Abstract
Similarities between the pathologic progression of cancer and the physiologic process of placentation (e.g., proliferation, invasion, and local/systemic tolerance) have been recognized for many years. Sex hormones such as human chorionic gonadotropin, estrogens, progesterone, and others contribute to induction of immunologic tolerance at the beginning of gestation. Sex hormones have been shown to play contributory roles in the growth of cancers such as breast, prostrate, endometrial and ovarian cancer, but their involvement as putative mediators of the immunologic escape of cancer are still being elucidated. Herein, we compare the emerging mechanism by which sex hormones modulate systemic immunity in pregnancy and their potentially similar role in cancer. To do this, we conducted a PubMed search using combinations of the following key words: immune regulation, sex hormones, pregnancy, melanoma and cancer. We did not limit our search to specific publication dates. Mimicking the maternal immune response to pregnancy, especially in late gestation, might aid in design of better therapies to reconstitute endogenous anti-tumor immunity and improve survival.
Keywords: Melanoma, Obstetrics, Endocrinology
INTRODUCTION
In 1948, Beard and Krebs acknowledged a striking similarity between a trophoblast and a tumor, publishing their observation titled “The Unitarian or Trophoblastic Thesis of Cancer.”1,2 Since then, these similarities have been extensively studied; many shared pathways and immunologic mediators have been identified.3,4 The purpose of this review is to take an in-depth look at existing research describing the role of sex hormones in the potentially parallel settings of reproductive and tumor immunology, with a focus on metastatic melanoma. Though imperfectly understood, sex hormones are important regulators of the immune system in both pregnancy and cancer.5,6 It is clear that they are involved in regulation and modifications of the immune system to allow invasion, proliferation and migration of tumor cells and trophoblasts.4 It is possible that an organ system-level view of the process of placentation as well as melanoma progression could yield additional insights into potential therapeutic targets for hormone-based immune modulation.7
The complexities and redundancies involved in orchestration of the maternal response to pregnancy as well as the host response to cancer are increasingly appreciated.3,8 Importantly, however, we and others have observed that neither pregnancy nor advanced cancers are static immunologic events.9–11 Oscillations in systemic immunity between inflammation and tolerance seen in patients with metastatic melanoma have been documented and seem to follow a biologically predictable pattern. When tolerance seen in malignant melanoma is disrupted and brought back to a state of inflammation, patients have a much better prognosis than those whose immune systems stay in an immunologically exhausted state. Pregnancy is also characterized by many hormonal fluctuations, although the time scale is for these hormonal and immunologic changes may be measured in weeks as opposed to days.12 (figure 1) While it seems intuitive to consider the involvement of sex hormones interacting with the maternal immune system during pregnancy, it is less obvious but just as possible that such hormones alter systemic immunity in the setting of cancers such as melanoma. We describe the observations and experimental evidence supporting such involvement in the following paragraphs.
Figure 1.
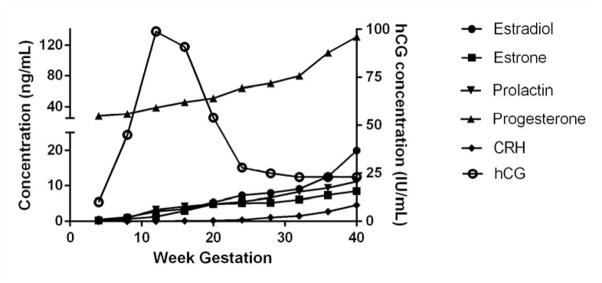
Hormonal changes in week of hormones important for the regulation of gestation in healthy pregnant women. CRH = corticotropin releasing hormone; hCG = human chorionic gonadotropin.
CLINICAL EVIDENCE OF HORMONAL REGULATION OF MELANOMA
The skin is capable of producing many neuroendocrine mediators such as melanin, steroids, thyroid hormones and sex hormones such as androgen, estrogen and progestin in order to maintain homeostasis; any failure to communicate between the skin, endocrine and immune system could result in deregulation and disease.13,14 Both melanocytes and melanoma tumors produce pigment in the melanosome that protects the skin against damaging ultraviolet rays through positive regulation by hormone like L-tyrosine and L-dihydroxyphenylalanine.15,16 Although the interplay between sex hormones and the immune system in melanoma remains poorly understood, several clinical observations support the role of sex hormones in melanoma development. Melanomas that are responsive to estrogens are associated with the superficial spreading melanoma subtype, a type of tumor with a much better prognosis. Additionally, estrogen exerts a proliferative effect on melanocytes and can lead to the development of hyperpigmentation in women using oral contraceptives (OC) or hormonal replacement therapy (HRT).17 Whether hormonal contraceptives increase the risk of melanoma is a matter of ongoing debate. Koomen et al has reported that high estrogens increase a woman’s risk for developing malignant melanoma,18 while Lens and Bataille have not observed a significant association.19 It may be no coincidence that melanoma is the most common form of cancer associated with pregnancy.20 This is believed to be due to the trophoblast’s increased need for lymphangiogenesis, which the melanoma then uses to promote its own growth. Complementary to this hypothesis, demographics and incidence may also provide an explanation as to this phenomenon. Additionally, pregnant women are more likely to be diagnosed with an invasive melanoma than their non-pregnant counterparts.
We have shown that aging in healthy subjects is associated with a Th2 bias.21 Women are more likely than men to develop melanoma before age 40, after which diagnosis of melanoma is observed at a much higher rate in males.22 However, females diagnosed with melanoma have a better prognosis than males, and premenopausal women have higher survival rates compared to postmenopausal women.23,24 Interestingly, melanoma metastasizes at a much slower rate in women compared to men, and the pattern of metastatic spread is also different, with more loco-regional recurrences observed in women.25,26 Still, reasons behind these sex differences have yet to be elucidated. With an extensive amount of emerging data demonstrating the importance of sex hormones on immune function, in the remainder of the review we will explore the mechanisms behind endocrine sex hormone mediated immunomodulation. First, we will highlight the potential involvement of hormones in tumor promotion, both via tolerance induction as well as chronic inflammation and angiogenesis. Next, we will describe hormones that may help improve anti-tumor immunity. Finally, we briefly discuss sexual dimorphism in immune responses, which may have implications for the development of personalized immunotherapy. A better understanding of immunological switches that control tolerance, immune activation, and immune reconstitution, of which all can be studied using the different phases of pregnancy as a model, could result in novel immunologic treatment strategies for melanoma and other malignancies.
PUTATIVE PRO-TUMOR/IMMUNE SUPPRESSIVE HORMONES: HUMAN CHRONIC GONADOTROPIN, PROGESTERONE, PLACENTAL GROWTH FACTOR, AND RELAXIN
Human chronic gonadotropin
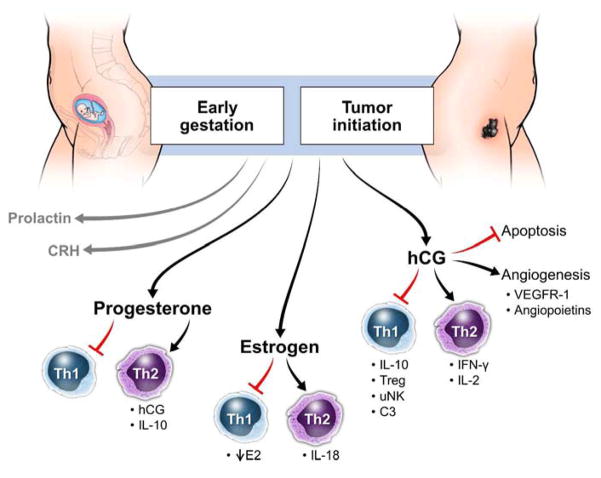
Soon after fertilization, the embryonic blastocyst begins secreting human chronic gonadotropin (hCG). HCG is a glycoprotein hormone primarily produced by trophoblasts, which promotes many processes including implantation, recognition, differentiation, angiogenesis and fetal-maternal homeostasis.27 (figure 2) Levels of hCG continue to rise until the 11th week of gestation and then slowly decreases through the remainder of the pregnancy. The main purpose of hCG in pregnancy is to prevent degradation of the corpus luteum and stimulate progesterone production.28 HCG also plays an important role in promoting immune suppression in the decidua by preventing maternal macrophage phagocytosis to the invading trophoblast in order to establish immune tolerance.29 The early trophoblast promotes fetal tolerance by secreting hCG which acts as a powerful chemoattractant for T regulatory cells (Treg) to migrate to the placenta after fertilization.30 Treg cells play an important role in maintaining self-tolerance and modulating tolerance to non-self antigen, such as those displayed by the fetus. Expression of complement component 3 (C3) was found to be upregulated by hCG on stromal cells of the baboon endometrium post ovulation, suggesting hCG is also able to modulate the decidual environment during the preimplantation stage.31,32
Figure 2.
Effects of sex hormones during early pregnancy to promote implantation and fetal tolerance and the proposed role in initiation of cancer. CRH = corticotropin releasing hormone; E2 = estradiol; hCG = human chorionic gonadotropin; IFN = interferon; IL = interleukin; uNK = uterine natural killer cell; VEGFR = vascular endothelial growth factor receptor.
