Abstract
Ontogenetic changes in habitat are driven by shifting life-history requirements and play an important role in population dynamics. However, large portions of the life history of many pelagic species are still poorly understood or unknown. We used a novel combination of stable isotope analysis of vertebral annuli, Bayesian mixing models, isoscapes and electronic tag data to reconstruct ontogenetic patterns of habitat and resource use in a pelagic apex predator, the salmon shark (Lamna ditropis). Results identified the North Pacific Transition Zone as the major nursery area for salmon sharks and revealed an ontogenetic shift around the age of maturity from oceanic to increased use of neritic habitats. The nursery habitat may reflect trade-offs between prey availability, predation pressure and thermal constraints on juvenile endothermic sharks. The ontogenetic shift in habitat coincided with a reduction of isotopic niche, possibly reflecting specialization upon particular prey or habitats. Using tagging data to inform Bayesian isotopic mixing models revealed that adult sharks primarily use neritic habitats of Alaska yet receive a trophic subsidy from oceanic habitats. Integrating the multiple methods used here provides a powerful approach to retrospectively study the ecology and life history of migratory species throughout their ontogeny.
Keywords: stable isotope analysis, elasmobranch, ontogenetic shift, satellite tagging, stable isotope mixing model
1. Introduction
Ontogenetic changes in habitat use and trophic ecology are ubiquitous in the natural world [1], and often reflect a shift in life-history priorities, from a juvenile strategy that maximizes growth and survival [2] to an adult strategy that includes reproduction [3,4]. Historically, studying pelagic species throughout ontogeny has been difficult given their migratory nature and inaccessibility of their habitat, leaving large portions of their life history poorly understood or unknown [5]. This lack of information about early life history and ontogenetic shifts in habitat is a concern given that juvenile survivorship and recruitment is vital in maintaining the health of populations of long-lived marine species, including elasmobranchs (sharks, skates and rays) [3,6]. Given the vulnerability of pelagic elasmobranchs to overexploitation, the general worldwide decline in elasmobranch populations [7] and their importance as apex predators [8], obtaining information on ecology of pelagic elasmobranchs throughout their ontogeny is critical for their conservation and management.
Salmon sharks (Lamna ditropis) are endothermic, apex predators in North Pacific ecosystems. Large salmon sharks are highly migratory, ranging from subtropical to subpolar waters [9], and smaller sharks are generally found in the southern extent of their range [10]. Two salmon shark nursery areas have been hypothesized to exist in the eastern North Pacific (ENP). One extends across the North Pacific in the North Pacific Transition Zone (NPTZ) and the other encompasses the California Current from British Columbia, Canada to Baja California, Mexico [11,12]. The relative importance of each nursery area is unclear.
Changes in the distribution of salmon sharks and other pelagic species over their life history have important conservation and management ramifications. Ontogenetic changes in habitat use would lead to varying degrees of vulnerability to fisheries or natural mortality. For example, prior to the 1992 ban on pelagic driftnets, high levels of bycatch of young salmon sharks were common in the high seas fisheries along the NPTZ [10,12] with potentially important direct impacts on salmon shark populations and indirect effects on North Pacific ecosystems.
The stable isotope composition of an organism's tissues is a reflection of its diet and environment [13]. Some accretionary structures, such as otoliths, vibrissae, baleen, feathers and shark vertebrae [14,15], are continuously growing, metabolically inert tissues that reflect the diet of the organism at the time of tissue synthesis, providing a continuous time series of diet and environment. Serially sampling accretionary structures for stable isotope analysis (SIA) or other biogeochemical tracers is an approach that can be used to study shifts in diet and habitat throughout the entire life history of individual animals, including sharks [14,16].
The stable isotope composition of primary producers varies spatially based on differences in oceanographic and biogeochemical processes [17,18], and these differences are propagated through local food webs such that consumers isotopically resemble the food webs in which they feed [17]. Therefore, the stable isotope compositions of different food webs provide the context within which a consumer's stable isotope composition can be used as tracers of habitat use, especially in migratory species [19–21]. This approach has been used to understand movements in marine organisms including marine mammals [22,23], seabirds [24], teleosts [25] and elasmobranchs [26]. The broad distribution of salmon sharks extends across several biogeographic provinces in the ENP [27]. Variability in oceanographic and biogeochemical processes [28] drives differences in baseline stable isotope values among these provinces [17]. This isoscape over which salmon sharks migrate and forage allows the stable isotope composition of their tissues to be used to discern broad-scale patterns of ecoregion use.
The goal of this study was to create an ontogenetic time series of δ13C and δ15N recorded in the vertebral annuli of salmon sharks to identify and understand ontogenetic patterns of habitat use. These time series were then used to: (i) elucidate the relative importance of different nursery areas; (ii) estimate the relative importance of different ecoregions to salmon sharks across their life history using Bayesian isotope mixing models and electronic tag data; (iii) identify shifts in resource use with age; and (iv) understand the variability in the breadth of resources used across the ontogeny of salmon sharks.
2. Material and methods
(a). Sample collection and preparation
Vertebrae were collected from 20 salmon sharks caught in the sport fishery in Prince William Sound, Alaska (figure 1) during the summers of 2007 and 2009 (electronic supplementary material, table S1) and stored frozen. Owing to the preponderance of females in the ENP [10], females comprised the bulk of the specimens collected. Sagittal sections (4 mm thick) were cut from each vertebra using a low-speed saw with diamond blades and then polished with a Buehler Ecomet III lapping wheel using 600 and 800 µm grit silicon-carbide wet/dry sandpaper. Sections were air-dried and images captured using a Leica dissecting microscope with an attached Spot RT video camera. Annuli (annual growth bands) were independently counted by two of the authors (A.B.C. and K.J.G.), and assessments compared and agreed upon.
