Abstract
Erucic acid is a valuable industrial fatty acid with many applications. The main producers of this acid are today high erucic rapeseed (Brassica napus) and mustard (Brassica juncea), which have 45%–50% of erucic acid in their seed oils. Crambe abyssinica is an alternative promising producer of this acid as it has 55%–60% of erucic acid in its oil. Through genetic modification (GM) of three genes, we have previously increased the level of erucic acid to 71% (68 mol%) in Crambe seed oil. In this study, we further investigated different aspects of oil biosynthesis in the developing GM Crambe seeds in comparison with wild-type (Wt) Crambe, rapeseed and safflower (Carthamus tinctorius). We show that Crambe seeds have very low phosphatidylcholine-diacylglycerol interconversion, suggesting it to be the main reason why erucic acid is limited in the membrane lipids during oil biosynthesis. We further show that GM Crambe seeds have slower seed development than Wt, accompanied by slower oil accumulation during the first 20 days after flowering (DAF). Despite low accumulation of erucic acid during early stages of GM seed development, nearly 86 mol% of all fatty acids accumulated between 27 and 50 DAF was erucic acid, when 40% of the total oil is laid down. Likely bottlenecks in the accumulation of erucic acid during early stages of GM Crambe seed development are discussed.
Keywords: erucic acid, seed oil, Crambe abyssinica, biosynthesis, acylation, enzymes
Introduction
There is an increasing utilization of seed oils as renewable resources in industrial feedstocks, especially those accumulating unusual fatty acids (FAs) that may vary in carbon chain length, number and position of unsaturated bonds or contain specialized functional groups (Dyer et al., 2008; Carlsson et al., 2011). Erucic acid (cis-13-docosenoic acid, 22:1), a very long-chain FA commonly found in seed oils of Brassicaceae, is an industrially important feedstock (Temple-Heald, 2004). High purity of desirable FAs is essential for the industrial application of target plant oils (Carlsson et al., 2011). Therefore, it is necessary to understand in detail the synthesis and metabolism of uncommon FAs, such as erucic acid in developing seeds in order to further increase its content by using gene technology and other plant breeding methods.
In developing oilseeds, triacylglycerols (TAG) biosynthesis is initiated with de novo FA synthesis in the plastid. In most oil seeds, the major forms of FAs exported from the plastid is oleoyl-CoA (18:1-CoA), palmitoyl-CoA (16:0-CoA) and stearoyl-CoA (18:0-CoA) (Bates et al., 2013). Erucic acid (22:1) is synthesized outside the plastid by the malonyl-CoA-dependent elongation of 18:1 catalysed by the condensing enzyme fatty acid elongase (FAE), a 3-keto reductase, a 3-hydroxy hydratase and an enoyl reductase. It has been suggested that part of the 18:1 exported from the plastid is diluted with a pre-existing oleate pool in the cytosol before elongation to erucic acid (Hlousek-Radojcic et al., 1995; Bao et al., 1998). From studies of pea leaves and soybean embryos, it has been concluded that there is major acyl flux of 18:1 from the plastid into phosphatidylcholine (PC) where further modification like desaturation and hydroxylation occurs before the acyl groups are used for de novo glycerol lipid synthesis (Bates et al., 2007, 2009; Bates and Browse, 2011; Bates et al., 2012, 2013).
The direct lipid precursor for TAG assembly is diacylglycerol (DAG) (Bates et al., 2013), which represents an important branch point between TAG and membrane lipids synthesis (Li-Beisson et al., 2013). Isotope labelling studies have shown that there are kinetically distinct DAG pools in developing soybeans used for PC and TAG synthesis (Bates et al., 2009; Bates and Browse, 2012).
De novo DAG synthesis is formed by the sequential esterification of glycerol-3-phosphate (G-3-P) at sn-1 and sn-2 position and forming phosphatidic acid (PA) which is then dephosphorylated. This process, known as Kennedy pathway (Kennedy, 1961), involves three key enzymes: glycerol-3-phosphate acyltransferase (GPAT), lysophosphatidic acid acyltransferase (LPAAT) and phosphatidic acid phosphatase (PAP). Specialized LPAATs have been cloned from coconut endosperm and Limnanthes douglasii seeds showing high specificities for lauroyl-CoA and 22:1-CoA, respectively (Hanke et al., 1995; Knutzon et al., 1999). Despite that plants in the Brassicaceae family accumulate high amounts of 22:1 in their seed oils, LPAATs from these species have been found incapable of acylating 22:1-CoA to lysophosphatidic acid (LPA) (Frentzen, 1993), and thus, this FA is virtually absent from the sn-2 position of their seed TAG. This block might partly explain why no Brassica species have more than two-thirds of the total oil as erucic acid.
Apart from de novo synthesis, DAG can also be derived from PC by interconversion between these two molecules catalysed by a phosphatidylcholine: diacylglycerol cholinephosphotransferase (PDCT) (Lu et al., 2009; Hu et al., 2012). Such an interconversion reaction has also been proposed to be catalysed by the reverse reaction of CDP-choline: diacylglycerol cholinephosphotransferase (CPT), the enzyme responsible for the net synthesis of PC (Slack et al., 1983, 1985). The polyunsaturated FAs (PUFA), linoleic and linolenic acids, are produced when the precursor FAs are esterified in PC (Bates et al., 2013). These acids could enter DAG by the interconversion between DAG and PC or by the transfer of acyl groups from PC to the acyl-CoA pool that is used in the G-3-P pathway. The latter transfer has been suggested to be catalysed by the reverse action of acyl-CoA: lysophosphatidylcholine acyltransferase (LPCAT) (Stymne and Stobart, 1984) or by phospholipase A (PLA) and subsequent activation to acyl-CoA by acyl-CoA synthetases (Bates et al., 2013). PC-DAG interconversion and LPCAT-mediated acyl-editing mechanism have been suggested to control the two-thirds of acyl fluxes of PUFA from PC to TAG in Arabidopsis (Bates et al., 2012).
The esterification of sn-3 position of DAG to form TAG is catalysed by diacylglycerol acyltransferase (DGAT) (Stymne and Stobart, 1987) or the acyl-CoA-independent phospholipid/diacylglycerol acyltransferase (PDAT) (Ståhl et al., 2004). Two different classes of DGATs have been identified and suggested to possess different substrate specificities, and their contribution to TAG biosynthesis may vary among different oilseed species (Bates et al., 2013). DGAT1 and PDAT1 have been found to have compensating functions in TAG production in Arabidopsis (Zhang et al., 2009). Castor bean PDAT has been shown to have high activity towards hydroxylated FAs (Dahlqvist et al., 2000), and its expression increased the accumulation of hydroxy FA in the seed oil of transgenic Arabidopsis expressing castor bean hydroxylase (Van Erp et al., 2011).
Although the FA compositions in PC and TAGs of the common five acyl groups, 16:0, 18:0, 18:1, 18:2 and 18:3, reflect each other in most oil seeds, this is not the case for many unusual FAs (Dyer et al., 2008). They could occur in very high amounts in TAG, whereas their contents are very low in PC. These unusual TAG-specific FAs can be divided into two groups. One group consists of those FAs that are synthesized while being esterified to PC, catalysed by divergent FAD2-like enzymes. These include hydroxylated, acetylenic, epoxygenated and conjugated FAs (Dyer et al., 2008). In these cases, there must be mechanisms of removing these FAs from PC with high selectivity and channelling them into TAG. The other group consists of unusual FAs that are not produced on PC, such as medium chain and very long-chain FAs. Erucic acid belongs to the second group as it is synthesized by elongation of 18:1-CoA to 22:1-CoA. The exclusion of medium chain FAs from PC has been studied in some details in Cuphea lanceolata, a species having TAG with about 85% carpric (10:0) FAs (Bafor et al., 1990). One of the mechanisms preventing medium chain fatty acid to enter PC from DAG in Cuphea seeds was suggested to be very low PC-DAG interconversion. Here, we report on the metabolism of erucoyl groups through analysing the enzymatic activities in microsomal fractions from developing seeds of wild-type (Wt) Crambe abyssinica, a species accumulating high amount of erucic acid in its seed oil, and a genetic modified (GM) high erucic acid Crambe line with over 70% of erucic acid in its seed oil. These results are compared with the metabolism in microsomal fractions from developing seeds of safflower and rapeseed. Further, in vivo [14C]glycerol-labelling data in rapeseed and Crambe as well as the FA amount and composition in PC, DAG and TAG at different stages of Crambe seed development are presented. Based on these results, we discuss possible mechanisms in the exclusion of erucoyl groups from PC in Crambe as well as limiting factors in producing oils with even higher amount of erucic acid in GM crambe.
