Abstract
There are no clinical studies on the effects of catheter‐based radiofrequency renal denervation (RDN) on renal artery structure using 64‐detector computed tomography (CT). A total of 39 patients with resistant hypertension received RDN and 38 patients received drug treatment. Mean systolic pressure and diastolic pressure in the RDN group decreased after 1, 3, 6, and 12 months of procedure (P<.05) and urinary protein level significantly decreased after 6 and 12 months (P<.05). The diameter, length, and sectional area of the renal artery; number of cases of atherosclerosis; and plaque burden of 64‐detector CT renal arteriography did not change at 12 months of follow‐up (P<.05), whereas the plaque burden increased significantly in the control group (P<.05). RDN significantly and persistently reduced blood pressure and decreased urinary protein excretion rate in patients with resistant hypertension and did not exhibit any adverse effect on renal function and renal artery structure.
Hypertension is one of the most common cardiovascular diseases, with an incidence rate in adults of approximately 18.8%. After nearly a century of hypertension studies, scientific understanding of the etiology, pathology, and pathogenesis of hypertension has made great progress. New studies on the role of the sympathetic adrenergic system and the renin‐angiotensin system in the pathogenesis, progression, and target organ damage of hypertension have grown increasingly deeper, and new perspectives on the goals of hypertension treatment have been proposed based on these studies. Krum and colleagues1, 2, 3 reported using catheter‐based radiofrequency renal denervation (RDN) technology to treat resistant hypertension and proved that RDN could cause persistent and long‐term blood pressure (BP) reduction in patients. This method was safe and did not cause obvious complications. However, there have been few reports on the effects of RDN on the renal function of patients, and the observation time has been short (only 6 months). In particular, there are no clinical reports on the effects of RDN on renal artery structure. This study used a nonrandomized concurrent control method to investigate the effectiveness and safety of catheter‐based RDN on resistant hypertension and its effect on renal function. This study also, for the first time, used multidetector spiral computed tomography angiography (MSCTA) to investigate the changes in renal artery structure, including changes in diameter, length, sectional area, atherosclerosis, and plaque burden.
Methods
Study Patients: Inclusion Criteria
A total of 81 patients with resistant hypertension who were consecutively treated in the Department of Cardiology of the Third Xiangya Hospital of Central South University from October 2011 to February 2013 were enrolled. Four patients were excluded for anatomical reasons (mainly on the basis of dual renal artery systems). A total of 39 cases received catheter‐based RDN, and 38 cases continuously received drug treatment. There were 40 men and 37 women, and the average age was 61.7±13.5 years. There were 13 patients with confirmed diabetes and 10 with sleep apnea syndrome. Resistant hypertension refers to patients with office BP (systolic pressure) ≥160 mm Hg (patients with type 2 diabetes ≥150 mm Hg) after improvements in lifestyle and taking sufficient dosages of at least 3 types of antihypertensive drugs, including diuretics. Exclusion criteria included age younger than 18 years, secondary hypertension, type 1 diabetes, or abnormal anatomy of the renal artery (multiple renal arteries with diameter <4 mm, trunk length of the main artery <20 mm, and dual renal artery systems). Patients with renal artery stenosis >50% or patients who had received renal artery balloon angioplasty or stenting were excluded. Patients with glomerular filtration rates (GFR) <45 mL/(min 1.73 m2) (calculated using the Modification of Diet in Renal Disease [MDRD] formula or represented by cystatin C) were also excluded. This study was approved by the ethics committee of the Third Xiangya Hospital of Central South University, and all enrolled patients signed informed consent forms.
RDN Procedure
An 8F RDC guiding catheter (Johnson and Johnson, New Brunswick, NJ) was introduced into the renal artery opening through the sheath. The patient underwent bilateral renal arteriography to confirm the location of the renal artery openings.1, 2 The 5F standard radiofrequency catheter (IBI, St. Jude Medical, St. Paul, MN) was introduced into the distal renal artery through the guiding catheter. The ablation was conducted at 4 spots from far to near in a spiral shape. Each ablation was conducted using 8 W for 2 minutes (Figure 1 and Figure 2). During ablation, the temperature and impedance of the tip of the catheter were monitored. The fluctuation of temperature during ablation was between 45°C and 55°C. Morphine and fentanyl were given during the procedure for analgesia. The patients were also given heparin 100 IU/kg to prevent coagulation.
Figure 1.
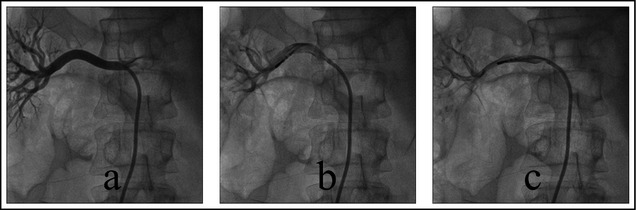
(a) Right renal arteriography before ablation. (b) Ablation of lower wall of right renal artery. (c) Ablation of anterior wall of right renal artery.
Figure 2.
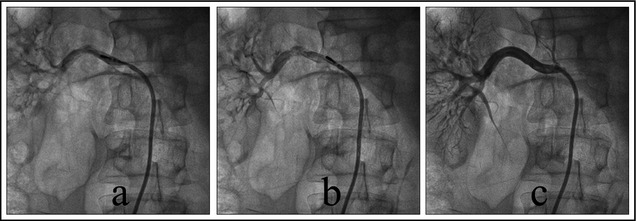
(a) Ablation of posterior wall of right renal artery. (b) Ablation of upper wall of right renal artery. (c) Right renal arteriography after ablation.
