Abstract
Schizophrenia is associated with increased cardiovascular disease morbidity and mortality. Schizophrenia is also associated with immune and inflammatory abnormalities, including aberrant blood levels of lymphocytes, cytokines and high-sensitivity C-reactive protein (hsCRP). The purpose of this study is to investigate the relationship between total and differential white blood cell (WBC) counts, hsCRP, and indices of cardiovascular disease risk in patients with schizophrenia and related non-affective psychoses. 108 inpatients and outpatients age 18–70 with non-affective psychoses and 44 controls participated in this cross-sectional study. Subjects had a fasting blood draw between 8 and 9 am for glucose, lipids, total and differential WBC counts, and hsCRP. Vital signs and medical history were obtained. Patients with non-affective psychosis had significantly higher hsCRP levels than controls (p=0.04). In linear regression analyses, lymphocyte and monocyte counts were a significant predictor of the total-to-HDL cholesterol ratio in subjects with non-affective psychosis (p≤0.02 for each). In binary logistic regression analyses, total WBC count was a significant predictor of an elevated 10-year estimated risk of myocardial infarction and cardiovascular disease in subjects with non-affective psychosis (p≤0.03 for each). Associations between total and differential WBC counts and cardiovascular disease risk indices were stronger in males than females with non-affective psychosis. Our findings provide further evidence that measurement of total and differential WBC counts may be germane to the clinical care of patients with schizophrenia and related disorders, and support an association between inflammation and cardiovascular disease risk in these patients.
Keywords: Schizophrenia, Non-affective psychosis, Leukocytes, Neutrophils, Monocytes, Lymphocytes, C-reactive protein, Inflammation, Cardiovascular disease, Risk assessment
1. Introduction
Cardiovascular disease is the leading cause of death in patients with schizophrenia and related disorders, and premature mortality is dramatically increased in this patient population (Brown 1997; Harris and Barraclough, 1998; Miller et al., 2006, Saha et al., 2007). A number of different assessment tools have been developed to predict cardiovascular disease risk in the general population, including the Framingham Risk Score (FRS), the Framingham General Cardiovascular Risk Score (FGRS), and the total-to-HDL cholesterol ratio. The FRS is a tool for estimating the 10-year risk of having a myocardial infarction (Wilson et al., 1998), and incorporates data on age, sex, total and HDL cholesterol, systolic blood pressure, smoking status and current anti-hypertensive medication. The FGRS is a tool for estimating the 10-year risk of all cardiovascular disease events (including coronary, cerebrovascular, and peripheral arterial disease and heart failure), and is comprised of the same variables as the FRS, plus the presence or absence of diabetes (D’Agostino et al. 2008). The total-to-HDL cholesterol ratio is also used as a predictor of cardiovascular disease risk (Conroy et al., 2003; Lemieux et al., 2001).
In a survey of 102 subjects with schizophrenia, McCreadie et al. (2003) found a 53% prevalence of an elevated total-to-HDL cholesterol ratio (defined as >5.0). Several previous studies have also found increased FGRS in patients with schizophrenia compared to controls (Jin et al., 2011; Ratliff et al., 2013; Said et al., 2012; Sicras-Mainar et al., 2013; Tay et al., 2013; Wysokinski et al., 2012; Yazici et al., 2011). Among 689 subjects from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) and matched controls from the National Health and Nutrition Examination Survey III, mean FGRS scores were significantly elevated in both males (9.4% vs 7.0%) and females (6.3% vs 4.2%) with schizophrenia (Goff et al., 2005). In several studies, presence of the metabolic syndrome was associated with increased cardiovascular disease risk (Said et al., 2012; Tay et al., 2013; Wysokinski et al., 2012; Yazici et al., 2011).
The metabolic syndrome is a constellation of metabolic risk factors associated with the development of atherosclerotic cardiovascular disease (Galassi et al., 2006; Grundy et al., 2005) and cardiovascular disease mortality (Galassi et al., 2006). The metabolic syndrome is common in patients with schizophrenia and related disorders, with a prevalence of 43%—based on American Heart Association criteria (Grundy et al., 2005)—in the CATIE trial (McEvoy et al., 2005). The metabolic syndrome is also associated with a state of chronic, low-grade inflammation (Devaraj et al., 2010). A meta-analysis found that the acute phase inflammatory marker high-sensitivity CRP (hsCRP) was an independent predictor of cardiovascular disease (Kaptoge et al., 2010). Schizophrenia is also associated with immune and inflammatory abnormalities, including aberrant blood levels of lymphocytes (Miller et al., 2013), cytokines (Miller et al., 2011) and hsCRP (Miller et al., 2014), including studies in patients with first-episode psychosis and minimal exposure to antipsychotics. The adverse effects of atypical antipsychotics further impact risk of the metabolic syndrome, and subsequently, cardiovascular disease risk in patients with schizophrenia.
Total and differential white blood cell (WBC) counts and hsCRP blood levels may predict metabolic syndrome in patients with schizophrenia and other non-affective psychoses (Fan et al., 2010; Miller et al., 2013b). Another recent study found that in patients with schizophrenia, CRP levels were linearly associated with 10-year cardiovascular disease risk (stratified by low, moderate, and high/very high; Sicras-Mainar et al., 2013). However, total and differential WBC counts have not been explored as a predictor of cardiovascular disease risk in schizophrenia. The purpose of the present study, therefore, is to investigate the relationship between total and differential WBC counts, hsCRP, and indices of cardiovascular disease risk in patients with schizophrenia and related non-affective psychoses. We hypothesize that these measures are associated with increased cardiovascular disease risk in subjects with non-affective psychosis.