Results
Acylation of 18:1-CoA to glycerol backbone in microsomes from developing oil seeds
Microsomal preparation from developing seeds of Crambe and rapeseed (20 days after flowering, DAF) and safflower (18 days after pollination, DAP, Stobart et al., 1997) were incubated with [14C]G-3-P and nonradioactive18:1-CoA for 20, 40 and 80 min, and the radioactivity in the different lipids was determined. We used microsomes from both Wt Crambe and a GM Crambe line expressing a LdLPAAT gene, a CaFAD2-RNAi and a BnFAE1 with increased erucic acid content (Li et al., 2012). Wt Crambe, GM Crambe and rapeseed microsomes all incorporated radioactivity in a very similar manner with PA being the dominant labelled lipid at all the time points. DAGs and TAGs showed a time-dependent increase but only very small amounts of radioactivity accumulated in PC (Figure1). A similar incorporation pattern was also seen in microsomes prepared from developing seeds of an erucic acid producing turnip rape (Brassica rapa), except that a significant higher amount of label was found in PC (Figure S1). In contrast, safflower microsomes showed quite different labelling pattern of the lipids. Radioactive PA was efficiently converted to DAG from which about equal amount of radioactivity was transferred to PC and TAG (Figure1). A similar incorporation pattern for the glycerol backbone from [14C]G-3-P and 18:1-CoA has previously been reported in microsomal preparation of safflower seeds (Griffiths et al., 1985).
Figure 1.
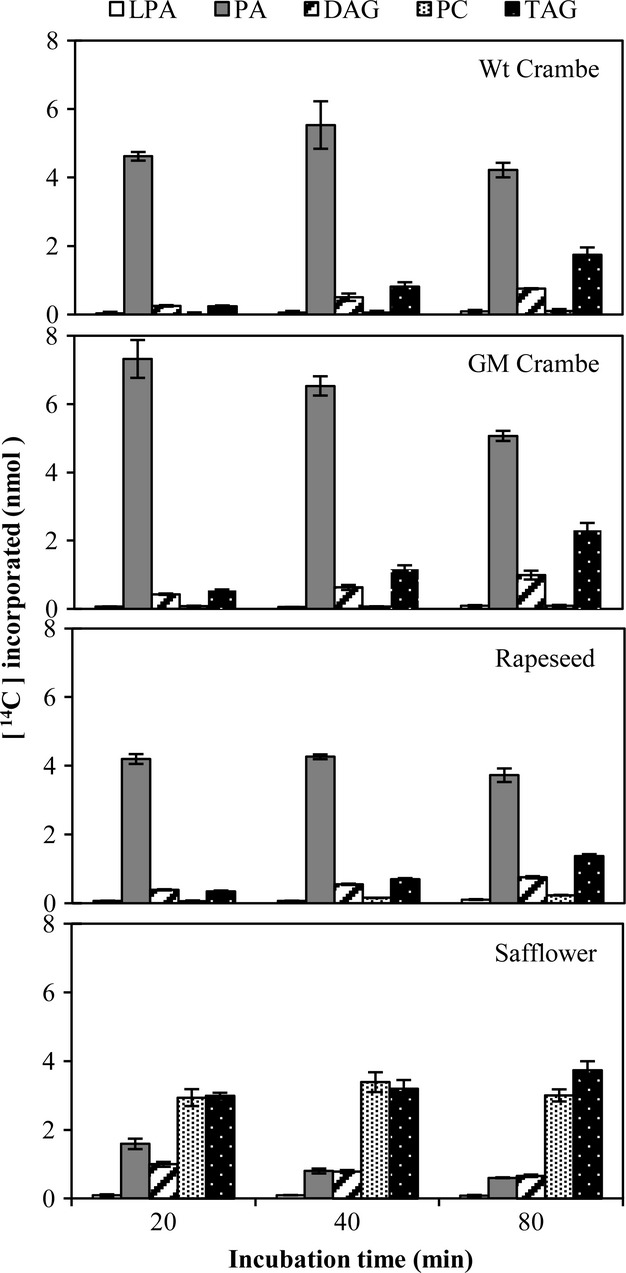
Time course incorporation of radioactivity into various lipids in microsomal preparations from developing seeds of different species, incubated with [14C]glycerol 3-phosphate and nonradioactive18:1-CoA. Wt, wild type; GM, genetically modified; LPA, lysophosphatidic acid; PA, phosphatidic acid; DAG, diacylglycerol; PC, phosphatidylcholine; TAG, triacylglycerols. Results are shown from triplicate samples ± SD.
The inefficient conversion of PA to DAG in Crambe, rapeseed and turnip rape microsomes (Figures1 and S1) indicates that the phosphatidic acid phosphohydrolase (PAP) has a significant control of flux of acyl groups into TAG. However, the PAP is only partially bound to the membranes, at least in safflower (Ichihara et al., 1990), and thus a substantial part of this activity might have been lost during preparation of the microsomal fractions.
The high proportion of radioactivity in DAG and TAG compared with PC in Wt crambe and rapeseed indicates much lower DAG-PC interconversion rates in these microsomes than in safflower and, presumably, low PDCT activities, the enzyme catalysing such interconversion (Lu et al., 2009).
We incubated the same microsomal fractions with glycerol-labelled [14C]18:1-LPA and nonradioactive18:1-CoA (Figure2). PA was rapidly formed in assays with all membranes and was very similar metabolized as in incubation with [14C]G-3-P and 18:1-CoA. We then compared the acylation of glycerol-labelled 18:1-LPA with incubations with glycerol-labelled [14C] 22:1-LPA with nonradioactive18:1-CoA (Figure3). Contrary to the metabolism of 18:1-LPA, the 22:1-LPA was acylated much slower to PA in microsomes for all the species (Figure3). The higher conversion into PA with the GM Crambe membranes than with the Wt Crambe and rapeseed indicates that the introduced Limnanthes LPAAT accepts 22:1-LPA somewhat better than the endogenous LPAATs in this species. However, it could also be due to general higher LPAAT activity, as indicated by more complete acylation of 18:1-LPA by the membranes of GM Crambe than Wt Crambe (Figure2). PC was hardly labelled except in safflower membranes, where it was the major metabolite after 80 min, indicating that the PDCT did not exclude DAG species with erucoyl groups.
Figure 2.
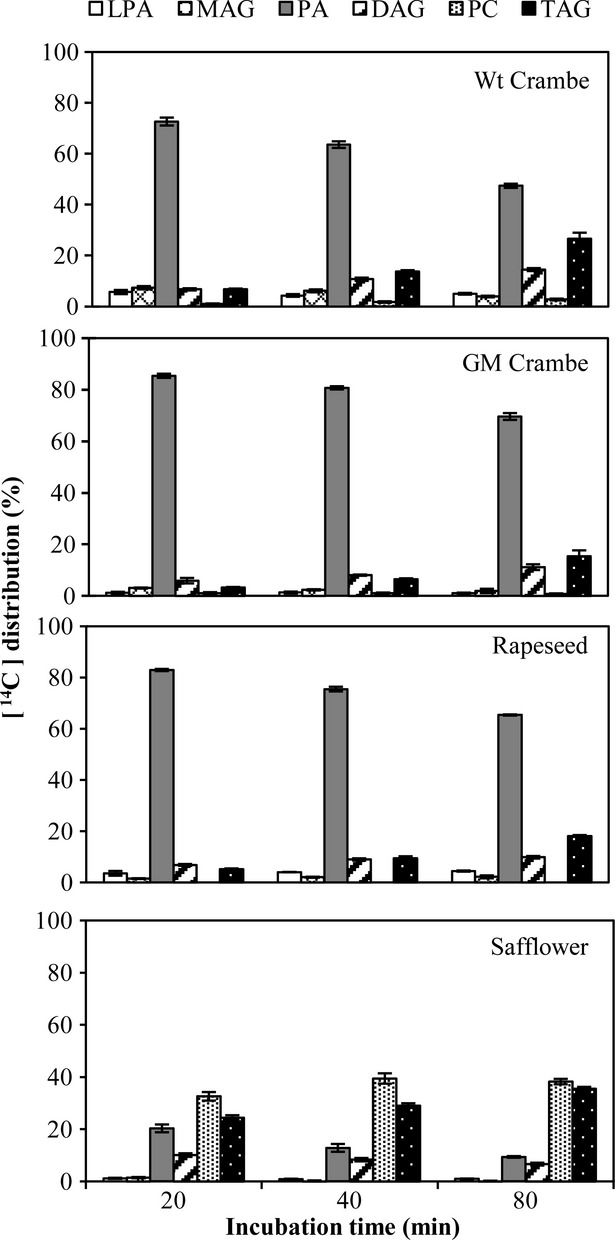
Time course incorporation of radioactivity into various lipids in microsomal preparations from developing seeds of different species, incubated with glycerol-labelled [14C]18:1-LPA and nonradioactive 18:1-CoA. Wt, wild type; GM, genetically modified; LPA, lysophosphatidic acid; MAG, monoacylglycerol; PA, phosphatidic acid; DAG, diacylglycerol; PC, phosphatidylcholine; TAG, triacylglycerols. Results are shown from triplicate samples ± SD.
Figure 3.
