Abstract
Salt marsh habitat loss to vegetation die-offs has accelerated throughout the western Atlantic in the last four decades. Recent studies have suggested that eutrophication, pollution and/or disease may contribute to the loss of marsh habitat. In light of recent evidence that predators are important determinants of marsh health in New England, we performed a total predator exclusion experiment. Here, we provide the first experimental evidence that predator depletion can cause salt marsh die-off by releasing the herbivorous crab Sesarma reticulatum from predator control. Excluding predators from a marsh ecosystem for a single growing season resulted in a >100% increase in herbivory and a >150% increase in unvegetated bare space compared to plots with predators. Our results confirm that marshes in this region face multiple, potentially synergistic threats.
Keywords: Trophic cascade, herbivore release, salt marsh die-off, predator depletion, experimental predator removal
Introduction
Understanding the vulnerability and resilience of marine ecosystems to human disturbances is one of the most pressing issues of our time (Jackson et al. 2001; Lotze et al. 2006). Coastal salt marsh wetlands are among the most valuable ecosystems per capita worldwide (Costanza et al. 1997), but many have become degraded by human impacts (Lotze et al. 2006). Over the next century coastal ecosystems, such as salt marshes, are predicted to be further threatened by climate change, sea-level rise, ocean acidification and overexploitation (Jackson et al. 2001; Hoegh-Guldberg & Bruno 2010), making preservation of coastal ecosystems one of conservation biology's greatest challenges (Biggs et al. 2009; Worm et al. 2009).
Throughout the western Atlantic, salt marsh creek bank die-off, the rapid regime shift from dense monocultures of marsh grass to unvegetated eroding mud flats, has become an increasingly pervasive threat (Bertness & Silliman 2008). Historically, it was assumed that salt marshes were exclusively governed by physical factors (e.g. Teal 1962; Valiela & Teal 1974), but recent experimental field studies suggest that both bottom up and top down control can cause rapid and dramatic shifts in salt marsh ecosystem structure (Bertness et al. 2002; Silliman & Bertness 2002; Jefferies et al. 2006; Bertness & Silliman 2008). These hypotheses include: (1) eutrophication decreases plant investment into belowground biomass causing creek bank collapse (Deegan et al. 2012), (2) harsh physical conditions such as hypersaline or anoxic sediments driven by climatic extremes cause plant mortality (Alber et al. 2008), (3) increased boating activity erodes creek banks and damaged vegetation (Smith & Carullo 2007), (4) pollution and disease adversely affect plant health (Smith & Carullo 2007; Elmer et al. 2013) and/or (5) predator depletion releases herbivorous crabs from predator control (Altieri et al. 2012; Coverdale et al. 2013a). While it is likely that multiple, simultaneous threats to marsh vegetation have played a role in recent die-offs, few have been experimentally tested.
In New England, recreational overfishing has recently been hypothesised to trigger die-off by targeting and depleting large predators, releasing the herbivorous purple marsh crab Sesarma reticulatum from predator control (Altieri et al. 2012; Coverdale et al. 2013a). This has led to large increases in S. reticulatum populations, expansion of communal S. reticulatum burrow complexes and overgrazing of Spartina alterniflora, the foundation species that builds and maintains these marshes (Altieri et al. 2012). Comparative data from salt marshes spanning the Atlantic coastline from Cape Cod to Long Island Sound (n = 35 sites) (Coverdale et al. 2013a) support the linkage between predator depletion, S. reticulatum release and salt marsh die-off: 80% of intersite variation in the extent of die-off is explained by intersite variation in S. reticulatum herbivory, which is negatively correlated with predator density (Altieri et al. 2012). Predator depletion has been similarly implicated as a source of habitat degradation in terrestrial ecosystems (Ripple et al. 2014), but support for this hypothesis across ecosystems is largely comparative and/or correlative; controlled field experiments are lacking.
Here, we explicitly examine the hypothesis that predators regulate marsh productivity by reducing herbivory. We use a total predator exclusion experiment to rigorously test the conclusions of previous comparative studies between sites with and without concentrated recreational fishing pressure (Altieri et al. 2012; Coverdale et al. 2013a). We also quantify wave exposure and eutrophication to determine potential small-scale contributions of bottom up factors. Manipulating the abundance of top predators in a field setting provides additional insight into the consequences of predator depletion in coastal ecosystems, which is one of the most pressing marine conservation issues worldwide (Myers & Worm 2003).
