Abstract
Culturally transmitted traits are observed in a wide array of animal species, yet we understand little about the costs of the behavioural patterns that underlie culture, such as innovation and social learning. We propose that infectious diseases are a significant cost associated with cultural transmission. We investigated two hypotheses that may explain such a connection: that social learning and exploratory behaviours (specifically, innovation and extractive foraging) either compensate for existing infection or increase exposure to infectious agents. We used Bayesian comparative methods, controlling for sampling effort, body mass, group size, geographical range size, terrestriality, latitude and phylogenetic uncertainty. Across 127 primate species, we found a positive association between pathogen richness and rates of innovation, extractive foraging and social learning. This relationship was driven by two independent phenomena: socially contagious diseases were positively associated with rates of social learning, and environmentally transmitted diseases were positively associated with rates of exploration. Because higher pathogen burdens can contribute to morbidity and mortality, we propose that parasitism is a significant cost associated with the behavioural patterns that underpin culture, and that increased pathogen exposure is likely to have played an important role in the evolution of culture in both non-human primates and humans.
Keywords: cultural evolution, parasitism, behavioural innovation, social learning, comparative study
1. Introduction
Cultural transmission has allowed humans and non-human animals to flexibly adapt to and shape their environments. The capacity to learn new behaviours—both individually through innovation and from others through social learning—allows flexibility in the face of changing environments [1–4]. Individual learning of a novel behaviour, or innovation, occurs through exploration and experimentation [2,5], whereas social learning occurs when one individual learns from the behaviour of another individual [3,6,7]. Much of the research on innovation and social learning has focused on the ecological and social benefits of behaviours acquired by these processes. For example, learned foraging behaviours can enable individuals to more effectively acquire energy, either by accessing new resources or by more efficiently exploiting existing food sources [2]. Similarly, extractive foraging (feeding on embedded or encased foods such as nutmeat, shellfish, bone marrow and buried tubers) can provide access to novel or nutritionally rich food resources. Extractive foraging has been linked to exploratory behaviour and innovation, and also has been proposed to be a condition favouring the development of complex culture [8–10].
Although many studies have focused on the benefits of innovation, extractive foraging and social learning, relatively few studies have considered the costs of these behaviours. Costs may include the immediate costs of performing a behaviour and constitutive costs associated with developing the ability to perform a behaviour [11]. In terms of immediate costs, innovation and extractive foraging may carry risks such as poisoning from eating toxic food sources, predation, or injury from interacting with new objects or individuals [5]. Additionally, social learning may favour increased proximity among individuals, which could increase competition for resources. In terms of constitutive costs, brain size may be a cost of innovation and social learning, with rates of both innovation and social learning positively correlated with brain volume across primates ([12], see [13] for discussion). Production and maintenance of energetically expensive brain tissue can be accommodated by decreasing energetic investment in functions such as digestion, locomotion or reproduction [14]. Larger brains may also generate life-history costs involving greater allocation of resources to large-brained offspring [15,16].
We tested the hypothesis that innovation, extractive foraging and social learning are associated with increased disease risk [17–19]. Higher rates of innovation and more varied extractive foraging, which are indicators of greater environmental exploration, may result in greater exposure to infectious agents in the environment. For example, primates foraging on insects can be exposed to acanthocephalans, and primates digging in soil may be exposed to helminths [20,21]. If social learning is associated with increased social contact either directly or via increased joint use of resources [22], more frequent social learners may be more exposed to socially contagious parasites. Because we were interested in how increases in species-level behavioural variation affected parasite variation within species, we used measures of richness—the number of observed unique behaviours or parasites for a species—as our main indicator variables. This decision was further motivated by our goal of capturing a wide diversity of parasites and behaviours, a goal that would be hindered if we were to test the hypotheses with single-parasite measures of prevalence (the proportion of individuals infected with a specified parasite in a single population) or infection intensity (the number of reproductive parasites present within a single infected individual at a point in time).
Given our hypotheses, we propose that social learning and exploration may have parasite-related fitness costs that, if great enough, would offset their benefits. These parasite-related costs could thus partially account for the observed interspecific variation in social learning and exploration across primates [8]. Beyond the obvious negative impacts of parasites on fitness via morbidity [23] and mortality [24–26], infectious diseases also have negative impacts on cognitive development and function [27], hinder growth [28] and require individuals to allocate more time and energy to resting and immune function [29]. These fitness consequences of parasitism are often amplified when a greater diversity of parasites are found in a host or species; for example, co-infection leads to higher host mortality in a wide variety of host and parasite taxa [30–32].
Previous research has linked higher rates of exploratory or innovative behaviours with higher levels of parasitism in rodents [17] and birds [18,19,33]. Additionally, indicators of social contact patterns such as group size, population density and social network properties are positively correlated with parasitism [34–37], although none of these patterns has been linked directly to social learning. Unfortunately, the causes behind these correlations are poorly understood, with two major competing hypotheses [5,17–19]. The first, the ‘exposure hypothesis’, suggests that increased exploration or social learning leads to increased exposure to parasites. The second, the ‘compensation hypothesis’, suggests that increased exploration and social learning are compensatory responses to higher parasite levels. No study on the correlation of parasitism and behaviours underpinning culture has yet included primates, which is remarkable given the large number of studies on social learning and innovation in primates and their importance for understanding the evolution of human culture [3,6].
Based on the general hypothesis that exploratory behaviour and social learning have parasite-related costs, we contrast these two specific, but not necessarily exclusive hypotheses (table 1). Under the ‘compensation hypothesis’, if parasite-related costs were driving a general need for further exploration and social learning in primates, we predict positive correlations between these behaviours and all measures of parasite richness, regardless of transmission mode. However, under the ‘exposure hypothesis’, we predict that richness of parasites transmitted through social contact will covary positively with rates of social learning, but not with our measures of environmental exploration (innovation and extractive foraging), while richness of parasites transmitted through contact with the environment is predicted to covary most strongly with rates of innovation and extractive foraging, but not with social learning.