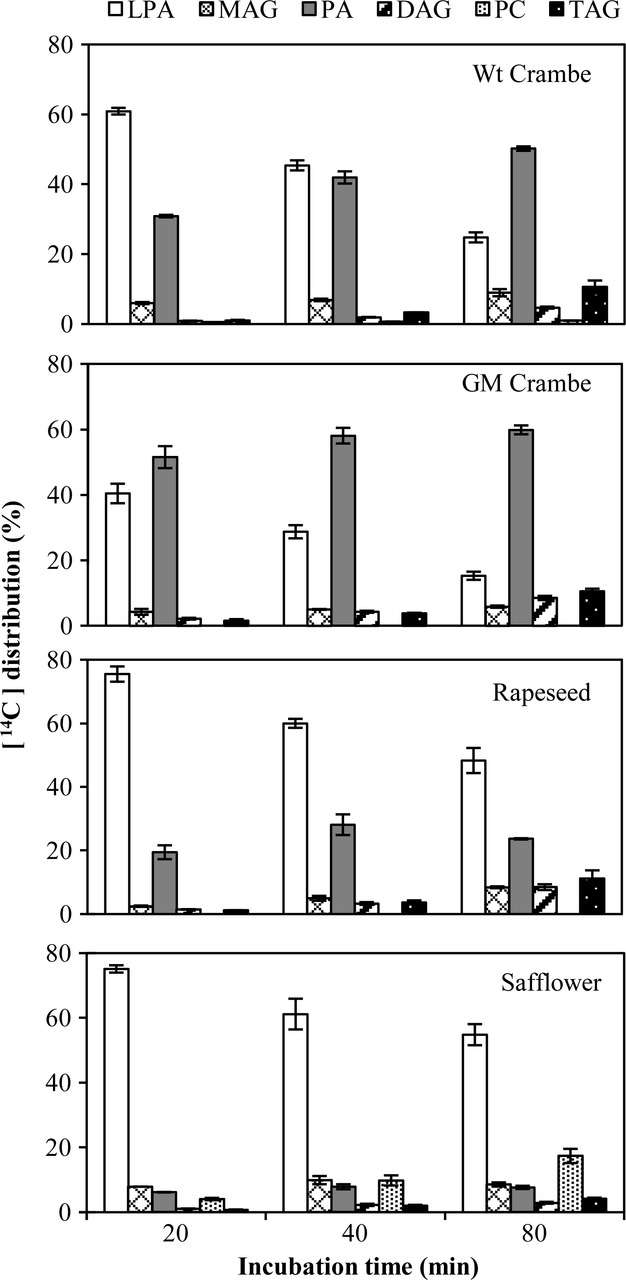
Time course incorporation of radioactivity into various lipids in microsomal preparations from developing seeds of different species, incubated with glycerol-labelled [14C]22:1-LPA and nonradioactive 18:1-CoA. Wt, wild type; GM, genetically modified; LPA, lysophosphatidic acid; MAG, monoacylglycerol; PA, phosphatidic acid; DAG, diacylglycerol; PC, phosphatidylcholine; TAG, triacylglycerols. Results are shown from triplicate samples ± SD.
Acylation of 22:1-CoA to the glycerol backbone in microsomes from developing oil seeds
Incubations of Wt and GM Crambe and safflower microsomes with [14C]G-3-P and 22:1-CoA yielded only 2.5%–3.5% of the radioactivity seen in complex lipids with 18:1-CoA and [14C]G-3-P substrates (compare Figures1 and 4). In Wt Crambe, the LPA substrate remained the most labelled lipid at all incubation times. The next highly labelled lipid was comigrating with monoacylglycerol (MAG) according to the authentic standard. The most likely origin of this compound is dephosphorylation of the formed lyso-PA by a phospholipase of C type. Only a very small amount of PA was produced. In GM Crambe, a substantial amount of the LPA was converted to PA, demonstrating that the introduced Limnanthes LPAAT was able to acylate 22:1-CoA to 22:1-LPA. No labelled MAG could be detected in the assays with the GM Crambe lines, indicating that the Limnanthes LPAAT efficiently outcompeted the enzyme responsible for the formation MAG in Wt membranes. The safflower microsomes could acylate 22:1-CoA to G-3-P to produce 22:1-LPA, of which a substantial part was converted to MAG, but, interestingly, a large proportion was also found in PA and even higher in PC. We could not detect any labelled DAG or TAG in any of these incubations for all the species (Figure4). However, it should be noted that the total amount of 14C incorporation into complex lipids was very low and the label in these lipids might be below the detection limit.
Figure 4.
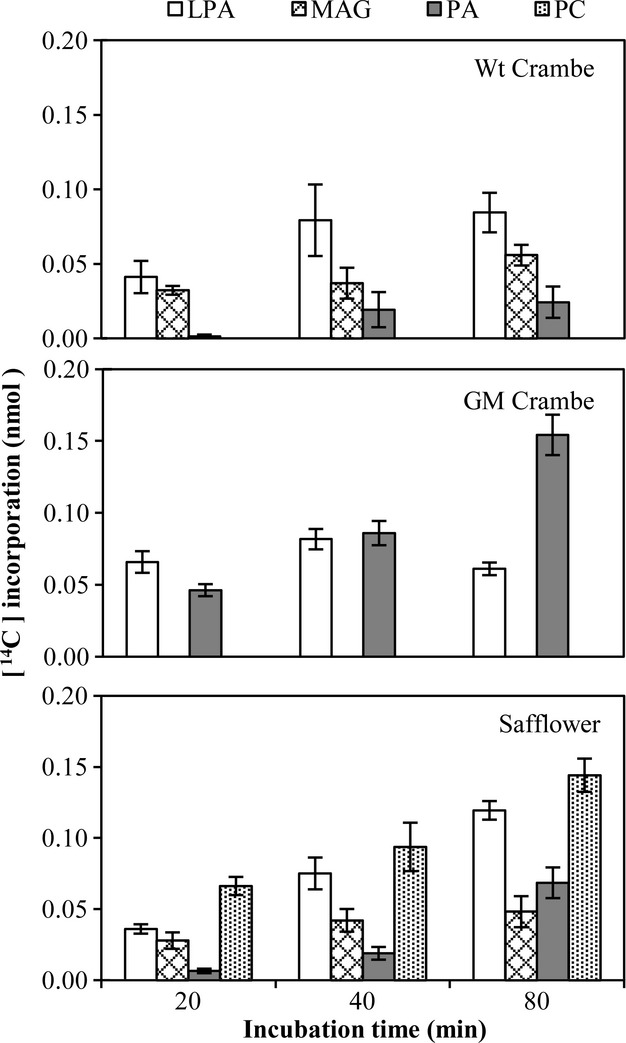
Time course incorporation of radioactivity into various lipids in microsomal preparations from developing seeds of different species, incubated with [14C]glycerol 3-phosphate and nonradioactive 22:1-CoA. Wt, wild type; GM, genetically modified; LPA, lysophosphatidic acid; MAG, monoacylglycerol; PA, phosphatidic acid; DAG, diacylglycerol; PC, phosphatidylcholine; TAG, triacylglycerols. Results are shown from triplicate samples ± SD.
Incubations with glycerol-labelled [14C]18:1-LPA and nonradioactive 22:1-CoA demonstrated a dramatic difference in the metabolism between GM and Wt Crambe (Figure5). Virtually, all LPA was acylated with 22:1 within 20 min with the membranes of GM Crambe, further confirming the functional role of introduced Limnanthes LPAAT gene in the acylation of 22:1 at the sn-2 position. In contrast, very little labelled PA was detected in Wt Crambe where a substantial amount of labelled MAG was formed. The rapeseed had somewhat better ability to acylate 22:1 than the Wt Crambe. Surprisingly, a substantial amount of 22:1 was acylated to 18:1-LPA in safflower microsomes and PA was efficiently converted to DAG and further to PC and TAG, again demonstrating no constrains in DAG-PC interconversion due to erucoyl groups in DAG in these microsomes.
Figure 5.