Materials and Methods
Experimental setup
This study was conducted at a marsh in North Bay, Osterville, Massachusetts bordering a marina that serves recreational fisherman and experienced severe creek bank cordgrass die-off in the early 2000s (Altieri et al. 2012). More recently, it has largely recovered from die-off (Altieri et al. 2013), potentially as a result of compensatory predation (Bertness & Coverdale 2013). Currently, creek banks at this site have only a small (<0.5 m) band of active die-off with fully recovered cordgrass above and below the remnant die-off zone. S. reticulatum herbivory is concentrated at the border of the high marsh, which is only inundated during monthly spring tides. Cordgrass in the low zone has reached a size refuge from herbivory (Coverdale et al. 2012) and low marsh substrate is too soft to support S. reticulatum burrows (Altieri et al. 2013).
We tested the impact of top predators on salt marsh vegetation with a predator exclusion experiment. Treatments included full predator exclusion cages, procedural controls and unmanipulated controls. Predator exclusion cages and cage controls measured 1.0 × 1.0 × 0.5 m (L × W × H) and were constructed of plastic coated 2 cm wire mesh. Eight replicates of each treatment were randomly assigned to plots on the terrestrial border of the low marsh where S. reticulatum burrowing and herbivory are concentrated. Exclusion cages were inserted 5 cm into the marsh surface, effectively preventing access to large recreationally fished predatory crabs and fish (e.g. striped bass, Morone saxatilis, blue crabs, Callinectes sapidus and smooth dogfish, Mustelus canis) and green crabs (Carcinus maenas), a compensatory predator of S. reticulatum (Bertness & Coverdale 2013). Exclusion cages allowed S. reticulatum to move in and out of cages freely via underground burrow networks. The 2-cm mesh also excluded all but the smallest juvenile predatory crabs (C. sapidus and C. maenas), which have previously been shown to be incapable of consuming adult S. reticulatum (Coverdale et al. 2013b). Predator exclusion cages also effectively excluded mammalian predators (e.g. raccoon, Procyon lotor) as well as birds (e.g. night heron, Nycticorax nycticorax). Cage controls were identical in dimension but lacked tops and mesh sides were elevated 15 cm over the substrate to allow predator access; access for the dominant S. reticulatum predators (C. sapidus and C. maenas) was not restricted by procedural control cages. Unmanipulated control plots were marked with corner posts. S. reticulatum burrow density, grazing pressure (per cent stems grazed) and per cent vegetation cover within each replicate plot were quantified at the initiation of the experiment to test whether starting conditions were similar across treatments. Variables were transformed when necessary to meet the assumptions of anova and were analysed with a one-way anova with treatment as the factor.
Effects of S. reticulatum herbivory and burrowing
To assess the effect of predator exclusion on S. reticulatum herbivory, we transplanted ungrazed cordgrass cores from a common source site in Narragansett Bay at the terrestrial border of each plot in May 2013. Cordgrass cores were observed weekly to ensure survival and stems were counted and scored for herbivore damage after 3 months, at which point the majority of the cordgrass had flowered. S. reticulatum grazing leaves characteristic rasped edges and clipped blades on cordgrass (Holdredge et al. 2008), therefore we were confident in concluding that S. reticulatum is the contributing grazer among potential salt marsh herbivores. The proportion of final stems grazed was analysed with a one-way anova (caged vs. cage control vs. control).
To avoid disturbing enclosures, we quantified per cent bare space and S. reticulatum burrow density within two 0.25 m2 quadrats divided into 100 5 x 5 cm subquadrats along the top and bottom edge of all replicates after cordgrass began flowering. S. reticulatum burrows are easily distinguished from other crab burrows such as fiddler crabs (Uca pugnax) and have been previously shown to be closely associated with S. reticulatum abundance and herbivory (Holdredge et al. 2008; Coverdale et al. 2012, 2013b). We monitored the upper and lower halves in each replicate to quantify elevation effects, as S. reticulatum grazing is concentrated at the terrestrial border of the cordgrass zone. Within each zone, we also harvested cordgrass in two centrally placed 10 x 10 cm subquadrats, and counted, dried and weighed them to quantify aboveground cordgrass biomass. All subquadrats within the upper and lower border of the plots were pooled to yield average values for each elevation. Per cent bare space, S. reticulatum burrow density, and aboveground biomass were transformed to meet the assumptions of anova and analysed with nested anovas [elevation (plot) and treatment].
