Highlights
-
•
Telomere elongation decreases human tumour cell viability after irradiation.
-
•
The longer the telomeres, the greater the sensitivity to sub-lethal irradiation.
-
•
Increased sensitivity to irradiation is independent of telomere signal-free ends.
Keywords: Telomeres, Telomerase, Human tumour cells, Gamma irradiation, DNA damage
Abbreviations: ALT, alternative lengthening of telomeres; ANOVA, analysis of variance; CST, CTC1, STN1 and TEN1 complex; DSB, double-stranded DNA break; hTR, human telomerase RNA; PDL, population doubling level; PE, plating efficiency; Q-FISH, quantitative fluorescence in situ hybridization; SF, survival fraction; SFE, signal-free end; SV40, simian virus 40; TERT, telomerase reverse transcriptase; TRF, telomere restriction fragment
Abstract
More than 85% of all human cancers possess the ability to maintain chromosome ends, or telomeres, by virtue of telomerase activity. Loss of functional telomeres is incompatible with survival, and telomerase inhibition has been established in several model systems to be a tractable target for cancer therapy. As human tumour cells typically maintain short equilibrium telomere lengths, we wondered if enforced telomere elongation would positively or negatively impact cell survival. We found that telomere elongation beyond a certain length significantly decreased cell clonogenic survival after gamma irradiation. Susceptibility to irradiation was dosage-dependent and increased at telomere lengths exceeding 17 kbp despite the fact that all chromosome ends retained telomeric DNA. These data suggest that an optimal telomere length may promote human cancer cell survival in the presence of genotoxic stress.
1. Introduction
The acquisition of telomerase reverse transcriptase (TERT) expression and its ability, together with the telomerase RNA, to maintain telomeres at chromosome ends is one of the hallmarks of cancer [1]. In the absence of telomerase function or upon enforced telomerase inhibition, telomere erosion eventually results in a telomere-specific DNA damage response that leads to chromosome end resection, loss, or fusion, and anaphase bridges (reviewed in [2,3,4]). Short telomeres, which are typical of human cancer cells [5,6], are a preferential substrate for telomere elongation by telomerase in many model systems. Indeed, the levels of the telomerase components TERT and its integral RNA, hTR, are limiting in many cell types including cancer cells (reviewed in [7]). Conversely, long telomeres inhibit access by telomerase, and in some instances excessively elongated telomeres are actively trimmed by complex recombination mechanisms [8–12] (reviewed in [13,14]). Thus, in many cell types, the homeostatic balance between these processes ensures that telomere length is maintained around a given equilibrium telomere length. We questioned why tumour cells tend to maintain short average telomere lengths, and whether long telomeres might be disadvantageous for cell survival in the presence of DNA damage.
2. Materials and methods
2.1. Cell culture and cell irradiation
Cell culture and population doubling level (PDL) calculations were performed as described [15]. Irradiation experiments were carried out according to Boyd et al. [16]. Cells were seeded in 25 cm2 flasks at a concentration of 1 × 105 cells/flask. After 24 h adherent cells were irradiated at a range of doses from 0 to 10 Gy using a 60Co irradiator, returned to a 37 °C incubator for 24 h, then resuspended in 0.5% w/v trypsin solution and counted on a haemocytometer. Cells were plated in triplicate at 2.5 × 103 per 75 cm2 flask and incubated at 37 °C for 7–10 days. Cells were fixed and stained with 10% w/v crystal violet in methanol. Clusters of approximately 50 or more cells were scored as a single colony. The SF was calculated by determining the plating efficiency (PE, defined as the number of colonies divided by the number of cells seeded), with the first PE value at 0 Gy (of n = 3) normalized to 1.0. Thus, the average SF at 0 Gy is near (but not exactly equal to) 1.0, and the SF of cells exposed to irradiation are expressed relative to the SF at 0 Gy. All the cell lines in each graph were subjected to irradiation and subsequent manipulations at the same time. Statistical analysis was carried out using Prism 5.0 (GraphPad, Inc.), in which the SF values (at least n = 3) of a cell line at a given irradiation dosage were assigned to a single column (e.g. in Fig. 2A, left, there were 12 columns, representing SF values for 17 PDL and 146 PDL lines at 0, 2, 4, 6, 8, and 10 Gy, respectively). All columns were compared simultaneously using one-way ANOVA (parametric, unmatched observations) followed by a Tukey post-test. The calculated p-values shown represent level of significance (** p < 0.01; *** p < 0.001) of a given PDL at a particular irradiation dosage relative to the earliest PDL at the same irradiation dosage.