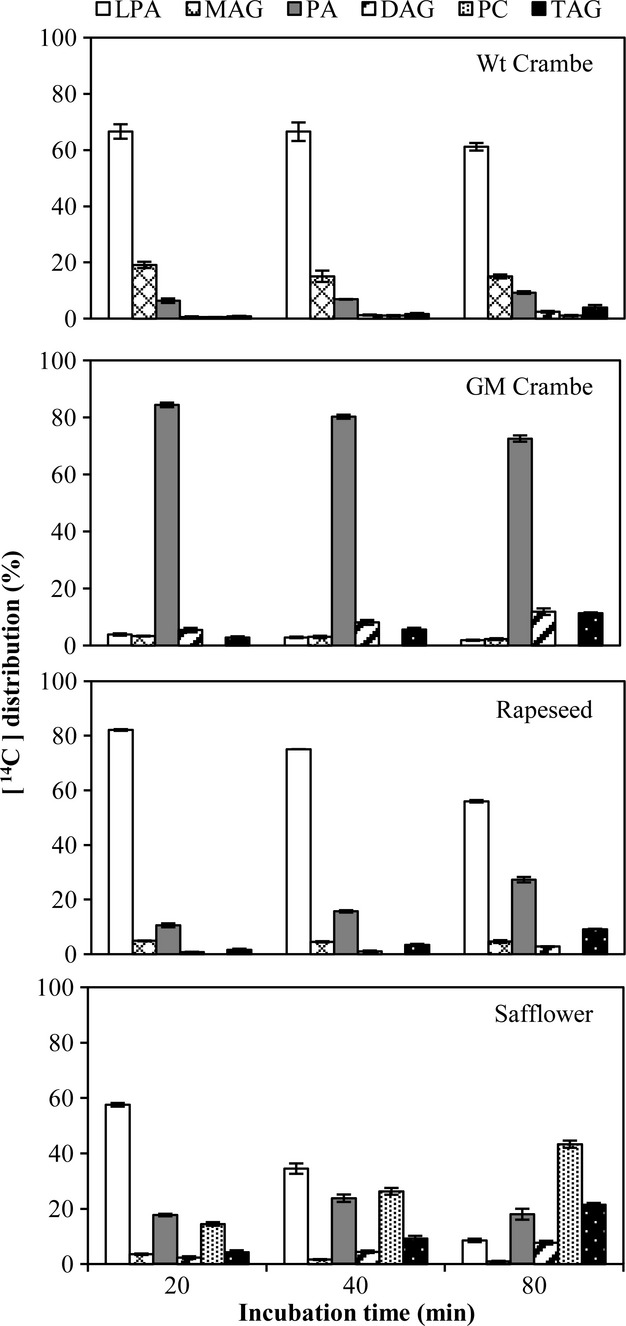
Time course incorporation of radioactivity into various lipids in microsomal preparations from developing seeds (from species indicated in the figure) incubated with glycerol-labelled [14C]18:1-LPA and nonradioactive 22:1-CoA. Wt, wild type; GM, genetically modified; LPA, lysophosphatidic acid; MAG, monoacylglycerol; PA, phosphatidic acid; DAG, diacylglycerol; PC, phosphatidylcholine; TAG, triacylglycerols. Results are shown from triplicate samples ± SD.
The next set of incubations tested were Wt Crambe, GM Crambe and safflower membranes with glycerol-labelled [14C]22:1-LPA and 22:1-CoA (Figure6). Very little PA was formed with these combination of substrates in Wt Crambe where MAG was the dominating metabolite. In contrast, the GM Crambe efficiently acylated 22:1 to this acyl acceptor, however, at lower rate than with 18:1-LPA (compare Figures5 and 6). The 22:1-LPA was also to some extent acylated with 22:1 in safflower membranes but with much lower efficiency than 18:1-LPA. Again, glycerol label was found in PC in safflower microsomes and was the major labelled metabolite after 80 min incubation.
Figure 6.
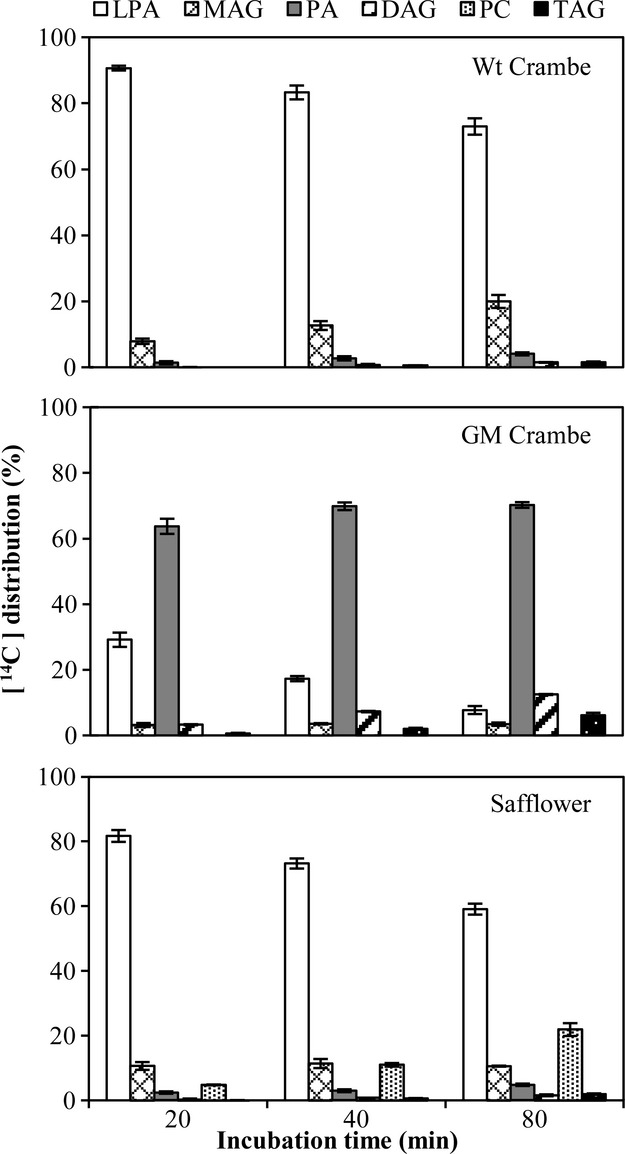
Time course incorporation of radioactivity into various lipids in microsomal preparations from developing seeds (from species indicated in the figure) incubated with glycerol-labelled [14C]22:1-LPA and nonradioactive 22:1-CoA. Wt, wild type; GM, genetically modified; LPA, lysophosphatidic acid; MAG, monoacylglycerol; PA, phosphatidic acid; DAG, diacylglycerol; PC, phosphatidylcholine; TAG, triacylglycerols. Results are shown from triplicate samples ± SD.
In vivo [14C]glycerol labelling
To correlate the detected in vitro activities of the flow of glycerol backbone towards TAGs in the microsomal membranes of the developing seeds with in vivo activities, we fed [14C]glycerol to detached developing embryos of rapeseed and Wt Crambe collected 20 DAF and determined the amount of radioactivity in the complex lipid at different time intervals. Only four lipids, PA, DAG, PC and TAG, contained a significant amount of radioactivity, of which PA accounted only for a very small portion of the total labelled lipids (Figure7). DAG and TAG were the main labelled lipids at all the time points in Crambe seeds, while the radioactivity in PC was two to three times less than DAG or TAG. In rapeseed, DAG was the highest labelled lipid, while TAG showed about half of the radioactivity compared with PC.
Figure 7.
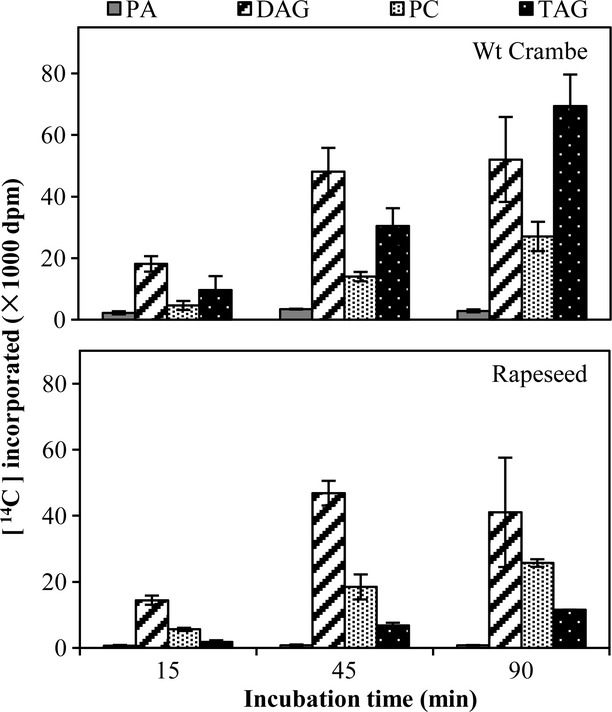
Time course incorporation of radioactivity into various lipids by detached developing wild-type Crambe and rapeseed embryos after incubation with [14C]glycerol. PA, phosphatidic acid; DAG, diacylglycerol; PC, phosphatidylcholine; TAG, triacylglycerols. Results are shown from triplicate samples ± SD.
Fatty acid composition of PC, DAG and TAG in developing Crambe seeds
Analysis of total FA composition and amount in the Wt and GM Crambe seeds as well as acyl composition of TAG, DAG and PC were conducted at 20, 27, 34, 41 and 50 DAF, and the complete analyses are presented in Supporting Information Tables S1 and S2. Some of these data are extracted in graphic form in Figure8. During the first 20 DAF, the rate of oil accumulation was about one-third in GM Crambe compared with Wt Crambe (Figure8a). Between 20 and 27 DAF, the rate of oil accumulation increased and was somewhat higher than in Wt during that period (Table1), thereafter the oil content continued to increase at a decreasing pace up to 50 DAF. The Wt seeds accumulated oil in a near linear fashion between 20 DAF and 34 DAF, where it had maximal amount of oil accumulation and with a slight decrease afterwards. It has been shown that oil content in rape seeds decline upon maturation and that a TAG lipase is involved in this process (Kelly et al., 2013). The Wt seeds lost chlorophyll between 20 and 27 DAF, whereas the GM seeds peaked in chlorophyll content at 27 DAF (Figure8a). At 50 DAF, both lines had completely lost all their chlorophyll and were regarded as fully matured. Thus, the GM seeds had a delayed seed development as well as a much longer period of oil accumulation than the Wt seeds. The mature GM seeds had 21% lower amount of oil per seed than the Wt, but were 16% less in weight, thus giving similar oil content per seed weight as the Wt seeds.