2. Methods
2.1. Subjects
108 inpatients and outpatients aged 18–70 and diagnosed with schizophrenia (n=66) and related non-affective psychoses, including schizoaffective disorder (n=39), psychotic disorder not otherwise specified (n=2), or brief psychotic disorder (n=1), and forty-four controls, who were part of studies of immune function in schizophrenia and related disorders, were recruited in the Augusta, Georgia area between July 2010 and June 2014. The broader category of non-affective psychosis, which includes schizoaffective disorder, appears to share characteristics of schizophrenia (Lichterman et al., 2000; Tamminga et al., 2013). Recruitment was non-randomized. Subjects were referred to the investigators by their inpatient or outpatient psychiatrist. Exclusion criteria for all subjects for the present study included alcohol withdrawal; pregnancy; current scheduled use of non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or other immunomodulatory agents; history of exposure to an antibiotic in the past 2 weeks (based on subject’s self-report plus a review of their electronic medical record); history of an immune disorder; current urinary tract infection; and illicit drug use in the past 30 days. “As needed” use of NSAIDs was not an exclusion criterion, although only one subject endorsed such use. No subject had a history of recent trauma or surgical intervention. All subjects had a negative urine drug screen. An additional exclusion criterion for patients was current use of clozapine. Additional exclusion criteria for controls were lifetime diagnosis of schizophrenia or related disorder; lifetime or current diagnosis of a manic, depressed, or mixed affective episode, or history of exposure to an antipsychotic, antidepressant, valproate, lithium or gabapentin. Antipsychotic medications were not standardized for subjects with non-affective psychoses. The majority of patients were treated with monotherapy with second-generation antipsychotics.
2.2. Procedures
After providing written informed consent, subjects underwent a laboratory, physical, and psychiatric diagnostic evaluation. Subjects had a blood draw between 8 and 9 am after a ten-hour fast. Vital signs, height/weight, and medical history were obtained. Diagnosis (or absence of a diagnosis in the controls) was verified using the Structured Clinical Interview for DSM-IV disorders (SCID) psychosis and mood disorders modules (Finnig et al., 1994). One rater (BJM) performed the SCID interviews. For patients, symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS). A total of four raters performed the PANSS interviews for subjects in this study, although one rater (BJM) performed the majority of the interviews (n=83, 77%). All raters were trained to perform the SCID and PANSS and inter-rater reliability with the principal investigator. Data on smoking (number of cigarettes per day) and other substance use was obtained using the Dartmouth Assessment of Lifestyle Inventory (DALI; Ford, 2013). Data on socioeconomic status (SES) was obtained using the Hollingshead-Redlich Scale (Hollingshead and Redlich, 1958). Data on current antipsychotic medications were used to calculate the subject’s current daily dose in chlorpromazine (CPZ) equivalents (Woods, 2003; Woods, 2010). The study was approved by the IRB’s of both Georgia Regents University and the Georgia Department of Community Health.
2.3. Laboratory evaluation
Blood analyses were performed at Clinical Pathology Laboratories Southeast (Augusta, Georgia). Complete blood counts with differential, fasting serum glucose, and lipid panels were analyzed by standard clinical laboratory assays. Glucose and lipids were measured using an Olympus AU2700 Chemistry-Immuno Analyzer (Olympus America, Inc., Melville, NY). CBC with differential was analyzed using a COULTER LH 750 Hematology Analyzer (Beckman Coulter, Inc., Brea, CA). hsCRP levels were measured using an enzyme-linked immunosorbent assay.
2.4. Indices of Cardiovascular Disease Risk
The FRS was calculated using the National Heart, Lung, and Blood Institute tool for estimating 10-year risk of having a myocardial infarction (Wilson et al., 1998). The FGRS was calculated using the tool developed by D’Agostino et al. (2008) for estimating 10-year risk of cardiovascular disease events. The total-to-HDL cholesterol ratio was calculated by dividing total cholesterol by HDL cholesterol.
2.5. Statistical analysis
The data were analyzed using SPSS version 22 (SPSS, Inc.; Chicago, Illinois). Patients with non-affective psychoses and controls were analyzed separately. A one-sample Kolmogorov-Smirnov test was used to examine each variable for normality. Age, PANSS scores, lymphocytes, and monocytes were normally distributed in subjects with non-affective psychoses; BMI and SES were normally distributed in controls. All other variables were not normally distributed, and were log transformed prior to the analyses. Demographic and clinical characteristics, and blood analyses in patients and controls were analyzed using either Student’s t-test (2-sided), Mann-Whitney U, or Fisher’s exact test (2-sided). Binary correlation coefficients (Spearman’s rho) were calculated between immune/inflammatory parameters (total WBC, neutrophils, lymphocytes, monocytes, and hsCRP), the three indices of cardiovascular disease risk, and other demographic and clinical variables, including inpatient versus outpatient status, illness duration, PANSS scores, antipsychotic dose (chlorpromazine units), age, sex, race, body mass index (BMI), smoking (number of cigarettes per day), SES, and alcohol use (DALI alcohol score). Given the large number of potential confounding variables and the number of subjects in the study, only those demographic and clinical variables correlated with indices of cardiovascular disease risk with a p-value of ≤0.10 were included in the regression models. Linear regression models were used to evaluate immune/inflammatory parameters as predictors of the cardiovascular disease risk indices, after controlling for potential confounding factors. For the FRS, potential confounders included PANSS total score, illness duration, and DALI alcohol score. For the FGRS, potential confounders included PANSS total score, illness duration, and BMI. For the total-to-HDL cholesterol ratio, potential confounders included PANSS positive score and BMI. Age, sex, and and smoking were three variables used in determining the FRS and FGRS, and so were not considered in the corresponding linear regression models. Binary logistic regression models were used to evaluate immune/inflammatory parameters as predictors of elevated FRS (10-year estimated risk >10%) and FGRS (10-year estimated risk >20%), and elevated total-to-HDL cholesterol ratio (>5.0). For the elevated FRS, illness duration was a potential confounder. For elevated FGRS, potential confounders included illness duration and race. For elevated total-to-HDL cholesterol ratio, none of the demographic and clinical variables met the p≤0.10 threshold for inclusion in logistic regression. For all regression models, a backwards model building strategy was used to arrive at a final model controlling for potential confounders. All potential confounders, regardless of statistical significance were included in a full regression model. The least significant potential confounder was removed from the model and a −2log likelihood test was performed to examine whether or not the variable was needed in the model or not. Additionally, the effect of removing the potential confounder on the association between a given immune/inflammatory parameter and cardiovascular disease risk index was assessed. Variables that did not result in a significant −2log likelihood test or did not change the estimated association between immune/inflammatory parameter and cardiovascular disease risk index were removed from the model. The final model resulted in those variables that were statistically significant confounders, changed the association significantly, or resulted in a significant −2log likelihood test. For all analyses, results were considered statistically significant at the α=0.05 level (two-sided). As FRS were significantly higher in males than females with non-affective psychosis, in post-hoc analyses we repeated the primary analyses stratified by sex.