HCG also regulates uterine natural killer (uNK) cells.33 This uNK subset makes up approximately 70% of the lymphocyte population in the endometrium and plays an important role in maintaining and regulating the uterine spiral arteries during the first trimester.34 UNKs stimulate decidual monocytes to secrete IFN-γ promoting Treg proliferation through indoleamine 2,3-Dioxygenase (IDO) and transforming growth factor beta (TGF-β).35 Interestingly, we have found uNK cells in the blood of patients with stage IV melanoma and they also positively correlated with TGF-β levels in plasma.36 HCG drives a systemic response during pregnancy. Before in vitro fertilization (IVF), women given hCG had increased levels of anti-inflammatory IL-27 and IL-10, reduced levels of pro-inflammatory IL-17, which resulted in elevated Tregs and a more receptive uterine wall for implantation.37 They also notice that hCG affected the maternal adaptive immune system, promoting a Th2 differentiated state by activating T cells that produce IL-4 while inhibiting T cells that secrete IFN-γ.37
Additionally, the formation of new blood vessels is also driven by hCG which acts upon pro-angiogenic molecules such as vascular endothelial growth factor receptor (VEGFR)-1 and angiopoietins.38 Soluble VEGF-C aids in immune tolerance by suppressing the cytotoxic activity of uNK cells at the fetal-maternal interface.39 HCG enhances VEGF production by endometrial cells through paracrine feedback, further promoting blood vessel formation to the fetus.40 IL-10, an inhibitor of inflammatory cytokines which is found at high levels near the beginning of pregnancy, induces the trophoblast to produce VEGF-C and stimulate placental angiogenesis.41 Also, endocrine gland-derived vascular endothelial growth factor (ED-VEGF) has been implicated as a negative regulator of trophoblast invasion in the placenta, as high levels of ED-VEGF are seen in preeclampsia, and has recently been hypothesized to be regulated by hCG.42 HCG prevents apoptosis of endometrial cells though the Fas-FasL pathway, which in turn drives maternal tolerance during early pregnancy.43
HCG plays very similar roles in the invasion and progression of cancer (melanoma). The hCG receptor was first discovered in trophoblastic neoplasms, which suggested that it has an important role in regulating growth and invasion in not only in pregnancy, but in cancer as well.44 Several studies have found that many cancers, including bladder cancer, cervical cancer, lung cancer, pancreatic cancer and colorectal cancer can be diagnosed by high levels of hCG in the serum.45,46 HCG mRNA has also been expressed in tumor cells from patients diagnosed with malignant melanoma and has been suggested for use as a biomarker for disease.47 Others have shown that up to 60% of active neoplasia will express high levels of hCG in serum.48 Antibodies against hCG have been detected during malignancy, but hCG levels are so high that the effect of these antibodies seems to be minimal, therefore allowing hCG to act as an autocrine growth factor to promote malignancy.49 Tumor secretion of hCG prevents apoptosis, allowing the cancer to become more resistant and aggressive.50 As a potent angiogenic factor, hCG secreted by the tumor stimulates sprout formation through vasodilation, maturation and increased vessel permeability, thus promoting tumor growth.51
Progesterone
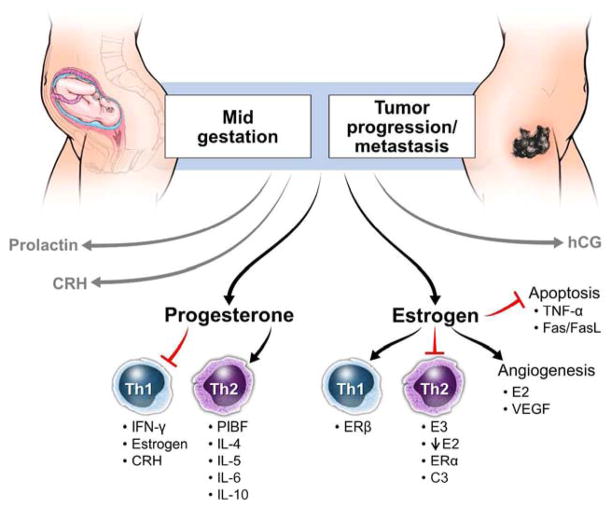
Initial progesterone production in pregnancy is induced by hCG. Progesterone secretion by the placenta continues to increase throughout pregnancy, decreasing approximately 4 weeks before labor onset. Progesterone is a potent immunomodulator that establishes Th2 bias in pregnancy by reducing the production of pro-inflammatory cytokines by macrophages in response to infectious stimuli, and altering cytokine secretion of T cell subsets towards IL-10 production.52 (figure 2 and 3) Further, it induces secretion of chemokines such as CXCL10, CX3CL1 and CCL2 that localize Th2 biased immune cells to the placenta and upregulates non-classical HLA-G.53 Interestingly, most progesterone receptor (PR) expressing decidual T cells express a γ/δ T cell receptor (TCR).54 This limits the number of ligands the TCR can recognize which provides protection to the growing fetus. These T cells are also thought to be able to identify antigens presented by trophoblasts.55 The progesterone receptor (PR) comes in two main isoforms, PR-A and PR-B, which compete with one another for progesterone binding.56 PR-B is expressed on myometrial cells during the majority of pregnancy and inhibits expression of pro-inflammatory genes; however, PR-A is expressed during labor and it in turn promotes pro-inflammatory gene activation.57 PR-A overexpression at the end of pregnancy also promotes the activation of estrogen by increasing estrogen receptor alpha (ER-α) expression.58 A smaller, soluble PR isoform, PR-C, has been found to compete specifically with PR-B for progesterone binding, by binding directly to progesterone and inhibiting PR-B signaling near parturition, therefore, promoting labor associated myometrium changes and activating pro-inflammatory pathways.59
Figure 3.
Maintenance and regulation of tolerance by sex hormones during mid-pregnancy and the proposed role of hormones in progression/metastasis of cancer. CRH = corticotropin releasing hormone; C3 = complement component 3; ER = estrogen receptor; E2 = estradiol; E3 = estrone; hCG = human chorionic gonadotropin; IFN= interferon; IL = interleukin; PlBF = placental binding factor; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
The immune effects of progesterone are exerted by inhibition of pro-inflammatory transcription factor NF-κB through IκB.60 Progesterone effects are also mediated by progesterone-induced blocking factor (PIBF), which is expressed on lymphocytes in the decidua and is important in maintaining pregnancy. Women who suffer from spontaneous miscarriages or have high stress levels have been found to have low PIBF levels, which was associated with pregnancy complications.61 Natural killer (NK) cells in pregnancy are very sensitive to progesterone: low levels of progesterone are required to achieve inhibition, compared to 100 fold higher levels needed to achieve similar NK cell inhibition in non-pregnant individuals.62 In mice, progesterone activates a Th2 immune biased response by inhibiting maturation of dendritic cells, which would more readily initiate a Th1 response through the promotion of cytotoxic T cell expansion.63
In cancer, progesterone modulates immune responses by inhibiting T cell proliferation64 and IFN-γ expression65 while enhancing IL-4, IL-5, IL-6 and IL-10 production, and promoting a humoral response.66 Interestingly, human melanocytes can produce steroids de novo through the metabolism of progesterone or cholesterol.67–69 Progesterone has been shown to inhibit proliferation of human melanocytes by blocking estrogen effects.70 The progesterone receptor has been identified in the cytoplasm and nucleus of melanocytes by immunohistochemistry.71 Melanoma cells that do not express the PR were still found to be regulated in the presence of progesterone, but are modulated through signal transduction versus transcription.72 Progesterone induced blocking factor (PIBF) has been suggested to play a role in cell cycle regulation and Th2 biased immunity through the IL-4 receptor.73 PIBF mRNA is constitutively expressed in tumor cells and does not require the presence of the progesterone receptor, which provides a mechanism for cancer to escape anti-tumor immune responses.55 In vitro experiments using WM266-4 cells showed that progesterone, 17beta-estradiol and dihydrotestosterone given together can inhibit tumor growth through the down-regulation of IL-8, which is a potent cytokine that induces melanoma cell growth.74
Placental growth factor
We have previously described a VEGF-driven state of chronic inflammation in metastatic melanoma.75 Placental growth factor (PlGF), a VEGF homologue, may play a similar role in perpetuating chronic inflammation. PlGF plays a significant role in embryogenesis, promoting both angiogenesis and vascularization to the fetus during inflammation.76 It was discovered that Flt-1, a VEGF receptor, binds PlGF and mediates recruitment of monocytes.77{ Peripheral blood monocytes (PBM) treated with PlGF showed increased expression of pro-inflammatory cytokines IL-1β and TNF-α, and chemokines MCP-1, IL-8 and MIP-1β, suggesting PlGF plays an important role in inducing inflammation.78 Melanocytes and melanoma tumors are also known to secrete PlGF, which makes them weakly responsive to anti-VEGF therapy.79 However, when PlGF was neutralized, even in tumors resistant to anti-VEGF therapy, the tumor could no longer signal through VEGFR-1, inhibiting growth during a pre-clinical study.80 Therefore, it has been hypothesized that PlGF plays a significant role in allowing a tumor to become drug resistant.81,82 Further studies need to be conducted to fully understand PlGF’s role in inflammation and tumor progression.
Relaxin
The role of relaxin in pregnancy (induction of MMPs, ECM remodeling, labor), as well as induction of pro-inflammatory cytokines IL-6 and IL-8, has been well documented in rhesus monkeys.83 While relaxin has not been studied in melanoma to date, its role in carcinogenesis of breast and prostate cancer has been well established. Relaxin was implicated in tumor growth, cell invasion during pregnancy and likely tumor invasion in carcinogenesis as well as in angiogenesis by induction of VEGF.84–86 Whether relaxin plays a role in melanoma development remains to be elucidated.
PUTATIVE ANTI-TUMOR/PRO-INFLAMMATORY HORMONES: CORTICOTROPIN-RELEASING HORMONE, PROLACTIN AND VISFATIN
CRH
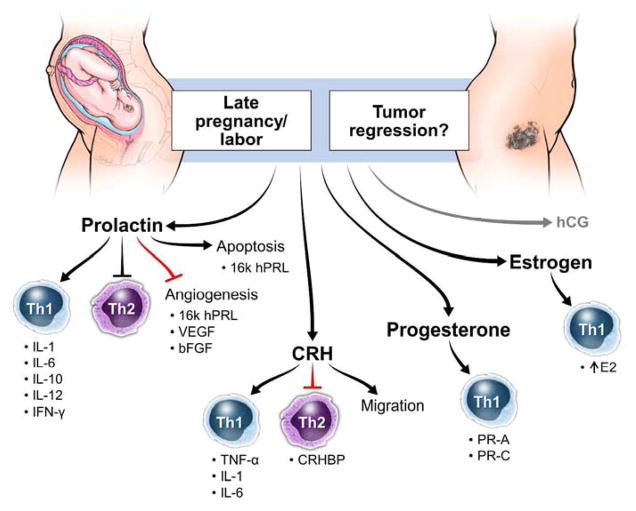
Corticotropin-releasing hormone (CRH) has been postulated to regulate the duration of gestation, with its levels being the highest during labor. (figure 4) CRH works directly on myometrial cells to facilitate the onset of labor, and it is tightly regulated by progesterone, estrogens, nitric oxide, IL-1β and tumor necrosis factor alpha (TNF-α).59 Corticotropin-releasing hormone binding protein (CRHBP) binds CRH and inactivates its ability to promote adrenocorticotropic hormone (ACTH) production.87 CRHBP levels decrease throughout pregnancy allowing high levels of CRH to accumulate and promote labor. Other actions of CRH include regulation of fetal blood flow, placental prostaglandin and cortisol production and uterine contractility.88
Figure 4.