Figure 8.
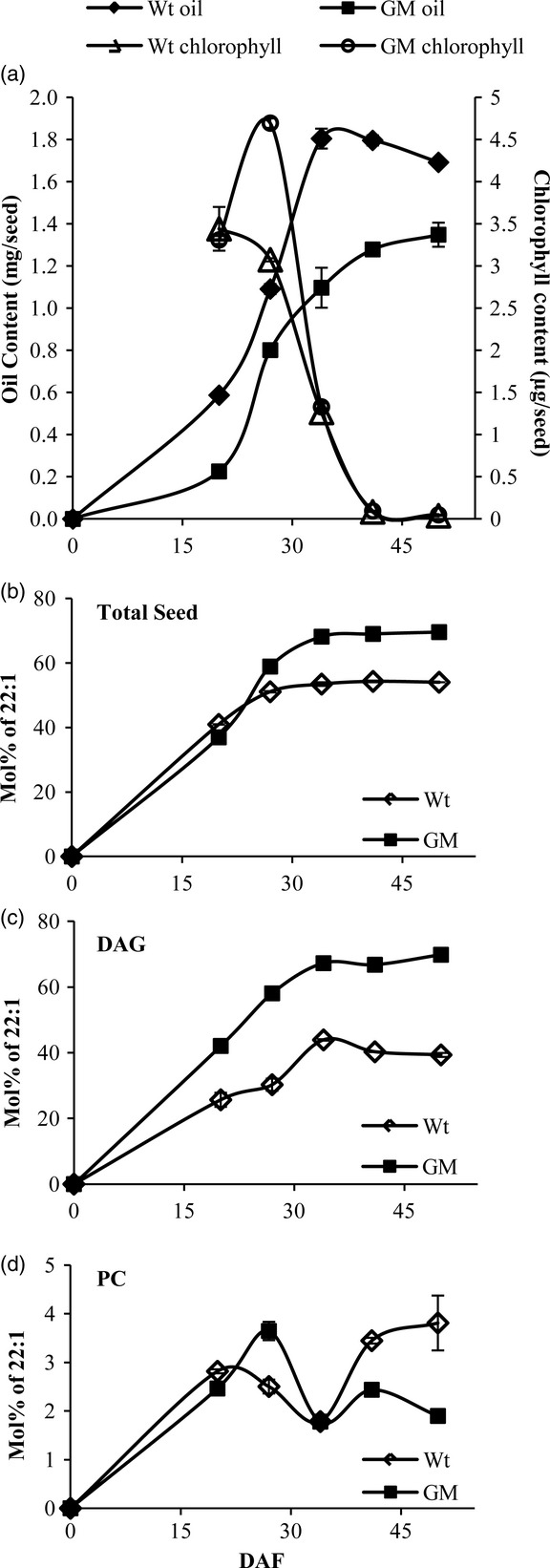
Oil accumulation and fatty acid composition of wild-type (Wt) and genetically modified (GM) Crambe seeds at different days after flowering (DAF). (a) Oil content and chlorophyll content; (b) Total seed fatty acid composition; (c) Fatty acid composition of diacylglycerols (DAG); (d) Fatty acid composition of phosphatidylcholine (PC). The data are mean ± SD of duplicate analyses presented in Supporting Information Tables S1 and S2.
Table 1.
Total fatty acid (FA) and erucic acid (22:1) content of wild-type (Wt) and genetically modified (GM) Crambe seeds at various days after flowering (DAF). The data (means ± SD) are duplicate analyses presented in Supporting Information Table S1
| DAF | Wt | GM | ||
|---|---|---|---|---|
| FA nmol/seed | 22:1 nmol/seed | FA nmol/seed | 22:1 nmol/seed | |
| 20 | 1822 ± 9 | 746 ± 5 | 704 ± 10 | 260 ± 4 |
| 27 | 3320 ± 74 | 1696 ± 45 | 2396 ± 37 | 1411 ± 22 |
| 50 | 5125 ± 32 | 2768 ± 17 | 3960 ± 168 | 2755 ± 118 |
| nmol of FA accumulated between 20 and 27 DAF | 1498 | 950 | 1692 | 1151 |
| mol% of 22:1 accumulated between 20 and 27 DAF | 63.4% | 68.0% | ||
| nmol of FA accumulated between 27 and 50 DAF | 1805 | 1072 | 1564 | 1344 |
| mol% of 22:1 accumulated between 27 and 50 DAF | 59.4% | 85.9% | ||
Erucic acid amounted to about 40 mol% of all acyl groups in both Wt and GM Crambe seeds at 20 DAF. At 34 DAF, GM and Wt seeds had about 68 and 53 mol% erucic acid, respectively, and this proportion remained quite stable during the rest of the seed development (Figure8b). It should be noted that the 68 mol% 22:1 in GM corresponds to about 71% by weight, as previously reported in this line (Li et al., 2012).
The nmol of total acyl groups and the nmol erucic acid per seed accumulated between 20, 27 and 50 DAF are shown in Table1. It can be concluded that of all FAs accumulated between 20 and 27 DAF, erucic acid accounted for 63 mol% in Wt and 68 mol% in GM. Between 27 and 50 DAF, 59 and 86 mol% of the accumulated FAs were erucic acid in Wt and GM lines, respectively, assuming that there is no conversion of oleic acid to erucic acid occurring on the acyl groups that had already accumulated at the first 27 DAF. The mol% of erucic acid in DAG at the different stages of seed development is presented in Figure8c. The proportion of erucic acid at 27 and 50 DAF amounted to 30 and 39 mol% in Wt and 58 and 70 mol% in GM, respectively (Figure8c). The much higher proportion of 22:1 in DAG also at 20 DAF in the GM line (Figure8c) indicates that the introduced Limnnathes LPAAT enzyme has high activity already at this early stage of seed development. It was previously reported that mature seeds of the high erucic GM Crambe line had half the amount of erucic acid in PC compared with Wt seeds (Li et al., 2012). The amount of erucic acid found in PC at the different stages of seed development is depicted in Figure8d. Although the level of 22:1 in GM seeds was half of that found in Wt seeds at 50 DAF, the levels fluctuated during the seed development and it was 40% higher than in Wt seeds at 27 DAF. It is particular noteworthy that the levels of erucic acid in PC doubled in PC in Wt seeds between 34 and 50 DAF, despite that there was no net synthesis of TAG during this period (compare Figure8a,d).
Discussion
GPAT and LPAAT activities towards erucoyl and oleoyl substrates
Crambe abyssinica is a species having 55%–60% of erucic acid in its seed oil (TAG). We have developed GM Crambe lines with over 70% of erucic acid by coexpressing a BnFAE1, a LdLPAAT and a CaFAD2-RNAi (Li et al., 2012). When measuring the activity of the acylation enzymes in the G-3-P pathway leading to TAG synthesis in microsomal preparations from developing Crambe seeds, we can conclude that 22:1-CoA is utilized much less efficient (2.5%–3.5%) than 18:1-CoA in the acylation of G-3-P by GPAT(s) and is not better utilized than by the safflower GPAT enzyme. This is quite unexpected in the view of the high amount of erucic acid (>85%) found in sn-1 + sn-3 positions of Wt Crambe TAGs (Li et al., 2012). It raises a question if there is a GPAT with high activity for erucoyl-CoA in Crambe that has been lost or inactivated during membrane preparations. It should be pointed out here that no plant gene for GPAT involved in either seed TAG or membrane lipid synthesis has with certainty been identified (Bates et al., 2013).
The very poor acylation of LPA with 22:1-CoA by Wt Crambe microsomal enzymes is expected as this acyl group is only found at low levels (8%) at position sn-2 in Crambe seed TAGs (Li et al., 2012). The enzymes in the membranes prepared from GM Crambe expressing the Limnanthes LPAAT gene, effectively acylated this acyl group to 18:1-LPA. The Limnanthes enzyme has previously been shown to efficiently utilize this acyl-CoA species (Hanke et al., 1995). Our results showed that 22:1-LPA was a much poorer substrate than 18:1-LPA in the acylation of 18:1-CoA in all membranes tested. In membranes of GM Crambe, 22:1-LPA was also a markedly poorer substrate than 18:1-LPA in acylation with 22:1-CoA. This indicates a bottleneck in the formation of PA with two 22:1 acyl groups, even with the Limnanthes LPAAT expressed. When studying the glycerol acylation enzymes in rapeseed, we obtained very similar results as with Wt Crambe. Moreover, we included microsomal preparations from developing safflower seeds in our assays. The lipid metabolizing enzymes in safflower microsomal membranes have been extensively studied (Stymne et al., 1983; Stymne and Stobart, 1984; Griffiths et al., 1985; Stobart and Stymne, 1985) and have significantly contributed to our present understanding of TAG biosynthesis in seeds (Stymne and Stobart, 1987; Bates et al., 2013). Unexpectedly, the safflower LPAAT(s) had much higher capacity to acylate 22:1 to 18:1-LPA than Wt Crambe and rapeseed enzymes although safflower does not produce 22:1 in its seed oil. Among the species accumulating high amounts of very long-chain FAs in their seed oil, it is only the Limnanthes species and Tropoleum majus that have been reported to have 22:1 in high amounts at the sn-2 position (Löhden and Frentzen, 1992). Our studies show that the safflower LPAAT had poor capacity to acylate 22:1 when 22:1-LPA was acyl acceptor compared with the introduced Limnanthes LPAAT in GM Crambe seeds.