3. Results
A total of 152 subjects—108 patients and 44 controls—were included in the study. Table 1 presents the demographic and clinical characteristics of the study sample. The patient and control groups did not differ with regard to age, sex, BMI, or alcohol use. Patients with non-affective psychosis were more likely to be of African descent, smoked more, and had lower SES than controls (p<0.05 for each). Patients with non-affective psychosis also had significantly higher hsCRP levels (p=0.04), but not total and differential WBC counts compared to controls. Patients with non-affective psychosis had significantly higher FRS and FGRS than controls (p≤0.01 for each), but there was no difference in the total-to-HDL cholesterol ratio between subject groups (p=0.56). The prevalence of elevated scores on the cardiovascular disease risk indices did not differ between patients and controls. Among patients with non-affective psychosis, 11% had a FRS of >10%, and 12% had a FGRS >20% (i.e., a >10% estimated risk of myocardial infarction or >20% estimated risk of cardiovascular disease, respectively, in the next 10 years).
Table 1.
Demographic and Clinical Characteristics of the Study Sample
| Variable | Patients | Controls | p-value+* | ||||
|---|---|---|---|---|---|---|---|
| All (n=108) | Males (n=59) | Females (n=49) | All (n=44) | Males (n=19) | Females (n=25) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 41.8 (12.6) | 38.4 (12.9) | 45.8 (10.9) | 37.3 (14.2) | 37.4 (13.7) | 37.2 (14.9) | 0.08 |
| Smoking (cigarettes/day) | 7.3 (10.3) | 8.9 (10.7) | 5.4 (10.7) | 1.5 (4.3) | 0.4 (1.2) | 2.3 (5.4) | <0.01 |
| BMI (kg/m2) | 30.9 (7.6) | 30.9 (8.1) | 31.0 (7.1) | 28.5 (5.2) | 29.6 (4.1) | 27.7 (5.8) | 0.14 |
| SES | 33 (15) | 30 (13) | 37 (16) | 53 (11) | 54 (10) | 53 (12) | <0.01 |
| DALI Alcohol Score | −1.6 (2.1) | −1.0 (2.1) | −2.3 (1.9) | −1.5 (2.1) | −0.5 (1.8) | −2.2 (2.0) | 0.78 |
| WBC (×103/μL) | 6.4 (2.2) | 6.6 (2.2) | 6.1 (2.1) | 5.9 (2.2) | 6.0 (1.5) | 5.8 (2.6) | 0.12 |
| Neutrophils (×103/μL) | 3.7 (1.8) | 3.8 (1.9) | 3.5 (1.7) | 3.4 (1.8) | 3.5 (1.2) | 3.4 (2.2) | 0.33 |
| Lymphocytes (×103/μL) | 2.0 (0.6) | 2.0 (0.6) | 2.0 (0.7) | 1.8 (0.5) | 1.8 (0.3) | 1.8 (0.6) | 0.10 |
| Monocytes (×103/μL) | 0.41 (0.17) | 0.45 (0.17) | 0.36 (0.16) | 0.40 (0.21) | 0.45 (0.21) | 0.36 (0.21) | 0.43 |
| High-sensitivity CRP (mg/L) | 7.3 (10.9) | 4.9 (6.9) | 10.2 (13.9) | 3.5 (3.9) | 4.5 (4.9) | 2.7 (2.6) | 0.04 |
| Framingham Risk Score (%) | 3.6 (5.9) | 5.2 (6.8) | 1.6 (3.6) | 2.2 (4.8) | 4.0 (6.7) | 0.7 (1.2) | 0.01 |
| Framingham General Cardiovascular Risk Score (%) | 8.7 (8.5) | 9.9 (9.5) | 7.2 (6.8) | 6.1 (8.4) | 8.2 (10.8) | 4.5 (5.7) | <0.01 |
| Total : HDL Cholesterol Ratio | 3.9 (1.6) | 4.0 (1.5) | 3.8 (1.7) | 3.7 (1.0) | 3.8 (0.8) | 3.6 (1.1) | 0.56 |
| Age at first hospitalization for psychosis | 25 (10) | 23 (8) | 29 (11) | <0.01 | |||
| Antipsychotic dose (CPZ equivalents) | 397 (467) | 418 (496) | 370 (431) | 0.93 | |||
| PANSS Positive | 19 (7) | 19 (7) | 19 (6) | 0.67 | |||
| PANSS Negative | 17 (6) | 17 (6) | 16 (5) | 0.22 | |||
| PANSS General | 37 (10) | 37 (10) | 38 (12) | 0.69 | |||
| PANSS Total | 73 (18) | 73 (17) | 73 (19) | 0.99 | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | p-value+** | |
|
| |||||||
| Sex (male) | 59 (55) | 19 (43) | 0.22 | ||||
| Race | |||||||
| Caucasian | 35 (32) | 18 (31) | 17 (35) | 22 (50) | 13 (68) | 9 (36) | 0.02 |
| African Descent | 69 (64) | 38 (64) | 31 (63) | 16 (36) | 5 (26) | 11 (44) | |
| Western Asian | 1 (1) | 1 (2) | 0 (0) | 2 (5) | 1 (5) | 1 (4) | |
| Hispanic | 2 (2) | 1 (2) | 1 (2) | 3 (7) | 0 (0) | 3 (12) | |
| Other | 1 (1) | 1 (2) | 0 (0) | 1 (2) | 0 (0) | 1 (4) | |
| Framingham Risk Score >10% | 11 (11) | 10 (17) | 1 (2) | 4 (10) | 4 (22) | 0 (0) | 1.00 |
| Framingham General Cardiovascular Risk Score >20% | 12 (12) | 10 (17) | 2 (4) | 4 (10) | 3 (17) | 1 (4) | 1.00 |
| Total : HDL Cholesterol Ratio >5.0 | 16 (15) | 10 (17) | 6 (13) | 5 (12) | 2 (11) | 3 (13) | 0.80 |
P-values are for comparisons between all patients and all controls
Mann-Whitney U test was used for all comparisons, except PANSS Total, Positive, and General scores, which were analyzed using Student’s t-test, 2-sided
Fisher’s exact test, 2-sided was used for all comparisons
Males with non-affective psychosis had significantly higher absolute and elevated FRS than females (p≤0.02 for each), but there were no differences in the FGRS and total-to-HDL cholesterol ratio by sex. Males with non-affective psychosis had significantly higher monocyte counts (p<0.01) than females, and females with non-affective psychosis had a trend for higher hsCRP levels than males (p=0.06); there were no other differences in immune/inflammatory parameters by sex. There was also no difference in antipsychotic dose (in CPZ equivalents) between males and females with non-affective psychosis. In subjects with non-affective psychosis, there were no differences in immune/inflammatory parameters or cardiovascular risk indices by race or clinical status (i.e., inpatient versus outpatients). In controls, there were no significant differences in immune/inflammatory parameters or cardiovascular risk indices by sex or race, except for higher monocytes in Caucasians versus subjects of African descent (p<0.01).