Effects of water movement
Previous studies suggest that wave exposure may contribute to die-off (Smith & Carullo 2007). Therefore, we tested time integrated wave exposure in each of our plots to see if there were inherent differences that might contribute to variations in die-off intensity. We deployed a magnesium calcite chalk block within the top edge of each plot to compare water movement among replicates in the first week of July (see Bertness et al. 1991). Chalk blocks were pre-weighed, glued to a hardware cloth base, pinned to the substrate with wire staples and left in the field for 5 weeks. Chalk blocks were then dried at 40 °C to a constant weight, and reweighed. Chalk loss/treatment was used as an integrated measure of wave exposure over time. Per cent chalk block dissolution was analysed with a one-way anova (caged vs. cage control vs. control).
Nutrient effects
Previous studies suggest that eutrophication can cause large-scale marsh die-off (Deegan et al. 2012). To determine if eutrophication was a driver of small-scale variation in die-off intensity, we quantified differences in ambient nitrogen levels across treatments by transplanting an undamaged caged common source cordgrass core on the terrestrial border of each plot in mid May 2013. Cores were caged above- and belowground with 1 cm galvanised hardware cloth to prevent herbivory (Coverdale et al. 2012). Hardware cloth cages do not influence cordgrass growth (Holdredge et al. 2008; Coverdale et al. 2012). The aboveground biomass of each core transplant was harvested, dried at 40 °C and weighed after cordgrass flowered in August.
Aboveground cordgrass transplant biomass was used to determine if cordgrass growth potential was similar across treatments; differences in growth potential may indicate local-scale pollution (Smith & Carullo 2007) or disease (Elmer et al. 2013) effects. To determine aboveground per cent leaf tissue nitrogen for each transplant core leaf tissue from each transplant core was dried in a convection oven, coarsely ground and dried at 60 °C before further grinding. Samples were analysed for per cent nitrogen using an elemental analyser (Model NC2100; ThermoQuest CE Instruments, San Jose, CA, USA). Aboveground biomass, and per cent nitrogen were analysed with separate one-way anovas (caged vs. cage control vs. control).
Due to concerns about winter storm damage disturbing experimental plots, we present data from within a single growing season.
Results
Initial vegetation cover, herbivory and burrow density did not differ across treatments (F2,21 = 1.02, P > 0.25; F2,21 = 1.45, P > 0.25; F2,21 = 0.30, P > 0.50 respectively).
Total predator removal from vegetated creek banks for a single growing season significantly increased S. reticulatum burrow density and bare space and decreased cordgrass abundance (Fig. 1). Final herbivory on uncaged cordgrass transplants varied across treatments: full predator exclusion plots had >100% more herbivory than cage control and control plots with predator access (F2,20 = 28.77, P < 0.0001; Fig. 2a). Aboveground cordgrass biomass mirrored differences in herbivory, with lower cordgrass biomass in high elevation subquadrats along the grazing border than in the lower elevation subquadrats (F24,21 = 3.37, P < 0.05; Fig. 2b). Overall, excluding predators led to a >60% decrease in aboveground cordgrass biomass compared to cage control and control plots (F2,21 = 23.18, P < 0.0001; Fig. 2b).
Figure 1.