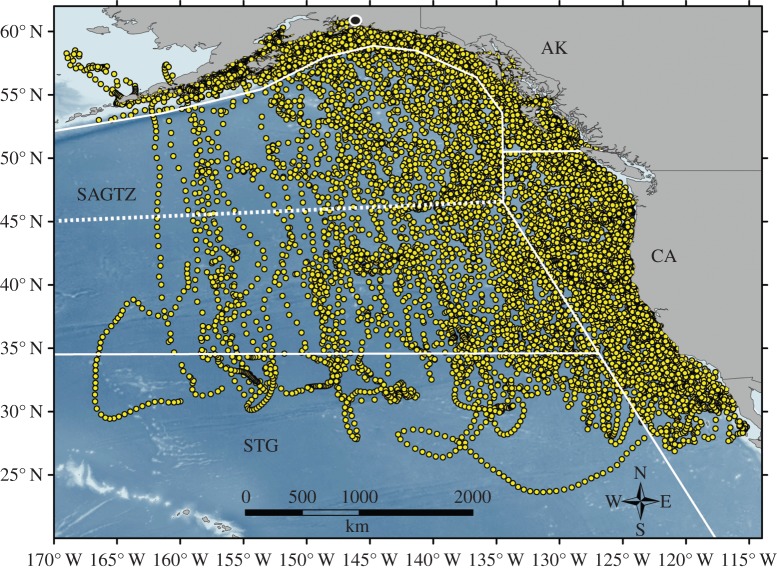
Figure 1.
Map of major ecoregions of the northeastern Pacific. Regions include Subtropical Gyre (STG), Alaska (AK) and California Current (CA). SAGTZ is a combination of the Subarctic Gyre (SAG) and NPTZ. The northern border of the NPTZ is marked by the dotted line. Tagging location of sharks is indicated with the black circle. Also shown are positions of sharks (n = 44) with 1 year of tracking data used as priors in isotope mixing model.
Tissue from each annulus, including inside the birthmark representing in utero growth (age U), was collected using a New Wave micro-mill with an 800 µm Brassler carbide dental drill bit. Because the amount of tissue available in a single annulus was limited, each annulus was sampled from both adjacent arms of the corpus calcareum (electronic supplementary material, figure S1). Samples were decalcified using EDTA following Kim and Koch [29]. As salmon sharks deposit growth bands annually [11] and the annuli of Lamna have been shown to be metabolically inert [30], the stable isotope composition of each annulus represents a record of diet and movements integrated over an entire year (electronic supplementary material). We refer to all samples for a particular age as ‘year class x’ or ‘age x’ sharks. Because annuli narrow as growth slows [31], it was difficult to collect enough tissue from older ages (approx. 11+ years), so the number of annuli sampled from each individual does not necessarily equal that individual's age.
In addition to analysing adult salmon sharks, vertebral samples were collected from 31 age 0 juveniles (hereafter referred to as California juveniles) stranded on beaches in Central California and Oregon between 2006 and 2010 and stored frozen. All material outside the vertebral birthmark was removed for analysis and, because tissue was not limited, decalcified using 0.25 N HCl [32]. All samples were analysed at the Stable Isotope Laboratory at University of California Santa Cruz using an elemental analyser coupled to an isotope ratio mass spectrometer (Delta XP-EA, Thermo-Finnagen IRMS) (electronic supplementary material).
(b). Isotopic characterization of salmon shark habitats
The range of salmon sharks in the ENP was divided into ecoregions based on the biogeographic provinces defined by Longhurst [28] (figure 1). These regions include the Alaska Coastal Downwelling Province (hereafter referred to as Alaska or AK), California Current Province (California or CA), Pacific Subarctic Gyre Province (Subarctic Gyre or SAG), North Pacific Polar Front Province, which is analogous to the NPTZ, and the Pacific Tropical Gyre Province (Subtropical Gyre or STG). Owing to a lack of data from the NPTZ, we were unable to isotopically characterize this province, and it was combined with the SAG to create the SAGTZ ecoregion. Because CA and AK ecoregions contain neritic habitats, we refer to them as neritic ecoregions (although they do contain pelagic habitats) and STG and SAGTZ as oceanic ecoregions.
We collected all published stable isotope values for known salmon shark prey identified in the literature [10,33–35] (electronic supplementary material, table S2) and grouped them based on the ecoregion within which the samples were collected. These values were used to calculate a mean ± s.d. stable isotope value for each ecoregion. Only values from species that are representative of known salmon shark prey that might be consumed in each ecoregion were used. By using the mean value of all potential prey species, we are assuming sharks are generalists across their range and ontogeny. Although there is evidence of dietary specialization within particular ecoregions, seasons and age classes, the information cannot be applied across all ecoregions and ages and are likely not relevant at the annual time scale at which annuli integrate dietary information (electronic supplementary material).
(c). Analysis
To identify ontogenetic shifts in resource use, we used the Bayesian mixing model mixsir v. 1.0.4 [36] to estimate the relative contribution of ecoregions to each year class. The model was run for each year class using discrimination factors (4.2 ± 0.7 for carbon, 2.5 ± 1.1 for nitrogen) from leopard shark vertebrae [16] and uninformative priors. Results using uninformative priors reflect the relative contribution of prey from a given ecoregion, regardless of whether an individual is foraging within that ecoregion or is consuming migratory prey originating from another region. Hence, model results are an estimate of the net contribution of different ecoregions to salmon shark diets and may not primarily reflect time spent in regions. To integrate known patterns of ecoregion residency based on tagging studies, we also ran the model using informative priors based on electronic tagging data [36]. Integrating tagging data resulted in a more refined a posteriori model that better reflect patterns of ecoregion residency.
Between 2002 and 2009, female salmon sharks in Port Gravina, Prince William Sound, Alaska, were tagged with satellite tags as part of the Tagging of Pacific Predators (TOPP) programme [37], resulting in 44 one-year-long or longer SPOT tracks (Smart Position or Temperature Transmitting tags, Wildlife Computers) that lasted from one winter to the next, corresponding to the period of deposition of one vertebral annulus [11]. The proportion of time each shark spent in each ecoregion was calculated. A Dirichlet distribution was fit to the distribution of time sharks spent in each ecoregion following Moore & Semmens [36], and the resulting α values were used to define prior information regarding the relative contribution of ecoregions based on time spent in each ecoregion (electronic supplementary material, figure S2). Because tag data were all from older sharks (ages 8+), the mixing model using informative priors was only used for ages 8+. Satellite tracking revealed that tagged salmon sharks spent the majority of their time in AK (mean 56%, median 56%, interquartile range (IQR) 28–71%), with 20% of tagged sharks spending more than 90% of their time in AK. Sharks spent less, but relatively similar, amounts of time in CA (mean 23%, median 6%, IQR 0–45%) and SAGTZ (16%, 10%, 1–23%) and the least time in the STG (5%, 1%, 0–9%). Overall, time spent in 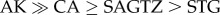 (electronic supplementary material, figure S2).
(electronic supplementary material, figure S2).