Role of sex hormones in the promotion of inflammation and labor as a natural method for provoking regression of tumor in cancer patients. bFGF = basic fibroblast growth factor; CRH = corticotropin releasing hormone; CRHBP = corticotropin releasing hormone binding protein; E2 = estradiol; hCG = human chorionic gonadotropin; hPRL = human prolactin; IFN = interferon; IL= interleukin; PR = progesterone receptor; VEGF= vascular endothelial growth factor.
CRH regulates the hypothalamic pituitary adrenal (HPA) axis, which allows cells to respond to environmental stresses.89 Melanocytes are known to secrete CRH, which permits them to produce cortisol and ACTH to maintain skin homeostasis.90 In cell culture, melanoma cells treated with CRH migrated further during stress, which was determined to be mediated by the ERK1/2 pathway.91 Increases in the levels of CRH and proopiomelanocortin (POMC) are strongly associated with malignant melanoma.92 Another function of CRH in the skin is to act as a growth regulator by both promoting and suppressing cell proliferation. CRH receptor 1 (CRH-R1) controls the action of CRH and can promote cAMP and IP3 synthesis in dermal and epidermal cells.93 Melanomas exclusively express CRH-R1 which plays an important role in proliferation and has become a agonist target.94 In a B16 mouse melanoma model, daily injections with CRH were found to reduce tumor volume by 30–60% compared to control animals.95 Research shows that CRH promotes survival of melanocytes during starvation and prevents cell proliferation by inhibiting growth factor signaling through cAMP and IP3 second messengers.96 TNF-α, IL-1 and IL-6 stimulate HPA axis to produce CRH which drives a pro- inflammatory, Th1 biased response.97 When peripheral CRH acts on the HPA axis in the brain, it triggers a classical feedback mechanism that leads to the secretion of cortisol, a steroid hormone with anti-inflammatory effects.98 Thus, CRH is a potential modulator of a Th1 biased response and its role should be further studied in cancer.
Prolactin
Prolactin (PRL) is a polypeptide hormone secreted by the syncytiotrophoblast that reaches its highest levels during late pregnancy. (figure 4) PRL acts on corpus luteum cells in the ovary to stimulate secretion of progesterone to maintain pregnancy and on epithelial cells of the mammary gland to initiate milk production.99 The amount of PRL secreted is directly proportional to the size of the fetus and it functions to provide energy for the mother and nutrients for the fetus. Through lipolysis and anti-insulin effects of prolactin action, the maternal insulin level increases, providing free fatty acids and amino acids to the growing fetus.100 Prolactin functions to increase β-islet cell proliferation, inhibits apoptosis and causes β-islet cells to become more responsive to glucose.101 Glucocorticoid expression of Rasd1 near the end of gestation changes insulin secretion during pregnancy through the inhibitory effects of prolactin on Rasd1 transcription.102 Activation of dopamine neurons suppresses PRL secretion through placental lactogens.103 The N-terminal 16K prolactin fragment had been determined to stop proliferation and migration of vascular endothelial cells and cause cell cycle arrest resulting in apoptosis.104 High levels of 16K hPRL can be detected in serum and urine of women suffering from preeclampsia.105
In melanoma, PRL drives Th1 biased immunity through the secretion of IL-1, IL-6, IL-10, IL-12 and IFN-γ by NK cells and B lymphocytes,106,107 and plasma cell activation.108 It increases T cell proliferation109 and decreases B cell apoptosis.110 Through animal studies, prolactin’s anti-tumor effect has been shown to promote tumor specific macrophages through IFN-γ and IL-12, and CT26 tumor bearing mice injected with recombinant human PRL and IL-15 had enhanced cytotoxic activity to the tumor resulting in fewer lung metastasis and longer overall survival.111,112 However, PRL does not have an anti-tumor effect in all cancers. In breast cancer, high levels of prolactin are associated with a greater risk for developing cancerous ERα+ tumors and is associated with much poorer outcomes for patients with these increased levels.113 This is likely due to the fact that PRL promotes mammary tumorigenesis independent of cyclin D1 activation.114 This has not yet been observed in melanoma. Pro-inflammatory cytokines such as IL-1, IL-2 and IL-6 produced by both pituitary and extrapituitary cells have a stimulatory effect on PRL secretion, while IFN-γ inhibits its production.115 Many immune cells express the prolactin receptor (PRLR) including monocytes, macrophages, B and T cells, granulocytes and NK cells.116 Prolactin secretion from the anterior pituitary gland is inhibited by the dopamine D2 receptor (D2R), which is widely expressed on melanoma cells and plays a key role in inhibiting adenylyl cyclase, which is necessary for cellular signal transduction.117,118 PRL also enhances the effect of immune cells, including CD34+ stem cells, in the blood through upregulation of major histocompatibility complex (MHC) class II expression on antigen presenting cells, T cell clonal expansion, antibody production, increased cytotoxicity of NK cells and microbe killing of macrophages.119 In mouse melanoma models, 16K hPRL can block angiogenesis and inhibit tumor growth by activating NF-κB causing tumor infiltrating lymphocytes to access and destroy cancer cells.120 This PRL peptide endogenously blocks Notch signaling which greatly impairs the tumor’s ability to vascularize.121 16K hPRL has also been shown to block angiogenesis through inhibition of basic fibroblast growth factor and VEGF, making it a target for anti-tumor therapies.122 Thus, better understanding of how PRL can promote cell-mediated immunity could help researchers design better ways to initiate tumor destruction.
Visfatin
Previously known as pre-B-cell colony enhancing factor (PBEF) or nicotinamide phosphoribosyltransferase (Nampt), visfatin is a visceral fat cytokine with an important role in the promotion of inflammation.123–125 Visfatin levels are highest at the end of gestation, and promote the secretion of IL-1β, IL-6, IL-8, COX-2, TNF-α and prostaglandin E2.26,127 Interestingly, infection associated preterm labor is concomitant with elevated visfatin circulating in maternal plasma.128 These findings suggest visfatin could be important for immune resolution back to a Th1 state. However, the role of visfatin in melanoma is poorly understood. It appears that visfatin is more highly expressed in melanoma lesions compared to benign lesions.129 Moreover, it seems that visfatin may promote melanoma cell growth in vitro. Namely, a study using melanoma Me45 cell line showed that visfatin increased proliferation of these tumor cells.130 In another study, neutrophils have been found to synthesize visfatin in response to inflammatory stimulus and inhibit apoptosis in vitro and in sepsis patients.131 Thus, it remains possible that there could be an additional regulatory level of visfatin during pregnancy, which might be lost during tumorigenesis, and thereby promotes melanoma proliferation, but more studies need to be conducted to elucidate visfatin role in melanoma.
ESTROGEN: A DOUBLE-EDGED SWORD MODULATING TH1/TH2 IMMUNITY
Women have high levels of estrogen, which drop dramatically when they reach menopause. Estrogen modulates the immune response by inducing peripheral T cells to secrete pro-inflammatory cytokines IFN-γ and IL-2,132 but also promotes tolerance by inducing IL-10 secretion.133 (figure 3 and 4) Estradiol-17β (E2) at high concentrations induces a Th1 response while at low concentrations it biases the system toward immune tolerance.134 Pro-inflammatory responses are regulated by E2 through NF-κB. On the other hand, estrone (E3) is only detectable in pregnant women because it is produced by the placenta and fetus. E3 is important for reducing the production of pro-inflammatory cytokines in circulation by decreasing IκB degradation which inhibits NF-κB activation and apoptosis (figure 3).135 Estrogens work through interaction with the estrogen receptor (ER) to induce transcriptional regulation. Immune cells including dendritic cells (DCs), natural killer (NK) cells, macrophages and lymphocytes express estrogen receptors, signifying that this hormone modulates their function.136 Indeed, the differentiation of DCs is regulated by E2 acting upon ERα. E2 levels are the highest during the third trimester, when immature DCs are high and actively presenting fetal antigen to the mother’s immune system to begin labor.137 Complement component 3 (C3), a protein that plays a central role in inducing inflammation in innate immunity, is increased by estrogen in oviductal epithelial cells.138 Angiogenesis in the uterus is also driven by estrogen. VEGF mRNA levels increase in the endometrium in the presence of E2.139 This increases placental blood flow and vasodilation which are both characteristic of angiogenesis. Cytotrophoblasts, but not syncytiotrophoblasts, stimulate VEGF production directly and this production is correlated to the levels of E2 in the serum.140 Thus, estrogen stimulates VEGF production and blood vessel formation, which is essential for establishment and maintenance of the fetus during pregnancy.