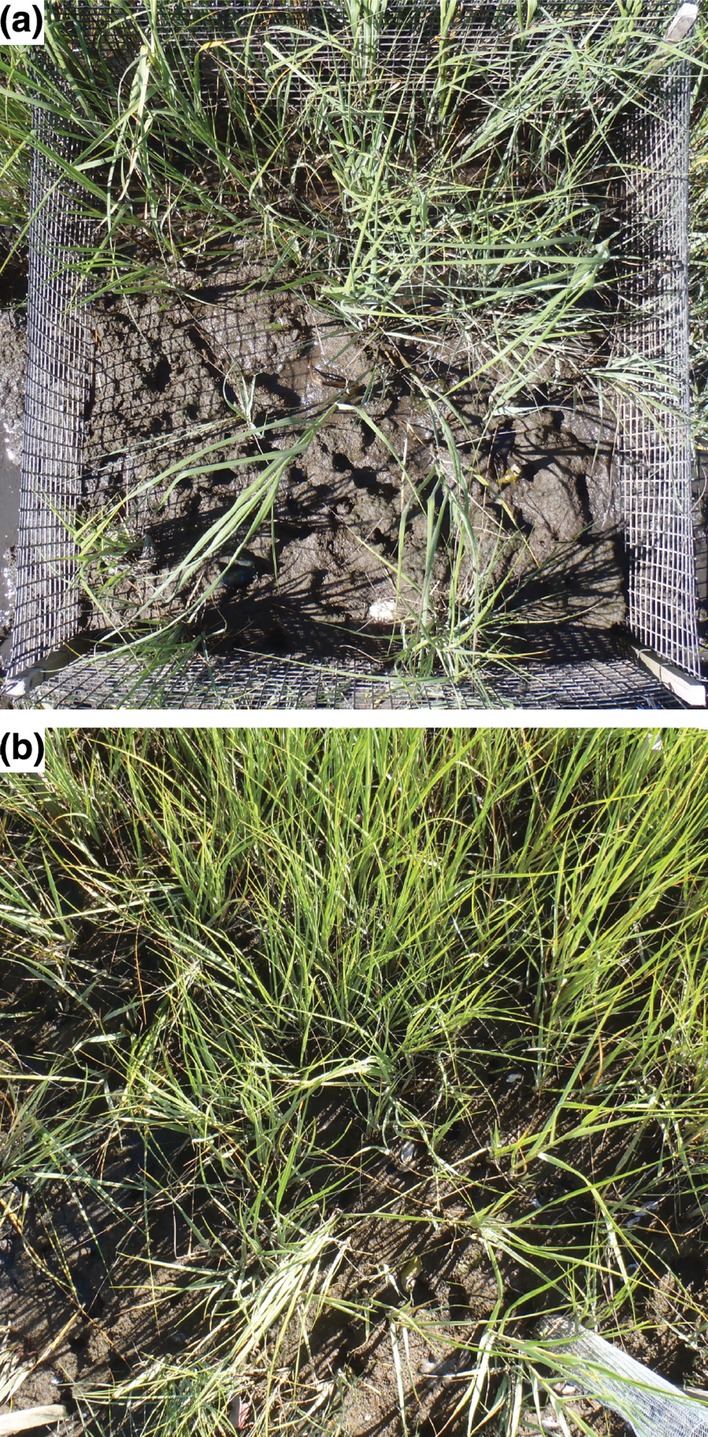
Predator exclusion (a) and open control (b) plots after 3 months of predator exclusion. All plots had similar initial cordgrass cover. In the predator exclusion plot (a) note the increased density of S. reticulatum burrows and thinned S. alterniflora from grazing. In the control (b) note the dense cordgrass monoculture, fewer S. reticulatum burrows and minimal bare space.
Figure 2.
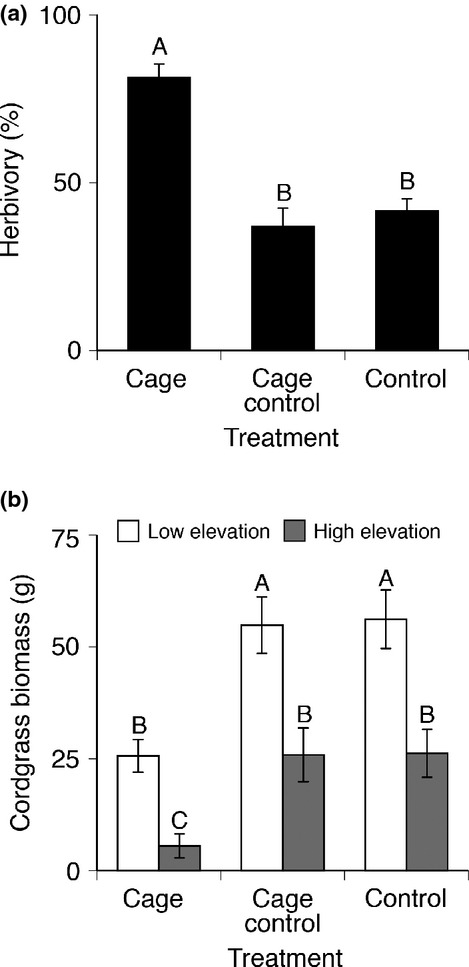
End of season herbivory (a) on common source cordgrass transplants across treatments, cordgrass transplants in predator exclusion cages experienced ∼2× higher herbivory than cordgrass transplants in the cage control and control plots. (Letters, Tukey HSD). Pooled aboveground cordgrass biomass (b) mirrored differences in herbivory, caged plots had less biomass than cage control and control plots and biomass was consistently lower at higher elevations along the grazing border (Letters, Tukey HSD).