We used discriminant analysis (systat v. 10.2), to classify age 0 sharks (n = 20) to one of two known nursery areas, the NPTZ and California Current. We used isotopic values of annuli for year class 1 and 2 (n = 39) from adult vertebrae to represent use of an oceanic nursery, hence the NPTZ [12], based on the high contribution from oceanic resources to these year classes from the mixing model (see Results). California juveniles were used to represent the California Current nursery because they were sampled in that ecoregion and their isotopic signatures were distinct from the NPTZ nursery group (age 1 and 2 sharks) and similar to neritic ecoregions (electronic supplementary material, figure S4). To investigate the timing of ontogenetic shifts, discriminant analysis was also used to classify sharks of intermediate ages (age 3–9) to either juvenile or adult habitats. For this analysis, we again used age 1 and 2 sharks (n = 39) to characterize juvenile habitat. Because all female salmon sharks are mature by age 10 [11], we used data for age 10+ (n = 38) to characterize adult habitat. Through classification (ages 3–9) or a reclassification (ages 1–2, ages 10+), all data for each year class were categorized as belonging to the adult or juvenile habitat group. A logistic regression model (Matlab R2009b) was then fit to the binomial classification data to identify when sharks transitioned from juvenile to adult habitat.
To examine how isotopic niche width may change ontogenetically, the standard ellipse area corrected for sample size (SEAc), implemented using SIBER [38], was calculated for each year class. This area represents an estimate of the core isotopic niche for each year class, and we hereafter refer to this metric as the isotopic niche. We did not attempt to account for ontogenetic shifts in trophic level in our analyses as all available information indicates that salmon sharks feed at a relatively consistent trophic level throughout their ontogeny (electronic supplementary material).
3. Results
A total of 251 annuli were sampled from 20 adult salmon sharks (18 F, 2 M, mean 185.6 cm, precaudal length ±6.5 s.d.; electronic supplementary material, table S1) with a maximum of 14 annuli sampled from each vertebra, not including tissue sampled from inside the birthmark. Some annuli were lost during decalcification due to lack of tissue.
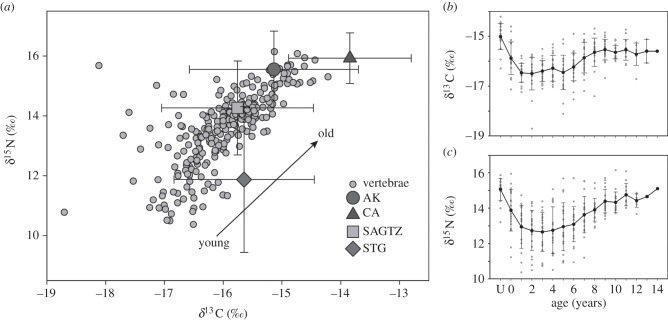
There was a clear ontogenetic shift in δ13C and δ15N values (figure 2; electronic supplementary material, table S3), with variable but low δ13C and δ15N values early in life transitioning to relatively consistent, higher values later in life. Tissue sampled from within the birthmark (age U), representing in utero growth, was enriched in 13C and 15N relative to the first several years of life and was generally similar to the oldest annuli (figure 2a,b), suggesting that age U values reflect maternal sources.
Figure 2.
(a) Stable isotope values of all annuli, ranging from in utero to 14 years of age. Mean (±s.d.) ecoregion estimates (grey symbols) are adjusted to account for trophic discrimination factors. Ontogenetic time series of δ13C (b) and δ15N (c) and mean (±s.d.) values for each year class. Age U reflects in utero salmon sharks.
(a). Mixing model
Stable isotope data for 52 prey groups from 33 studies were grouped by ecoregion [28] (electronic supplementary material, table S4). The δ13C and δ15N values of prey clustered into relatively distinct regional groups (figure 2a; electronic supplementary material, table S5). The California (δ13C –18.04 ± 1.04 s.d., δ15N 13.43 ± 0.84) and Alaska ecoregions (−19.34 ± 1.44, 13.05 ± 1.28) have the highest mean δ13C and δ15N. The Subarctic Gyre and Transition Zone (−19.96 ± 1.29, 11.77 ± 1.57) and Subtropical Gyre (−19.84 ± 1.19, 9.38 ± 2.44) have slightly lower δ13C values than AK and lower δ15N values than the other regions. The resulting regional values generally bounded the vertebral data, though some low δ13C and δ15N vertebral values fell outside regional values (figure 2a). The trends that we observed in mean δ13C and δ15N ecoregion values are concordant with differences in primary production and biogeochemical and oceanographic processes across the different ecoregions [18,28,39–41].
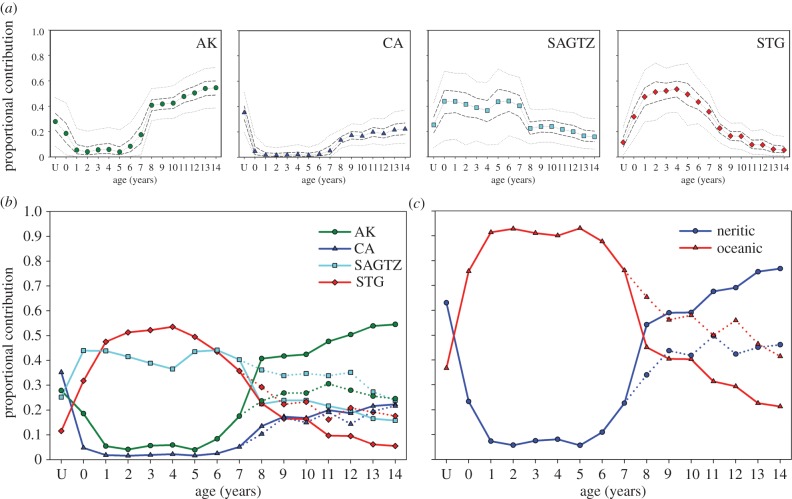
Mixing model results showed an ontogenetic shift in salmon shark habitat (figure 3; electronic supplementary material, figure S3). CA, AK and SAGTZ contributed roughly equivalent amounts to age U sharks, with STG contributing the least (figure 3; electronic supplementary material, figure S3). During juvenile years (age 1 to age 6), sharks primarily used STG and SAGTZ resources. Neritic ecoregions (AK and CA) contributed relatively little to salmon sharks until around age 7, at which point the contribution of these two regions started to increase.
Figure 3.