Table 2 presents the binary correlations between immune/inflammatory parameters and cardiovascular disease risk indices in patients and controls. In subjects with non-affective psychosis, the FGRS was significantly positively correlated with hsCRP levels (ρ=0.23, p=0.02), and lymphocyte and monocyte counts were significantly positively correlated with the total-to-HDL cholesterol ratio (p≤0.02 for each). The FRS was positively correlated with monocyte counts at the trend level (p=0.07). In controls, there were no significant correlations between immune/inflammatory parameters and cardiovascular risk indices. The pattern of the correlational results was similar when restricting to subjects with schizophrenia.
Table 2.
Binary correlations between immune/inflammaotry parameters and cardiovascular risk inices in patients with non-affective psychoses
| 2a. All subjects | |||||||
|---|---|---|---|---|---|---|---|
| Subject Group | Parameter | Framingham Risk Score | Framingham General Cardiovascular Risk Score | Total : HDL Cholesterol Ratio | |||
| ρ | p-value | ρ | p-value | ρ | p-value | ||
| Patients | WBC | 0.12 | 0.24 | 0.12 | 0.22 | 0.15 | 0.14 |
| Neutrophils | 0.09 | 0.35 | 0.11 | 0.27 | 0.03 | 0.74 | |
| Lymphocytes | 0.12 | 0.23 | 0.12 | 0.23 | 0.28 | <0.01 | |
| Monocytes | 0.18 | 0.07 | 0.09 | 0.35 | 0.23 | 0.02 | |
| hsCRP | 0.06 | 0.55 | 0.23 | 0.02 | 0.16 | 0.10 | |
|
| |||||||
| Controls | WBC | 0.08 | 0.60 | 0.07 | 0.67 | 0.22 | 0.16 |
| Neutrophils | 0.24 | 0.14 | 0.26 | 0.10 | 0.31 | 0.05 | |
| Lymphocytes | −0.28 | 0.08 | −0.29 | 0.06 | −0.05 | 0.76 | |
| Monocytes | 0.01 | 0.95 | −0.04 | 0.82 | 0.07 | 0.66 | |
| hsCRP | 0.15 | 0.34 | 0.30 | 0.06 | 0.23 | 0.14 | |
| 2b. Males | |||||||
|---|---|---|---|---|---|---|---|
| Subject Group | Parameter | Framingham Risk Score | Framingham General Cardiovascular Risk Score | Total : HDL Cholesterol Ratio | |||
| ρ | p-value | ρ | p-value | ρ | p-value | ||
| Patients | WBC | 0.26 | 0.05 | 0.27 | 0.04 | 0.42 | <0.01 |
| Neutrophils | 0.26 | 0.05 | 0.28 | 0.03 | 0.24 | 0.07 | |
| Lymphocytes | 0.14 | 0.29 | 0.13 | 0.33 | 0.49 | <0.01 | |
| Monocytes | 0.21 | 0.12 | 0.17 | 0.20 | 0.49 | <0.01 | |
| hsCRP | 0.20 | 0.13 | 0.26 | 0.05 | 0.23 | 0.08 | |
|
| |||||||
| Controls | WBC | 0.22 | 0.39 | 0.49 | 0.04 | 0.60 | 0.01 |
| Neutrophils | 0.26 | 0.30 | 0.51 | 0.03 | 0.46 | 0.06 | |
| Lymphocytes | −0.39 | 0.11 | −0.18 | 0.47 | 0.48 | 0.04 | |
| Monocytes | 0.21 | 0.41 | 0.36 | 0.14 | 0.27 | 0.29 | |
| hsCRP | −0.02 | 0.93 | 0.20 | 0.44 | 0.21 | 0.41 | |
| 2c. Females | |||||||
|---|---|---|---|---|---|---|---|
| Subject Group | Parameter | Framingham Risk Score | Framingham General Cardiovascular Risk Score | Total : HDL Cholesterol Ratio | |||
| ρ | p-value | ρ | p-value | ρ | p-value | ||
| Patients | WBC | −0.10 | 0.50 | −0.08 | 0.59 | −0.13 | 0.39 |
| Neutrophils | −0.15 | 0.33 | −0.11 | 0.49 | −0.21 | 0.17 | |
| Lymphocytes | 0.13 | 0.39 | 0.10 | 0.53 | 0.01 | 0.98 | |
| Monocytes | 0.10 | 0.51 | −0.06 | 0.72 | −0.08 | 0.61 | |
| hsCRP | 0.03 | 0.87 | 0.23 | 0.13 | 0.10 | 0.53 | |
|
| |||||||
| Controls | WBC | −0.13 | 0.57 | −0.21 | 0.34 | −0.06 | 0.77 |
| Neutrophils | 0.10 | 0.66 | 0.05 | 0.83 | 0.08 | 0.70 | |
| Lymphocytes | −0.29 | 0.18 | −0.34 | 0.12 | −0.29 | 0.18 | |
| Monocytes | −0.26 | 0.24 | −0.34 | 0.11 | −0.04 | 0.85 | |
| hsCRP | 0.29 | 0.18 | 0.37 | 0.08 | 0.03 | 0.88 | |
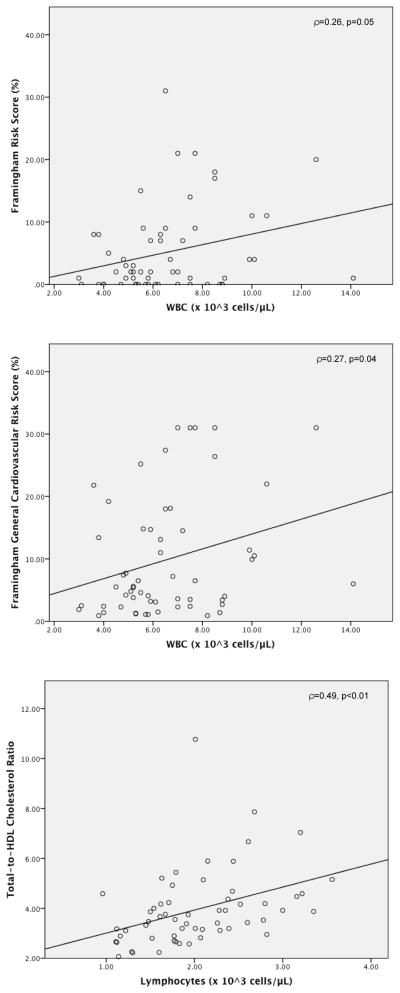
Post-hoc analyses demonstrated stronger associations between immune/inflammatory parameters and cardiovascular risk indices in males, as shown in Table 2 and Figure 1. In males with non-affective psychosis, both the FRS and FGRS were significantly positively correlated with total WBC and neutrophil counts (p<0.05 for each). The FGRS was also significantly positively correlated with hsCRP levels (p<0.05). The FGRS was also significantly positively correlated with total WBC (ρ=0.25, p<0.05) and neutrophil (ρ=0.26, p=0.03) counts. Total WBC, lymphocyte, and monocyte counts were all significantly positively correlated with the total-to-HDL cholesterol ratio (p<0.01 for each). In male controls, the FGRS was significantly positively correlated with total WBC and neutrophil counts (p≤0.04 for each). Total WBC and lymphocyte counts were significantly positively correlated with the total-to-HDL cholesterol ratio (p≤0.04 for each). By contrast, in female patients and controls, there were no significant correlations between any immune/inflammatory parameters and cardiovascular disease risk indices.