Table 1.
Predicted associations between parasite richness and behavioural richness under the competing hypotheses: (a) the ‘exposure hypothesis' and (b) the ‘compensation hypothesis’.
| (a) ‘exposure hypothesis' | socially transmitted parasites | environmentally transmitted parasites |
|---|---|---|
| social learning | positive association | no association |
| exploration | no association | positive association |
| (b) ‘compensation hypothesis’ | socially transmitted parasites | environmentally transmitted parasites |
|---|---|---|
| social learning | positive association | positive association |
| exploration | positive association | positive association |
2. Material and methods
(a). Overview
To test our competing hypotheses, we examined three behavioural measures of social learning and environmental exploration, namely the number of reports of social learning, innovation and extractive foraging per species, with innovation and extractive foraging together indexing ‘exploratory behaviour’, as they both relate to the exploration and exploitation of an animal's environment. We use ‘parasite’ to refer to any infectious disease-causing agent, ranging from macro-parasites like helminths and arthropods to micro-parasites, or pathogens, such as viruses, bacteria, protozoa and fungi. In addition to a general analysis of all parasites, we investigated social learning and exploratory behaviour in relation to parasites that are either socially transmitted or environmentally transmitted, and thus relevant to our two specific hypotheses. Socially transmitted parasites rely on direct host-to-host contact for their transmission, such as viruses that cause respiratory infections and are spread through sneezing, coughing and physical contact. Environmentally transmitted parasites spread through environmental substrates such as soil and water, in which parasite infectious stages are found (e.g. Giardia spp.; [20]). Thus, we ran five analyses: one global analysis testing for an association between all behaviours and all parasites, and four sub-analyses addressing each of the predictions of our specific hypotheses.
(b). Datasets
Behavioural data for each primate species were retrieved from an existing database based on a search of approximately 4000 primate and behavioural articles for examples of innovation, social learning and extractive foraging [8]. The database provides a measure of the variety of reports within each behavioural category for each species. The majority of these reports were observational, and thus reports of social learning (which is difficult to characterize without controlled experiments) should be interpreted with caution, while innovation and extractive foraging are more easily characterized by observational studies [8,12,22]. In this respect, experimental investigation of species differences would be valuable, but such data are challenging to gather objectively for the large number of species investigated here [8]. To avoid double-counting reports, cases that simultaneously qualified as more than one behavioural category (e.g. a case of extractive foraging that is also an innovation) were excluded from the behavioural database (as in [8]), apart from instances of social learning and any other measure, since the social learner and individual learned from are different individuals. Examples of innovative tool use and of extractive foraging with tools were also excluded because we lacked a clear hypothesis linking tool use to parasitism. Behavioural data were summarized as the total number of distinct behaviours (a measure of behavioural richness) that could be categorized as social learning, innovation or extractive foraging for each primate species in the dataset. Identical findings emerged when innovation and extractive foraging were analysed separately (electronic supplementary material, table S1). The compilation was primarily composed of wild observations but also included a substantial number of records from captivity. Previous research has shown that removal of data from captive and wild populations with human intervention does not affect the relationships among our behavioural variables [12]. Thus to maximize the size of the dataset, both captive and wild data were included.
We extracted parasite species richness from the Global Mammal Parasite Database (GMPD) [38], which is based on published literature on wild populations (electronic supplementary material, S1). After compiling data from both databases, 127 primate species were found to co-occur between datasets and were thus included in this study. Following the classification system of Corbett & Hill (C&H; [39]), the 127 species consisted of 26 strepsirrhines, 1 tarsier, 38 New World monkeys, 53 Old World monkeys, 5 gibbons and 4 great apes (excluding humans) (electronic supplementary material, appendix S-A). Seventy-four per cent of all primate species were sampled from the C&H taxonomy.
(c). Control variables
We controlled for four variables that may influence parasite richness [40–42]: (i) average body size of a species, because larger-bodied individuals consume more resources and provide more niches for parasites [40]; (ii) average group size for a species, because larger groups are more likely to maintain a parasite than are smaller ones [34,36,37]; (iii) geographical range of the species, because species that cover more area are more likely to encompass the ranges of multiple parasites, likely have larger populations to sustain more parasites and are more likely to encounter greater variation in habitat types that could support different parasites [20,43] and may show greater behavioural diversity [44], and (iv) the absolute value of the latitudinal mid-point of each species' range (henceforth ‘absolute latitude’), because previous studies have shown that parasite richness decreases as host species move away from the equator [45]. An additional analysis that also included a binary variable for substrate use (arboreal versus terrestrial, because terrestrial species would be expected to encounter a greater variety of environmentally transmitted parasites) revealed an identical, albeit weaker, pattern of results, perhaps due to the decrease in power or the lack of resolution that a binary variable can provide. Owing to these various issues with this variable, we left substrate use out of our main multivariate analyses, but we present and discuss additional results involving associations between learning categories, substrate use and parasite transmission in the electronic supplementary material, S1. Data on mean adult body mass, mean group size, total geographical range and absolute latitude were collected from the PanTHERIA database [46], and, when data were unavailable, from the All the World's Primates database [47].
More intensive sampling could lead to higher counts of both parasites and behaviours [48]. We controlled for differences in research effort by regressing parasite and behaviour counts on citation counts, and using the residuals from these models in our analyses. When testing for a correlation between two sets of residuals obtained with identical x-variables, spurious positive results can arise due to measurement error in x [49]. To control for this so-called ‘Economos problem’ of correlated residuals, we used separate, independent sources to estimate sampling effort for the parasite and behavioural data [50]. We collated our parasite richness data with data on the number of references for each host species using the Primate Information Network's ‘PrimateLit’ bibliographic database (http://primatelit.library.wisc.edu/), accessed in May 2010. Similarly, we collated our behavioural richness data with data on the number of references for each species using the Zoological Record citation index for 1993–2001 (see [8] for details). Both bibliographic databases cover a range of subject areas and both field and captive studies, and they were chosen to ascertain general research effort in the study of a given species. Log10-transformed parasite richness and behavioural richness data were then regressed against the independently obtained measures of sampling effort while controlling for phylogeny, with residuals from these regressions used in the analyses.