(a) Ontogenetic time series of proportional contribution of the different ecoregions to salmon shark vertebral tissue based on MixSIR using informative priors. Median (symbols), IQR (dashed lines) and 95% credible intervals (dotted lines) are shown. Priors were based on time spent in each ecoregion by satellite-tagged sharks and only used for ages 8+. (b) Time series of median proportional contribution of different ecoregions to salmon shark annuli (solid lines: informative priors, dotted lines: uninformative priors). (c) Cumulative proportional input of oceanic (median STG + SAGTZ) and neritic ecoregions (median AK + CA) (solid lines for ages 8+: informative priors, dotted lines for ages 8+: uninformative priors). Note that salmon sharks mature between 6 and 9 years of age [11].
The relative contribution of different ecoregions to older year classes (age 8+) varied based on whether uninformative or informative priors were used; yet, the overall pattern remained consistent, with AK and SAGTZ having the greatest contribution (figure 3b). Using uninformative priors, AK and SAGTZ contributed most and CA and STG contributed a lower amount (figure 3b; electronic supplementary material, figure S3). Using informative priors, the contribution of AK was greatest, the contribution of CA and SAGTZ were roughly equivalent, and the STG contributed little. Overall, this represents an ontogenetic shift in primary salmon shark habitat from oceanic ecoregions (STG and SAGTZ) during early life to increased utilization of neritic resources (AK and CA) as adults (figure 3c).
(b). Discriminant analysis
Discriminant analysis showed a significant difference between isotopic values of the two nursery groups (squared canonical correlation = 0.69, eigenvalue 2.18, Wilks' λ = 0.32, F2,56 = 60.97, p = 0), with a high degree of reclassification success (90% CA, 100% NPTZ for both reclassification and jackknife validation). Six age 0 sharks (30%) were classified to the California Current nursery and 14 age 0 sharks (70%) to the NPTZ nursery (electronic supplementary material, figure S4).
Discriminant analysis suggests that salmon sharks shift from juvenile oceanic habitats to adult neritic habitats around age 6. Isotopic values of the juvenile and adult groups were significantly different (squared canonical correlation = 0.71, eigenvalue 2.42, Wilks' λ = 0.29, F2,63 = 76.34, p = 0), and discriminant analysis successfully reclassified the juvenile and adult data at an average rate of 94% (89% juvenile, 97% adult for both reclassification and jackknife validation). Fitting a logistic model (Wald statistic 47.359, p < 0.001) to these data indicated that sharks transitioned to adult habitats by age 6 (electronic supplementary material, figures S5 and S6).
(c). Isotopic niche
An ontogenetic shift in isotopic niche area was also apparent (figure 4). For the first several years (age 0–4), niche area was relatively large, with age 5 having the largest area (figure 4, inset), representing use of a wide range of resources (e.g. habitat and diet). Following age 5, niche area declined and was relatively consistent and small in ages 8+, suggesting a decline in diversity of resources used. Age U sharks had niche areas similar to the oldest age classes.
Figure 4.
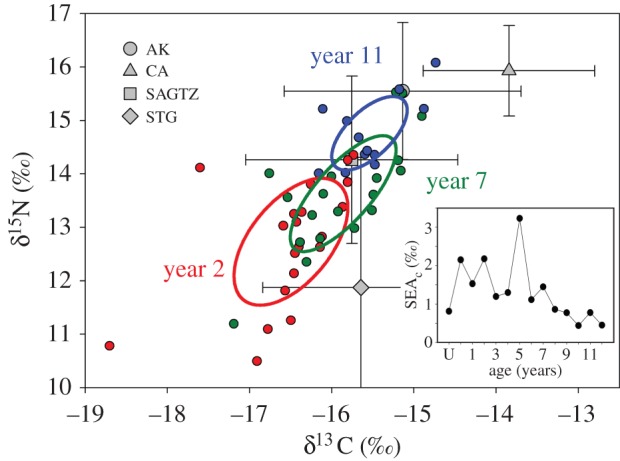
SEAc of different year classes, showing three representative years (ages 2, 7 and 11). Inset shows the time series of SEAc area.
4. Discussion
Stable isotope analysis of shark vertebrae, Bayesian isotope mixing models, isoscapes and electronic tag data were used to reconstruct ontogenetic patterns of habitat and resource use in a pelagic shark for the first time, revealing new insights into the life history of the salmon shark, including insight into their early life history and ontogenetic shifts in habitat and isotopic niche width. This integrative approach provides a framework that can be used to retrospectively study the early life history and ontogenetic shifts in habitat of highly mobile and wide ranging elasmobranchs, or other species with serially accreting structures, providing valuable insight into the life history of difficult to study and increasingly threatened pelagic elasmobranchs [7].
(a). Nursery and juvenile habitats
Mixing model results indicated that juvenile salmon sharks primarily used oceanic resources (figure 3). This suggests that stable isotope composition of juvenile sharks reflects use of the NPTZ nursery. The NPTZ is a dynamic and productive oceanic region bounded by the subarctic and subtropical frontal zones that has been shown to be an important foraging area and migratory corridor for a variety of pelagic predators [42], including juvenile salmon sharks [12,35].
Nakano & Nagasawa [12] noted that juveniles are most abundant in 14–16°C waters of the NPTZ, which is characteristic of habitats north of the transition zone chlorophyll front (TZCF), and rarely occur to the south. The TZCF, a major feature of the NPTZ, is a sharp transition in chlorophyll concentration which separates the more productive subarctic gyre from the oligotrophic subtropical gyre [42]. This front annually shifts as much as 10° in latitude through the NPTZ [43] and is tightly coupled with the 18°C surface isotherm [44]. Some annuli with the lowest δ15N and δ13C values (figure 2), primarily from sharks youngers than 6, were not well bounded by mean SAGTZ or STG values, suggesting that prey from the vicinity of the NPTZ have not been adequately characterized or represented in the mean regional values.
How δ13C and δ15N values of primary producers and prey change across the strong environmental gradient present in the NPTZ is unknown; however, they may change significantly, as has been demonstrated in other areas with strong productivity gradients [17,45]. The low variability in δ13C and high variability in δ15N in juvenile salmon sharks (figure 2b,c), may reflect foraging across the TZCF and a latitudinal gradient in δ15N. The high variability in δ15N may also reflect the longitudinal gradient in baseline δ15N values along the NPTZ, where the lowest δ15N occurs in the central Pacific [46,47]. This suggests that an individual residing exclusively within the NPTZ could be foraging across a strong gradient in baseline stable isotope values. Thus, location of foraging relative to the TZCF and movement of prey across this dynamic boundary could play an important role in determining the isotopic composition of juvenile salmon sharks.
(b). Why use the North Pacific Transition Zone as a nursery?