Figure 1.
Correlations between immune parameters and cardiovascular risk scores in male patients with non-affective psychoses
Table 3 shows the results of the linear regression analyses. After controlling for potential confounders, in subjects with non-affective psychosis, monocyte (p=0.02) and lymphocyte (p=0.01) counts were significant predictors of the total-to-HDL cholesterol ratio. In controls, there were no significant correlations between immune/inflammatory parameters and cardiovascular risk indices.
Table 3.
Linear regression anlayses of immune/inflammatory parameters as a predictor of cardiovascular risk indices in patients with non-affective psychoses
| 3a. All subjects | |||||||
|---|---|---|---|---|---|---|---|
| Subject Group | Parameter | Framingham Risk Score | Framingham General Cardiovascular Risk Score | Total : HDL Cholesterol Ratio | |||
| Beta | p-value | Beta | p-value | Beta | p-value | ||
| Patients | WBC | −0.12 | 0.66 | 0.08 | 0.38 | 0.07 | 0.50 |
| Neutrophils | −0.14 | 0.56 | 0.06 | 0.49 | −0.03 | 0.78 | |
| Lymphocytes | 0.11 | 0.55 | 0.06 | 0.52 | 0.28 | 0.01 | |
| Monocytes | 0.10 | 0.60 | 0.02 | 0.86 | 0.22 | 0.02 | |
| hsCRP | 0.28 | 0.11 | 0.11 | 0.28 | 0.09 | 0.43 | |
|
| |||||||
| Controls | WBC | −0.04 | 0.91 | −0.01 | 0.94 | 0.01 | 0.94 |
| Neutrophils | 0.10 | 0.79 | 0.16 | 0.31 | 0.15 | 0.36 | |
| Lymphocytes | −0.45 | 0.20 | −0.29 | 0.07 | −0.22 | 0.17 | |
| Monocytes | −0.17 | 0.66 | −0.03 | 0.85 | −0.07 | 0.66 | |
| hsCRP | −0.13 | 0.74 | 0.29 | 0.07 | 0.10 | 0.56 | |
| 3b. Males | |||||||
|---|---|---|---|---|---|---|---|
| Subject Group | Parameter | Framingham Risk Score | Framingham General Cardiovascular Risk Score | Total : HDL Cholesterol Ratio | |||
| Beta | p-value | Beta | p-value | Beta | p-value | ||
| Patients | WBC | −0.05 | 0.84 | 0.18 | 0.14 | 0.33 | 0.01 |
| Neutrophils | −0.17 | 0.53 | 0.15 | 0.18 | 0.07 | 0.62 | |
| Lymphocytes | 0.19 | 0.34 | 0.09 | 0.44 | 0.46 | <0.01 | |
| Monocytes | 0.14 | 0.50 | 0.01 | 0.91 | 0.46 | <0.01 | |
| hsCRP | 0.22 | 0.31 | 0.14 | 0.26 | 0.18 | 0.19 | |
|
| |||||||
| Controls | WBC | −0.69 | 0.23 | 0.34 | 0.17 | 0.46 | 0.04 |
| Neutrophils | −0.37 | 0.56 | 0.43 | 0.08 | 0.46 | 0.01 | |
| Lymphocytes | −0.53 | 0.22 | −0.24 | 0.33 | 0.33 | 0.13 | |
| Monocytes | −0.45 | 0.36 | 0.24 | 0.34 | 0.10 | 0.66 | |
| hsCRP | −0.33 | 0.50 | 0.10 | 0.70 | 0.21 | 0.36 | |
In males with non-affective psychosis, total WBC, monocyte, and lymphocyte counts were significant predictors of the total-to-HDL cholesterol ratio FRS (p≤0.01 for each). In male controls, WBC and neutrophil counts were significant predictors of the total-to-HDL cholesterol ratio (p≤0.04 for each). Linear regression analyses were not performed in females, since there were no significant bivariate correlations.
Table 4 shows the results of the binary logistic regression analyses. After controlling for potential confounders, in subjects with non-affective psychosis, total WBC, neutrophil, monocyte, and lymphocyte counts were all significant predictors of elevated FRS (p≤0.04 for each). Total WBC count was a significant predictor of elevated FGRS (p=0.03). Monocyte counts were a predictor of elevated total-to-HDL cholesterol ratio (p=0.02). A similar pattern of findings was observed in males with non-affective psychosis: a) Total WBC, neutrophil, monocyte, and lymphocyte counts were significant predictors of elevated FRS (p≤0.03 for each), b) WBC counts predicted elevated FGRS at the trend level (p=0.08), and c) WBC, monocyte, and lymphocyte counts were significant predictors of an elevated total-to-HDL cholesterol ratio (p≤0.04 for each). Binary logistic regression analyses were not performed in male controls, as the estimates were unstable due to the small sample size.
Table 4.