The metabolism of DAG in microsomal membranes
As reported previously (Stobart and Stymne, 1985), microsomal preparations from safflower seeds effectively channelled DAG produced from PA into both TAG and PC. With 18:1 containing substrates, about equal proportions of the label entered these two lipids. The main enzyme responsible for the channelling of DAG into PC is believed to be PDCT, interconverting the two molecules by transferring a phosphocholine group to DAG (Lu et al., 2009). It should be noted that when the 22:1 substrates were used, the safflower enzymes readily incorporated DAG with one or two erucoyl groups into PC, indicating that safflower PDCT does not discriminate against these DAG species.
The amount of label entering PC in in vitro assays was very low in membranes of both Wt Crambe and rapeseed, indicating that the PDCT activity in these membranes is much lower compared with that in safflower. It is a likely that the low DAG-PC interconversion is limiting the amount of erucoyl groups entering the membrane lipids in Crambe. The low flow of glycerol backbone into PC in Wt Crambe was confirmed by in vivo labelling of developing Crambe seeds with [14C]glycerol. The relative incorporation [14C]glycerol into DAG, PC and TAG in the Crambe is very similar to that reported for [14C]glycerol-labelling experiment with developing seeds from the rod1 (PDCT) mutant of Arabidopsis (Lu et al., 2009) and radically different from Wt Arabidopsis (Lu et al., 2009; Bates and Browse, 2012). The developing rapeseed incorporated relatively more [14C]glycerol label in PC than in TAG compared with Crambe but significantly less than Wt Arabidopsis (Lu et al., 2009; Bates and Browse, 2012), suggesting that rapeseeds has intermediate DAG-PC interconversion activity compared with Arabidopsis and Crambe.
Apart from PDCT, there is also a net synthesis of PC from DAG catalysed by DAG/CDP-choline choline phosphotransferase (CPT) (Li-Beisson et al., 2013). It has been shown that when a FAE1 is expressed constitutively in Arabidopsis, up to 20% of very long-chain FAs are accumulated in the leaf PC despite the very low amount of very long-chain FAs in seed PC, indicating that CPT does, to some extent, accept very long chain containing DAG in Arabidopsis (Millar et al., 1998). In studies of acyl fluxes in detached developing soybeans, Bates et al. (2009) showed that there were at least two kinetically distinct DAG pools. The high amount of erucic acid detected in total DAG in Wt Crambe seeds at different developmental stages might therefore not reflect the DAG pool used by CPT in the net synthesis of PC. Thus, it is likely that Crambe has at least two distinct DAG pools, one with low amounts of erucic acid used for net synthesis of PC and one highly enriched in erucic acid, used for TAG synthesis.
It is clear from the lipid analyses of the developing Crambe seeds in this study that some erucic acid is accumulating in PC, but the level oscillates in a way that is difficult to explain with present knowledge. The most remarkable difference between the Wt and GM lines in this respect is over doubling of the mol% of erucic acid in PC in Wt between 34 and 50 DAF, when no net synthesis of TAG is taken place, whereas there are only small changes in the erucic acid levels in PC in the GM line during the same period. Because the GM seeds, unlike the Wt seeds, continue to accumulate oil during this period, it can be speculated that there is a mechanism editing out erucic acid from PC operating only during active oil deposition, keeping the erucic acid level low close to maturity in the GM line. Editing out mechanisms for medium chain and oxygenated acyl groups from PC have been suggested to be catalysed by specific phospholipases (Ståhl et al., 1995), but proofs of their in vivo functions are still lacking.
Oil accumulation during seed development
In GM Crambe seeds, the oil accumulation occurred at a much slower rate during the first 20 DAF and proceeded over longer period than in Wt seeds. The fact that the chlorophyll content of the GM seeds started to decline at least 7 days later than in the Wt seeds indicates that the whole seed development was delayed. The proportion of erucic acid was about half of that of mature seeds in the GM line at 20 DAF and about 30% lower than in mature seeds in the Wt seeds. It has previously been reported that the proportion of erucic acid increase with seed development and low at the onset on TAG accumulation in erucic acid producing rapeseed (Bhardwaj and Hamama, 2003). It is clear from the biochemical data on LPAAT activity and from the much higher proportion of erucic acid in DAG in GM seeds than in Wt at 20 DAF that the introduced Limnanthes LPAAT has already good activity at this early stage. All the three genes, BnFAE1, LdLPAAT and CaFAD2-RNAi, introduced into GM Crambe, are under control of the seed-specific napin promoter and can be anticipated to be simultaneously expressed. It is therefore likely that the enzyme BnFAE1 is active at a stage where endogenous FAE enzyme is not fully expressed. Because the elongation of oleic acid to erucic acid needs four enzymes, that is, the condensing enzyme FAE, a 3-keto reductase, a 3-hydroxy hydratase and an enoyl reductase, these enzymes can be assumed to be coordinately produced in the Wt Crambe. It is therefore tempting to speculate that the much slower initial oil accumulation in GM seeds compared with Wt is due to an imbalanced activity of the FAE activity compared with the reducing enzymes, leading to a release of a 3-keto and/or 3-hydroxy intermediates that will be shunted to beta-oxidation and thereby creating a futile cycle. However, the transgenes could also have pleiotropic effects, for example, by disturbing basal membrane lipid metabolism at early stages of seed development. Regardless of the reason for the initial slower oil accumulation in the GM seeds, it is clear that the bottleneck in achieving even higher amount than 72% of erucic acid is probably due to a lower accumulation of erucic acid during the first 27 DAF when about 60% of the oil is laid down. Between 20 and 27 DAF, when the endogenous enzymes involved in erucic acid synthesis can be anticipated to be fully expressed, the amount of erucic acid accounted to 68 mol% of all the FAs accumulated in GM seeds but between 27 and 50 DAF 86 mol% of all FAs accumulated was erucic acid. In contrast, the proportion of erucic acid of all FAs accumulated between 20 and 50 DAF remained rather constant in Wt seeds (59%–63%). This suggests that there is a limiting factor in erucic acid synthesis between 20 and 27 DAF in the GM seeds. As the FA synthesis slows down after 27 days, the proportion of erucic acid produced sharply increases. Because such bottleneck(s) does not impair oil accumulation, they can be expected to be different from the bottlenecks occurred during the first 20 DAF. Such later factors might be inadequate supply of cytosolic malonyl-CoA and/or not enough activity of the introduced genes. If the bottleneck(s) in the production of erucic acid during the first 27 DAF can be solved, it should be possible to develop Crambe lines with over 80% mol% of erucic acid in the oil. It should be noted that single GM Crambe seeds could accumulate up to 77% of erucic acid (Li et al., 2012), demonstrating a certain plasticity in the maximal amount of 22:1 that could be accumulated in the seeds in the studied line. However, seeds from subsequent generations raised by half-seed techniques from seeds with over 73% of 22:1 did show the same distribution of erucic acid among the seeds with a mean of 71–73% (Li et al. unpublished results).
Concluding remarks
We show that Crambe seeds have very low DAG-PC interconversion and this can be assumed to be the major reason of the low amount of erucic acid found in PC. Our results also indicate that the GPAT acylating erucic acid to G-3-P is a different enzyme than the GPAT acylating oleic acid that is lost or inactivated in the microsomal membranes from the developing seeds. We further show that GM Crambe seeds, expressing three genes for increased erucic acid synthesis, have a slower seed development than Wt with a slower oil accumulation during the first 20 DAF. Erucic acid accounted for 86 mol% of all FAs accumulated in the GM between 27 and 50 DAF when about 40% of the final oil is laid down. In order to further increase in erucic acid above the 68 mol% achieved in the GM lines, the bottlenecks in the production of erucic acid at the first 27 DAF must be identified and alleviated. Future comparative transcriptomic, lipidomic and in vivo isotope labelling studies on developing GM and Wt seeds are likely to confirm or disapprove this hypothesis.
Experimental procedures
Plant materials
Wt Crambe (Crambe abyssinica Hochst. cv. Galactica), the transgenic high erucic acid Crambe line 3G7-6-13 (Li et al., 2012), rapeseed (Brassica napus L. cv. Mosaik), turnip (Brassica rapa L. cv. Bele) and safflower (Carthamus tinctorius L. cv. Centennial) were used in this study. All the plant materials were grown in a controlled growth chamber with photoperiod of 16 h (250 μmol/m2/s), temperature of 23/15 °C (day/night) and 60% humidity.