Women at risk for familial breast cancer have increased risk for developing melanoma and vice versa, suggesting that estrogens can promote tumorigenesis.141 It has been known for many years that melanoma tumor cells express estrogen receptors.142 Estrogens affect lymphocytes by initiating the secretion of IL-10, IL-12 and IFN-γ and inhibition of TNF-α,143 stimulation of antibody production,144 and the reduction of macrophage and dendritic cell apoptosis.145,146 E2 has been proven to inhibit melanoma growth by obstructing receptor binding of IL-8.74 Chronic exposure to estrogens was suggested to increase NF-κB stimulation and induce pro-inflammatory responses by macrophages.147 It has been shown that acute and chronic inflammation leads to the upregulation of ERα, but not ERβ, thus impacting the effects of estrogens on T cells, as T cells express ERα, while B cells express ERβ.148 It was also reported that estrogen decreases apoptosis, as well as TNF-α production.149 E2 and E3 each have a strong affinity for binding ERα,150 which is why ERα is commonly associated with tumor promotion. Yet, ERβ has been found on malignant melanoma cells that are negative for ERα.151 Loss of ERβ expression correlated with increased invasiveness of the tumor,152 suggesting that loss of ERβ expression increases malignant transformation in melanoma. The most important prognostic factors in melanoma are tumor thickness (Breslow depth) and invasive level (Clark level); as each increase, the patient’s prognosis decreases.153 Interestingly, increased ERβ expression is not found on non-malignant cells surrounding the tumor, only on the tumor cells themselves.154 Another role of ERβ is regulation of monocyte apoptosis through the Fas/FasL signaling pathway.155 It has been shown that myeloid progenitor cells exposed to GM-CSF differentiate into immature dendritic cells through expression of ERα and increased levels of estrogens.156,157 Recently, 2–methoxyestradiol (2ME2), an estrogen derivative, has been determined to be a potent inhibitor of angiogenesis and melanoma growth in a mouse model.158 Taken together, this indicates that estrogens can both promote or hinder tumor growth and monitoring ER expression could help clinicians determine the patient prognosis to the disease.
SEXUAL DIMORPHISM IN RESPONSE TO HORMONES
In this review, we have discussed existing data supporting a role for sex hormones and their ability to manipulate the immune system in both pregnancy and melanoma. Beyond these observations, several studies have identified sexual dimorphism with respect to inflammatory responses in a variety of settings. For example, sex-based differences in vascular function have been described and show the development of early atherosclerotic lesions and plaques with increased production of inflammatory mediators (IL-10 and TNF-α) in women compared to men.159–161 That the state of pregnancy, and not a general state of tolerance in women, accounts for additional sex-based differences in immune function is supported by the fact that autoimmune diseases are more prevalent in women.162 Moreover, autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematous (SLE) are diagnosed more frequently in women and are strongly correlated with sex hormones.163,164 Interestingly, some autoimmune diseases such as arthritis remit with pregnancy, but SLE has been found to often worsen in severity during gestation.163 Women also exhibit enhanced innate immune responses to infections,165,166 not blunted ones, and survive episodes of severe sepsis to a greater degree than men.167 It is clear that sex plays a role in risk, severity and prognosis of many diseases, including cancer, and research in this area has just begun. It may one day become important to consider the sex of the cancer patient when personalizing cancer immunotherapies.
REVERSING TOLERANCE IN MELANOMA
In order to positively affect survival of patients with cancer, it appears critical to find a way to break immune tolerance to the malignancy. Insights into achieving this goal in metastatic melanoma may lie in well recognized abnormalities of pregnancy (e.g. spontaneous abortions). For example, serum taken from women suffering from recurrent spontaneous abortion have a cytokine profile indicative of a Th1 response compared to healthy pregnant women.168 To counter this, intravenous immunoglobin (IVIG) given to women, with reported previous recurrent miscarriages and/or suffering from recurrent miscarriages after IVF, every 3–4 weeks starting from conception or 24 hours before embryo transfer showed a decrease of natural killer cells and a implantation success rate of 92.5%,compared to 25% for women not receiving IVIG.169 Moreover, new immunotherapies for promoting tolerance to the fetus during gestation are being studied in animal models and could have important implications in melanoma. In mice, researchers have shown that blocking CD80 and CD86 enhances maternal tolerance decreasing implantation failure through the increase of Tregs and development of Th2 cells.170 The use of anti-CTLA4 antibody therapy with CD80/CD86 blockade has been found to regulate the Th2/Th1 balance in PBMCs isolated from women with recurrent spontaneous abortion, which lead to the design of an adenoviral cytotoxic T lymphocyte antigen 4 (CTLA4) antibody that showed improved pregnancy outcomes in a mouse model of spontaneous abortion.171,172
The opposite, immune activating effect is desired in metastatic melanoma. Recent FDA approval of the anti-CTLA4 antibody, ipilimumab, was developed to promote T cell activation and tumor destruction by promoting cytotoxic capacity of naturally occurring tumor specific T cells thereby overcoming, in part, their state of immune tolerance.173 Metastatic melanoma patients treated with ipilimumab given alone or in combination with a peptide vaccine (gp100) exhibited a median overall survival time (OS) of approximately 10 months, an improvement over the 6.4 month OS observed in patients receiving gp100 vaccine alone.174 As breakthrough as this drug is, it comes at a cost of significant immune related adverse events in up to 15% of patients.175 Another new and promising antibody targets program death 1 (PD-1), an additional receptor found on activated cells which is critical for immune regulation, has been shown to promote a proinflammatory environment by IFN-γ and IL-2 secretion.176 In clinical trials, this drug, following treatment with or without the anti-CTLA inhibitor, showed a high rate of sustained tumor regression and median overall survival of 11 months in patients with metastatic melanoma.177 This lead to administering both ipilimumab and nivolumab (anti-PD-1) concurrently, resulting in 53% of patients obtaining an 80% reduction in tumor volume.178 Unfortunately, the reported increase in objective response rate was paralleled with a similar increase in severe toxicity. Other promising immunotherapies for melanoma patients are also being studied. Anti-tumor immunity has been shown by utilizing an antibody to CCR4 a marker found on immune suppressive Treg cells, which when given to a T-cell leukemia-lymphoma patient resulted in a CD8+ T cell response to the tumor.179 HER2/neu, an antibody commonly used to treat breast cancers, has recently shown positive effect on a number of melanoma cell lines and xenograft models.180 Bevacizumab, an anti-VEGF antibody, given to metastatic melanoma patients in combination with albumin-bound paclitaxel and carboplatin, resulted in an increase of CD8+ lymphocytes but did not affect the Th1/Th2 ratio.181
Albeit promising, the clinical successes of present day immunotherapeutic strategies for metastatic cancer fail short of their preclinical results. Many share the belief that the reason for this discrepancy in clinical translation is the result of tumor driven immune tolerance of human cancer.182 Another reason for this discrepancy is that malignant melanocytes utilize melanogenesis, which is a normal metabolic process that generates a local immunosuppressive environment through POMC derived peptides and steroids.16 It was discovered that inhibiting melanogenesis increases the potency of the immune system and chemotherapy against tumors.183 Overcoming the tumor induced modulation of systemic immunity in order to recover the ability of endogenously generated immune cells to effectively destroy the malignancy will be a significant challenge. An interesting concept in pregnancy, which could lead to better understanding of malignancy, is the spontaneous return to cytotoxicity near the end of parturition.137 Near parturition, an unknown event results in the reactivation of Th1 maternal immunity and the initiation of labor (rejection). Characterizing this phenomenon, by further studying the role cytokines, cells and hormones play could translate into different methods for breaking tolerance in cancer patients, which could ultimately improve therapeutic efficacy and overall survival. The skin is a steroidogenic organ; it synthesizes its own steroids and sex hormones and can regulate local immune activities along with affecting the function of the epidermis.184 CRH has been found to block human melanoma cell proliferation in vitro96 and could provide additional therapeutic value when paired with a targeted agent. As progesterone promotes a tolerant state in pregnancy, treating a stable melanoma patient with progesterone was found to cause proliferation of dormant micrometastases by tipping the immune system back to a Th2 state.185 B7-H1 (PD-L1), is a molecule expressed on antigen presenting cells and contributes to tumor evasion and expansion of Tregs.186,187 In a B7-H1 knockout mouse model of melanoma, it was discovered that females had superior tumor growth resistance compared to males due to estrogen mediated suppression of Treg cells.188 Clinically, in a phase I study of advanced cancers, including melanoma, patients treated with PD-1 antibody showed a significant response rate (28%) which was durable in only those patients expressing PD-L1, making it a potential biomarker for anti-PD-1 treatment response.189 However, the authors did not compare men to women to look at potential differences due to sex. Altogether, in order to improve outcomes for melanoma patients treated with immunotherapeutics we must more completely understand the process of normal pregnancy immunoregulation, specifically the return to cytotoxicity (Th1 bias) at the end of gestation.
CONCLUSION
The dynamic maternal immune responses to normal pregnancy have evolved out of the need to support a semi-allogenic fetus over the duration of the pregnancy, without significant infectious or inflammatory impediment to mother . In turn, the maternal immune system is tightly regulated by hormone release and cytokine action to protect the developing fetus. Cancers, including melanoma, appear to induce similar tolerogenic immune programs through a variety of mechanisms, including paracrine secretion of sex hormones, to drive angiogenesis required for oxygen and nutrient supply, all while evading immune attack in a manner similar to the process of placentation. A better understanding of the molecular switches involved in the induction and reversal of immune tolerance in the setting of pregnancy may help identify new methods for targeted immune modulation for patients with melanoma.
Acknowledgments
This project was supported by the Office of Research in Women’s Health and the National Institute of Child Health and Human Development, Oregon BIRCWH Award Number 2K12HD043488-11 (SGH).