Binary logistic regression of immune/inflammatory parameters as a predictor of cardiovascular risk indices in patients with non-affective psychoses
| 4a. All subjects | ||||||
|---|---|---|---|---|---|---|
| Parameter | Framingham Risk Score > 10% | Framingham General Cardiovascular Risk Score > 20% | Total : HDL Cholesterol Ratio > 5.0 | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| WBC | 80.8 (2.8–2341) | 0.01 | 13.51 (1.25–146) | 0.03 | 2.54 (0.50–12.91) | 0.26 |
| Neutrophils | 7.84 (1.14–55.43) | 0.04 | 3.60 (0.77–16.78) | 0.10 | 1.52 (0.53–4.36) | 0.44 |
| Lymphocytes | 4.00 (1.23–12.99) | 0.02 | 2.21 (0.84–5.83) | 0.11 | 1.81 (0.81–4.07) | 0.15 |
| Monocytes | 757 (6.6–86699) | 0.01 | 23.3 (0.60–902) | 0.09 | 51.2 (2.1–1247) | 0.02 |
| hsCRP | 0.84 (0.48–1.46) | 0.54 | 1.21 (0.72–2.02) | 0.47 | 1.13 (0.77–1.65) | 0.54 |
| 4b. Males | ||||||
|---|---|---|---|---|---|---|
| Parameter | Framingham Risk Score > 10% | Framingham General Cardiovascular Risk Score > 20% | Total : HDL Cholesterol Ratio > 5.0 | |||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| WBC | 2448 (5.1–1170597) | 0.01 | 16.47 (0.75–364) | 0.08 | 14.11 (1.27–158) | 0.03 |
| Neutrophils | 29.2 (1.5–586) | 0.03 | 3.17 (0.44–22.94) | 0.25 | 3.24 (0.68–15.48) | 0.14 |
| Lymphocytes | 28.4 (2.3–351) | 0.01 | 6.86 (0.93–50.81) | 0.06 | 3.27 (1.06–10.05) | 0.04 |
| Monocytes | 1436 (2.3–909554) | 0.03 | 6.35 (0.02–1775) | 0.52 | 1867 (10.5–330811) | <0.01 |
| hsCRP | 0.96 (0.48–1.93) | 0.91 | 1.27 (0.59–2.72) | 0.55 | 1.35 (0.77–2.38) | 0.30 |
Post-hoc analyses were also performed to evaluate the potential influence of outlying values of WBC and hsCRP on results. Two patients with non-affective psychosis and one control had elevated WBC counts (>11×103/μL). Excluding these subjects did not alter the results. hsCRP levels >3 mg/L are considered elevated. Fifty-nine subjects with non-affective psychosis (55%) and 17 controls (40%) had a hsCRP >3 mg/L. Furthermore, hsCRP levels >10 mg/L may suggest the presence of an underlying inflammatory disease, although levels in this range can be seen on a genetic basis in ~6% of apparently healthy individuals (Kushner et al., 2006). Remarkably, 24 subjects with non-affective psychosis (22%) and 4 controls (9%) in our sample had a hsCRP >10 mg/L, in the absence of any evidence of intercurrent bacterial infection. After excluding these subjects, in non-affective psychosis, hsCRP levels were significantly positively correlated with FGRS (ρ=0.28, p=0.01) and the total-to-HDL cholesterol ratio (ρ=0.23, p=0.03). In linear regression, hsCRP levels were a significant predictor of FRS scores (p=0.03). In binary logistic regression, hsCRP levels were a significant predictor of an elevated total-to-HDL cholesterol ratio (OR=1.91, 95% CI=1.00–3.65, p=0.05). In males with non-affective psychosis, hsCRP levels were significant predictors of FGRS scores (p=0.04) and an elevated total-to-HDL cholesterol ratio (p=0.04), and predicted an elevated FGRS at the trend level (p=0.07).
4. Discussion
Subjects with non-affective psychoses had significantly higher hsCRP levels than controls. After controlling for potential confounders, monocyte and lymphocyte counts were a significant predictor of the total-to-HDL cholesterol ratio in subjects with non-affective psychosis. Furthermore, after controlling for potential confounders, total WBC count was a significant predictor of an elevated 10-year estimated risk of myocardial infarction and cardiovascular disease in subjects with non-affective psychosis. Associations between total and differential WBC counts and cardiovascular risk indices were stronger in males than females with non-affective psychosis. Total WBC and neutrophil counts were significant predictors of the total-to-HDL cholesterol ratio in male controls. In the primary analysis, hsCRP levels were not associated with cardiovascular disease risk indices in either patients with non-affective psychosis or controls. However, in a post-hoc analysis excluding subjects with a hsCRP >10 mg/L, hsCRP was associated with cardiovascular disease risk indices in subjects with non-affective psychosis.
We observed a somewhat discordant pattern of results in healthy controls, although our small sample size may have limited statistical power. Outside of schizophrenia in the general population, total WBC (Orakzai et al., 2007; Park et al., 2010; Spencer et al., 2007) and monocyte (Waterhouse et al., 2008) counts, as well as C-reactive protein levels (Choi et al., 2004; Park et al., 2010), have been associated with cardiovascular disease risk. In the Framingham Offspring study, increased total WBC counts were associated with incident atrial fibrillation (Rienstra et al., 2012). Taken, together, these findings suggesting the observed relationships may not be specific to subjects with non-affective psychoses.
The strengths of the present study include consideration of multiple potential confounding factors. We also extended our previous findings, showing that total and differential WBC counts and hsCRP levels are associated not only with metabolic syndrome, but also cardiovascular risk in non-affective psychosis. To our knowledge, ours is the first study to consider total and differential WBC counts as a predictor of cardiovascular disease risk indices in patients with schizophrenia and related disorders. Several potential limitations of the present study are the heterogeneity of the sample with respect to clinical status (although we did not find any differences in immune/inflammatory parameters or cardiovascular disease risk indices in inpatients versus outpatients), and the non-standardized antipsychotic treatment. Another limitation is the small sample size, particularly in healthy controls, which may have limited statistical power. Data were also not available to assess the contributions of diet, exercise, and family history of heart disease to cardiovascular disease risk. Additional studies in both patients and controls are needed to evaluate whether or not the observed relationships are specific to subjects with non-affective psychoses. Due to the cross-sectional design, our study does not permit inferences regarding the ability of baseline total and differential WBC counts (or hsCRP levels) to predict incident cardiovascular disease.