Chemicals
[U-14C] Glycerol-sn-3-phosphate and [U-14C]glycerol were products from Perkin-Elmer. Nonradioactive glycerol-3-phosphate, bovine serum albumin (BSA, essentially fatty acid free) and catalase were products from Sigma Chemical Company. Coenzyme A, oleic acid and erucic acid were products from Larodan (Malmö, Sweden). Acyl-CoAs (18:1-CoA, 22:1-CoA) were prepared according to the method described by Sánchez et al. (1973). [14C]Glycerol-labelled oleoyl-lysophosphatidic acid (18:1- LPA) and erucoyl-lysophosphatidic acid (22:1- LPA) were synthesized by acylating [14C] glycerol-3-phosphate with oleic acid and erucic acid mixed anhydride, respectively, according to the study described by Kanda and Wells (1981) and isolated from the reaction mixture by separation on TLC plates (Silica 60, Merck). The LPAs were eluted from the gel with methanol/chloroform (2:1, v/v) and left at room temperature for 24 h to allow for isomerization of the sn-2 isomer to the stable sn-1 form. The LPAs were then extracted into chloroform, evaporated and dissolved in water before used in assays. LPA concentrations were measured by quantification of the acyl groups relative to methyl-heptadecanoic acid by GLC analysis after methylation. Specific radioactivity of the substrate was calculated by measuring the radioactivity by scintillation counting and the amount of acyl groups as determined by GLC.
Microsomal preparations
Developing cotyledons were harvested at 20 days after flowering (DAF), except for safflower seeds that was harvested 18 days after pollination. Embryos with cotyledons were excised from the seed coat and collected in ice-cold 0.1 m potassium phosphate buffer before homogenized, and all the manipulation was carried out at 4 °C. The tissues were ground in 0.1 m potassium phosphate buffer, pH 7.2, containing 1% BSA, 1000 units of catalase/mL and 0.33 m sucrose. The supernatant was filtered through a double layer of Miracloth® and centrifuged at 20 000 g for 10 min. The supernatant was filtered through a single layer of Miracloth® and centrifuged at 105 000 g for 90 min. The resulting microsomal pellets were resuspended in a small volume of potassium phosphate buffer with 1000 units of catalase/mL, flash frozen in liquid nitrogen and stored at −80 °C until used. Protein content of the microsomal preparations was measured with BCA® reagents (Pierce Chemical Company) with BSA as standard.
Enzyme assays
Microsomal preparations were incubated at 30 °C with gentle shaking in 0.1 m Tris buffer pH 7.2, containing 4 mm MgCl2, 1 mg BSA, 25 nmol 18:1-CoA or 22:1-CoA, 25 nmol of [14C]glycerol-3-phosphate or 9 nmol [14C]glycerol-labelled 18:1-LPA or 22:1-LPA in a final assay volume of 100 μL. The assays were initiated by addition of microsomal preparations (equivalent to 100 μg microsomal protein) into the reaction buffer, and terminated by addition of 100 μL 0.15 m acetic acid and 500 μL of methanol/chloroform (2:1, by vol.), followed by extraction of the lipids into a chloroform phase according to the method described by Bligh and Dyer (1959). Assays were performed in triplicate and the values shown in Figures and Tables are mean values ± standard deviation (SD).
Analysis of radio-labelled lipids
After counting an aliquot of the lipid containing chloroform extract from the assays by liquid scintillation, the rest of the lipids were separated on TLC (Silica 60, Merck). The plates were first developed to 3/4th of their height in chloroform/methanol/acetic acid/water, (90/15/10/3, by vol.) to separate the polar lipids. After drying, the plates were redeveloped to the top in n-hexane/diethyl ether/acetic acid, (70/30/1, by vol.) to separate the neutral lipids. The radioactive spots on the TLC plates were visualized by electronic autoradiography Instant Imager® (Canberra Packard Instruments) and identified by migration of authentic radioactive standard lipid and the relative radioactivity in each lipid was calculated by the Instant Imager® software. The absolute amount of radioactivity in each lipid was calculated based on its relative amount on the plate and the total amount of 14C-activity in the chloroform phase.
In vivo [14C] glycerol labelling
Developing seeds at 20 DAF were harvested and kept on ice. Ten dissected embryos were pooled for each incubation in 300 μL prechilled medium (pH 5.8, 2% sucrose, 0.5x Murashige and Skoog medium). After 20 min preincubation under light at room temperature, labelling was initiated by removing the media and replacing with 100 μL fresh media containing 106 dpm [14C]glycerol. At time intervals, the incubations were terminated by first removing the media and washing with fresh media twice, and then adding 0.15 m acetic acid/(50 mm EDTA + 0.1 m glycerol) (1:5, by vol.). The total lipids were then rapidly extracted from the embryos into a chloroform phase by homogenizing the tissues with Ultra-Turrax® according to the method described by Bligh and Dyer (1959). The radio-labelled lipid analysis was carried out as described above. All assays were performed in triplicate.
Fatty acid composition and chlorophyll determination
A hundred developing Crambe seeds including the pod (each seed is surrounded by a pod) were collected from both the Wt and the GM Crambe lines, at 20, 27, 34, 41 and 50 DAF. The total lipids were then rapidly extracted from the seeds into a chloroform phase by homogenizing the seeds with Ultra-Turrax® as described above. The chloroform phase was evaporated under nitrogen, and the lipids were redissolved in 2 mL chloroform. In order to determine the total amount of FAs and FA composition in the total lipids, 20 μL of extract was redissolved in 2 mL methylation solution (2% H2SO4 in water-free methanol) and methylated at 90 °C for 1 h. Heptadecanoic acid (17:0) was added as the internal standard. After methylation, 2 mL water and 2 mL hexane were added, followed by brief vortexing and centrifugation. The hexane phase containing FA methyl esters (FAMEs) was analysed by gas chromatography according to the method described by Li et al. (2012). Two hundred μL and 100 μL of each total chloroform phase were loaded on TLC and developed in chloroform/methanol/acetic acid/water, (90/15/10/3, by vol.) for polar lipids and in n-hexane/diethyl ether/acetic acid, (70/30/1, by vol.) for neutral lipids, respectively. PC, DAG and TAG spots were identified under UV light after staining with 0.05% primuline in acetone/water (80/20, by vol.), by migration relative to standard 18:1-PC, di-18:1-sn-1,2-DAG and 18:1-TAG. Each lipid spot corresponding to PC, DAG and TAG was scraped from TLC and methylated in situ on the gel as described above. All analyses were carried out in duplicate from the same chloroform extract.
For chlorophyll measurements, duplicate samples of 40 μL of chloroform extracts were dried under nitrogen and redissolved in 1 mL 96% ethanol. The chlorophyll content was determined in replicate by the UV/VIS spectrophotometer (Multiskan GO, Thermo Scientific) according to the equation (Arnon, 1949).
Acknowledgments
Financial support from the European Commission FP7 project ICON, the Swedish research Council FORMAS and Vinnova are gratefully acknowledged.
Supporting Information
Additional Supporting information may be found in the online version of this article
Time course incorporation of radioactivity into various lipids in microsomal preparations from developing turnip incubated with [14C]glycerol 3-phosphate and nonradioactive 18:1-CoA.
Total fatty acid (FA) composition and content in wild-type (Wt) and genetically modified (GM) Crambe seeds at different days after flowering (DAF).
Table S2Fatty acid (FA) composition of diacylglycerols (DAG), triacylgclycerols (TAG) and phosphatidylcholine (PC) in wild-type (Wt) and genetically modified (GM) Crambe seeds at different days after flowering (DAF).