Our results indicate that the NPTZ is the primary nursery area for salmon sharks in the ENP. A smaller proportion of age 0 sharks (30%) were assigned to the California Current nursery, and by age 1 these sharks had shifted to the oceanic nursery area where they remained for several years. Historically, juvenile salmon sharks were caught in high numbers in the open ocean driftnet fisheries for salmon and flying squid in the NPTZ [10,12,33], with estimates ranging as high as 100 000 individuals caught annually as bycatch [10]. This high level of mortality would be of concern for the salmon shark population, given the great importance of juvenile survivorship to population growth rate [48]. However with the 1992 ban on pelagic driftnets, this source of mortality was greatly reduced [49].
It is hypothesized that use of nursery areas by juvenile sharks is generally related to increased prey availability and/or a reduction in predation [2,33]. The NPTZ is a productive pelagic habitat and likely provides an abundant prey field for young salmon sharks [42]. However, there is increasing evidence that many elasmobranchs use nurseries to reduce predation [50,51], especially from larger sharks [52]. Nagasawa [33] suggested that juveniles in the NPTZ may experience reduced rates of predation from other shark species, such as adult blue sharks (Prionace glauca) and mako sharks (Isurus oxyrinchus), which are generally more southerly distributed.
However, as an endothermic shark, the distribution of salmon sharks may be uniquely influenced by temperature. Along with porbeagles (Lamna nasus), salmon sharks are the most endothermic of all the lamnid sharks [53]. The extensive anatomical and physiological adaptations that salmon sharks possess allow adults to defend a specific body core temperature despite large fluctuations in ambient temperature [54]. However, little is known about how endothermic capacity changes during ontogeny. The salmon shark's red muscle is specialized to function at elevated temperatures between 20 and 30°C [55], and rapidly loses function as it cools to temperature below 20°C. The inability to conserve heat and maintain an elevated body core temperature could have significant consequences in endothermic sharks.
Because thermal inertia of sharks will change with size [54], small salmon sharks with a high surface area to volume ratio may be restricted to habitats of more moderate temperature ranges until they attain a mass where heat production and loss can be balanced in order to maintain an optimal body core temperature. Within the NPTZ juvenile salmon sharks can experience consistent moderate thermal conditions by migrating latitudinally with the TZCF. This would provide juvenile salmon sharks with a relatively consistent thermal habitat, high concentration of food resources [42] and low predation risk [12].
The California Current is highly productive [28], and though there are more prey, there likely are more potential predators as well, including a variety of large pelagic and coastal shark species. Additionally, as an eastern boundary current CA experiences seasonal upwelling, which can drop temperatures as low as 3–4°C below normal [56], suggesting that it may present a more thermally challenging environment. Small numbers of juvenile salmon sharks consistently strand along the west coast of North America [57], generally during periods of upwelling. This suggests that thermal factors may play a role in many of these strandings (AB Carlisle 2014, unpublished data). Indeed, several pelagic species in the California Current are known to migrate in response to upwelling [37]. Thus, the NPTZ may represent a trade-off between prey availability, predation pressure and thermal conditions for salmon sharks.
(c). Shift to adult habitat and adult habitat use
Although ontogenetic shifts in habitat have been well studied in coastal elasmobranchs [3,52], these shifts are less well described in pelagic species. Our results showed a general ontogenetic shift from use of oceanic habitats to increased use of neritic habitats. Blue sharks exhibit an ontogenetic shift in distribution, with juveniles being primarily found at higher latitudes than adults [12]. North Pacific spiny dogfish (Squalus suckleyi) live in mid-water, offshore habitats as juveniles and transition to neritic, demersal habitats as adults [58]. White sharks exhibit the opposite pattern as salmon sharks, shifting from coastal juvenile habitats [59] to increased use of oceanic habitats as they mature [19,60].
Our results indicate that salmon sharks primarily use oceanic habitats until approximately age 6 and then increasingly use neritic ecoregions. The timing of the shift corresponds with the onset of maturity for female salmon sharks (6–9 years) [11] and with the minimum reported age of salmon sharks in Alaska. The smallest shark measured and tagged by the TOPP programme [37] in Prince William Sound, AK was estimated to be 6 years old, and Goldman & Musick [11] reported that the youngest shark aged from Alaskan waters was 5 years old (electronic supplementary material, figure S6).
Once adult salmon sharks begin to use neritic ecoregions, they may become more specialized on particular habitats or prey as demonstrated by the reduction in isotopic niche in the oldest year classes. Older salmon sharks exhibit a high degree of site fidelity to particular neritic habitats [37] and exhibit consistent patterns of habitat use in neritic regions [61], suggesting consistent foraging behaviour which may reduce the breadth of resources used. For example, salmon sharks exhibit a high degree of site fidelity to Prince William Sound, AK, where they aggregate in the summer to forage extensively on large concentrations of Pacific salmon gathering at the mouths of their natal rivers [34]. This type of consistent behaviour may help to account for the reduced isotopic niche in adult sharks, and although an increase in dietary niche breadth is often associated with increased size in elasmobranchs and other predatory fish [3], niche contraction has been documented as larger individuals become more specialized on particular prey [62].
The ontogenetic shift in habitat may play a role in reducing competition with younger conspecifics, as has been noted in other elasmobranchs [63], or reflect changes in energetic requirements or endothermic capacity. The fact that the shift corresponds with age at maturity suggests this change may be related to reproduction. For female salmon sharks, with maturity comes the increased energetic demands associated with reproduction, so use of productive neritic habitats may increase the availability of resources necessary for reproduction [64]. Additionally, by the time they reach maturity, salmon sharks are likely large enough to have the thermoregulatory capacity to exploit colder, more dynamic and productive coastal waters while maintaining their elevated body temperatures.
(d). Incorporation of satellite tag data into mixing models
Incorporating electronic tag data into mixing models helped disentangle the interactive effects of shark migrations and movements of their prey, or more generally to unravel the influence of the provenance of resources used by sharks from where sharks actually feed. Integrating movement data with SIA helped elucidate the relationship between time spent in a region and amount of feeding within that region, as has been done with white sharks [19]. Uninformative mixing model results appear to provide a reasonable estimate of the net contribution of different ecoregions to adult salmon sharks, independent of shark movements.