(d). Phylogeny and phylogenetic uncertainty
In all analyses reported—including those controlling for sampling effort—we incorporated uncertainty in primate phylogeny and the underlying evolutionary model by using Bayesian phylogenetic comparative methods (Markov chain Monte Carlo Phylogenetic Generalized Least Squares models, or MCMC PGLS), as implemented in BayesTraits [51] and assuming flat priors. Because we have imperfect knowledge of the exact evolutionary history of living primates, our analyses controlled for phylogenetic uncertainty by using a set of 100 dated, bifurcating phylogenies, downloaded from 10kTrees Version 3 for the 125 species identified in the C&H taxonomy, plus two additional species not identified in the C&H taxonomy [52]. Regression models were run for 3 300 000 iterations, with a 300 000 iteration burn-in, and sampled every 100 iterations. Rate deviation parameters were set to maintain acceptance rates between 25 and 35%, and we estimated λ, which scales the internal branch lengths of a phylogeny and is generally used to quantify phylogenetic signal [51,53]. A value of λ = 1 indicates that evolution of a given trait has occurred according to a Brownian motion model of evolution and thus shows phylogenetic signal; a value of λ = 0 indicates that trait variation is independent of phylogeny and values of λ between zero and one indicate an intermediate phylogenetic signal. We included an estimate of λ to control for the effect of phylogeny in the statistical models; this is preferable to using phylogenetic independent contrasts (PICs) because PIC assumes a λ of 1, rather than allowing λ to take intermediate values. Three runs of each model were tested to ensure convergence to common values and plateaued likelihood, and consistent findings were confirmed before reporting results. All models reported in this study resulted in convergence to common values for all variables tested. Regression coefficients used for controlling sampling effort were obtained as the mean of the posterior distribution.
(e). Bayesian statistical models and evaluation criteria
First, we investigated the effect of all different behaviours on all parasites, which we will refer to as the ‘total’ model, using the following linear model: Residual[PSR] ∼ intercept + βResidual[BR] × Residual[BR] + βBM × Body Mass + βGS × Group Size + βGR × Geographical Range + βAL × Absolute Latitude + error (where PSR is parasite species richness, BR is behaviour richness, BM is body mass, GS is group size, GR is geographical range and AL is absolute latitude). The ‘total’ model included many parasites that were documented as being transmitted by both social and environmental contact.
Second, we extracted richness of exclusively socially transmitted and exclusively environmentally transmitted parasites from the GMPD. Fifty-four host species (11 strepsirhines, 13 Old World monkeys, 26 New World monkeys and 4 great apes) were found to have sufficient data for testing our hypotheses (i.e. for each species, at least one parasite species was present for each of the two categories of mutually exclusive transmission modes). We controlled for sampling effort, and re-estimated parameters for the statistical model as used in the ‘total’ model. We also investigated the association between social learning and exploratory behaviours using our methods, and, in line with previous work [8,12], found that these two measures were positively correlated (electronic supplementary material, figure S1).
Levels of support for an association between two variables were based on the proportion of regression coefficients with slopes in the predicted direction, assigned as follows: more than 95% of slopes in the predicted direction were interpreted as ‘strong support’ and 90% to 95% as ‘likely support’. Variance inflation factors (VIFs) of all models were tested to detect multicollinearity in the statistical models, with critical values set at greater than or equal to 10 [54]. All VIFs for predictors across all models were well below the critical value, with a maximum value of 1.51.
3. Results
(a). Total richness results
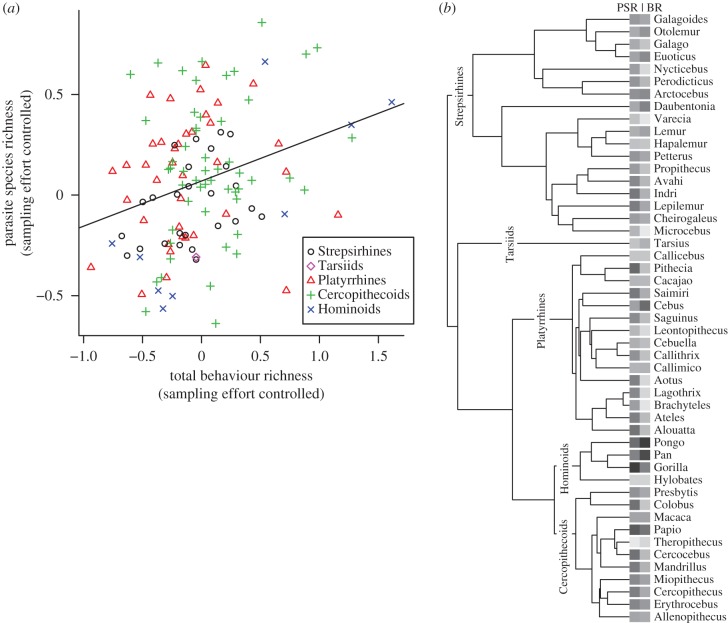
Across primate species, the number of reports of social learning, innovation and extractive foraging (‘total behaviour richness') covaried positively with total parasite richness (figure 1), with both variables controlled for sampling effort. We found ‘strong support’ (see §2(e) for definitions of support) for this association in our MCMC Bayesian PGLS model, with over 99% of sampled iterations exhibiting positive slopes. Our models also included additional controls for body mass, geographical range, absolute latitude and group size, which are commonly investigated as predictors of parasite richness [20,40]. We found ‘likely support’ for a positive association between total parasite richness and body mass, ‘strong support’ for a positive association between total parasite richness and geographical range, and ‘strong support’ for a negative association between total parasite richness and absolute latitude. The model revealed intermediate phylogenetic signal (mean λ = 0.29; see §2(d) for explanation of λ) and despite strong support for several key variables, fit the data modestly, with mean R2 = 0.15 (table 2).