References
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafor M, Jonsson L, Stobart A, Stymne S. Regulation of triacylglycerol biosynthesis in embryos and microsomal preparations from the developing seeds of Cuphea lanceolata. Biochem. J. 1990;272:31–38. doi: 10.1042/bj2720031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Pollard M, Ohlrogge J. The biosynthesis of erucic acid in developing embryos of Brassica rapa. Plant Physiol. 1998;118:183–190. doi: 10.1104/pp.118.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Browse J. The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual fatty acids in transgenic seeds. Plant J. 2011;68:387–399. doi: 10.1111/j.1365-313X.2011.04693.x. [DOI] [PubMed] [Google Scholar]
- Bates PD, Browse J. The significance of different diacylgycerol synthesis pathways on plant oil composition and bioengineering. Front. Plant Sci. 2012;3:147. doi: 10.3389/fpls.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Ohlrogge JB, Pollard M. Incorporation of newly synthesized fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. J. Biol. Chem. 2007;282:31206–31216. doi: 10.1074/jbc.M705447200. [DOI] [PubMed] [Google Scholar]
- Bates PD, Durrett TP, Ohlrogge JB, Pollard M. Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol. 2009;150:55–72. doi: 10.1104/pp.109.137737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Fatihi A, Snapp AR, Carlsson AS, Lu C. Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol. 2012;160:1530–1539. doi: 10.1104/pp.112.204438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Stymne S, Ohlrogge J. Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 2013;16:358–364. doi: 10.1016/j.pbi.2013.02.015. [DOI] [PubMed] [Google Scholar]
- Bhardwaj HL, Hamama AA. Accumulation of glucosinolate, oil, and erucic acid in developing Brassica seeds. Ind. Crop Prod. 2003;17:47–51. [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Carlsson AS, Yilmaz JL, Green AG, Stymne S, Hofvander P. Replacing fossil oil with fresh oil–with what and for what? Eur. J. Lipid Sci. Technol. 2011;113:812–831. doi: 10.1002/ejlt.201100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist A, Ståhl U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Stymne S. Phospholipid: diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc. Natl Acad. Sci. USA. 2000;97:6487–6492. doi: 10.1073/pnas.120067297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer JM, Stymne S, Green AG, Carlsson AS. High-value oils from plants. Plant J. 2008;54:640–655. doi: 10.1111/j.1365-313X.2008.03430.x. [DOI] [PubMed] [Google Scholar]
- Frentzen M. Acyltransferases and triacylglycerols. In: Moore TS Jr, editor. Lipid Metabolism in Plants. Boca Raton, FL: CRC Press; 1993. pp. 195–220. [Google Scholar]
- Griffiths G, Stobart AK, Stymne S. The acylation of sn-glycerol 3-phosphate and the metabolism of phosphatidate in microsomal preparations from the developing cotyledons of safflower (Carthamus tinctorius L.) seed. Biochem. J. 1985;230:379–388. doi: 10.1042/bj2300379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke C, Wolter FP, Coleman J, Peterek G, Frentzen M. A plant acyltransferase involved in triacylglycerol biosynthesis complements an Escherichia Coli sn-1-acylglycerol-3-phosphate acyltransferase mutant. Eur. J. Biochem. 1995;232:806–810. [PubMed] [Google Scholar]
- Hlousek-Radojcic A, Imai H, Jaworski JG. Oleoyl-CoA is not an immediate substrate for fatty acid elongation in developing seeds of Brassica napus. Plant J. 1995;8:803–809. [Google Scholar]
- Hu Z, Ren Z, Lu C. The phosphatidylcholine diacylglycerol cholinephosphotransferase is required for efficient hydroxy fatty acid accumulation in transgenic Arabidopsis. Plant Physiol. 2012;158:1944–1954. doi: 10.1104/pp.111.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara K, Murota N, Fujii S. Intracellular translocation of phosphatidate phosphatase in maturing safflower seeds: a possible mechanism of feedforward control of triacylglycerol synthesis by fatty acids. Biochim. Biophys. Acta. 1990;1043:227–234. doi: 10.1016/0005-2760(90)90021-o. [DOI] [PubMed] [Google Scholar]
- Kanda P, Wells MA. Facile acylation of glycerophosphocholine catalyzed by trifluoroacetic anhydride. J. Lipid Res. 1981;22:877–879. [PubMed] [Google Scholar]
- Kelly AA, Shaw E, Powers SJ, Kurup S, Eastmond PJ. Suppression of the SUGAR-DEPENDENT1 triacylglcycerol lipase family during seed development enhances oil uield in oil seed rape (Brassica napus L.) Plant Biotechnol. J. 2013;11:355–361. doi: 10.1111/pbi.12021. [DOI] [PubMed] [Google Scholar]
- Kennedy EP. Biosynthesis of complex lipids. Fed. Proc. 1961;20:934–940. [PubMed] [Google Scholar]
- Knutzon DS, Hayes TR, Wyrick A, Xiong H, Davies HM, Voelker TA. Lysophosphatidic acid acyltransferase from coconut endosperm mediates the insertion of laurate at the sn-2 position of triacylglycerols in lauric rapeseed oil and can increase total laurate levels. Plant Physiol. 1999;120:739–746. doi: 10.1104/pp.120.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, van Loo E, Gruber J, Fan J, Guan R, Frentzen M, Stymne S, Zhu L. Development of ultra-high erucic acid oil in the industrial oil crop Crambe abyssinica. Plant Biotechnol. J. 2012;10:862–870. doi: 10.1111/j.1467-7652.2012.00709.x. [DOI] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, Franke RB, Graham IA, Katayama K, Kelly AA, Larson T, Markham JE, Miquel M, Molina I, Nishida I, Rowland O, Samuels L, Schmid KM, Wada H, Welti R, Xu C, Zallot R, Ohlrogge J. Acyl-lipid metabolism. Arabidopsis Book. 2013;11:e0161. doi: 10.1199/tab.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhden I, Frentzen M. Triacylglycerol biosynthesis in developing seeds of Tropaeolum majus L. and Limnanthes douglasii R. Br. Planta. 1992;188:215–224. doi: 10.1007/BF00216816. [DOI] [PubMed] [Google Scholar]
- Lu C, Xin Z, Ren Z, Miquel M. An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc. Natl Acad. Sci. USA. 2009;106:18837–18842. doi: 10.1073/pnas.0908848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AA, Wrischer M, Kunst L. Accumulation of very-long-chain fatty acids in membrane glycerolipids is associated with dramatic alterations in plant morphology. Plant Cell. 1998;10:1889–1902. doi: 10.1105/tpc.10.11.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M, Nicholls DG, Brindley DN. The relationship between palmitoylcoenzymeA synthetase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria. Biochem. J. 1973;132:697–706. doi: 10.1042/bj1320697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack CR, Campbell LC, Browse JA, Roughan PG. Some evidence for the reversibility of the cholinephosphotransferase-catalysed reaction in developing linseed cotyledons in vivo. Biochim. Biophys. Acta. 1983;754:10–20. [Google Scholar]
- Slack CR, Roughan PG, Browse JA, Gardiner SE. Some properties of cholinephosphotransferase from developing safflower cotyledons. Biochim. Biophys. Acta. 1985;833:438–448. [Google Scholar]
- Ståhl U, Banas A, Stymne S. Plant microsomal phospholipid acyl hydrolases have selectivities for uncommon fatty acids. Plant Physiol. 1995;107:953–962. doi: 10.1104/pp.107.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhl U, Carlsson AS, Lenman M, Dahlqvist A, Huang B, Banaś W, Banaś A, Stymne S. Cloning and functional characterization of a phospholipid: diacylglycerol acyltransferase from Arabidopsis. Plant Physiol. 2004;135:1324–1335. doi: 10.1104/pp.104.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart AK, Stymne S. The regulation of the fatty-acid composition of the triacylglycerols in microsomal preparations from avocado mesocarp and the developing cotyledons of safflower. Planta. 1985;163:119–125. doi: 10.1007/BF00395905. [DOI] [PubMed] [Google Scholar]
- Stobart AK, Mancha M, Lenman M, Dahlqvist A, Stymne S. Triaclglycerols are synthesised and utilized by transacylation reactions in microsomal preparations of developing safflower (Carthamus tinctorius L.) seeds. Planta. 1997;203:58–66. [Google Scholar]
- Stymne S, Stobart AK. Evidence for the reversibility of the acyl-CoA: lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem. J. 1984;223:305–314. doi: 10.1042/bj2230305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stymne S, Stobart AK. Triacylglycerol biosynthesis. In: Stumpf PK, Conn EE, editors. The Biochemistry of Plants. Vol. 9. New York: Academic Press; 1987. pp. 175–214. [Google Scholar]
- Stymne S, Stobart AK, Glad G. The role of the acyl-CoA pool in the synthesis of polyunsaturated 18-carbon fatty acids and triacylglycerol production in the microsomes of developing safflower seeds. Biochim. Biophys. Acta. 1983;752:198–208. doi: 10.1016/0005-2760(83)90113-3. [DOI] [PubMed] [Google Scholar]
- Temple-Heald C. High erucic oil: its production and uses. In: Gunstone FD, editor. Rapeseed and Canola Oil: Productions, Properties and Uses. Oxford: Blackwell Publishing, CRC Press; 2004. pp. 111–129. [Google Scholar]
- Van Erp H, Bates PD, Burgal J, Shockey J. Castor phospholipid: diacylglycerol acyltransferase facilitates efficient metabolism of hydroxy fatty acids in transgenic Arabidopsis. Plant Physiol. 2011;155:683–693. doi: 10.1104/pp.110.167239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Fan J, Taylor DC, Ohlrogge JB. DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell. 2009;21:3885–3901. doi: 10.1105/tpc.109.071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time course incorporation of radioactivity into various lipids in microsomal preparations from developing turnip incubated with [14C]glycerol 3-phosphate and nonradioactive 18:1-CoA.
Total fatty acid (FA) composition and content in wild-type (Wt) and genetically modified (GM) Crambe seeds at different days after flowering (DAF).
Table S2Fatty acid (FA) composition of diacylglycerols (DAG), triacylgclycerols (TAG) and phosphatidylcholine (PC) in wild-type (Wt) and genetically modified (GM) Crambe seeds at different days after flowering (DAF).