Fig. 2.
Tumour cell viability after treatment with ionizing radiation. (A) Independently derived TERT-positive (Vec-1, Vec-2) or TERT-excised lines (Cre-4, Cre-1) (as in Fig. 1A) were subjected to 0–10 Gy ionizing radiation and analyzed for clonogenic survival (see Section 2 for details). (B) A third representative experiment with a TERT-positive (Vec-2) or a TERT-excised line (Cre-1). PDL are indicated in red; average telomere length in blue; n.d. not determined. p-Values (**p < 0.01, ***p < 0.0001) are relative to the earliest PDL at the same irradiation dosage. y-Axis, log10 of the survival fraction; x-axis, dosage of irradiation in Gy. Error bars indicate standard deviation. (C) Plot of survival fraction (SF) as a function of average telomere length, using the data in panel (A) (upper 2 graphs), and (D), plot of the survival fraction (SF) as a function of average telomere length, using the data shown in panel (B), to demonstrate that the maximal SF occurred across a similar range of telomere lengths in independent experimental series.
2.2. Telomere length measurements
Q-FISH analysis and Southern blots to assess telomere length were performed as described previously [15]. Q-FISH analysis employed an automated Metafer Slide Scanning platform (Metasystems, Inc.) capable of automated analysis of 8 slides under identical conditions. Unless otherwise stated, Q-FISH profiles shown in the same figure panel were analyzed simultaneously in this manner. Average telomere lengths at a given PDL were determined via linear regression analysis of average telomere signal intensity obtained via 3 independent Q-FISH measurements verified against TRF length determined by southern blot where a.u. (y) were converted to kbp (x) using the formula y = 64x − 106. This linear regression analysis yielded an R-squared co-efficient of 0.97 (data not shown). In Fig. 1C, signal-free ends were defined as chromosome ends that yielded no detectable telomere fluorescence signal, from a total of 920 ends analyzed for each sample; the data from one representative experiment is shown (n = 3). p-values of p < 0.05 (*) or p < 0.0001 (****) were calculated using Fisher's exact test (Prism 5.0, GraphPad Inc.).
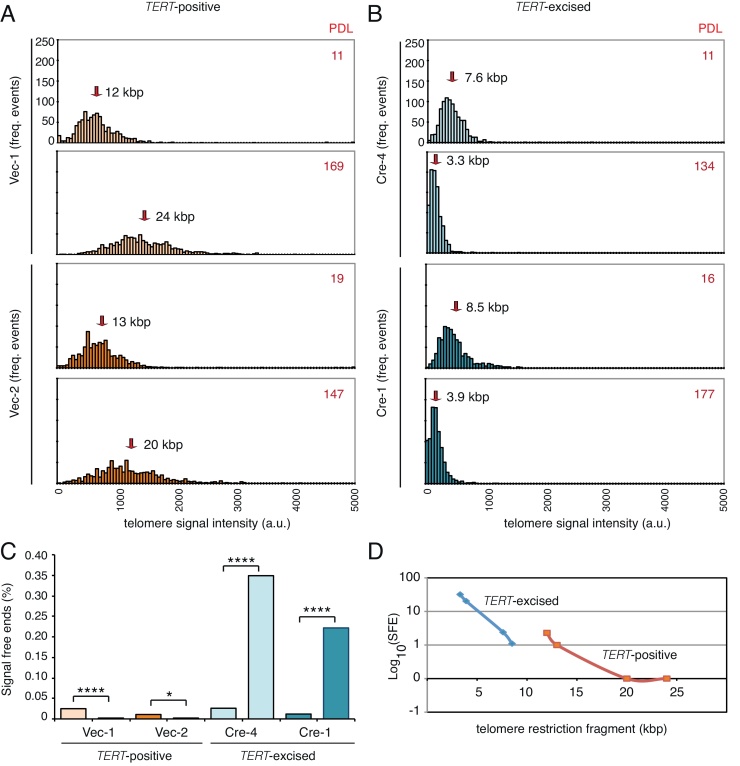
Fig. 1.