Abbreviations
- CRH
corticotrophin releasing hormone
- IFN-γ
interferon gamma
- IL
interleukin
- DC
dendritic cells
- E2
estradiol-17b
- E3
estrone
- ER
estrogen receptor
- HCG
human chronic gonadotrophin
- PIBF
progesterone induced blocking factor
- PlGF
placental growth factor
- PR
progesterone receptor
- PRL
prolactin
- Treg
T regulatory cell
- uNK
uterine natural killer cell
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of Interest Statement: No conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beard HH. Correlation of the unitarian or trophoblastic thesis with the biological test of malignancy. Fed Proc. 1948;7(1 Pt 1):145. [PubMed] [Google Scholar]
- 2.Krebs ET., Jr Carcinogenesis; in the light of the trophoblastic or unitarian thesis of cancer. Int Rec Med Gen Pract Clin. 1951;164(3):141–169. contd. [PubMed] [Google Scholar]
- 3.Ferretti C, Bruni L, Dangles-Marie V, Pecking AP, Bellet D. Molecular circuits shared by placental and cancer cells, and their implications in the proliferative, invasive and migratory capacities of trophoblasts. Hum Reprod Update. 2007;13(2):121–141. doi: 10.1093/humupd/dml048. [DOI] [PubMed] [Google Scholar]
- 4.Holtan SG, Creedon DJ, Haluska P, Markovic SN. Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin Proc. 2009;84(11):985–1000. doi: 10.1016/S0025-6196(11)60669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park DW, Yang KM. Hormonal regulation of uterine chemokines and immune cells. Clin Exp Reprod Med. 2011;38(4):179–185. doi: 10.5653/cerm.2011.38.4.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belfiore A, Perks CM. Grand challenges in cancer endocrinology: endocrine related cancers, an expanding concept. Front Endocrinol. 2013;4:141. doi: 10.3389/fendo.2013.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtan SG, Mansfield AS, Creedon DJ, et al. An organ system based approach to prognosis in advanced melanoma. Front Biosci. 2012;4:2823–2833. doi: 10.2741/e586. [DOI] [PubMed] [Google Scholar]
- 8.Soeters PB, Grimble RF. The conditional role of inflammation in pregnancy and cancer. Clin Nutr. 2012;32(3):460–465. doi: 10.1016/j.clnu.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Coventry BJ, Ashdown ML, Quinn MA, Markovic SN, Yatomi-Clarke SL, Robinson AP. CRP identifies homeostatic immune oscillations in cancer patients: a potential treatment targeting tool? J Transl Med. 2009;7:102. doi: 10.1186/1479-5876-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leontovich AA, Dronca RS, Suman VJ, et al. Fluctuation of systemic immunity in melanoma and implications for timing of therapy. Front Biosci. 2012;4:958–975. doi: 10.2741/E433. [DOI] [PubMed] [Google Scholar]
- 11.Dronca RS, Leontovich AA, Nevala WK, Markovic SN. Personalized therapy for metastatic melanoma: could timing be everything? Future Oncol. 2012;8(11):1401–1406. doi: 10.2217/fon.12.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkpatrick HF, Robertson JD. Hormonal changes in pregnancy. Med Illus. 1953;7(7):553–555. [PubMed] [Google Scholar]
- 13.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zouboulis CC, Chen WC, Thornton MJ, Qin K, Rosenfield R. Sexual hormones in human skin. Horm Metab Res. 2007;39(2):85–95. doi: 10.1055/s-2007-961807. [DOI] [PubMed] [Google Scholar]
- 15.Slominski A, Zmijewski MA, Pawelek J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Mel Res. 2012;25(1):14–27. doi: 10.1111/j.1755-148X.2011.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 17.Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. III. Hormonal and reproductive factors in women. Int J Cancer. 1988;42(6):821–824. doi: 10.1002/ijc.2910420603. [DOI] [PubMed] [Google Scholar]
- 18.Koomen ER, Joosse A, Herings RM, Casparie MK, Guchelaar HJ, Nijsten T. Estrogens, oral contraceptives and hormonal replacement therapy increase the incidence of cutaneous melanoma: a population-based case-control study. Ann Onc: J Eur Soc Med Onc. 2009;20(2):358–364. doi: 10.1093/annonc/mdn589. [DOI] [PubMed] [Google Scholar]
- 19.Lens M, Bataille V. Melanoma in relation to reproductive and hormonal factors in women: current review on controversial issues. Cancer Causes Control. 2008;19(5):437–442. doi: 10.1007/s10552-008-9110-4. [DOI] [PubMed] [Google Scholar]
- 20.Stensheim H, Moller B, van Dijk T, Fossa SD. Cause-specific survival for women diagnosed with cancer during pregnancy or lactation: a registry-based cohort study. J Clin Oncol. 2009;27(1):45–51. doi: 10.1200/JCO.2008.17.4110. [DOI] [PubMed] [Google Scholar]
- 21.Mansfield AS, Nevala WK, Dronca RS, Leontovich AA, Shuster L, Markovic SN. Normal ageing is associated with an increase in Th2 cells, MCP-1 (CCL1) and RANTES (CCL5), with differences in sCD40L and PDGF-AA between sexes. Clin Exp Immunol. 2012;170(2):186–193. doi: 10.1111/j.1365-2249.2012.04644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beddingfield FC., 3rd The melanoma epidemic: res ipsa loquitur. Oncologist. 2003;8(5):459–465. doi: 10.1634/theoncologist.8-5-459. [DOI] [PubMed] [Google Scholar]
- 23.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 24.Kemeny MM, Busch E, Stewart AK, Menck HR. Superior survival of young women with malignant melanoma. Am J Surg. 1998;175(6):437–444. doi: 10.1016/s0002-9610(98)00070-1. discussion 444–435. [DOI] [PubMed] [Google Scholar]
- 25.Shaw HM, Milton GW, Farago G, McCarthy WH. Endocrine influences on survival from malignant melanoma. Cancer. 1978;42(2):669–677. doi: 10.1002/1097-0142(197808)42:2<669::aid-cncr2820420238>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 26.Mervic L. Time course and pattern of metastasis of cutaneous melanoma differ between men and women. PloS one. 2012;7(3):e32955. doi: 10.1371/journal.pone.0032955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ticconi C, Zicari A, Belmonte A, Realacci M, Rao Ch V, Piccione E. Pregnancy-promoting actions of HCG in human myometrium and fetal membranes. Placenta. 2007;28 (Suppl A):S137–143. doi: 10.1016/j.placenta.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Keay SD, Vatish M, Karteris E, Hillhouse EW, Randeva HS. The role of hCG in reproductive medicine. BJOG. 2004;111(11):1218–1228. doi: 10.1111/j.1471-0528.2004.00412.x. [DOI] [PubMed] [Google Scholar]
- 29.RCWHVMCWLPKNBRK. Chorionic gonadotrophin can enhance innate immunity by stimulating macrophage function. J Leukoc Biol. 2007;82(4):926–933. doi: 10.1189/jlb.0207092. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher A, Brachwitz N, Sohr S, et al. Human chorionic gonadotropin attracts regulatory T cells into the fetal-maternal interface during early human pregnancy. J Immunol. 2009;182(9):5488–5497. doi: 10.4049/jimmunol.0803177. [DOI] [PubMed] [Google Scholar]
- 31.Tsampalas M, Gridelet V, Berndt S, Foidart JM, Geenen V, Perrier d'Hauterive S. Human chorionic gonadotropin: a hormone with immunological and angiogenic properties. J Reprod Immunol. 2010;85(1):93–98. doi: 10.1016/j.jri.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Sherwin JR, Sharkey AM, Cameo P, et al. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology. 2007;148(2):618–626. doi: 10.1210/en.2006-0832. [DOI] [PubMed] [Google Scholar]
- 33.Kane N, Kelly R, Saunders PT, Critchley HO. Proliferation of uterine natural killer cells is induced by human chorionic gonadotropin and mediated via the mannose receptor. Endocrinology. 2009;150(6):2882–2888. doi: 10.1210/en.2008-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nature Review Immunol. 2006;6(8):584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 35.Vacca P, Moretta L, Moretta A, Mingari MC. Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol. 2011;32(11):517–523. doi: 10.1016/j.it.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Holtan SG, Creedon DJ, Thompson MA, Nevala WK, Markovic SN. Expansion of CD16-negative natural killer cells in the peripheral blood of patients with metastatic melanoma. Clin Dev Immunol. 2011;2011:316314. doi: 10.1155/2011/316314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koldehoff M, Katzorke T, Wisbrun NC, et al. Modulating impact of human chorionic gonadotropin hormone on the maturation and function of hematopoietic cells. J Leukoc Biol. 2011;90(5):1017–1026. doi: 10.1189/jlb.0910520. [DOI] [PubMed] [Google Scholar]
- 38.Reisinger K, Baal N, McKinnon T, Munstedt K, Zygmunt M. The gonadotropins: tissue-specific angiogenic factors? Mol Cell Endocrinol. 2007;269(1–2):65–80. doi: 10.1016/j.mce.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Kalkunte SS, Mselle TF, Norris WE, Wira CR, Sentman CL, Sharma S. Vascular Endothelial Growth Factor C Facilitates Immune Tolerance and Endovascular Activity of Human Uterine NK Cells at the Maternal-Fetal Interface. J Immunol. 2009;182(7):4085–4092. doi: 10.4049/jimmunol.0803769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berndt S, Perrier d'Hauterive S, Blacher S, et al. Angiogenic activity of human chorionic gonadotropin through LH receptor activation on endothelial and epithelial cells of the endometrium. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20(14):2630–2632. doi: 10.1096/fj.06-5885fje. [DOI] [PubMed] [Google Scholar]
- 41.Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol. 2010;63(6):482–491. doi: 10.1111/j.1600-0897.2010.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brouillet S, Hoffmann P, Chauvet S, et al. Revisiting the role of hCG: new regulation of the angiogenic factor EG-VEGF and its receptors. Cell Mol Life Sci. 2012;69(9):1537–1550. doi: 10.1007/s00018-011-0889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kayisli UA, Selam B, Guzeloglu-Kayisli O, Demir R, Arici A. Human chorionic gonadotropin contributes to maternal immunotolerance and endometrial apoptosis by regulating Fas-Fas ligand system. J Immunol. 2003;171(5):2305–2313. doi: 10.4049/jimmunol.171.5.2305. [DOI] [PubMed] [Google Scholar]
