0.01 | |
| voyage route | 56 | 56562.9 | 12.3 | <0.01 | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| BWE status | — | — | — | — | — | — | — | — | 5 | 7.7 | 4.2 | <0.01 |
| time × voyage route | 56 | −2093.2 | −3.2 | <0.01 | — | — | — | — | n.a. | n.a. | n.a. | n.a. |
| BWE status × voyage route | 56 | −25 323.3 | −8.7 | <0.01 | 6 | 33 191.8 | 5.8 | <0.01 | n.a. | n.a. | n.a. | n.a. |
Final CP and total PP varied across voyage routes, taxonomic groups and BWE status (figure 1b and table 4). Both variables were higher for Atlantic than for Pacific voyages (figure 1b and table 4). Invertebrates exhibited the lowest final CP, followed by diatoms and dinoflagellates (Bonferroni post hoc tests, p < 0.01 in all pairwise comparisons). Total PP of invertebrates was also lower than those of diatoms and dinoflagellates (Bonferroni post hoc tests, p < 0.01 for both pairwise comparisons), though the latter groups did not differ (p = 1.00). Final CP was higher in exchanged than in control tanks, particularly for dinoflagellates on Atlantic voyages (figure 1b and table 4). While final total PP was lower in exchanged tanks when compared with control ones for all taxonomic groups on Atlantic voyages, it was higher in exchanged than in control tanks for invertebrates and similar between treatments for diatoms (figure 1b and table 4).
Table 4.
Results of MANOVA addressing the effects of voyage route, taxonomic group, BWE status and their interactions on final CP and total PP.
| variables | d.f. | F | p |
|---|---|---|---|
| voyage route | |||
| Wilks's λ | 2 | 28.2 | <0.01 |
| univariate F-tests | |||
| CP | 1 | 57.5 | <0.01 |
| total PP | 1 | 23.2 | <0.01 |
| taxonomic group | |||
| Wilks’s λ | 4 | 34.3 | <0.01 |
| univariate F-tests | |||
| CP | 2 | 9.9 | <0.01 |
| total PP | 2 | 130.9 | <0.01 |
| BWE status | |||
| Wilks’s λ | 2 | 14.4 | <0.01 |
| univariate F-tests | |||
| CP | 1 | 29.9 | <0.01 |
| total PP | 1 | 7.8 | <0.01 |
| taxonomic group × voyage route | |||
| Wilks’s λ | 2 | 24.0 | <0.01 |
| univariate F-tests | |||
| CP | 1 | 5.0 | 0.03 |
| total PP | 1 | 20.9 | <0.01 |
| BWE status × voyage route | |||
| Wilks’s λ | 2 | 13.1 | <0.01 |
| univariate F-tests | |||
| CP | 1 | 15.4 | <0.01 |
| total PP | 1 | 24.7 | <0.01 |
| BWE status × taxonomic group | |||
| Wilks’s λ | 4 | 2.7 | 0.04 |
| univariate F-tests | |||
| CP | 2 | 3.3 | 0.05 |
| total PP | 2 | 3.9 | 0.03 |
Our comparison of rank-abundance distributions with more traditional summary methods indicated that total PP was strongly influenced by the most dominant species, and did not provide any information regarding abundances of moderately common or rare species (figures 2 and 3; electronic supplementary material, figures S1–S8). Mean PP (±s.e.m.) summarized variation in abundances among species, with greater variation associated with more uneven assemblages, though it typically underestimated abundance of dominant species while greatly overestimating that of rare ones (figures 2 and 3; electronic supplementary material, figures S1–S8).