The increased importance of oceanic resources to large salmon sharks in the uninformative mixing model results may reflect movements of prey from oceanic habitats to neritic habitats. Despite many sharks spending the majority of their time in Alaska, salmon sharks likely receive an important trophic subsidy from offshore waters in the form of migratory prey. Pacific salmon mature in oceanic habitats and reflect SAGTZ resources [65]. The salmon targeted by salmon shark foraging aggregations in coastal Alaska [34] are likely an important seasonal source of energy and would represent an important trophic subsidy from oceanic waters to salmon sharks in neritic habitats. Therefore, salmon sharks occurring in AK may be using both AK and SAGTZ resources despite spending most of their time in AK, in effect integrating resources from across the North Pacific while spending the majority of their time within Alaskan waters.
Our results demonstrate that retrospective SIA can be used to reconstruct broad-scale patterns of habitat and resource use for highly migratory pelagic species throughout their ontogeny. Knowledge of ontogenetic patterns of habitat use, in conjunction with an understanding of the relative importance of survivorship of different age classes to population growth rate and persistence, is crucial for effective management of these long-lived species. For salmon sharks, population growth rates are most sensitive to juvenile survivorship. Hence, management efforts that reduce juvenile mortality in the NPTZ, which we demonstrated as their primary nursery habitat, are crucial for population persistence. As the persistence of other species may be sensitive to mortality at other life stages, our results emphasize the need to broaden our understanding of the habitat use patterns of highly migratory species across all life stages in order to guide their successful management.
Supplementary Material
Supplementary Material
Acknowledgements
We acknowledge B. Semmens, J. Dale, K. James, T. Booth, G. Somero, A. Andrews and G. Cailliet for their valuable advice and help with this project. We also thank B. Failor (ADF&G) for supplying the adult salmon shark vertebrae for this study as well as three anonymous reviewers who helped improve the manuscript. A.C. designed the study, collected and analysed data, and wrote the manuscript. K.G. and J.B. collected data. S.L. and A.S. analysed data. S.L., K.G., D.M., T.K. and B.B. helped with data interpretation. All authors helped write the manuscript and gave final approval for publication.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Funding statement
Funding was provided by PADI Foundation, the Alfred P. Sloan Foundation, the David and Lucile Packard Foundation, the Gordon and Betty Moore Foundation, Monterey Bay Aquarium Foundation and the Office of Naval Research.
Competing interests
We have no competing interests.
References
- 1.Morris DW. 2003. Toward an ecological synthesis: a case for habitat selection. Oecologia 136, 1–13. ( 10.1007/s00442-003-1241-4) [DOI] [PubMed] [Google Scholar]
- 2.Heupel MR, Carlson JK, Simpfendorfer CA. 2007. Shark nursery areas: concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 337, 287–297. ( 10.3354/meps337287) [DOI] [Google Scholar]
- 3.Grubbs RD. 2010. Ontogenetic shifts in movements and habitat use. In Sharks and their relatives II: biodiversity, physiology, and conservation (eds Carrier JC, Musick JA, Heithaus MR.), pp. 319–350. Boca Raton, FL: CRC Press. [Google Scholar]
- 4.Werner EE, Gilliam JF. 1984. The ontogenetic niche and species interactions in size-structured populations. Annu. Rev. Ecol. Syst. 15, 393–425. ( 10.1146/annurev.es.15.110184.002141) [DOI] [Google Scholar]
- 5.Hazen EL, Maxwell SM, Bailey H, Bograd SJ, Hamman M, Gaspar P, Godley BJ, Shillinger GL. 2012. Ontogeny in marine tagging and tracking science: technologies and data gaps. Mar. Ecol. Prog. Ser. 457, 221–240. ( 10.3354/meps09857) [DOI] [Google Scholar]
- 6.Cortes E. 2002. Incorporating uncertainty into demographic modeling: application to shark populations and their conservation. Conserv. Biol. 16, 1048–1062. ( 10.1046/j.1523-1739.2002.00423.x) [DOI] [Google Scholar]
- 7.Dulvy NK, et al. 2008. You can swim but you can't hide: the global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 18, 459–482. ( 10.1002/aqc.975) [DOI] [Google Scholar]
- 8.Heithaus MR, Frid A, Wirsing AJ, Worm B. 2008. Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210. ( 10.1016/j.tree.2008.01.003) [DOI] [PubMed] [Google Scholar]
- 9.Weng KC, Castilho PC, Morrissette JM, Landeira-Fernandez AM, Holts DB, Schallert RJ, Goldman KJ, Block BA. 2005. Satellite tagging and cardiac physiology reveal niche expansion in salmon sharks. Science 310, 104–106. ( 10.1126/science.1114616) [DOI] [PubMed] [Google Scholar]
- 10.Goldman KJ, Musick JA. 2008. The biology and ecology of the salmon shark, Lamna ditropis. In Sharks of the open ocean: biology, fisheries and conservation (eds Camhi MD, Pikitch EK, Babcock EA.), pp. 95–104. Oxford, UK: Blackwell Publishing. [Google Scholar]
- 11.Goldman KJ, Musick JA. 2006. Growth and maturity of salmon sharks (Lamna ditropis) in the eastern and western North Pacific, and comments on back-calculation methods. Fish. Bull. 104, 278–292. [Google Scholar]
- 12.Nakano H, Nagasawa K. 1996. Distribution of pelagic elasmobranchs caught by salmon research gillnets in the North Pacific. Fish. Sci. 62, 860–865. [Google Scholar]
- 13.Peterson BJ, Fry B. 1987. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 18, 293–320. ( 10.1146/annurev.es.18.110187.001453) [DOI] [Google Scholar]