- 44.Lei ZM, Rao CV, Ackerman DM, Day TG. The expression of human chorionic gonadotropin/human luteinizing hormone receptors in human gestational trophoblastic neoplasms. J Clin Endocrinol Metab. 1992;74(6):1236–1241. doi: 10.1210/jcem.74.6.1592864. [DOI] [PubMed] [Google Scholar]
- 45.Sarandakou A, Protonotariou E, Rizos D. Tumor markers in biological fluids associated with pregnancy. Crit Rev Clin Lab Sci. 2007;44(2):151–178. doi: 10.1080/10408360601003143. [DOI] [PubMed] [Google Scholar]
- 46.Cole LA. hCG, the wonder of today's science. Reproductive Biol Endocrinol. 2012;10:24. doi: 10.1186/1477-7827-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doi F, Chi DD, Charuworn BB, et al. Detection of beta-human chorionic gonadotropin mRNA as a marker for cutaneous malignant melanoma. Int J Cancer. 1996;65(4):454–459. doi: 10.1002/(SICI)1097-0215(19960208)65:4<454::AID-IJC11>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 48.Ayala AR, Saad A, Vazquez X, Ramirez-Wiella G, Perches RD. Human chorionic gonadotropin immunoreactivity in serum of patients with malignant neoplasms. American J Reprod Immunol. 1983;3(3):149–151. doi: 10.1111/j.1600-0897.1983.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 49.Akhvlediani L, Nagervadze M, Dumbadze G. Detection of tolerance against human chorionic gonadotropin at malignant and benign tumors of female reproduction system. Georgian Med News. 2009;(171):20–24. [PubMed] [Google Scholar]
- 50.Butler SA, Ikram MS, Mathieu S, Iles RK. The increase in bladder carcinoma cell population induced by the free beta subunit of human chorionic gonadotrophin is a result of an anti-apoptosis effect and not cell proliferation. Br J Cancer. 2000;82(9):1553–1556. doi: 10.1054/bjoc.2000.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zygmunt M, Herr F, Keller-Schoenwetter S, et al. Characterization of human chorionic gonadotropin as a novel angiogenic factor. J Clin Endocrinol Metab. 2002;87(11):5290–5296. doi: 10.1210/jc.2002-020642. [DOI] [PubMed] [Google Scholar]
- 52.Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol. 1993;151(9):4562–4573. [PubMed] [Google Scholar]
- 53.Szekeres-Bartho J, Halasz M, Palkovics T. Progesterone in pregnancy; receptor-ligand interaction and signaling pathways. J Reprod Immunol. 2009;83(1–2):60–64. doi: 10.1016/j.jri.2009.06.262. [DOI] [PubMed] [Google Scholar]
- 54.Szekeres-Bartho J, Barakonyi A, Polgar B, et al. The role of gamma/delta T cells in progesterone-mediated immunomodulation during pregnancy: a review. Am J Reprod Immunol. 1999;42(1):44–48. doi: 10.1111/j.1600-0897.1999.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 55.Szekeres-Bartho J, Polgar B. PIBF: the double edged sword. Pregnancy and tumor. Am J Reprod Immunol. 2010;64(2):77–86. doi: 10.1111/j.1600-0897.2010.00833.x. [DOI] [PubMed] [Google Scholar]
- 56.Gadkar-Sable S, Shah C, Rosario G, Sachdeva G, Puri C. Progesterone receptors: Various forms and functions in reproductive tissues. Front Biosci. 2005;10:2118–2130. doi: 10.2741/1685. [DOI] [PubMed] [Google Scholar]
- 57.Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97(5):E719–730. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87(6):2924–2930. doi: 10.1210/jcem.87.6.8609. [DOI] [PubMed] [Google Scholar]
- 59.Vrachnis N, Malamas FM, Sifakis S, Tsikouras P, Iliodromiti Z. Immune aspects and myometrial actions of progesterone and CRH in labor. Clin Dev Immunol. 2012;2012:937618. doi: 10.1155/2012/937618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishida M, Choi JH, Hirabayashi K, et al. Reproductive phenotypes in mice with targeted disruption of the 20alpha-hydroxysteroid dehydrogenase gene. J Reprod Dev. 2007;53(3):499–508. doi: 10.1262/jrd.18125. [DOI] [PubMed] [Google Scholar]
- 61.Arck PC, Rucke M, Rose M, et al. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod Biomed. 2008;17(1):101–113. doi: 10.1016/s1472-6483(10)60300-8. [DOI] [PubMed] [Google Scholar]
- 62.Szekeres-Bartho J, Hadnagy J, Pacsa AS. The suppressive effect of progesterone on lymphocyte cytotoxicity: unique progesterone sensitivity of pregnancy lymphocytes. J Reprod Immunol. 1985;7(2):121–128. doi: 10.1016/0165-0378(85)90066-x. [DOI] [PubMed] [Google Scholar]
- 63.Liang J, Sun L, Wang Q, Hou Y. Progesterone regulates mouse dendritic cells differentiation and maturation. Int Immunopharmacol. 2006;6(5):830–838. doi: 10.1016/j.intimp.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 64.De Leon-Nava MA, Nava K, Soldevila G, et al. Immune sexual dimorphism: effect of gonadal steroids on the expression of cytokines, sex steroid receptors, and lymphocyte proliferation. J Steroid Biochem Mol Biol. 2009;113(1–2):57–64. doi: 10.1016/j.jsbmb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Piccinni MP, Giudizi MG, Biagiotti R, et al. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol. 1995;155(1):128–133. [PubMed] [Google Scholar]
- 66.Canellada A, Blois S, Gentile T, Margni Idehu RA. In vitro modulation of protective antibody responses by estrogen, progesterone and interleukin-6. Am J Reprod Immunol. 2002;48(5):334–343. doi: 10.1034/j.1600-0897.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- 67.Slominski A, Gomez-Sanchez CE, Foecking MF, Wortsman J. Metabolism of progesterone to DOC, corticosterone and 18OHDOC in cultured human melanoma cells. FEBS Lett. 1999;455(3):364–366. doi: 10.1016/s0014-5793(99)00889-3. [DOI] [PubMed] [Google Scholar]
- 68.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81(7):2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 69.Slominski A, Zjawiony J, Wortsman J, et al. A novel pathway for sequential transformation of 7-dehydrocholesterol and expression of the P450scc system in mammalian skin. Eur J Biochem. 2004;271(21):4178–4188. doi: 10.1111/j.1432-1033.2004.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiedemann C, Nagele U, Schramm G, Berking C. Inhibitory effects of progestogens on the estrogen stimulation of melanocytes in vitro. Contraception. 2009;80(3):292–298. doi: 10.1016/j.contraception.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 71.Im S, Lee ES, Kim W, et al. Donor specific response of estrogen and progesterone on cultured human melanocytes. J Korean Med Sci. 2002;17(1):58–64. doi: 10.3346/jkms.2002.17.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang X, Zhang X, Zhou M, Li J. Effects of progesterone on the growth regulation in classical progesterone receptor-negative malignant melanoma cells. J Huazhong Univ Sci Tech. 2010;30(2):231–234. doi: 10.1007/s11596-010-0220-3. [DOI] [PubMed] [Google Scholar]
- 73.Kozma N, Halasz M, Polgar B, et al. Progesterone-induced blocking factor activates STAT6 via binding to a novel IL-4 receptor. J Immunol. 2006;176(2):819–826. doi: 10.4049/jimmunol.176.2.819. [DOI] [PubMed] [Google Scholar]
- 74.Kanda N, Watanabe S. 17beta-estradiol, progesterone, and dihydrotestosterone suppress the growth of human melanoma by inhibiting interleukin-8 production. J Invest Dermatol. 2001;117(2):274–283. doi: 10.1046/j.1523-1747.2001.01422.x. [DOI] [PubMed] [Google Scholar]
- 75.Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(6):1931–1939. doi: 10.1158/1078-0432.CCR-08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 77.Clauss M, Weich H, Breier G, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271(30):17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 78.Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood. 2003;102(4):1515–1524. doi: 10.1182/blood-2002-11-3423. [DOI] [PubMed] [Google Scholar]
- 79.Bagley RG, Ren Y, Weber W, et al. Placental growth factor upregulation is a host response to antiangiogenic therapy. Clin Cancer Res. 2011;17(5):976–988. doi: 10.1158/1078-0432.CCR-10-2687. [DOI] [PubMed] [Google Scholar]
- 80.Fischer C, Jonckx B, Mazzone M, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131(3):463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 81.Eriksson A, Cao R, Pawliuk R, et al. Placenta growth factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell. 2002;1(1):99–108. doi: 10.1016/s1535-6108(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 82.Loges S, Schmidt T, Carmeliet P. “Antimyeloangiogenic” therapy for cancer by inhibiting PlGF. Clin Cancer Res. 2009;15(11):3648–3653. doi: 10.1158/1078-0432.CCR-08-2276. [DOI] [PubMed] [Google Scholar]
- 83.Bryant-Greenwood GD, Yamamoto SY, Sadowsky DW, Gravett MG, Novy MJ. Relaxin stimulates interleukin-6 and interleukin-8 secretion from the extraplacental chorionic cytotrophoblast. Placenta. 2009;30(7):599–606. doi: 10.1016/j.placenta.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 84.Tashima LS, Mazoujian G, Bryant-Greenwood GD. Human relaxins in normal, benign and neoplastic breast tissue. J Mol Endocrinol. 1994;12(3):351–364. doi: 10.1677/jme.0.0120351. [DOI] [PubMed] [Google Scholar]
- 85.Mazoujian G, Bryant-Greenwood GD. Relaxin in breast tissue. Lancet. 1990;335(8684):298–299. doi: 10.1016/0140-6736(90)90124-n. [DOI] [PubMed] [Google Scholar]
- 86.Hansell DJ, Bryant-Greenwood GD, Greenwood FC. Expression of the human relaxin H1 gene in the decidua, trophoblast, and prostate. J Clin Endocrinol Metab. 1991;72(4):899–904. doi: 10.1210/jcem-72-4-899. [DOI] [PubMed] [Google Scholar]
- 87.Perkins AV, Wolfe CD, Eben F, Soothill P, Linton EA. Corticotrophin-releasing hormone-binding protein in human fetal plasma. J Endocrinol. 1995;146(3):395–401. doi: 10.1677/joe.0.1460395. [DOI] [PubMed] [Google Scholar]
- 88.Grammatopoulos DK, Hillhouse EW. Role of corticotropin-releasing hormone in onset of labour. Lancet. 1999;354(9189):1546–1549. doi: 10.1016/S0140-6736(99)03418-2. [DOI] [PubMed] [Google Scholar]