4. Discussion
Understanding the dynamics of a species assemblage during the transport stage prior to introduction can provide insights into how invasion risk changes temporally [2,3]. Characterization of assemblages in invasion vectors has typically relied on CP and total PP [6,7]. Here, we extended this approach to examine rank-abundance distributions and CP : total PP relationships for a variety of common taxa found in ships' ballast water and to explore the importance of voyage route and BWE. Our results suggest that rank-abundance distributions and CP : total PP in control tanks vary greatly by voyage route (Atlantic versus Pacific). Responses in rank-abundance distributions and CP : total PP for control tanks also differed by taxonomic group. Paradoxically, in certain cases, BWE actually increased CP and/or total PP by adding new species or increasing the abundance of existing dominant species, thereby increasing overall introduction risk. Our study thus provides a useful application of rank-abundance distributions in invasion ecology to characterize transported assemblages and to examine efficacy of management strategies.
Differences in source inocula and voyage length can both influence responses in assemblage structure during transport. When voyage length was standardized for Atlantic and Pacific trips, route predicted CP or total PP, depending on the taxonomic group. It is apparent that initial rank-abundance distributions differed between Atlantic and Pacific routes within a particular taxonomic group, consistent with resports on differences in richness and abundance of ballast water organisms among source regions [6]. When full voyages were considered, voyage route again was a significant predictor of CP or total PP. Furthermore, CP and/or total PP declined at faster rates during Pacific than Atlantic voyages, suggesting that trip length was also important. Overall, we observed stronger reductions in CP and/or total PP in control tanks for Pacific than Atlantic routes. Long transit times prolong organisms' exposure to biotic and abiotic stressors in ballast water [6]. Therefore, longer trips are generally associated with lower species richness and abundance of ballast water organisms [6,11].
Differences between the two voyage routes are also reflected in final CP : total PP, in which CP and total PP were higher for Atlantic voyages than for Pacific ones in both control and exchanged tanks. All things being equal (e.g. shipping traffic, environmental suitability and study effort), our results suggest that vessels arriving with ballast to the Atlantic coast of Canada present a higher risk of ballast-mediated introductions by transoceanic vessels. This finding is consistent with the occurrence of more non-indigenous species on the Atlantic than the Pacific coast of Canada (i.e. 112 versus 94) [20]. However, other factors such as introduction of new species by other vectors (e.g. hull fouling and aquaculture), secondary spread by natural and anthropogenic (e.g. domestic shipping) means and other site- and species-specific attributes may obscure the pattern between regions [15].
Relative dynamics for CP and total PP among taxonomic groups may be attributed to taxon-specific tolerance to abiotic and biotic stresses. We observed significant attenuation of total PP, or both CP and total PP in some cases, for invertebrates. Conversely, diatoms and dinoflagellates exhibited significant reductions in CP but no changes in total PP. These findings suggest that invertebrate species generally have broad tolerance, though members of moderately common species exhibited the highest mortality. By contrast, tolerance varies by species for diatoms and dinoflagellates, in which mortality was selectively high for rare species, while moderately common and dominant species mostly survived or even reproduced during transportation [12,13]. Our results corroborate those described by Briski et al.'s [2] conceptual model, with a few exceptions. Cases that deviated from the conceptual model were mostly limited to Pacific voyages, further supporting our previous findings of the effect of voyage route on assemblage dynamics.
BWE appeared to increase introduction risk in certain cases by augmenting existing populations and/or adding new species. We observed elevated risk for both invertebrates and diatoms after BWE on Pacific voyages, the former owing to increased total PP and the latter to increased CP. Conversely, only dinoflagellates demonstrated elevated risk as a result of increased CP following BWE on Atlantic voyages. Increases in total PP but not CP following BWE indicate increased population abundance for species already present in ballast water tanks, possibly as a result of hatching from diapausing eggs promoted by loading of ‘fresh’ oceanic water [21] and/or uptake of individuals of existing species during BWE [22]. We attribute increases in CP after BWE to uptake of new species during BWE [12,13]. Our findings accord with previous studies that reported increased species richness due to the addition of new species and persistence of original ones following BWE [22,23]. This finding is of particular concern for marine and coastal systems because new open-ocean species, in addition to original coastal ones, could potentially survive if released into saline or strongly brackish waters. By contrast, these species are unlikely to survive in freshwater environments, thus posing a much lower invasion potential.