S. reticulatum burrow density and bare space also varied with visibly striking differences between control and predator exclusion plots after only 3 months (Fig. 1). S. reticulatum burrow densities were similar across elevations (F24,21 = 1.44, P > 0.10); however, predator exclusion led to a >95% overall increase in burrow density relative to cage control and control plots (F2,21 = 10.62, P < 0.001; Fig. 3a). Within replicates, the amount of bare space was higher in high elevation subquadrats along the grazing border than low elevations (F24,21 = 2.40, P < 0.05) and excluding predators led to >150% increase in bare space (F2,21 = 27.88, P < 0.0001; Fig. 3b).
Figure 3.
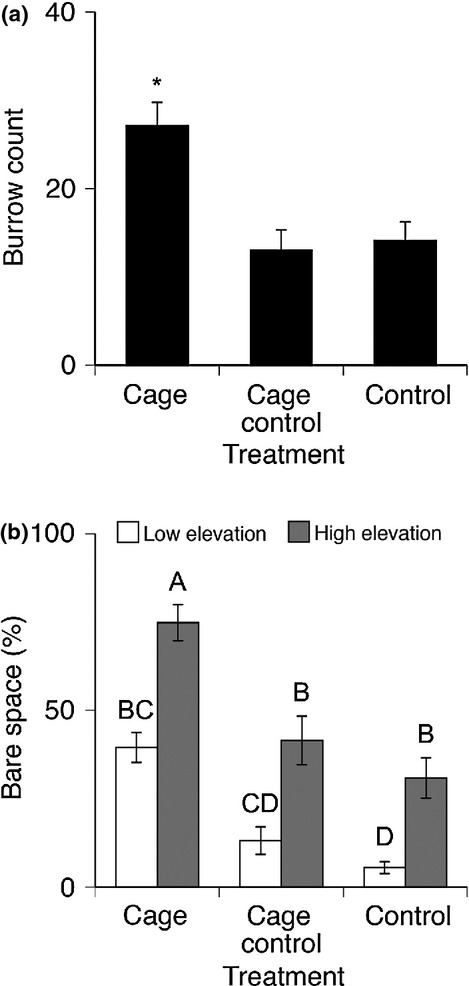
End of season pooled subquadrat (0.5 m2) burrow counts (a) revealed that there were significantly more burrows in caged plots than cage control and control plots (*Denotes significant difference, Tukey HSD). Bare space (b) was also higher in caged plots than cage control and control plots, and overall bare space was higher at high elevations along the grazing border (Letters, Tukey HSD).
The biomass and nitrogen content of caged cordgrass core transplants did not vary across treatments (F2,21 = 1.51, P > 0.05; F2,21 = 0.57, P > 0.05 respectively). There were also no differences in chalk block dissolution across treatments (F2,21 = 0.98, P > 0.25).
Discussion
Our experimental predator exclusion provides substantial evidence for the potential importance of top down control in triggering rapid shifts in New England salt marshes. As predicted by comparative studies of sites with and without predator depletion and recreational overfishing (Altieri et al. 2012), excluding predators for a single growing season led to a >100% increase in S. reticulatum herbivory (Fig. 2a), a >60% decrease in aboveground cordgrass biomass (Fig. 2b), a >95% increase in S. reticulatum substrate disturbance (Fig. 3a) and a >150% increase in unvegetated bare space relative to control plots (Fig. 3b). We found no differences in wave exposure or nitrogen among our plots, suggesting that neither wave exposure nor nutrients contributed to the variation in experimentally triggered die-off. These results suggest that predator depletion, over the course of a single growing season, is capable of causing rapid, dramatic shifts in the biotic and abiotic condition of New England salt marshes.