- 89.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80(3):979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 90.Slominski A, Zbytek B, Szczesniewski A, et al. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288(4):E701–706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- 91.Yang Y, Park H, Kim TS, Bang SI, Cho D. Enhancement of cell migration by corticotropin-releasing hormone through ERK1/2 pathway in murine melanoma cell line, B16F10. Exp Dermatol. 2007;16(1):22–27. doi: 10.1111/j.1600-0625.2006.00511.x. [DOI] [PubMed] [Google Scholar]
- 92.Kim MH, Cho D, Kim HJ, et al. Investigation of the corticotropin-releasing hormone-proopiomelanocortin axis in various skin tumours. Br J Dermatol. 2006;155(5):910–915. doi: 10.1111/j.1365-2133.2006.07442.x. [DOI] [PubMed] [Google Scholar]
- 93.Slominski A, Zbytek B, Zmijewski M, et al. Corticotropin releasing hormone and the skin. Front Biosci. 2006;11:2230–2248. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Slominski AT, Zmijewski MA, Zbytek B, Tobin DJ, Theoharides TC, Rivier J. Key Role of CRF in the Skin Stress Response System. Endocr Rev. 2013 doi: 10.1210/er.2012-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carlson KW, Nawy SS, Wei ET, et al. Inhibition of mouse melanoma cell proliferation by corticotropin-releasing hormone and its analogs. Anticancer Res. 2001;21(2A):1173–1179. [PubMed] [Google Scholar]
- 96.Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006;206(3):780–791. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332(20):1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 98.Ilias I, Mastorakos G. The emerging role of peripheral corticotropin-releasing hormone (CRH) J Endocrinol Invest. 2003;26(4):364–371. doi: 10.1007/BF03345186. [DOI] [PubMed] [Google Scholar]
- 99.Ciereszko RE, Petroff BK, Ottobre AC, Guan Z, Stokes BT, Ottobre JS. Assessment of the mechanism by which prolactin stimulates progesterone production by early corpora lutea of pigs. J Endocrinol. 1998;159(2):201–209. doi: 10.1677/joe.0.1590201. [DOI] [PubMed] [Google Scholar]
- 100.Herrera E, Munoz C, Lopez-Luna P, Ramos P. Carbohydrate-lipid interactions during gestation and their control by insulin. Braz J Med Biol Res. 1994;27(11):2499–2519. [PubMed] [Google Scholar]
- 101.Sorenson RL, Brelje TC. Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res. 1997;29(6):301–307. doi: 10.1055/s-2007-979040. [DOI] [PubMed] [Google Scholar]
- 102.Lellis-Santos C, Sakamoto LH, Bromati CR, et al. The Regulation of Rasd1 Expression by Glucocorticoids and Prolactin Controls Peripartum Maternal Insulin Secretion. Endocrinology. 2012;153(8):3668–3678. doi: 10.1210/en.2012-1135. [DOI] [PubMed] [Google Scholar]
- 103.Voogt JL, Lee Y, Yang S, Arbogast L. Regulation of prolactin secretion during pregnancy and lactation. Prog Brain Res. 2001;133:173–185. doi: 10.1016/s0079-6123(01)33013-3. [DOI] [PubMed] [Google Scholar]
- 104.Martini JF, Piot C, Humeau LM, Struman I, Martial JA, Weiner RI. The antiangiogenic factor 16K PRL induces programmed cell death in endothelial cells by caspase activation. Mol Endocrinol. 2000;14(10):1536–1549. doi: 10.1210/mend.14.10.0543. [DOI] [PubMed] [Google Scholar]
- 105.Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128(3):589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 106.Matera L, Contarini M, Bellone G, Forno B, Biglino A. Up-modulation of interferon-gamma mediates the enhancement of spontanous cytotoxicity in prolactin-activated natural killer cells. Immunology. 1999;98(3):386–392. doi: 10.1046/j.1365-2567.1999.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peeva E, Zouali M. Spotlight on the role of hormonal factors in the emergence of autoreactive Blymphocytes. Immunol Lett. 2005;101(2):123–143. doi: 10.1016/j.imlet.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 108.Chavez-Rueda K, Hernandez J, Zenteno E, Leanos-Miranda A, Legorreta-Haquet MV, Blanco-Favela F. Identification of prolactin as a novel immunomodulator on the expression of costimulatory molecules and cytokine secretions on T and B human lymphocytes. Clin Immunol. 2005;116(2):182–191. doi: 10.1016/j.clim.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 109.Yu-Lee LY. Prolactin modulation of immune and inflammatory responses. Recent Prog Horm Res. 2002;57:435–455. doi: 10.1210/rp.57.1.435. [DOI] [PubMed] [Google Scholar]
- 110.Orbach H, Zandman-Goddard G, Amital H, et al. Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin D, and TPA levels in autoimmune diseases. Ann N Y Acad Sci. 2007;1109:385–400. doi: 10.1196/annals.1398.044. [DOI] [PubMed] [Google Scholar]
- 111.Majumder B, Biswas R, Chattopadhyay U. Prolactin regulates antitumor immune response through induction of tumoricidal macrophages and release of IL-12. Int J Cancer. 2002;97(4):493–500. doi: 10.1002/ijc.1624. [DOI] [PubMed] [Google Scholar]
- 112.Sun R, Wei H, Zhang J, Li A, Zhang W, Tian Z. Recombinant human prolactin improves antitumor effects of murine natural killer cells in vitro and in vivo. Neuroimmunomodulation. 2002;10(3):169–176. doi: 10.1159/000067179. [DOI] [PubMed] [Google Scholar]
- 113.Swaminathan G, Varghese B, Fuchs SY. Regulation of prolactin receptor levels and activity in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(1):81–91. doi: 10.1007/s10911-008-9068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Asher JM, O'Leary KA, Rugowski DE, Arendt LM, Schuler LA. Prolactin promotes mammary pathogenesis independently from cyclin d1. Am J Pathol. 2012;181(1):294–302. doi: 10.1016/j.ajpath.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chikanza IC. Prolactin and neuroimmunomodulation: in vitro and in vivo observations. Ann N Y Acad Sci. 1999;876:119–130. doi: 10.1111/j.1749-6632.1999.tb07629.x. [DOI] [PubMed] [Google Scholar]
- 116.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19(3):225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 117.Peverelli E, Mantovani G, Vitali E, et al. Filamin-A is essential for dopamine d2 receptor expression and signaling in tumorous lactotrophs. J Clin Endocrinol Metab. 2012;97(3):967–977. doi: 10.1210/jc.2011-2902. [DOI] [PubMed] [Google Scholar]
- 118.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22(6):724–763. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- 119.Richards SM, Murphy WJ. Use of human prolactin as a therapeutic protein to potentiate immunohematopoietic function. J Neuroimmunol. 2000;109(1):56–62. doi: 10.1016/s0165-5728(00)00303-9. [DOI] [PubMed] [Google Scholar]
- 120.Tabruyn SP, Sabatel C, Nguyen NQ, et al. The angiostatic 16K human prolactin overcomes endothelial cell anergy and promotes leukocyte infiltration via nuclear factor-kappaB activation. Mol Endocrinol. 2007;21(6):1422–1429. doi: 10.1210/me.2007-0021. [DOI] [PubMed] [Google Scholar]
- 121.Nguyen NQ, Castermans K, Berndt S, et al. The antiangiogenic 16K prolactin impairs functional tumor neovascularization by inhibiting vessel maturation. PloS one. 2011;6(11):e27318. doi: 10.1371/journal.pone.0027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 123.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14(2):1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rongvaux A, Shea RJ, Mulks MH, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32(11):3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 125.Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178(3):1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 126.Ognjanovic S, Tashima LS, Bryant-Greenwood GD. The effects of pre-B-cell colony-enhancing factor on the human fetal membranes by microarray analysis. Am J Obstet Gynecol. 2003;189(4):1187–1195. doi: 10.1067/s0002-9378(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 127.Lappas M. Visfatin regulates the terminal processes of human labour and delivery via activation of the nuclear factor-kappaB pathway. Mol Cell Endocrinol. 2012;348(1):128–134. doi: 10.1016/j.mce.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 128.Mazaki-Tovi S, Romero R, Vaisbuch E, et al. Maternal plasma visfatin in preterm labor. J Matern Fetal Neonatal Med. 2009;22(8):693–704. doi: 10.1080/14767050902994788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maldi E, Travelli C, Caldarelli A, et al. Nicotinamide phosphoribosyltransferase (NAMPT) is over-expressed in melanoma lesions. Pigment Cell Mel Res. 2013;26(1):144–146. doi: 10.1111/pcmr.12037. [DOI] [PubMed] [Google Scholar]
- 130.Buldak RJ, Buldak L, Polaniak R, et al. Visfatin affects redox adaptative responses and proliferation in Me45 human malignant melanoma cells: an in vitro study. Oncol Rep. 2013;29(2):771–778. doi: 10.3892/or.2012.2175. [DOI] [PubMed] [Google Scholar]
- 131.Jia SH, Li Y, Parodo J, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113(9):1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tanriverdi F, Silveira LF, MacColl GS, Bouloux PM. The hypothalamic-pituitary-gonadal axis: immune function and autoimmunity. J Endocrinol. 2003;176(3):293–304. doi: 10.1677/joe.0.1760293. [DOI] [PubMed] [Google Scholar]
- 133.Cohen-Solal JF, Jeganathan V, Grimaldi CM, Peeva E, Diamond B. Sex hormones and SLE: influencing the fate of autoreactive B cells. Curr Top Microbiol Immunol. 2006;305:67–88. doi: 10.1007/3-540-29714-6_4. [DOI] [PubMed] [Google Scholar]
- 134.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 135.Zang YC, Halder JB, Hong J, Rivera VM, Zhang JZ. Regulatory effects of estriol on T cell migration and cytokine profile: inhibition of transcription factor NF-kappa B. J Neuroimmunol. 2002;124(1–2):106–114. doi: 10.1016/s0165-5728(02)00016-4. [DOI] [PubMed] [Google Scholar]