Rank-abundance distributions provide a more accurate description of entrained assemblages than summary statistics including total and mean PP. Our study demonstrated that rank-abundance distributions effectively characterized variation in abundance across species, making it possible to visualize and examine assemblage dynamics over time, particularly before and after vector management, thereby allowing refined estimation of introduction risk. Conversely, total or mean PP consistently over- or underestimated abundances of most species because entrained assemblages were generally uneven, with extent of unevenness depending on voyage route, taxonomic group and vector management strategy. Over- and underestimation of abundances can have strong ecological consequences, respectively leading to misidentification of high- or low-risk introduction events.
Characterizing assemblage dynamics during transport and identifying factors that influence changes can improve our ability to forecast invasions. We are not aware of studies on temporal changes in assemblage structure during transport in vectors other than ballast water. We propose that the use of rank-abundance distributions—a tool commonly used to characterize communities in natural habitats—and the analysis of CP and total PP relationships be extended to assemblages entrained by myriad invasion vectors. Rank-abundance distributions, CP : total PP relationships and introduction risk may vary among vectors. For example, biofouling communities on a ship's hull are exposed to very hostile transport conditions due to shear stresses and changing environmental conditions, thus introduction risk probably changes over time. By contrast, species assemblages associated with live algae used as bait worm packaging should be exposed to low selection pressures during transport because environmental conditions are optimized to prevent worm mortality, thus we expect little to no change in introduction risk.
Supplementary Material
Acknowledgements
We thank two anonymous referees for constructive comments. We are grateful to Drs C. DiBacco and I. Kaczmarska for data.
Funding statement
Funding was provided by Transport Canada, Fisheries and Oceans Canada, Discovery and Canadian Aquatic Invasive Species Network grants to S.A.B. and H.J.M., NSERC Visiting Fellow Stipends at Fisheries and Oceans Canada and the Alexander von Humboldt Sofja Kovalevskaja Award to E.B., NSERC CGSD to F.T.C. and NSERC PGSD to J.B.
References
- 1.McGill BJ, et al. 2007. Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 10, 995–1015. ( 10.1111/j.1461-0248.2007.01094.x) [DOI] [PubMed] [Google Scholar]
- 2.Briski E, Chan FT, MacIsaac HJ, Bailey SA. 2014. A conceptual model of community dynamics during the transport stage of the invasion process: a case study of ships’ ballast. Divers. Distrib. 20, 236–244. ( 10.1111/ddi.12154) [DOI] [Google Scholar]
- 3.Drake DAR, Chan FT, Briski E, Bailey SA, MacIsaac HJ. 2014. Assemblage structure: an overlooked component of human-mediated species movements among freshwater ecosystems. J. Limnol. 73, 108–115. ( 10.4081/jlimnol.2014.802) [DOI] [Google Scholar]
- 4.Hulme PE, et al. 2008. Grasping at the routes of biological invasions: a framework for integrating pathways into policy. J. Appl. Ecol. 45, 403–414. ( 10.1111/j.1365-2664.2007.01442.x) [DOI] [Google Scholar]
- 5.Lockwood JL, Cassey P, Blackburn TM. 2009. The more you introduce the more you get: the role of colonization pressure and propagule pressure in invasion ecology. Divers. Distrib. 15, 904–910. ( 10.1111/j.1472-4642.2009.00594.x) [DOI] [Google Scholar]