Figure 1.
The ‘total’ Bayesian PGLS model reveals an association between behaviour and parasite richness. (a) Parasite species richness (PSR) covaries positively with behaviour richness (BR) per primate species. Species are coloured and grouped by monophyletic taxa, with a line-of-best-fit indicating the regression after controlling for body mass, group size, geographical range, absolute latitude, phylogeny and sampling effort. (b) 10kTrees [55] consensus phylogeny of genera included in the analysis, with relative measures of parasite species richness (first column) and behaviour richness (BR, second column) indicated at the tips, along with genus name. Shades of blocks indicate magnitude of a genus' PSR or BR, with darker shades representing greater relative values. (Online version in colour.)
Table 2.
Results of the five Bayesian PGLS models tested in this study. Parasite species richness was the response variable and all others (behaviour richness, body mass, group size, geographical range and absolute latitude) were predictors. Results involving substrate use are included in the electronic supplementary material, S1. Reported outputs for each predictor are the mean slopes (β) and proportion of models with predicted slopes (‘support’) sampled from 3 000 000 iterations. Model mean R2 and mean λ were estimated as the means of all iterations and 95% highest posterior density credibility intervals (95% HPD CI) values for λ were calculated from all results.
| parasite transmission mode | behavioural measure | behaviour richness |
body mass |
group size |
geographic range |
absolute latitude |
λ |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean β | support (%) | mean β | support (%) | mean β | support (%) | mean β | support (%) | mean β | support (%) | mean λ | 95% HPD CI | mean R2 | ||
| social | exploration | −0.02 | 36.5 | 0.18 | 99.8 | −0.08 | 10.4 | 0.09 | 98.8 | −0.01 | 64.2 | 0.26 | <0.01–0.54 | 0.20 |
| social | social learning | 0.16 | 98.0 | 0.14 | 99.3 | −0.06 | 17.2 | 0.08 | 98.3 | −0.01 | 73.7 | 0.20 | <0.01–0.47 | 0.27 |
| environmental | exploration | 0.13 | 92.9 | 0.12 | 94.3 | 0.05 | 71.2 | −0.01 | 42.3 | −0.03 | 84.9 | 0.19 | <0.01–0.50 | 0.11 |
| environmental | social learning | −0.04 | 65.5 | 0.15 | 96.8 | 0.04 | 66.2 | <0.01 | 50.0 | −0.03 | 80.4 | 0.21 | <0.01–0.53 | 0.08 |
| total | total | 0.19 | 99.7 | 0.09 | 91.1 | 0.05 | 74.3 | 0.07 | 95.7 | −0.04 | 98.3 | 0.29 | <0.01–0.56 | 0.15 |
(b). Transmission mode results
To focus specifically on whether particular types of exploration and learning influence exposure to particular parasites, we isolated exclusively socially transmitted and exclusively environmentally transmitted parasites in tests for associations with socially learned behaviours and exploratory behaviours. This isolation was important, because many parasites exhibited multiple transmission modes, which could lead to spurious correlations in the tests of our specific predictions [12,56]. If we had included parasites that were transmitted by both social and environmental contact, then we would much more likely to observe results in support of the ‘compensation hypothesis’, because such inclusions would lead to a convergence in our results for the four specific models.
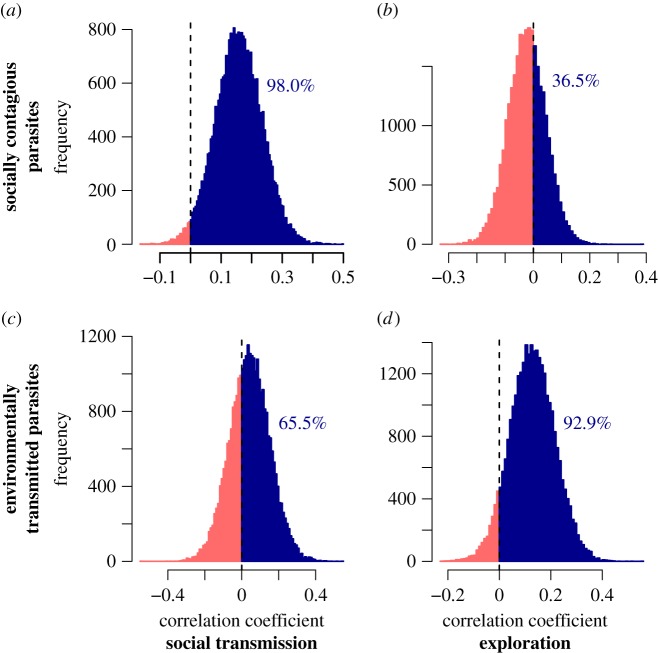
Tests of socially transmitted parasites supported the ‘exposure hypothesis’. Specifically, the number of reports of social learning was positively associated with measures of exclusively socially transmitted parasite richness, again in models that controlled for sampling effort and the other previously mentioned controls. This association between social learning and socially transmitted parasite richness received ‘strong support’, with 98% of iterations sampled exhibiting positive slopes (figure 2a). However, we found no support for an association between the richness of exploratory behaviour and exclusively socially transmitted parasite richness, with only 36.5% of iterations sampled exhibiting positive slopes for counts of exploratory behaviours (figure 2b). In both social transmission parasite models, body mass and geographical range were also ‘strong’ predictors of positive associations with socially transmitted parasite richness. Group size and absolute latitude were not clearly associated with socially transmitted parasite richness (table 2).
Figure 2.