- 14.Estrada JA, Rice AN, Natanson LJ, Skomal GB. 2006. Use of isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in white sharks. Ecology 87, 829–834. ( 10.1890/0012-9658(2006)87[829:UOIAOV]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 15.Koch PL. 2007. Isotopic study of the biology of modern and fossil vertebrates. In Stable isotopes in ecology and environmental science (eds Michener R, Lajtha K.), pp. 99–154. Boston, MA: Blackwell Publishing. [Google Scholar]
- 16.Kim SL, Tinker MT, Estes JA, Koch PL. 2012. Ontogenetic and among-individual variation in foraging strategies of Northeast Pacific white sharks based on stable isotope analysis. PLoS ONE 7, e45068 ( 10.1371/journal.pone.0045068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham BS, Koch PL, Newsome SD, McMahon KW, Aurioles D. 2010. Using isoscapes to trace the movements and foraging behavior of top predators in oceanic ecosystems. In Isoscapes: understanding movement, pattern, and process on earth through isotope mapping (eds West JB, Bowen GJ, Dawson TE, Tu KP.), pp. 299–318. New York, NY: Springer. [Google Scholar]
- 18.Michener RH, Kaufman L. 2007. Stable isotope ratios as tracers in marine food webs: an update. In Stable isotopes in ecology and environmental science (eds Michener R, Lajtha K.), pp. 238–282. Boston, MA: Blackwell. [Google Scholar]
- 19.Carlisle AB, et al. 2012. Using stable isotope analysis to understand the migration and trophic ecology of northeastern Pacific white sharks (Carcharodon carcharias). PLoS ONE 7, e30492 ( 10.1371/journal.pone.0030492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobson KA. 1999. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120, 314–326. ( 10.1007/s004420050865) [DOI] [PubMed] [Google Scholar]
- 21.Ramos R, Gonzalez-Solis J. 2012. Trace me if you can: the use of intrinsic biogeochemical markers in marine top predators. Front. Ecol. Environ. 10, 258–266. ( 10.1890/110140) [DOI] [Google Scholar]
- 22.Best PB, Schell DM. 1996. Stable isotopes in southern right whale (Eubalaena australis) baleen as indicators of seasonal movements, feeding and growth. Mar. Biol. 124, 483–494. ( 10.1007/BF00351030) [DOI] [Google Scholar]
- 23.Mendes S, Newton J, Reid RJ, Zuur AF, Pierce GJ. 2007. Stable carbon and nitrogen isotope ratio profiling of sperm whale teeth reveals ontogenetic movements and trophic ecology. Oecologia 151, 605–615. ( 10.1007/s00442-006-0612-z) [DOI] [PubMed] [Google Scholar]
- 24.Cherel Y, Hobson KA. 2007. Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar. Ecol. Prog. Ser. 329, 281–287. ( 10.3354/meps329281) [DOI] [Google Scholar]
- 25.Trueman CN, MacKenzie KM, Palmer MR. 2012. Identifying migrations in marine fishes through stable isotope analysis. J. Fish. Biol. 81, 826–847. ( 10.1111/j.1095-8649.2012.03361.x) [DOI] [PubMed] [Google Scholar]
- 26.Hussey NE, MacNeil MA, Olin JA, McMeans BC, Kinney MJ, Chapman DD, Fisk AT. 2012. Stable isotopes and elasmobranchs: tissue types, methods, applications and assumptions. J. Fish. Biol. 80, 1449–1484. ( 10.1111/j.1095-8649.2012.03251.x) [DOI] [PubMed] [Google Scholar]
- 27.Weng KC, Foley DG, Ganong JE, Perle C, Shillinger GL, Block BA. 2008. Migration of an upper trophic level predator, the salmon shark Lamna ditropis, between distant ecoregions. Mar. Ecol. Prog. Ser. 372, 253–264. ( 10.3354/meps07706) [DOI] [Google Scholar]
- 28.Longhurst AR. 2007. Ecological geography of the sea. Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 29.Kim SL, Koch PL. 2011. Methods to collect, preserve, and prepare elasmobranch tissues for stable isotope analysis. Environ. Biol. Fish. 95, 53–63. ( 10.1007/s10641-011-9860-9) [DOI] [Google Scholar]
- 30.Campana SE, Natanson LJ, Myklevoll S. 2002. Bomb dating and age determination of large pelagic sharks. Can. J. Fish. Aquat. Sci. 59, 450–455. ( 10.1139/f02-027) [DOI] [Google Scholar]
- 31.Goldman KJ, Cailliet GM, Natanson LJ, Andrews A. 2012. Assessing the age and growth of chondrichthyan fishes. Second edition. In The biology of sharks and their relatives (eds Carrier JC, Musick JA, Heithaus MR.), pp. 423–451. Boca Raton, FL: CRC Press. [Google Scholar]
- 32.Brown TA, Nelson DE, Vogel JS, Southon JR. 1988. Improved collagen extraction by modified Longin method. Radiocarbon 32, 171–177. [Google Scholar]
- 33.Nagasawa K. 1998. Predation by salmon sharks (Lamna ditropis) on Pacific salmon (Onchorhynchus spp.) in the North Pacific Ocean. North Pac. Anadromous Fish Comm. Bull. 1, 419–433. [Google Scholar]
- 34.Hulbert LB, Aires-Da-Silva AM, Gallucci VF, Rice JS. 2005. Seasonal foraging movements and migratory patterns of female Lamna ditropis tagged in Prince William Sound, Alaska. J. Fish Biol. 67, 490–509. ( 10.1111/j.0022-1112.2005.00757.x) [DOI] [Google Scholar]
- 35.Kubodera T, Watanabe H, Ichii T. 2007. Feeding habits of the blue shark, Prionace glauca, and salmon shark, Lamna ditropis, in the transition region of the Western North Pacific. Rev. Fish Biol. Fish. 17, 111–124. ( 10.1007/s11160-006-9020-z) [DOI] [Google Scholar]
- 36.Moore JW, Semmens BX. 2008. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 11, 470–480. ( 10.1111/j.1461-0248.2008.01163.x) [DOI] [PubMed] [Google Scholar]
- 37.Block BA, et al. 2011. Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90. ( 10.1038/nature10082) [DOI] [PubMed] [Google Scholar]
- 38.Jackson AL, Inger R, Parnell AC, Bearhop S. 2011. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602. ( 10.1111/j.1365-2656.2011.01806.x) [DOI] [PubMed] [Google Scholar]
- 39.Montoya JP. 2007. Natural abundance of 15N in marine planktonic ecosystems. In Stable isotopes in ecology and environmental science (eds Michener R, Lajtha K.), pp. 176–201. Boston, MA: Blackwell. [Google Scholar]
- 40.Clementz MT, Koch PL. 2001. Differentiating aquatic mammal habitat and foraging ecology with stable isotopes in tooth enamel. Oecologia 129, 461–472. [DOI] [PubMed] [Google Scholar]