- 6.Cordell JR, Lawrence DJ, Ferm NC, Tear LM, Smith SS, Herwig RP. 2009. Factors influencing densities of non-indigenous species in the ballast water of ships arriving at ports in Puget Sound, Washington, United States. Aquat. Conserv. 19, 322–343. ( 10.1002/aqc) [DOI] [Google Scholar]
- 7.Sylvester F, Kalaci O, Leung B, Lacoursière-Roussel A, Murray CC, Choi FM, Bravo MA, Therriault TW, MacIsaac HJ. 2011. Hull fouling as an invasion vector: can simple models explain a complex problem? J. Appl. Ecol. 48, 415–423. ( 10.1111/j.1365-2664.2011.01957.x) [DOI] [Google Scholar]
- 8.Simberloff D. 2009. The role of propagule pressure in biological invasions. Annu. Rev. Ecol. Evol. Syst. 40, 81–102. ( 10.1146/annurev.ecolsys.110308.120304) [DOI] [Google Scholar]
- 9.Bradie J, Chivers C, Leung B. 2013. Importing risk: quantifying the propagule pressure-establishment relationship at the pathway level. Divers. Distrib. 19, 1020–1030. ( 10.1111/ddi.12081) [DOI] [Google Scholar]
- 10.Briski E, et al. 2012. Relationship between propagule pressure and colonization pressure in invasion ecology: at test with ships’ ballast. Proc. R. Soc. B 279, 2990–2997. ( 10.1098/rspb.2011.2671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan FT, Briski E, Bailey SA, MacIsaac HJ. 2014. Richness-abundance relationships for zooplankton in ballast water: temperate versus Arctic comparisions. ICES J. Mar. Sci. 71, 1876–1884. ( 10.1093/icesjms/fsu020) [DOI] [Google Scholar]
- 12.Simard N, Plourde S, Gilbert M, Gollasch S. 2011. Net efficacy of open ocean ballast water exchange on plankton communities. J. Plankton Res. 33, 1378–1395. ( 10.1093/plankt/fbr038) [DOI] [Google Scholar]
- 13.DiBacco C. 2007. Transpacific voyages 2007 data. Canadian Aquatic Invasive Species Network See http://www.isdm-gdsi.gc.ca/ais-eae/goHome-allerAccueil.do?lang=en.
- 14.Klein G, MacIntosh K, Kaczmarska I, Ehrman JM. 2010. Diatom survivorship in ballast water during trans-Pacific crossings. Biol. Invasions 12, 1031–1044. ( 10.1007/s10530-009-9520-6) [DOI] [Google Scholar]
- 15.DiBacco C, Humphrey DB, Nasmith LM, Levings CD. 2012. Ballast water transport of non-indigenous zooplankton to Canadian ports. ICES J. Mar. Sci. 69, 483–491. ( 10.1093/icesjms/fsr133) [DOI] [Google Scholar]
- 16.R Development Core Team. 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 17.Chao A, Shen TJ. 2010. Program SPADE (Species Prediction And Diversity Estimation) See http://chao.stat.nthu.edu.tw.
- 18.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R, 1st edn New York, NY: Springer. [Google Scholar]
- 19.Cnaan A, Laird NM, Slasor P. 1997. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat. Med. 16, 2349–2380. () [DOI] [PubMed] [Google Scholar]
- 20.Casas-Monroy O, Linley RD, Adams JK, Chan FT, Drake DAR, Bailey SA. 2014. National risk assessment for introduction of aquatic nonindigenous species to Canada by ballast water. DFO Can. Sci. Advis. Sec. Res. Doc. 2013/128.
- 21.Bailey SA, Duggan IC, van Overdijk CDA, MacIsaac HJ. 2005. Viability of invertebrate diapausing eggs collected from residual ballast sediment. Limnol. Oceanogr. 48, 11 701–11 710. ( 10.4319/lo.2003.48.4.1701) [DOI] [Google Scholar]
- 22.Choi KH, Kimmerer W, Smith G, Ruiz GM, Lion K. 2005. Post-exchange zooplankton in ballast water of ships entering the San Francisco Estuary. J. Plankton Res. 27, 707–714. ( 10.1093/plankt/fbi044) [DOI] [Google Scholar]
- 23.Taylor MD, MacKenzie LM, Dodgshun TJ, Hopkins GA, de Zwart EJ, Hunt CD. 2007. Trans-Pacific shipboard trails on planktonic communities as indicators of open ocean ballast water exchange. Mar. Ecol. Prog. Ser. 350, 41–54. ( 10.3354/meps07016) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.