Telomere length and integrity in human tumour cell lines of varying telomere lengths and TERT status. Four independent clones from the same parental line in which (A) TERT was not excised and cells remained telomerase-positive (TERT-positive; Vec-1, Vec-2) or (B) TERT was excised and cells became telomerase-negative (TERT-excised; Cre-1, Cre-4), were propagated for the population doubling levels indicated (PDL, in red), and quantified for telomere signal intensity using Q-FISH. y-Axis, frequency of events; x-axis, telomere signal intensity in arbitrary units; each tick represents events across 50 a.u. (each 1000 a.u. marked as indicated). The scales for all graphs are equivalent; the y-axis labels are shown only for the top two graphs. Red arrows indicate average telomere length (see Section 2). (C) The percentage of chromosome ends lacking a telomere signal in metaphase preparations (as in A and B). y-Axis, percentage of telomere signal-free ends (total: 920 per sample); x-axis, labels as in (A), with earliest PDL at left. One representative experiment (of n = 3) shown where all samples were analyzed under identical conditions using an automated Metafer system. *p < 0.05, ****p < 0.001 (Fisher's exact test). (D) The incidence of telomere signal-free ends (total n = 920 for all samples) as a function of telomere length. y-Axis, log10 of the incidence of signal-free ends; x-axis, average telomere length in kbp. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Results
3.1. Prolonged TERT expression and its effect upon telomere length and integrity
In a previous study, we generated and characterized human tumour cell lines derived from a human embryonic kidney parental line containing the SV40 early region and oncogenic Ras, that either expressed exogenous TERT, possessed telomerase activity, and underwent telomere elongation (TERT-positive) or had TERT excised, lost telomerase activity, and underwent telomere erosion (TERT-excised) [17]. TERT-excised cells remained viable and capable of tumour formation for prolonged periods, and succumbed to apoptosis only upon significant accumulation of telomere signal-free ends [17]. In this study, we separately propagated these cell lines with or without TERT, in order to enable an assessment of relative DNA damage sensitivity of clonal isolates that were derived from the same parental line. In TERT-positive tumour cells between population doubling levels of 11 and 169, the mean telomere length continuously increased from 12 kbp to 24 kbp (Fig. 1A). Telomere elongation was assessed in two independently derived TERT-positive clones and telomere length increased by 54–76 bp per PDL (Fig. 1A and B, data not shown). In two other clonal lines, TERT was excised and the rate of telomere erosion ranged between 35 and 38 bp per PDL (Fig. 1A and B, data not shown). As expected, the frequency of telomere signal-free ends correlated positively with the acquisition of very short telomeres (Fig. 1C and D). TERT-positive cells, on the other hand, exhibited a statistically significant decrease and eventual elimination in the incidence of SFE as average telomere lengths increased (Fig. 1C and D). TERT-positive cells at late passages also did not exhibit evidence of extensive telomere trimming, as judged by the absence of accumulation of shorter telomeres (Fig. 1A: e.g. compare Q-FISH profiles in Vec-1 line at PDL 11 and 169).