Matrix of histograms displaying posterior distributions of βBR (model coefficient for behaviour richness) for all transmission mode-specific models. These models each also account for body mass, group size, geographical range, absolute latitude, sampling effort and phylogenetic uncertainty. Values marked in dark blue represent estimates for βBR that indicate positive associations, light red represents negative associations and percentages above histograms indicate percentage of positive values estimated in each model (measure of support). Y-axes indicate the parasite transmission mode for which parasite richness was collected, and x-axes the type of behaviour examined for a given model. Predicted associations of the ‘exposure hypothesis' (socially contagious parasite richness and social learning; environmental parasite richness and exploration) show support for positive associations, while non-predicted associations under this hypothesis show no support. (Online version in colour.)
Analyses of environmentally transmitted parasites provided additional support for the ‘exposure hypothesis’. The richness of exploratory behaviour was positively associated with measures of exclusively environmentally transmitted parasite richness, showing ‘likely support’ with nearly 93% of MCMC samples exhibiting positive slopes (figure 2d). Conversely, we found no evidence for an association between social learning and measures of exclusively environmentally transmitted parasite richness, with just under 66% of iterations sampled exhibiting positive slopes for relative counts of social learning (figure 2c). Body mass was a supported predictor of environmentally transmitted parasite richness (94–97% support), while group size, absolute latitude and geographical range were not clearly associated with environmentally transmitted parasite richness (table 2).
We conducted further analyses examining how terrestriality correlated with our measures of behavioural and parasite richness (electronic supplementary material, S1). We discovered more reports of social learning in terrestrial species than arboreal species, and a similar, although smaller, difference in exploratory behaviour (electronic supplementary material, figure S2). We also found support for more socially transmitted parasites in terrestrial than arboreal primates (electronic supplementary material, table S2).
4. Discussion
(a). Support for the ‘exposure hypothesis'
Much previous research on innovation, extractive foraging and social learning has focused on the benefits of these behaviours, yet they may also be associated with considerable costs. Here, the associations we report provide evidence for disease-related costs of behavioural flexibility in primates. Specifically, the total number of parasites covaried positively with richness of reports of innovation, extractive foraging and social learning. Moreover, parasite transmission mode was linked to the behavioural subcategories we addressed in this study: greater richness of socially learned behaviours was associated with a higher number of socially transmitted parasites, and greater richness of exploratory behaviours was associated with a higher number of environmentally transmitted parasites. Our analyses thus revealed support for the ‘exposure hypothesis' in primates, and are consistent with the idea that some aspects of social learning and exploration lead to greater exposure to different types of parasites.
Because the richness of socially learned behaviours was positively associated with socially transmitted parasites but not with environmentally transmitted parasites, we propose that social learning either requires, causes or motivates increased social contact and proximity, leading to the increased spread of socially transmitted parasite species within primate host populations. Alternatively, it could be that some other factor influences both social learning and the transmission of socially transmitted infections. For example, certain parasites may lead to higher rates of social contact [57], or contagious disease and social learning may covary with other factors that influence social contact, such as grouping and mating patterns [58], fission–fusion dynamics [59] or social network structure [35]. Furthermore, since the majority of behavioural reports included in this study were observational rather than experimental, the reports of social learning should be interpreted with caution, as wild studies of this phenomenon are often conducted without sufficient controls to unambiguously identify this learning process [22]. Clearly, experimental data, particularly from wild populations, would be valuable in further tests of our findings.
Because exploratory behaviour richness was positively associated with environmentally transmitted parasite richness but not with socially transmitted parasite richness, we propose that increased exploration exposes the host to new infectious diseases. We used innovation and extractive foraging to index exploratory behaviour, and thus either these behaviours themselves or their correlates could increase parasite exposure. Exploration was not associated with socially transmitted parasite richness, thus providing no support for the ‘compensation hypothesis’. Similarly, our findings are not consistent with the idea that environmentally and socially transmitted parasites provoke compensatory responses to a different degree, that exploratory behaviour and social learning differ in their efficacy as compensatory responses or these two possibilities in combination. Thus, overall, our analyses support the ‘exposure hypothesis' and not the ‘compensation hypothesis’.
That social learning and exploration are independently associated with parasites that show distinctly different transmission modes might seem counterintuitive given that social learning, innovation and extractive foraging are positively correlated in primates [8,12], a result that we replicated using our methods (see the electronic supplementary material, figure S1), again finding a modest correlation coefficient (R2 = 0.23). Thus, social learning and exploratory behaviour are correlated but not collinear, leaving ample independent variation that can be accounted for by factors such as parasitism. Moreover, the disassociation between the results presented in our study concerning exploratory behaviour and social learning provide reassurance that the associations with parasite richness are not the result of an unmeasured variable that correlates equally with both behavioural and parasite richness. Furthermore, the finding that social learning and exploratory behaviour differentially predict parasite richness provides evidence for divergent validity of these two measures. If measures of social learning and exploratory behaviour were confounded, for example through shared sampling biases, we would not expect support for the ‘exposure hypothesis’. Instead, our data suggest that species characterized by high levels of both exploration and social learning (e.g. Hominoidea, Macaca, Cebus and Papio; [8]) may pay a ‘double cost’ of both socially and environmentally transmitted parasites.
(b). Socio-ecological predictors of parasite richness
We also found support for some of the additional ecological, demographic and geographical hypotheses that we investigated. First, all of our analyses provided support for a positive association between mean body mass and parasite richness. This may reflect that larger-bodied organisms have more ‘niches' available for colonization or that larger-bodied organisms are exposed to more parasites through greater food intake. Second, we found positive associations of geographical range size with total and socially transmitted parasite richness, suggesting that socially contagious parasites have the strongest association with expanding range, perhaps driven by increased contact with other closely related species as ranges expand or larger population sizes being able to support more parasites.