- 136.Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97(1):107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 137.Holtan SG, Creedon DJ. Mother knows best: lessons from fetomaternal tolerance applied to cancer immunity. Front Biosci. 2011;3:1533–1540. doi: 10.2741/243. [DOI] [PubMed] [Google Scholar]
- 138.Lee YL, Cheong AW, Chow WN, Lee KF, Yeung WS. Regulation of complement-3 protein expression in human and mouse oviducts. Mol Reprod Dev. 2009;76(3):301–308. doi: 10.1002/mrd.20955. [DOI] [PubMed] [Google Scholar]
- 139.Shifren JL, Tseng JF, Zaloudek CJ, et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Enndocrinol Metab. 1996;81(8):3112–3118. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- 140.Hildebrandt VA, Babischkin JS, Koos RD, Pepe GJ, Albrecht ED. Developmental regulation of vascular endothelial growth/permeability factor messenger ribonucleic acid levels in and vascularization of the villous placenta during baboon pregnancy. Endocrinology. 2001;142(5):2050–2057. doi: 10.1210/endo.142.5.8174. [DOI] [PubMed] [Google Scholar]
- 141.Schoenberg BS, Christine BW. Malignant melanoma associated with breast cancer. South Med J. 1980;73(11):1493–1497. doi: 10.1097/00007611-198011000-00023. [DOI] [PubMed] [Google Scholar]
- 142.Walker MJ, Beattie CW, Patel MK, Ronan SM, Das Gupta TK. Estrogen receptor in malignant melanoma. J Clin Oncol. 1987;5(8):1256–1261. doi: 10.1200/JCO.1987.5.8.1256. [DOI] [PubMed] [Google Scholar]
- 143.Zen M, Ghirardello A, Iaccarino L, et al. Hormones, immune response, and pregnancy in healthy women and SLE patients. Swiss Med Wkly. 2010;140(13–14):187–201. doi: 10.4414/smw.2010.12597. [DOI] [PubMed] [Google Scholar]
- 144.Grimaldi CM, Cleary J, Dagtas AS, Moussai D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J Clin Invest. 2002;109(12):1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cutolo M, Sulli A, Craviotto C, et al. Modulation of cell growth and apoptosis by sex hormones in cultured monocytic THP-1 cells. Ann N Y Acad Sci. 2002;966:204–210. doi: 10.1111/j.1749-6632.2002.tb04216.x. [DOI] [PubMed] [Google Scholar]
- 146.Hughes GC, Clark EA. Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity. 2007;40(6):470–481. doi: 10.1080/08916930701464764. [DOI] [PubMed] [Google Scholar]
- 147.Calippe B, Douin-Echinard V, Laffargue M, et al. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol. 2008;180(12):7980–7988. doi: 10.4049/jimmunol.180.12.7980. [DOI] [PubMed] [Google Scholar]
- 148.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38(1):13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 149.Evans MJ, MacLaughlin S, Marvin RD, Abdou NI. Estrogen decreases in vitro apoptosis of peripheral blood mononuclear cells from women with normal menstrual cycles and decreases TNF-alpha production in SLE but not in normal cultures. Clin Immunol Immunopathol. 1997;82(3):258–262. doi: 10.1006/clin.1996.4300. [DOI] [PubMed] [Google Scholar]
- 150.Kuiper GG, Carlsson B, Grandien K, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 151.Ohata C, Tadokoro T, Itami S. Expression of estrogen receptor beta in normal skin, melanocytic nevi and malignant melanomas. J Dermatol. 2008;35(4):215–221. doi: 10.1111/j.1346-8138.2008.00447.x. [DOI] [PubMed] [Google Scholar]
- 152.Schmidt AN, Nanney LB, Boyd AS, King LE, Jr, Ellis DL. Oestrogen receptor-beta expression in melanocytic lesions. Exp Dermatol. 2006;15(12):971–980. doi: 10.1111/j.1600-0625.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 153.Scolyer RA, Judge MJ, Evans A, et al. Data Set for Pathology Reporting of Cutaneous Invasive Melanoma: Recommendations From the International Collaboration on Cancer Reporting (ICCR) Am J Surg Pathol. 2013 doi: 10.1097/PAS.0b013e31829d7f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.de Giorgi V, Mavilia C, Massi D, et al. Estrogen receptor expression in cutaneous melanoma: a real-time reverse transcriptase-polymerase chain reaction and immunohistochemical study. Arch Dermatol. 2009;145(1):30–36. doi: 10.1001/archdermatol.2008.537. [DOI] [PubMed] [Google Scholar]
- 155.Mor G, Sapi E, Abrahams VM, et al. Interaction of the estrogen receptors with the Fas ligand promoter in human monocytes. J Immunol. 2003;170(1):114–122. doi: 10.4049/jimmunol.170.1.114. [DOI] [PubMed] [Google Scholar]
- 156.Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172(3):1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- 157.Mao A, Paharkova-Vatchkova V, Hardy J, Miller MM, Kovats S. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175(8):5146–5151. doi: 10.4049/jimmunol.175.8.5146. [DOI] [PubMed] [Google Scholar]
- 158.Mabjeesh NJ, Escuin D, LaVallee TM, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer cell. 2003;3(4):363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 159.Miller VM. Sex-based differences in vascular function. Women's health. 2010;6(5):737–752. doi: 10.2217/whe.10.53. [DOI] [PubMed] [Google Scholar]
- 160.Raaz-Schrauder D, Klinghammer L, Baum C, et al. Association of systemic inflammation markers with the presence and extent of coronary artery calcification. Cytokine. 2012;57(2):251–257. doi: 10.1016/j.cyto.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 161.Hu FB, Stampfer MJ, Manson JE, et al. Trends in the incidence of coronary heart disease and changes in diet and lifestyle in women. J Engl J Med. 2000;343(8):530–537. doi: 10.1056/NEJM200008243430802. [DOI] [PubMed] [Google Scholar]
- 162.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. 2012;38(2–3):J282–291. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 163.Whitacre CC. Sex differences in autoimmune disease. Nature Immunol. 2001;2(9):777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- 164.Ostensen M, Villiger PM. Immunology of pregnancy-pregnancy as a remission inducing agent in rheumatoid arthritis. Transpl Immunol. 2002;9(2–4):155–160. doi: 10.1016/s0966-3274(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 165.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118(22):5918–5927. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34(3):177–192. doi: 10.1385/IR:34:3:177. [DOI] [PubMed] [Google Scholar]
- 167.Adrie C, Azoulay E, Francais A, et al. Influence of gender on the outcome of severe sepsis: a reappraisal. Chest. 2007;132(6):1786–1793. doi: 10.1378/chest.07-0420. [DOI] [PubMed] [Google Scholar]
- 168.Hill JA, Polgar K, Anderson DJ. T-helper 1-type immunity to trophoblast in women with recurrent spontaneous abortion. JAMA. 1995;273(24):1933–1936. [PubMed] [Google Scholar]
- 169.Moraru M, Carbone J, Alecsandru D, et al. Intravenous immunoglobulin treatment increased live birth rate in a Spanish cohort of women with recurrent reproductive failure and expanded CD56(+) cells. Am J Reprod Immunol. 2012;68(1):75–84. doi: 10.1111/j.1600-0897.2012.01135.x. [DOI] [PubMed] [Google Scholar]
- 170.Jin LP, Zhou YH, Wang MY, Zhu XY, Li DJ. Blockade of CD80 and CD86 at the time of implantation inhibits maternal rejection to the allogeneic fetus in abortion-prone matings. J Reprod Immunol. 2005;65(2):133–146. doi: 10.1016/j.jri.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 171.Fan W, Li SW, Liu XR. Influence of blockade of costimulation on Th1/Th2 cytokines shift in unexplained early recurrent spontaneous. J Sichuan Univ, Med Sci Ed. 2006;37(5):773–775. [PubMed] [Google Scholar]
- 172.Li W, Li B, Fan W, et al. CTLA4Ig gene transfer alleviates abortion in mice by expanding CD4+CD25+ regulatory T cells and inducing indoleamine 2,3-dioxygenase. J Reprod Immunol. 2009;80(1–2):1–11. doi: 10.1016/j.jri.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 173.Weber J. Overcoming immunologic tolerance to melanoma: targeting CTLA-4 with ipilimumab (MDX-010) Oncologist. 2008;13 (Suppl 4):16–25. doi: 10.1634/theoncologist.13-S4-16. [DOI] [PubMed] [Google Scholar]
- 174.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Trinh VA, Hagen B. Ipilimumab for advanced melanoma: a pharmacologic perspective. J Oncol Pharm Pract. 2013;19(3):195–201. doi: 10.1177/1078155212459100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dulos J, Carven GJ, van Boxtel SJ, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35(2):169–178. doi: 10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- 177.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A. 2013;110(44):17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ma J, Han H, Liu D, et al. HER2 as a promising target for cytotoxicity T cells in human melanoma therapy. PloS one. 2013;8(8):e73261. doi: 10.1371/journal.pone.0073261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mansfield AS, Nevala WK, Lieser EA, Leontovich AA, Markovic SN. The immunomodulatory effects of bevacizumab on systemic immunity in patients with metastatic melanoma. Oncoimmunology. 2013;2(5):e24436. doi: 10.4161/onci.24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 183.Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Intl J Cancer. 2009;124(6):1470–1477. doi: 10.1002/ijc.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: Implications for local immune functions. J Steroid Biochem Mol Biol. 2013;137:107–123. doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mordoh J, Tapia IJ, Barrio MM. A word of caution: do not wake sleeping dogs; micrometastases of melanoma suddenly grew after progesterone treatment. BMC cancer. 2013;13:132. doi: 10.1186/1471-2407-13-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 187.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105(27):9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lin PY, Sun L, Thibodeaux SR, et al. B7-H1-dependent sex-related differences in tumor immunity and immunotherapy responses. J Immunol. 2010;185(5):2747–2753. doi: 10.4049/jimmunol.1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]