We found an association between hsCRP and FGRS, though only in a post-hoc analysis excluding subjects with substantially elevated hsCRP (>10 mg/L). Sicras-Mainar et al. (2013) found an association between CRP levels and FGRS. However, this study did not model the FGRS as a continuous variable, but rather trichotomized cardiovascular disease risk as low (<15%), moderate (15–20%), or high/very high (>20%). This study also had a much larger sample size (n=705) than ours. A potentially important difference is that we excluded subjects taking scheduled NSAIDs, whereas Sicras-Mainar et al. (2013) statistically controlled for inflammatory disease in their analysis. It is also possible that different polymorphisms in the gene for CRP moderately contributed to discordant findings. Another recent study found that higher blood levels of brain-derived neurotrophic factor (BDNF) were associated with higher FRS in 61 subjects with schizophrenia (Nurjono et al., 2013). They found that subjects in the highest quartile of BDNF levels had a 3.3-fold higher mean FRS than subjects in the lowest quartile. This finding is intriguing given potential relationships between BDNF and inflammation in schizophrenia (Hsu et al., 2009)
Mechanistically, inflammation is a leading hypothesis for the association between WBC counts, hsCRP levels and cardiovascular disease risk. Total WBC—even within the normal range—is a marker of (low-grade) inflammation, which can promote vascular injury and atherosclerosis (Madjid et al., 2004). Prospective studies provide evidence for an association between total and differential WBC counts and increased cardiovascular disease risk in healthy individuals (Wheeler et al., 2004). Endothelial dysfunction, which is induced by CRP, also plays a central role in atherosclerotic cardiovascular disease and is associated with all individual criteria for the metabolic syndrome (Devaraj et al., 2010). We found significantly higher hsCRP levels in subjects with non-affective psychosis compared to controls, consistent with findings from our previous meta-analysis (Miller et al., 2014).
Future studies in a larger sample are needed to replicate our findings. Leukocytes may be a source of pro-inflammatory cytokines, which are abnormal in schizophrenia (Miller al., 2011). Thus, future studies could investigate the relationship between individual cytokines and cytokine networks and cardiovascular disease risk indices in non-affective psychosis. There is a need for prospective, longitudinal studies to examine the capacity of these immune/inflammatory parameters to predict future development of incident cardiovascular disease in patients. Another potentially important issue is whether genetic polymorphisms in CRP and/or immune-related genes moderate cardiovascular disease risk in patients with non-affective psychosis.
A recent meta-analysis of 77 publications found that approximately one-third of patients with schizophrenia meet criteria for the metabolic syndrome (Mitchell et al., 2011), a major risk factor for cardiovascular disease. Despite the American Diabetes Association/American Psychiatric Association consensus guidelines for metabolic monitoring of patients on antipsychotic drugs (2004), monitoring rates in clinical practice remain alarmingly low (Mitchell et al., 2012). Our findings provide additional evidence that measurement of blood total and differential WBC counts and hsCRP levels may be germane to the clinical care of patients with schizophrenia and related disorders. Advantages of WBC counts include that this marker is widely available, routinely ordered, inexpensive, and easy to interpret. Taken together, our results provide contribute to a growing body of evidence for an association between inflammation and cardiovascular disease risk in these patients.
Acknowledgments
The authors thank Courtney Caulder, Laura Meyer, Dawn Montoya, Becca Nichols, Niju Philip, Edna Stirewalt, and Christy Wise for assistance.
Funding
This study was funded, in part, by National Institute of Mental Health grant K23MH098014 (to Dr. Miller).
Footnotes
Disclosures
Dr. Miller has received grant/research support from the National Institute of Mental Health (NIMH), the American Psychiatric Association, and Georgia Regents University; and honoraria from Insight Consulting Group, Medscape, and Psychiatric Times.
Ms. Kandhal has nothing to disclose.
Dr. Rapaport is a member of the scientific advisory board for Pax, Inc. (unpaid) and the Depression and Bipolar Alternative Therapies Foundation, and a consultant for the American Psychiatric Association.
Dr. Mellor received funding support from the NIH, the Juvenile Diabetes Research Foundation, and the Carlos and Marguerite Mason Trust. Dr. Mellor is a member of the Scientific Advisory Board of NewLink Genetics Inc., and receives compensation for this service.
Dr. Buckley has served as a consultant for the NIMH, and has received grant/research support from Sunovion and the NIMH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65:267–272. doi: 10.4088/jcp.v65n0219. [DOI] [PubMed] [Google Scholar]
- Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry. 1997;171:502– 508. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- Choi H, Cho DH, Shin HH, Park JB. Association of high sensitivity C-reactive protein with coronary heart disease prediction, but not with carotid atherosclerosis, in patients with hypertension. Circ J. 2004;68:297–303. doi: 10.1253/circj.68.297. [DOI] [PubMed] [Google Scholar]
- Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, Njølstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM SCORE project group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Valleggi S, Siegel D, Jialal I. Role of C-reactive protein in contributing to increased cardiovascular risk in metabolic syndrome. Curr Atheroscler Rep. 2010;12:110–118. doi: 10.1007/s11883-010-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Liu EY, Freudenreich O, Park JH, Liu D, Wang J, Yi Z, Goff D, Henderson DC. Higher white blood cell counts are associated with an increased risk for metabolic syndrome and more severe psychopathology in non-diabetic patients with schizophrenia. Schizophr Res. 2010;118:211–217. doi: 10.1016/j.schres.2010.02.1028. [DOI] [PubMed] [Google Scholar]
- Fennig S, Craig T, Lavelle J, Kovasznay B, Bromet EJ. Best-Estimate Versus Structured Interview-Based Diagnosis in First-Admission Psychosis. Comprehensive Psychiatry. 1994;35:341–348. doi: 10.1016/0010-440x(94)90273-9. [DOI] [PubMed] [Google Scholar]