- 41.Goericke R, Fry B. 1994. Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Glob. Biogeochem. Cycles 8, 85–90. ( 10.1029/93GB03272) [DOI] [Google Scholar]
- 42.Polovina JJ, Howell E, Kobayashi DR, Seki MP. 2001. The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog. Oceanogr. 49, 469–483. ( 10.1016/S0079-6611(01)00036-2) [DOI] [Google Scholar]
- 43.Ayers JM, Lozier MS. 2010. Physical controls on the seasonal migration of the North Pacific transition zone chlorophyll front. J. Geophys. Res. 115, C05001 ( 10.1029/2009JC005596) [DOI] [Google Scholar]
- 44.Bograd SJ, Foley DG, Schwing FB, Wilson C, Laurs RM, Polovina JJ, Howell EA, Brainard RE. 2004. On the seasonal and interannual migrations of the transition zone chlorophyll front. Geophys. Res. Lett. 31, L17204 ( 10.1029/2004GL020637) [DOI] [Google Scholar]
- 45.Kline TC. 2009. Characterization of carbon and nitrogen stable isotope gradients in the northern Gulf of Alaska using terminal feed stage copepodite-V Neocalanus cristatus. Deep Sea Res. Part II Top. Stud. Oceanogr. 56, 2537–2552. ( 10.1016/j.dsr2.2009.03.004) [DOI] [Google Scholar]
- 46.Somes CJ, et al. 2010. Simulating the global distribution of nitrogen isotopes in the ocean. Glob. Biogeochem. Cycles 24, GB4019 ( 10.1029/2009GB003767) [DOI] [Google Scholar]
- 47.Chiba S, Sugisaki H, Kuwata A, Tadokoro K, Kobari T, Yamaguchi A, Mackas DL. 2012. Pan-North Pacific comparison of long-term variation in Neocalanus copepods based on stable isotope analysis. Prog. Oceanogr. 97–100, 63–75. ( 10.1016/j.pocean.2011.11.007) [DOI] [Google Scholar]
- 48.Goldman KJ. 2002. Aspects of age, growth, demographics and thermal biology of two Lamniform shark species, p. 220, College of William and Mary, Williamsburg, VA, USA. [Google Scholar]
- 49.McKinnell S, Seki MP. 1998. Shark bycatch in the Japanese high seas squid driftnet fishery in the North Pacific Ocean. Fish Res. 39, 127–138. ( 10.1016/S0165-7836(98)00179-9) [DOI] [Google Scholar]
- 50.Duncan KM, Holland KN. 2006. Habitat use, growth rates and dispersal patterns of juvenile scalloped hammerhead sharks Sphyrna lewini in a nursery habitat. Mar. Ecol. Prog. Ser. 312, 211–221. ( 10.3354/meps312211) [DOI] [Google Scholar]
- 51.Heupel MR, Hueter RE. 2002. Importance of prey density in relation to the movement patterns of juvenile blacktip sharks (Carcharhinus limbatus) within a coastal nursery area. Mar. Freshw. Res. 53, 543–550. ( 10.1071/MF01132) [DOI] [Google Scholar]
- 52.Springer S. 1967. Social organization of shark populations. In Sharks, skates, and rays (eds Gilbert PW, Mathewson RF, Rall DP.), pp. 149–174. Baltimore, MD: Johns Hopkins Press. [Google Scholar]
- 53.Carey FG, Casey JG, Pratt HL, Urquhart D, McCosker JE. 1985. Temperature, heat production, and heat exchange in lamnid sharks. South. Calif. Acad. Sci. Memoirs 9, 92–108. [Google Scholar]
- 54.Goldman KJ, Anderson SD, Latour RJ, Musick JA. 2004. Homeothermy in adult salmon sharks, Lamna ditropis. Environ. Biol. Fish. 71, 403–411. ( 10.1007/s10641-004-6588-9) [DOI] [Google Scholar]
- 55.Bernal D, Donley JM, Shadwick RE, Syme DA. 2005. Mammal-like muscles power swimming in a cold-water shark. Nature 437, 1349–1352. ( 10.1038/nature04007) [DOI] [PubMed] [Google Scholar]
- 56.Schwing FB, Moore CS, Ralston S, Sakuma KM. 2000. Record coastal upwelling in the California Current in 1999. CalCOFI Rep. 41, 148–160. [Google Scholar]
- 57.Schaffer PA, Lifland B, Van Sommeran S, Casper DR, Davis CR. 2012. Meningoencephalitis associated with Carnobacterium maltaromaticum-like bacteria in stranded juvenile salmon sharks (Lamna ditropis). Vet. Pathol. 50, 412–417. ( 10.1177/0300985812441033) [DOI] [PubMed] [Google Scholar]
- 58.Ketchen KS. 1986. The spiny dogfish (Squalus acanthias) in the northeast Pacific and a history of its utilization. Can. Spec. Publ. Fish. Aquat. Sci. 88, 78 p. [Google Scholar]
- 59.Weng KC, O'Sullivan JB, Lowe CG, Winkler CE, Dewar H, Block BA. 2007. Movements, behavior and habitat preferences of juvenile white sharks Carcharodon carcharias in the eastern Pacific. Mar. Ecol. Prog. Ser. 338, 211–224. ( 10.3354/meps338211) [DOI] [Google Scholar]
- 60.Hussey N, McCann H, Cliff G, Dudley S, Wintner S, Fisk A. 2012. Size-based analysis of diet and trophic position of the white shark, Carcharodon carcharias, in South African waters. In Global perspectives on the biology and life history of the white shark (ed. Domeier ML.), pp. 27–50. Boca Raton, FL: CRC Press. [Google Scholar]
- 61.Carlisle AB, Perle CR, Goldman KJ, Block BA. 2011. Seasonal changes in depth distribution of salmon sharks (Lamna ditropis) in Alaskan waters: implications for foraging ecology. Can. J. Fish. Aquat. Sci. 68, 1905–1921. ( 10.1139/f2011-105) [DOI] [Google Scholar]
- 62.Bethea DM, Buckel JA, Carlson JK. 2004. Foraging ecology of the early life stages of four sympatric shark species. Mar. Ecol. Prog. Ser. 268, 245–264. ( 10.3354/meps268245) [DOI] [Google Scholar]
- 63.Ebert DA. 2002. Ontogenetic changes in the diet of the sevengill shark (Notorynchus cepedianus). Mar. Freshw. Res. 53, 517–523. ( 10.1071/MF01143) [DOI] [Google Scholar]
- 64.Alonso MK, Crespo EA, Garcia NA, Pedraza SN, Mariotti PA, Mora NJ. 2002. Fishery and ontogenetic driven changes in the diet of the spiny dogfish, Squalus acanthias, in Patagonian waters, Argentina. Environ. Biol. Fish. 63, 193–202. ( 10.1023/A:1014229432375) [DOI] [Google Scholar]
- 65.Satterfield FR, Finney BP. 2002. Stable isotope analysis of Pacific salmon: insight into trophic status and oceanographic conditions. Prog. Oceanogr. 53, 231–246. ( 10.1016/S0079-6611(02)00032-0) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.