No supported positive associations between mean group size and measures of parasite richness were detected in any model tested in this study, consistent with previous comparative work on primates with an earlier version of this database [41]. Our results involving social learning and socially transmitted parasites suggest that more refined measures of sociality and social contact within groups may prove more useful for investigating socially transmitted infectious agents [22,35,55].
(c). Ameliorating the costs of parasitism
Increased parasitism may have profound impacts on host fitness; hence, species expressing greater behavioural flexibility may also possess mechanisms for ameliorating these costs, including through behaviourally flexible traits [23]. These coping mechanisms fall into two broad categories: physiological/immunological adaptations and avoidance/elimination/self-medication behaviours, such as grooming and the ingestion of medicinal plants or their addition to shelters [20,60]. Some of these anti-parasite strategies may themselves be facilitated by social processes (such as ectoparasite removal during allogrooming) or be socially learned [20]. Comparisons have previously been made between animal self-medication behaviours and human medicine [61], and further study of animal behavioural responses to disease may shed light on the evolution of human medical practices. Additionally, we only investigated one aspect of parasitism; other measures such as prevalence, intensity or virulence of parasites could provide further insights to the hypotheses that we tested [20].
Based on our findings, we propose that parasites and the infectious diseases that they cause pose substantial costs to behavioural patterns that underlie both human culture and animal traditions. Enhanced behavioural flexibility may have involved the evolution of counterstrategies to overcome these costs, such as medicative behaviours, or ways to increase the benefits of behavioural flexibility, such as increased cognitive sophistication in social learning [62]. As humans, we have experienced a marked increase over recent evolutionary history in our learned behavioural repertoires and in the diversity of parasites that infect us [63]. Further comparative and experimental investigation into infectious diseases as a constraint on the evolution of culture may therefore broaden our understanding of cognitive and cultural evolution both in humans and in other animals.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank C. Brezine for extracting parasite species richness data from the Global Mammal Parasite Database, and Y. Hager and K. Laland for contributing behavioural databases. We thank A. Georgiev, N. Cooper, R. Griffin, A. Navarrete, L. Jacquin, the Harvard Comparative Primatology and McGill Animal Cognition Research Groups for providing helpful feedback on early drafts. We also thank two anonymous reviewers for their insightful comments and suggestions.
Data accessibility
Phylogenetic treeblock files uploaded as electronic supplementary material (S2 and S3). Parasite richness, behaviour counts, body mass, social group size, geographical range, absolute latitude, terrestriality and citation index data: Dryad doi: http://dx.doi.org/10.5061/dryad.8kh92.
Funding statement
Training in phylogenetic comparative methods was provided by the AnthroTree Workshop, which is supported by the NSF (BCS-0923791) and the National Evolutionary Synthesis Center (NSF grant no. EF-0905606). C.M.M. was supported by the NSF Graduate Research Fellowship Program (grant no. DGE-1144152). C.L.N. was supported by Harvard University and the NSF (BCS-0923791 and EF-0723939/0904359). S.M.R. was supported by NSERC, McGill University, Utrecht University's High Potentials Programme and the Netherlands Organisation for Scientific Research (NWO) Evolution and Behaviour Programme.
References
- 1.Sih A. 2013. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 85, 1077–1088. ( 10.1016/j.anbehav.2013.02.017) [DOI] [Google Scholar]
- 2.Reader SM, Laland KN. (eds). 2003. Animal innovation. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Whiten A, Hinde RA, Stringer CB, Laland KN. (eds). 2012. Culture evolves. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L. 2005. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl Acad. Sci. USA 102, 5460–5465. ( 10.1073/pnas.0408145102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reader SM, Laland KN. 2001. Primate innovation: sex, age and social rank differences. Int. J. Primatol. 22, 787–805. ( 10.1023/A:1012069500899) [DOI] [Google Scholar]
- 6.Tomasello M, Call J. 1997. Primate cognition. New York, NY: Oxford University Press. [Google Scholar]
- 7.Dunbar RIM, Shultz S. 2007. Evolution in the social brain. Science 317, 1344–1347. ( 10.1126/science.1145463) [DOI] [PubMed] [Google Scholar]
- 8.Reader SM, Hager Y, Laland KN. 2011. The evolution of primate general and cultural intelligence. Phil. Trans. R. Soc. B 366, 1017–1027. ( 10.1098/rstb.2010.0342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day RL, Coe RL, Kendal JR, Laland KN. 2003. Neophilia, innovation and social learning: a study of intergeneric differences in callitrichid monkeys. Anim. Behav. 65, 559–571. ( 10.1006/anbe.2003.2074) [DOI] [Google Scholar]
- 10.van Schaik CP, Deaner RO, Merrill MY. 1999. The conditions for tool use in primates: implications for the evolution of material culture. J. Hum. Evol. 36, 719–741. ( 10.1006/jhev.1999.0304) [DOI] [PubMed] [Google Scholar]