3.2. The impact of telomere elongation upon survival after gamma irradiation
We tested the impact of telomere length on the response to ionizing radiation in two independently generated TERT-positive and TERT-excised cell populations. Without irradiation, all cell types exhibited an equivalent ability to form colonies regardless of telomere length or telomerase status (Fig. 2A, data not shown). Also as expected, at high irradiation doses (8–10 Gy) all cell types underwent significant cell death, with a low or zero survival fraction (SF) (Fig. 2A and B). After exposure to intermediate dosages of irradiation (2–6 Gy), TERT-excised cell populations with short telomeres exhibited a significant and dosage-dependent decline in SF relative to the same cell line with longer telomeres (Fig. 2A and B, right; compare blue lines at increasing PDL). The decreased survival of cells with short telomeres after exposure to gamma radiation is in keeping with previous studies showing that short telomeres and DNA damaging agents act synergistically to induce apoptosis [18–25]. However, cell populations with telomeres over 17 kbp in length also demonstrated a statistically significant decrease in SF at intermediate doses of gamma irradiation (Fig. 2A and B, left; compare orange lines at increasing PDL). Specifically, two independently derived TERT-positive cell clones with TRF lengths of approximately 24 or 20 kbp (Fig. 2A; 153 and 146 PDL, respectively) exhibited a significant decrease in SF after exposure to 4 or 6 Gy irradiation, compared with the same clones with TRF lengths of 12 or 13 kbp (Fig. 2A; 11 and 17 PDL, respectively). This observation was reproduced in another independent experimental series, where TERT-positive populations (PDL 58, 17 kbp; or PDL 142, 20 kbp) exhibited a statistically significant decrease in SF at 4 Gy or 6 Gy compared to the same population with shorter telomeres (PDL 15, 13 kbp) (Fig. 2B). This difference did not appear to be a function of the inherent ability to respond to DNA damage, since we observed similar levels of 53BP1 and γ-H2AX foci in untreated cells and in foci induction after one hour of exposure to 10 Gy in all TERT-positive lines, regardless of telomere length (data not shown). A plot of survival fraction against average telomere length demonstrated that average telomere lengths of >12 kbp or <17 kbp yielded a maximal SF across a range of sub-lethal irradiation doses using two independently derived datasets (Fig. 2C and D derived from data in Fig. 2A and B, respectively). This data demonstrates that an optimal range of telomere lengths was associated with an increased resistance to irradiation.
4. Discussion and conclusions
The mechanisms that lead to an increased irradiation sensitivity of cells with very long telomeres may be different than cells with very short telomeres. In primary cells where telomeres are critically short and thus possess signal-free ends, the presence of telomerase activity – accompanied by telomere elongation – rescues cells from the deleterious effects of ionizing radiation [26]. Similarly, the reactivation of telomerase and telomere elongation in a murine cancer model with critically short telomeres leads to rapid tumour progression [27,28], and TERT promoter mutations have been identified in melanoma and other aggressive human cancer types (reviewed in [29,30]). In these instances, telomere elongation occurs in a context where telomeres are initially short, and thus telomerase induction may permit SFE repair. However, in TERT-positive tumour cells with telomeres exceeding 17 kbp, there were no detectable SFE prior to irradiation, suggesting that the sensitizing mechanism(s) are SFE-independent. This observation is also distinct from telomere damage induced by oxidative stress, which is cumulative across increasing telomere lengths (6–9 kbp) despite the fact that sensitivity to ionizing radiation remained constant [26].
Further study is necessary to determine the exact mechanisms that lead to the irradiation vulnerability of cells with long telomeres. In the absence of DNA damage, TERT-positive cells with very long telomeres did not exhibit a difference in clonogenic survival compared with TERT-positive cells with shorter telomeres (Fig. 2, data not shown), and we showed previously that tumour formation in a xenograft model was comparable regardless of telomere length [17]. ALT cells are telomerase-negative, tumour-forming cells with very long telomeres, yet they too are sensitive to DNA damage [31], and ALT-like characteristics have been observed in normal cells with long telomeres [10,32,33]. Possible explanations for the problematic nature of long telomeres after irradiation are an increase in stalled forks or DSBs within the telomeric tract after DNA damage, the inherent irreparability of telomeric DNA that might be accentuated after DNA damage [34,35], or the potential for limiting availability of factors that protect telomeres from a DDR (e.g. TRF2) [36] or that promote replication fork restart, C-strand fill-in, and the repair of single-stranded DNA, such as CST (CTC1, STN1, TEN1) [37–41]. Indeed, replication stress and a deficiency in DSB repair has been cited as an explanation for the high rate of telomere loss observed in human cancer cells [4]; these deficiencies may become insurmountable in cancer cells with very long telomeres that are burdened with a significant induction of DSBs.
The deleterious effects of long telomeres on cell growth and DNA damage resistance has also been documented in other organisms such as Kluyveromyces lactis, Tetrahymena thermophila, and Arabidopsis thaliana, although in these instances there were concomitant mutations in genes encoding the telomerase RNA or the DNA repair proteins Ku70 or Mre11, which may complicate the direct relationship between the phenotype and the long telomeres themselves [42–48]. Our data suggest that very long telomeres may also be deleterious to human cancer cells when subjected to irradiation. It will be interesting to determine the mechanisms that increase the irradiation sensitivity of cells with long telomeres, and whether this is a general feature of human cancers.