Many of the predators of S. reticulatum are recreationally harvested, and depletion of these species (e.g. M. saxatilis, C. sapidus, M. canis; Altieri et al. 2012) is a pressing regional conservation concern. Previous work indicates that the biomass of non-harvested species (spider crabs, Libinia emarginata, diamondback terrapins, Malaclemys terrapin and mummichog fish, Fundulus heteroclitus) do not differ between healthy and die-off salt marshes, suggesting that predator depletion in this system is the result of targeted overfishing and not a more general fisheries decline (Altieri et al. 2012).
Interactions between predators and S. reticulatum have been shown to be both consumptive and non-consumptive in nature. Non-consumptive effects have strong, cascading effects on S. reticulatum behaviour, including foraging and herbivory (Bertness & Coverdale 2013; Coverdale et al. 2013b). As a result of perceived risk, S. reticulatum at our study site likely migrated locally to the refugia provided by full predator exclusion cages, potentially contributing significantly to the rapid change observed in this study. Natural refugia, including Sesarma burrows, have been shown to cause dense Sesarma aggregations and localised depletion of above- and belowground cordgrass biomass (Coverdale et al. 2012). Similar examples of non-consumptive effects in rocky shores (Trussell et al. 2002), freshwater lakes (Peacor & Werner 1997) and terrestrial grasslands (Schmitz 1998) suggest that non-consumptive effects may be a ubiquitous driver of trophic cascades and that effective predator conservation is urgent (Ripple et al. 2014).
Similar predator removal experiments are rare in salt marsh ecosystems and have generally focused on the effects of intermediate predators on marsh invertebrates (e.g. Vince et al. 1976; Kneib & Stiven 1982). To our knowledge, this is the first experimental study to investigate trophic cascades initiated by top predators in salt marshes. All previous experimental studies examining the potential for trophic cascades impacting salt marsh foundation species have been comparative studies manipulating grazers at grazed and ungrazed sites (Silliman & Bertness 2002; Altieri et al. 2012; Coverdale et al. 2013a). This is in stark contrast to the abundance of experimental top predator removal studies in rocky shore (e.g. Paine 1976), soft sediment (e.g. Wilson 1991) and coral reef habitats (e.g. Hughes 1994). Such studies are critical to understanding the causes and consequences of marsh die-off throughout the western Atlantic.
The last 60 years has seen a dramatic shift in ecosystems thought to be controlled exclusively by bottom up forces. Many ecosystems, including kelp forests, coral reefs and salt marshes have been shown to shift from bottom up to top down control after human disturbance (Estes & Palmisano 1994; Hughes 1994; Jackson et al. 2001; Bertness et al. 2008). The idea that these shifts represent real responses to changing conditions and not simply an increased focus on top down processes is becoming increasingly clear.
Salt marsh die-offs resulting from dysfunctional food webs and runaway consumption have also been documented in the Canadian subarctic (Jefferies et al. 2006), and the South-eastern and Gulf Coasts of the United States (Silliman & Bertness 2002). With the mounting number of comparative and experimental studies indicating strong consumer control in salt marshes, it is imperative that the multiplicative and potentially interactive effects of both top down and bottom up forces be included in the management and conservation of salt marsh ecosystems (Costanza et al. 1997). This is particularly pressing given the environmental challenges salt marshes are predicted to face over the next century (Jackson et al. 2001; Gedan et al. 2009; Hoegh-Guldberg & Bruno 2010) and the valuable ecosystem services they provide. Furthermore, there is increasing evidence that salt marsh productivity is driven by interactions between physical factors and consumer pressure, which may operate at different spatial and temporal scales (Jefferies et al. 2006; Bertness et al. 2008; Deegan et al. 2012). While our results indicate that predator depletion can cause rapid, dramatic shifts in the biotic and abiotic condition of New England salt marshes, increasing population density and human activity in coastal areas suggests that multiple interacting threats are likely to become increasingly common (Jackson et al. 2001), with the potential to fundamentally alter ecosystems worldwide.