- 11.Mery F, Kawecki TJ. 2003. A fitness cost of learning ability in Drosophila melanogaster. Proc. R. Soc. Lond. B 270, 2465–2469. ( 10.1098/rspb.2003.2548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reader SM, Laland KN. 2002. Social intelligence, innovation, and enhanced brain size in primates. Proc. Natl Acad. Sci. USA 99, 4436–4441. ( 10.1073/pnas.062041299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chittka L, Niven J. 2009. Are bigger brains better? Curr. Biol. 19, R995–R1008. ( 10.1016/j.cub.2009.08.023) [DOI] [PubMed] [Google Scholar]
- 14.Navarrete A, van Schaik CP, Isler K. 2011. Energetics and the evolution of human brain size. Nature 480, 91–93. ( 10.1038/nature10629) [DOI] [PubMed] [Google Scholar]
- 15.Barrickman NL, Bastian ML, Isler K, van Schaik CP. 2008. Life history costs and benefits of encephalization: a comparative test using data from long-term studies of primates in the wild. J. Hum. Evol. 54, 568–590. ( 10.1016/j.jhevol.2007.08.012) [DOI] [PubMed] [Google Scholar]
- 16.Bordes F, Morand S, Krasnov BR. 2011. Does investment into ‘expensive’ tissue compromise anti-parasitic defence? Testes size, brain size and parasite diversity in rodent hosts. Oecologia 165, 7–16. ( 10.1007/s00442-010-1743-9) [DOI] [PubMed] [Google Scholar]
- 17.Boyer N, Réale D, Marmet J, Pisanu B, Chapuis J-L. 2010. Personality, space use and tick load in an introduced population of Siberian chipmunks Tamias sibiricus. J. Anim. Ecol. 79, 538–547. ( 10.1111/j.1365-2656.2010.01659.x) [DOI] [PubMed] [Google Scholar]
- 18.Garamszegi LZ, Erritzøe J, Møller AP. 2007. Feeding innovations and parasitism in birds. Biol. J. Linnean Soc. 90, 441–455. ( 10.1111/j.1095-8312.2007.00733.x) [DOI] [Google Scholar]
- 19.Vas Z, Lefebvre L, Johnson KP, Reiczigel J, Rózsa L. 2011. Clever birds are lousy: co-variation between avian innovation and the taxonomic richness of their amblyceran lice. Int. J. Parasitol. 41, 1295–1300. ( 10.1016/j.ijpara.2011.07.011) [DOI] [PubMed] [Google Scholar]
- 20.Nunn CL, Altizer SM. 2006. Infectious disease in primates: behavior, ecology and evolution. New York, NY: Oxford University Press. [Google Scholar]
- 21.Ghai RR, Chapman CA, Omeja PA, Davies TJ, Goldberg TL. 2014. Nodule worm infection in humans and wild primates in Uganda: cryptic species in a newly identified region of human transmission. PLoS Neglect Trop. Dis. 8, e2641 ( 10.1371/journal.pntd.0002641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reader SM, Biro D. 2010. Experimental identification of social learning in wild animals. Learn. Behav. 38, 265–283. ( 10.3758/LB.38.3.265) [DOI] [PubMed] [Google Scholar]
- 23.Bush AO, Fernandez JA, Esch GW, Seed JR. 2001. Parasitism: the diversity and ecology of animal parasites. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 24.Formenty P, Boesch C, Wyers M, Steiner C, Donati F, Dind F, Walker F, Le Guenno B. 1999. Ebola virus outbreak among wild chimpanzees living in a rain forest of Côte d'Ivoire. J. Infect. Dis. 179, S120–S126. ( 10.1086/514296) [DOI] [PubMed] [Google Scholar]
- 25.Walsh PD, et al. 2003. Catastrophic ape decline in western equatorial Africa. Nature 422, 611–614. ( 10.1038/nature01566) [DOI] [PubMed] [Google Scholar]
- 26.Milton K. 1996. Effects of bot fly (Alouattamyia baeri) parasitism on a free-ranging howler monkey (Alouatta palliata) population in Panama. J. Zool. 239, 39–63. ( 10.1111/j.1469-7998.1996.tb05435.x) [DOI] [Google Scholar]
- 27.Kavaliers M, Colwell DD. 1995. Reduced spatial learning in mice infected with the nematode, Heligmosomoides polygyrus. Parasitology 110, 591–597. ( 10.1017/S0031182000065318) [DOI] [PubMed] [Google Scholar]
- 28.Checkley W, et al. 2008. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int. J. Epidemiol. 37, 816–830. ( 10.1093/ije/dyn099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart BL. 1990. Behavioral adaptations to pathogens and parasites: five strategies. Neurosci. Biobehav. Rev. 14, 273–294. ( 10.1016/S0149-7634(05)80038-7) [DOI] [PubMed] [Google Scholar]
- 30.Ezenwa VO, Etienne RS, Luikart G, Beja-Pereira A, Jolles AE. 2010. Hidden consequences of living in a wormy world: nematode-induced immune suppression facilitates tuberculosis invasion in African buffalo. Am. Nat. 176, 613–624. ( 10.1086/656496) [DOI] [PubMed] [Google Scholar]
- 31.Jolles AE, Ezenwa VO, Etienne RS, Turner WC, Olff H. 2008. Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology 89, 2239–2250. ( 10.1890/07-0995.1) [DOI] [PubMed] [Google Scholar]
- 32.Lohm J, Grahn M, Langefors Å, Andersen Ø, Storset A, von Schantz T. 2002. Experimental evidence for major histocompatibility complex-allele-specific resistance to a bacterial infection. Proc. R. Soc. Lond. B 269, 2029–2033. ( 10.1098/rspb.2002.2114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soler JJ, Peralta-Sánchez JM, Martín-Vivaldi M, Martín-Platero AM, Flensted-Jensen E, Møller AP. 2011. Cognitive skills and bacterial load: comparative evidence of costs of cognitive proficiency in birds. Naturwissenschaften 99, 111–122. ( 10.1007/s00114-011-0875-z) [DOI] [PubMed] [Google Scholar]
- 34.Altizer SM, et al. 2003. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol. Evol. Syst. 34, 517–547. ( 10.1146/annurev.ecolsys.34.030102.151725) [DOI] [Google Scholar]
- 35.Griffin RH, Nunn CL. 2012. Community structure and the spread of infectious disease in primate social networks. Evol. Ecol. 26, 779–800. ( 10.1007/s10682-011-9526-2) [DOI] [Google Scholar]