Conflict of interest statement
The authors declare no conflicts of interest.
Acknowledgements
This research was funded by the Wellcome Trust (WT84637). We thank Dean Betts, Yie Liu, Dan Nussey, Carolyn Price, Mike Tyers, and members of the Harrington lab for constructive discussions and suggestions. We dedicate this work to the late Dr. Michael Taboski.
References
- 1.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Harley C.B. Telomerase and cancer therapeutics. Nat. Rev. Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 3.Artandi S.E., DePinho R.A. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murnane J.P. Telomere dysfunction and chromosome instability. Mutat. Res. 2012;730:28–36. doi: 10.1016/j.mrfmmm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lange T., Shiue L., Myers M.R., Cox D.R., Naylor S.L., Killery A.M., Varmus H.E. Structure and variability of human chromosome ends. Mol. Cell. Biol. 1990;10:518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu L., Zhang C., Zhu G., Irwin M., Risch H., Menato G., Mitidieri M., Katsaros D., Yu H. Telomerase expression and telomere length in breast cancer and their associations with adjuvant treatment and disease outcome. Breast Cancer Res. 2011;13:R56. doi: 10.1186/bcr2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington L. Haploinsufficiency and telomere length homeostasis. Mutat. Res. 2012;730:37–42. doi: 10.1016/j.mrfmmm.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Compton S.A., Choi J.H., Cesare A.J., Ozgur S., Griffith J.D. Xrcc3 and Nbs1 are required for the production of extrachromosomal telomeric circles in human alternative lengthening of telomere cells. Cancer Res. 2007;67:1513–1519. doi: 10.1158/0008-5472.CAN-06-3672. [DOI] [PubMed] [Google Scholar]
- 9.Oganesian L., Karlseder J. Mammalian 5′ C-rich telomeric overhangs are a mark of recombination-dependent telomere maintenance. Mol. Cell. 2011;42:224–236. doi: 10.1016/j.molcel.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickett H.A., Henson J.D., Au A.Y., Neumann A.A., Reddel R.R. Normal mammalian cells negatively regulate telomere length by telomere trimming. Hum. Mol. Genet. 2011;20:4684–4692. doi: 10.1093/hmg/ddr402. [DOI] [PubMed] [Google Scholar]
- 11.Wang R.C., Smogorzewska A., Lange de T. Homologous recombination generates T-loop-sized deletions at human telomeres. Cell. 2004;119:355–368. doi: 10.1016/j.cell.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Watson J.M., Shippen D.E. Telomere rapid deletion regulates telomere length in Arabidopsis thaliana. Mol. Cell. Biol. 2007;27:1706–1715. doi: 10.1128/MCB.02059-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lustig A.J. Clues to catastrophic telomere loss in mammals from yeast telomere rapid deletion. Nat. Rev. Genet. 2003;4:916–923. doi: 10.1038/nrg1207. [DOI] [PubMed] [Google Scholar]
- 14.Bhattacharyya M.K., Lustig A.J. Telomere dynamics in genome stability. Trends Biochem. Sci. 2006;31:114–122. doi: 10.1016/j.tibs.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Taboski M.A., Sealey D.C., Dorrens J., Tayade C., Betts D.H., Harrington L. Long telomeres bypass the requirement for telomere maintenance in human tumorigenesis. Cell Rep. 2012;1:91–98. doi: 10.1016/j.celrep.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyd M., Ross S.C., Dorrens J., Fullerton N.E., Tan K.W., Zalutsky M.R., Mairs R.J. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and auger electron-emitting radionuclides. J. Nucl. Med. 2006;47:1007–1015. [PubMed] [Google Scholar]