Acknowledgments
We thank R.T. Paine and E. Sanford for comments on the manuscript, the Cape Cod National Seashore for access to field sites and logistic support. This research was made possible by grant NSF BIO OCE-0927090, the Brown University Undergraduate Teaching and Research Award Program, and the Voss Environmental Fellowship Program.
Authorship
MDB designed the project with help from CPB and TCC; all authors performed the experiments; MDB and CPB wrote the manuscript; CPB, MCB, SMC and ERS conducted the analyses; all authors edited the manuscript.
References
- Alber ME, Swenson M, Adamowicz SC. Mendelssohn IA. Salt marsh dieback: an overview of recent events in the US. Estuar. Coast. Shelf S. 2008;80:1–11. [Google Scholar]
- Altieri AH, Bertness MD, Coverdale TC, Herrmann NC. Angelini C. A trophic cascade triggers collapse of a salt marsh ecosystem with intensive recreational fishing. Ecology. 2012;93:1402–1410. doi: 10.1890/11-1314.1. [DOI] [PubMed] [Google Scholar]
- Altieri AH, Bertness MD, Coverdale TC, Axelman EE, Hermann NC, Szathmary PL. Feedbacks underlie the resilience of salt marshes and rapid reversal of consumer-driven die-off. Ecology. 2013;94:1647–1657. doi: 10.1890/12-1781.1. [DOI] [PubMed] [Google Scholar]
- Bertness MD. Coverdale TC. An invasive species facilitates the recovery of salt marsh ecosystems on Cape Cod. Ecology. 2013;94:1937–1943. doi: 10.1890/12-2150.1. [DOI] [PubMed] [Google Scholar]
- Bertness MD. Silliman BR. Consumer control of salt marshes driven by human disturbance. Conserv. Biol. 2008;22:618–623. doi: 10.1111/j.1523-1739.2008.00962.x. [DOI] [PubMed] [Google Scholar]
- Bertness MD, Gaines SD, Bermudez D. Sanford E. Extreme spatial variation in the growth and reproductive output of the acorn barnacle Semibalanus balanoides. Mar. Ecol. Prog. Ser. 1991;75:91–100. [Google Scholar]
- Bertness MD, Ewanchuk P. Silliman BR. Anthropogenic modification of New England salt marsh landscapes. Proc. Nat. Acad. Sci. 2002;99:1395–1398. doi: 10.1073/pnas.022447299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertness MD, Crain CM, Holdredge C. Sala N. Eutrophication triggers consumer control of New England salt marsh primary production. Conserv. Biol. 2008;22:131–139. doi: 10.1111/j.1523-1739.2007.00801.x. [DOI] [PubMed] [Google Scholar]
- Biggs R, Carpenter SR. Brock WA. Turning back from the brink: detecting an impending regime shift in time to avert it. Proc. Natl Acad. Sci. USA. 2009;106:826–831. doi: 10.1073/pnas.0811729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza R, d'Arge R, de Groot R, Farber S, Grasso M, Hannon B, et al. The value of the world's ecosystem services and natural capital. Nature. 1997;387:253–260. [Google Scholar]
- Coverdale TC, Altieri AH. Bertness MD. Belowground herbivory increases vulnerability of New England salt marshes to die-off. Ecology. 2012;93:2085–2094. doi: 10.1890/12-0010.1. [DOI] [PubMed] [Google Scholar]
- Coverdale TC, Bertness MD. Altieri AH. Regional ontogeny of New England salt marsh die-off. Consery. Biol. 2013a;27:1041–1048. doi: 10.1111/cobi.12052. [DOI] [PubMed] [Google Scholar]
- Coverdale TC, Axelman EE, Brisson CP, Young EW, Altieri AH. Bertness MD. New England salt marsh recovery: opportunistic colonization of an invasive species and its non-consumptive effects. PLoS ONE. 2013b;8:e73823. doi: 10.1371/journal.pone.0073823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deegan LA, Johnson DS, Warren RS, Peterson BJ, Fleeger JW, Fagherazzi S, et al. Coastal eutrophication as a driver of salt marsh loss. Nature. 2012;490:388–392. doi: 10.1038/nature11533. [DOI] [PubMed] [Google Scholar]
- Elmer WH, Useman S, Schneider RW, Marra RE, LaMondia JA, Mendelssohn IA, et al. Sudden vegetation dieback in Atlantic and Gulf Coast salt marshes. Plant Dis. 2013;97:436–445. doi: 10.1094/PDIS-09-12-0871-FE. [DOI] [PubMed] [Google Scholar]