- Ford P. An evaluation of the Dartmouth Assessment of Lifestyle Inventory and the Leeds Dependence Questionnaire for use among detained psychiatric inpatients. Addiction. 2003;98:111–118. doi: 10.1046/j.1360-0443.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. 2006;119:812–819. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, D’Agostino RB, Stroup TS, Davis S, Lieberman JA. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11– 53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social Class and Mental Illness. New York: Wiley; 1958. pp. 387–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Folsom D, Sasaki A, Mudaliar S, Henry R, Torres M, Golshan S, Glorioso DK, Jeste D. Increased Framingham 10-year risk of coronary heart disease in middle-aged and older patients with psychotic symptoms. Schizophr Res. 2011;125:295–299. doi: 10.1016/j.schres.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119:e17–28. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, Dagenais GR, Després JP. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: the Quebec Cardiovascular Study. Arch Intern Med. 2001;161:2685–2692. doi: 10.1001/archinte.161.22.2685. [DOI] [PubMed] [Google Scholar]
- Lichtermann D, Karbe E, Maier W. The genetic epidemiology of schizophrenia and of schizophrenia spectrum disorders. Eur Arch Psychiatry Clin Neurosci. 2000;250:304–310. doi: 10.1007/s004060070005. [DOI] [PubMed] [Google Scholar]
- Madjid M, Awan I, Willerson JT, Casscells SW. Leukocyte count and coronary heart disease. Implications for risk assessment. J Am Coll Cardiol. 2004;44:1946–1956. doi: 10.1016/j.jacc.2004.07.056. [DOI] [PubMed] [Google Scholar]
- McCreadie RG Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry. 2003;183:534–539. doi: 10.1192/bjp.183.6.534. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Scott Stroup T, Lieberman JA. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80m:19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Paschall CB, Svendsen DP. Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv. 2006;57:1482–1487. doi: 10.1176/ps.2006.57.10.1482. [DOI] [PubMed] [Google Scholar]
- Miller B, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biological Psychiatry. 2013;73:993–999. doi: 10.1016/j.biopsych.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Mellor A, Buckley P. Total and Differential White Blood Cell Counts, High- Sensitivity C-Reactive Protein, and the Metabolic Syndrome in Non-Affective Psychoses. Brain, Behavior, and Immunity. 2013b;31:82–89. doi: 10.1016/j.bbi.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Culpepper N, Rapaport MH. C-Reactive Protein in Schizophrenia: A Review and Meta-Analysis. Clinical Schizophrenia and Related Psychoses. 2014;7:223–230. [PubMed] [Google Scholar]
- Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42:125–147. doi: 10.1017/S003329171100105X. [DOI] [PubMed] [Google Scholar]
- Nurjono M, Tay YH, Lee J. The relationship between serum brain-derived neurotrophic factor (BDNF) and cardiometabolic indices in schizophrenia. Schizophr Res. 2014 doi: 10.1016/j.schres.2014.05.024. [DOI] [PubMed] [Google Scholar]
- Orakzai SH, Orakzai RH, Nasir K, Carvalho JA, Blumenthal RS, Santos RD. Relationship between white blood cell count and Framingham Risk Score in asymptomatic men. Arch Med Res. 2007;38:386–391. doi: 10.1016/j.arcmed.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Park CS, Ihm SH, Yoo KD, Kim DB, Lee JM, Kim HY, Chung WS, Seung KB, Kim JH. Relation between C-reactive protein, homocysteine levels, fibrinogen, and lipoprotein levels and leukocyte and platelet counts, and 10-year risk for cardiovascular disease among healthy adults in the USA. Am J Cardiol. 2010;105:1284–1288. doi: 10.1016/j.amjcard.2009.12.045. [DOI] [PubMed] [Google Scholar]
- Ratliff JC, Palmese LB, Reutenauer EL, Srihari VH, Tek C. Obese schizophrenia spectrum patients have significantly higher 10-year general cardiovascular risk and vascular ages than obese individuals without severe mental illness. Psychosomatics. 2013;54:67–73. doi: 10.1016/j.psym.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienstra M, Sun JX, Magnani JW, Sinner MF, Lubitz SA, Sullivan LM, Ellinor PT, Benjamin EJ. White blood cell count and risk of incident atrial fibrillation (from the Framingham Heart Study) Am J Cardiol. 2012;109:533–537. doi: 10.1016/j.amjcard.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- Said MA, Sulaiman AH, Habil MH, Das S, Bakar AK, Yusoff RM, Loo TH, Bakar SA. Metabolic syndrome and cardiovascular risk among patients with schizophrenia receiving antipsychotics in Malaysia. Singapore Med J. 2012;53:801–807. [PubMed] [Google Scholar]
- Sicras-Mainar A, Rejas-Gutiérrez J, Navarro-Artieda R, Blanca-Tamayo M. C-reactive protein as a marker of cardiovascular disease in patients with a schizophrenia spectrum disorder treated in routine medical practice. Eur Psychiatry. 2013;28:161–167. doi: 10.1016/j.eurpsy.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Spencer CG, Felmeden DC, Blann AD, Lip GY. Haemorheological, platelet and endothelial indices in relation to global measures of cardiovascular risk in hypertensive patients: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial. J Intern Med. 2007;261:82–90. doi: 10.1111/j.1365-2796.2006.01735.x. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013;170:1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay YH, Nurjono M, Lee J. Increased Framingham 10-year CVD risk in Chinese patients with schizophrenia. Schizophr Res. 2013;147:187–192. doi: 10.1016/j.schres.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Waterhouse DF, Cahill RA, Sheehan F, McCreery C. Prediction of calculated future cardiovascular disease by monocyte count in an asymptomatic population. Vasc Health Risk Manag. 2008;4:177–187. doi: 10.2147/vhrm.2008.04.01.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler JG, Mussolino ME, Gillum RF, Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30,374 individuals. Eur Heart J. 2004;25:1287–1292. doi: 10.1016/j.ehj.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of Coronary Heart Disease Using Risk Factor Categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine Equivalent Doses for Atypical Antipsychotics: An Update 2003–2010. 2011 doi: 10.4088/jcp.v64n0607. http://scottwilliamwoods.com/equivalencesupdate.php. [DOI] [PubMed]
- Wysokiński A, Kowman M, Kłoszewska I. The prevalence of metabolic syndrome and Framingham cardiovascular risk scores in adult inpatients taking antipsychotics - a retrospective medical records review. Psychiatr Danub. 2012;24:314–322. [PubMed] [Google Scholar]
- Yazici MK, Anil Yağcioğlu AE, Ertuğrul A, Eni N, Karahan S, Karaağaoğlu E, Tokgözoğlu SL. The prevalence and clinical correlates of metabolic syndrome in patients with schizophrenia: findings from a cohort in Turkey. Eur Arch Psychiatry Clin Neurosci. 2011;261:69–78. doi: 10.1007/s00406-010-0118-x. [DOI] [PubMed] [Google Scholar]