- 36.Côte IM, Poulin R. 1995. Parasitism and group size in social animals: a meta-analysis. Behav. Ecol. 6, 159–165. ( 10.1093/beheco/6.2.159) [DOI] [Google Scholar]
- 37.Rifkin JL, Nunn CL, Garamszegi LZ. 2012. Do animals living in larger groups experience greater parasitism? A meta-analysis. Am. Nat. 180, 70–82. ( 10.1086/666081) [DOI] [PubMed] [Google Scholar]
- 38.Nunn CL, Altizer SM. 2005. The global mammal parasite database: an online resource for infectious disease records in wild primates. Evol. Anthropol. 14, 1–2. ( 10.1002/evan.20041) [DOI] [Google Scholar]
- 39.Corbet GB, Hill JE. 1991. A world list of mammalian species. New York, NY: Oxford University Press. [Google Scholar]
- 40.Morand S. 2000. Wormy world: comparative tests of theoretical hypotheses on parasite species richness. In Evolutionary biology of host-parasite relationships (eds Poulin R, Morand S, Skorping A.), pp. 63–79. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 41.Nunn CL, Altizer SM, Jones KE, Sechrest W. 2003. Comparative tests of parasite species richness in primates. Am. Nat. 162, 597–614. ( 10.1086/378721) [DOI] [PubMed] [Google Scholar]
- 42.Poulin R. 1995. Phylogeny, ecology, and the richness of parasite communities in vertebrates. Ecol. Monogr. 65, 283–302. ( 10.2307/2937061) [DOI] [Google Scholar]
- 43.Gregory RD. 1990. Parasites and host geographic range as illustrated by waterfowl. Funct. Ecol. 4, 645–654. ( 10.2307/2389732) [DOI] [Google Scholar]
- 44.Kamilar JM, Marshack JL. 2012. Does geography or ecology best explain ‘cultural’ variation among chimpanzee communities? J. Hum. Evol. 62, 256–260. ( 10.1016/j.jhevol.2011.11.008) [DOI] [PubMed] [Google Scholar]
- 45.Nunn CL, Altizer SM, Sechrest W, Cunningham AA. 2005. Latitudinal gradients of parasite species richness in primates. Divers. Distrib. 11, 249–256. ( 10.1111/j.1366-9516.2005.00160.x) [DOI] [Google Scholar]
- 46.Jones KE, et al. 2009. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648 ( 10.1890/08-1494.1) [DOI] [Google Scholar]
- 47.Rowe N, Myers M. 2011. All the World's Primates. Primate Conservation, Inc; See http://www.alltheworldsprimates.org. [Google Scholar]
- 48.Cooper N, Nunn CL. 2013. Identifying future zoonotic disease threats: where are the gaps in our understanding of primate infectious diseases? Evol. Med. Public Health 2013, 27–36. ( 10.1093/emph/eot001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunn CL. 2002. Spleen size, disease risk and sexual selection: a comparative study in primates. Evol. Ecol. Res. 4, 91–107. [Google Scholar]
- 50.Deaner RO, Barton RA, van Schaik CP. 2003. Primate brains and life histories: renewing the connection. In Primate life histories and socioecology (eds Kappeler PM, Pereira ME.), pp. 233–265. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 51.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884. ( 10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 52.Arnold C, Matthews LJ, Nunn CL. 2010. The 10kTrees website: a new online resource for primate phylogeny. Evol. Anthropol. 19, 114–118. ( 10.1002/evan.20251) [DOI] [Google Scholar]
- 53.Freckleton RP, Harvey PH, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726. ( 10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 54.Petraitis PS, Dunham AE, Niewiarowski PH. 1996. Inferring multiple causality: the limitations of path analysis. Funct. Ecol. 10, 421–431. ( 10.2307/2389934) [DOI] [Google Scholar]
- 55.Nunn CL. 2012. Primate disease ecology in comparative and theoretical perspective. Am. J. Primatol. 74, 497–509. ( 10.1002/ajp.21986) [DOI] [PubMed] [Google Scholar]
- 56.Pedersen AB, Altizer SM, Poss M, Cunningham AA, Nunn CL. 2005. Patterns of host specificity and transmission among parasites of wild primates. Int. J. Parasitol. 35, 647–657. ( 10.1016/j.ijpara.2005.01.005) [DOI] [PubMed] [Google Scholar]
- 57.Bouwman KM, Hawley DM. 2010. Sickness behaviour acting as an evolutionary trap? Male house finches preferentially feed near diseased conspecifics. Biol. Lett. 6, 462–465. ( 10.1098/rsbl.2010.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terborgh J, Janson CH. 1986. The socioecology of primate groups. Annu. Rev. Ecol. Syst. 17, 111–136. ( 10.2307/2096991) [DOI] [Google Scholar]
- 59.Aureli F, et al. 2008. Fission–fusion dynamics: new research frameworks. Curr. Anthropol. 49, 627–654. ( 10.1086/586708) [DOI] [Google Scholar]
- 60.Huffman MA. 1997. Current evidence for self-medication in primates: a multidisciplinary perspective. Yearb. Phys. Anthropol. 40, 171–200. () [DOI] [Google Scholar]
- 61.Hart BL. 2011. Behavioural defences in animals against pathogens and parasites: parallels with the pillars of medicine in humans. Phil. Trans. R. Soc. B 366, 3406–3417. ( 10.1098/rstb.2011.0092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rendell L, Fogarty L, Hoppitt WJE, Morgan TJH, Webster MW, Laland KN. 2011. Cognitive culture: theoretical and empirical insights into social learning strategies. Trends Cogn. Sci. 15, 68–76. ( 10.1016/j.tics.2010.12.002) [DOI] [PubMed] [Google Scholar]
- 63.Barrett R, Kuzawa CW, McDade T, Armelagos GJ. 1998. Emerging and re-emerging infectious diseases: the third epidemiologic transition. Annu. Rev. Anthropol. 27, 247–271. ( 10.1146/annurev.anthro.27.1.247) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Phylogenetic treeblock files uploaded as electronic supplementary material (S2 and S3). Parasite richness, behaviour counts, body mass, social group size, geographical range, absolute latitude, terrestriality and citation index data: Dryad doi: http://dx.doi.org/10.5061/dryad.8kh92.