- 17.Taboski M.A.S., Sealey D.C.F., Dorrens J., Tayade C., Betts D.H., Harrington L. Long telomeres bypass the requirement for long telomeres in human tumorigenesis. Cell Rep. 2012;1:91–98. doi: 10.1016/j.celrep.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraemer K., Fuessel S., Kotzsch M., Ning S., Schmidt U., Wirth M.P., Meye A. Chemosensitization of bladder cancer cell lines by human telomerase reverse transcriptase antisense treatment. J. Urol. 2004;172:2023–2028. doi: 10.1097/01.ju.0000138157.46464.6e. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z., Koeneman K.S., Corey D.R. Consequences of telomerase inhibition and combination treatments for the proliferation of cancer cells. Cancer Res. 2003;63:5917–5925. [PubMed] [Google Scholar]
- 20.Cerone M.A., Londono-Vallejo J.A., Autexier C. Telomerase inhibition enhances the response to anticancer drug treatment in human breast cancer cells. Mol. Cancer Ther. 2006;5:1669–1675. doi: 10.1158/1535-7163.MCT-06-0033. [DOI] [PubMed] [Google Scholar]
- 21.Tauchi T., Ohyashiki J.H., Ohyashiki K. Telomerase inhibition combined with other chemotherapeutic reagents to enhance anti-cancer effect. Methods Mol. Biol. 2007;405:181–189. doi: 10.1007/978-1-60327-070-0_14. [DOI] [PubMed] [Google Scholar]
- 22.Misawa M., Tauchi T., Sashida G., Nakajima A., Abe K., Ohyashiki J.H., Ohyashiki K. Inhibition of human telomerase enhances the effect of chemotherapeutic agents in lung cancer cells. Int. J. Oncol. 2002;21:1087–1092. [PubMed] [Google Scholar]
- 23.Poynter K.R., Sachs P.C., Bright A.T., Breed M.S., Nguyen B.N., Elmore L.W., Holt S.E. Genetic inhibition of telomerase results in sensitization and recovery of breast tumor cells. Mol. Cancer Ther. 2009;8:1319–1327. doi: 10.1158/1535-7163.MCT-08-0849. [DOI] [PubMed] [Google Scholar]
- 24.Dong X., Liu A., Zer C., Feng J., Zhen Z., Yang M., Zhong L. siRNA inhibition of telomerase enhances the anti-cancer effect of doxorubicin in breast cancer cells. BMC Cancer. 2009;9:133. doi: 10.1186/1471-2407-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djojosubroto M.W., Chin A.C., Go N., Schaetzlein S., Manns M.P., Gryaznov S., Harley C.B., Rudolph K.L. Telomerase antagonists GRN163 and GRN163L inhibit tumor growth and increase chemosensitivity of human hepatoma. Hepatology. 2005;42:1127–1136. doi: 10.1002/hep.20822. [DOI] [PubMed] [Google Scholar]
- 26.Rubio M.A., Davalos A.R., Campisi J. Telomere length mediates the effects of telomerase on the cellular response to genotoxic stress. Exp. Cell Res. 2004;298:17–27. doi: 10.1016/j.yexcr.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Ding Z., Wu C.J., Jaskelioff M., Ivanova E., Kost-Alimova M., Protopopov A., Chu G.C., Wang G., Lu X., Labrot E.S., Hu J., Wang W., Xiao Y., Zhang H., Zhang J., Zhang J., Gan B., Perry S.R., Jiang S., Li L., Horner J.W., Wang Y.A., Chin L., DePinho R.A. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell. 2012;148:896–907. doi: 10.1016/j.cell.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu J., Hwang S.S., Liesa M., Gan B., Sahin E., Jaskelioff M., Ding Z., Ying H., Boutin A.T., Zhang H., Johnson S., Ivanova E., Kost-Alimova M., Protopopov A., Wang Y.A., Shirihai O.S., Chin L., DePinho R.A. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell. 2012;148:651–663. doi: 10.1016/j.cell.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patton E.E., Harrington L. Cancer: trouble upstream. Nature. 2013;495:320–321. doi: 10.1038/495320a. [DOI] [PubMed] [Google Scholar]
- 30.Heidenreich B., Rachakonda P.S., Hemminki K., Kumar R. TERT promoter mutations in cancer development. Curr. Opin. Genet. Dev. 2014;24:30–37. doi: 10.1016/j.gde.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Lovejoy C.A., Li W., Reisenweber S., Thongthip S., Bruno J., de Lange T., De S., Petrini J.H., Sung P.A., Jasin M., Rosenbluh J., Zwang Y., Weir B.A., Hatton C., Ivanova E., Macconaill L., Hanna M., Hahn W.C., Lue N.F., Reddel R.R., Jiao Y., Kinzler K., Vogelstein B., Papadopoulos N., Meeker A.K. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 2012;8:e1002772. doi: 10.1371/journal.pgen.1002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neumann A.A., Watson C.M., Noble J.R., Pickett H.A., Tam P.P.L., Reddel R.R. Alternative lengthening of telomeres in normal mammalian somatic cells. Genes Dev. 2013;27:18–23. doi: 10.1101/gad.205062.