- Estes JA. Palmisano JF. Sea otters: their role in structuring nearshore communities. Science. 1994;185:1058–1060. doi: 10.1126/science.185.4156.1058. [DOI] [PubMed] [Google Scholar]
- Gedan KB, Silliman BR. Bertness MD. Centuries of human-driven change in salt marsh ecosystems. Ann. Rev. Mar. Sci. 2009;1:117–141. doi: 10.1146/annurev.marine.010908.163930. [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O. Bruno JF. The impact of climate change on the world's marine ecosystems. Science. 2010;328:1523–1528. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- Holdredge C, Bertness MD. Altieri AH. Role of crab herbivory in die-off of New England salt marshes. Conserv. Biol. 2008;23:672–679. doi: 10.1111/j.1523-1739.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- Hughes TP. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- Jefferies RL, Jano AP. Abraham KF. A biotic agent promotes large-scale catastrophic change in the coastal marshes of Hudson Bay. J. Ecol. 2006;94:234–242. [Google Scholar]
- Kneib RT. Stiven AE. Benthic invertebrate responses to size and density manipulations of the common mummichog, Fundulus Heteroclitus, in an intertidal salt marsh. Ecology. 1982;63:1518–1532. [Google Scholar]
- Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, Kay MC, et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 2006;312:1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- Myers RA. Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- Paine RT. Size-limited predation: an observational and experimental approach with the Mytilus-Pisaster interaction. Ecology. 1976;57:858–873. [Google Scholar]
- Peacor SD. Werner EE. Trait-mediated indirect interactions in a simple aquatic food web. Ecology. 1997;73:1146–1156. [Google Scholar]
- Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, et al. Status and ecological effects of the world's largest carnivores. Science. 2014;343:6167. doi: 10.1126/science.1241484. doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- Schmitz OJ. Direct and indirect effects of predation and predation risk in old-field interaction webs. Amer. Nat. 1998;151:327–342. doi: 10.1086/286122. [DOI] [PubMed] [Google Scholar]
- Silliman BR. Bertness MD. A trophic cascade regulates salt marsh primary production. Proc. Natl Acad. Sci. USA. 2002;99:10500–10505. doi: 10.1073/pnas.162366599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP. Carullo M. Survey of potential marsh dieback sites in coastal Massachusetts. Boston, MA: MA Bays National Estuary Program and MA Office of Coastal Zone Management; 2007. [Google Scholar]
- Teal JM. Energy flow in the salt marsh ecosystem of Georgia. Ecology. 1962;43:614–624. [Google Scholar]
- Trussell GC, Ewanchuk PJ. Bertness MD. Field evidence of trait-mediated indirect effects in a rocky intertidal food web. Ecol. Lett. 2002;5:241–245. [Google Scholar]
- Valiela I. Teal JM. Nutrient limitation in salt marsh vegetation. In: Queen WH, editor; Reimold J, editor. Ecology of Halophytes. New York: Academic Press; 1974. pp. 543–563. [Google Scholar]
- Vince S, Valiela I. Backus N. Predation by the salt marsh killifish Fundulus heteroclitus (L.) in relation to prey size and habitat structure: consequences for prey distribution and abundance. J. Exp. Mar. Biol. Ecol. 1976;23:255–266. [Google Scholar]
- Wilson WH. Competition and predation in marine soft-sediment communities. Annu. Rev. Ecol. Syst. 1991;21:221–241. [Google Scholar]
- Worm BR, Hilborn R, Baum JK, Branch TA, Collie JS, Costello C, et al. Rebuilding Global Fisheries. Science. 2009;325:578–585. doi: 10.1126/science.1173146. [DOI] [PubMed] [Google Scholar]