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickett H.A., Cesare A.J., Johnston R.L., Neumann A.A., Reddel R.R. Control of telomere length by a trimming mechanism that involves generation of t-circles. EMBO J. 2009;28:799–809. doi: 10.1038/emboj.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fumagalli M., Rossiello F., Clerici M., Barozzi S., Cittaro D., Kaplunov J.M., Bucci G., Dobreva M., Matti V., Beausejour C.M., Herbig U., Longhese M.P., d’Adda di F., Fagagna Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat. Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hewitt G., Jurk D., Marques F.D., Correia-Melo C., Hardy T., Gackowska A., Anderson R., Taschuk M., Mann J., Passos J.F. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat. Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takai K.K., Hooper S., Blackwood S., Gandhi R., Lange de T. In vivo stoichiometry of shelterin components. J. Biol. Chem. 2010;285:1457–1467. doi: 10.1074/jbc.M109.038026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart J.A., Wang F., Chaiken M.F., Kasbek C., Chastain P.D., 2nd, Wright W.E., Price C.M. Human CST promotes telomere duplex replication and general replication restart after fork stalling. EMBO J. 2012;31:3537–3549. doi: 10.1038/emboj.2012.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang F., Stewart J.A., Kasbek C., Zhao Y., Wright W.E., Price C.M. Human CST has independent functions during telomere duplex replication and C-strand fill-in. Cell Rep. 2012;2:1096–1103. doi: 10.1016/j.celrep.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L.Y., Lingner J. CST for the grand finale of telomere replication. Nucleus. 2013;4:277–282. doi: 10.4161/nucl.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L.Y., Redon S., Lingner J. The human CST complex is a terminator of telomerase activity. Nature. 2012;488:540–544. doi: 10.1038/nature11269. [DOI] [PubMed] [Google Scholar]
- 41.Gao H., Cervantes R.B., Mandell E.K., Otero J.H., Lundblad V. RPA-like proteins mediate yeast telomere function. Nat. Struct. Mol. Biol. 2007;14:208–214. doi: 10.1038/nsmb1205. [DOI] [PubMed] [Google Scholar]
- 42.McEachern M.J., Blackburn E.H. Runaway telomere elongation caused by telomerase RNA gene mutations. Nature. 1995;376:403–409. doi: 10.1038/376403a0. [DOI] [PubMed] [Google Scholar]
- 43.Yu G.L., Bradley J.D., Attardi L.D., Blackburn E.H. In vivo alteration of telomere sequences and senescence caused by mutated tetrahymena telomerase RNAs. Nature. 1990;344:126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- 44.Bundock P., Hooykaas P. Severe developmental defects, hypersensitivity to DNA-damaging agents, and lengthened telomeres in Arabidopsis MRE11 mutants. Plant Cell. 2002;14:2451–2462. doi: 10.1105/tpc.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bundock P., van Attikum H., Hooykaas P. Increased telomere length and hypersensitivity to DNA damaging agents in an Arabidopsis KU70 mutant. Nucleic Acids Res. 2002;30:3395–3400. doi: 10.1093/nar/gkf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riha K., Watson J.M., Parkey J., Shippen D.E. Telomere length deregulation and enhanced sensitivity to genotoxic stress in Arabidopsis mutants deficient in Ku70. EMBO J. 2002;21:2819–2826. doi: 10.1093/emboj/21.11.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akimcheva S., Zellinger B., Riha K. Genome stability in Arabidopsis cells exhibiting alternative lengthening of telomeres. Cytogenet Genome Res. 2008;122:388–395. doi: 10.1159/000167827. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed S., Sheng H., Niu L., Henderson E. Tetrahymena mutants with short telomeres. Genetics. 1998;150:643–650. doi: 10.1093/genetics/150.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]