Monitoring Indicators and Follow‐Up
After admission, the patients rested for approximately 15 minutes. The sitting BP of the patients was measured 3 times using an Omron BP monitor, and the mean of these 3 BP results was calculated. The morning after admission, venous blood was collected to perform examinations such as routine blood tests, blood electrolyte tests, and liver function tests. The levels of serum creatinine were measured using the chemiluminescence method. The GFR was calculated using the MDRD equation or using cystatin C to represent the estimated GFR (eGFR). The simplified modification of diet in the renal disease study equation was: eGFR = 186 × [serum creatinine (μm)/88.4]−1.154 × age−0.203 × (0.742 if female). Further monitoring included 24‐hour urinary protein quantitation, electrocardiography (ECG), ambulatory BP monitoring, echocardiogram, urinary tract ultrasonography, and MSCTA of the renal artery. The BP of patients at 1 week and at 1, 3, 6, and 12 months after RDN were measured through outpatient follow‐up. The serum creatinine levels and 24‐hour urinary protein quantitation of the patients were measured at 1, 3, 6, and 12 months after RDN. Renal color ultrasound was performed again 6 months after RDN, and MSCTA of the renal artery was re‐examined 12 months after RDN.
64‐Detector MSCTA
The 64‐detector MSCTA of the renal artery was performed using a Philips Brilliance 64‐detector spiral CT (Philips, Amsterdam, The Netherlands). The contrast agent was iohexol (350 mg/mL), the dose was 11.5 of 21.0 mL/kg body mass, and the injection speed was 15–21.5 mL/s. At 15 to 20 seconds after the injection, retrospective ECG‐gated scanning of the renal artery was performed using artificial intelligence trigger scanning. The post‐processing method used the preview function to confirm the best imaging phase of the renal artery to obtain cross‐sectional images of layers 0.75 mm in thickness. In the synchronized workstation, reconstruction was performed using the methods of volume imaging, thin‐section maximum‐intensity projection, and surface reconstruction. After reconstruction, all data measurements were performed using the workstation: the diameter of the lumen of the main renal artery (the average of the diameter of the blood vessel at the opening of the renal artery and the diameter of the blood vessel before the main branch), the length, and cross‐sectional area of the lumen (L‐CSA) (the average of the cross‐sectional area of the lumen at the opening of the renal artery and the cross‐sectional area of the lumen before the first level of the main branch). If renal atherosclerosis was present, then the following measurements were determined: stenosis degree = lumen area of stenosis location/lumen area of reference blood vessel × 100%, plaque area = blood vessel area‐lumen area, and plaque burden = plaque area/blood vessel area × 100%. Selection of the reference blood vessel: The ideal was to select the location that was at the proximal or distal end of the lesion section, was nearest to normal, and did not have large branching from that point; the average value was the area of the reference blood vessel within 10 mm of the measurement point. The measurements were performed by two physicians with extensive experience in abdominal vascular imaging diagnosis (Figure 3).
Figure 3.
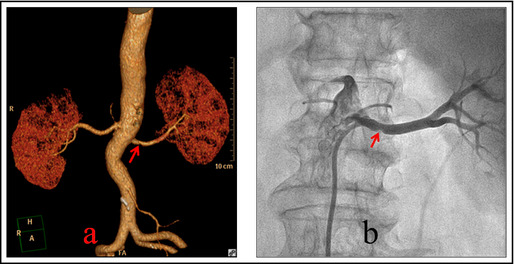
(a) Multidetector computed tomography angiography of bilateral renal arteries and 3‐dimensional imaging of renal artery. The arrow points to the middle section of the right renal artery where atherosclerosis plaque could be observed. The stenosis of the renal artery was 32.8% and plaque was present. (b) The right renal arteriography during radiofrequency renal denervation of the same patient. The arrow points to the middle section of the right renal artery where atherosclerosis plaque could be observed.
Adverse Reaction
Three cases had femoral artery hematoma; after treatment with compression bandaging, the hematoma did not increase. Re‐examination after 1 month showed that the hematoma was completely absorbed and did not leave any sequelae. Two cases had frequent vomiting, which was considered to be associated with fentanyl; the symptom was treated and disappeared.
Statistical Analysis
Statistical analysis was performed using SPSS 17.0 software (SPSS Inc, Chicago, IL). All data were presented as the mean±standard deviation. The change in BP after RDN was analyzed to estimate the effectiveness of the experiment. The mean BP change was calculated using the baseline BP and 95% confidence interval. The BP changes at different time intervals before and after radiofrequency was analyzed using repeated‐measures analysis of variance. The diameter, length, and sectional area of the renal artery and the changes in plaque burden before and after RDN were confirmed using the paired t test.
Results
Baseline Condition of Patients
The baseline conditions of age, sex, body weight index, mean arterial pressure, heart rate, creatinine, and medication of enrolled patients are shown in Table 1. The differences in baseline data such as age, diabetes, BP, types of antihypertensive drugs, creatinine clearance rate, eGFR, and urinary protein between these two groups were not statistically significant. The baseline condition of antihypertensive drug treatment was as follows: 100% of patients received diuretics; 95% of patients received angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, or both; 80% of patients received calcium channel antagonists; 70% of patients received β‐receptor blockers; and 48% of patients received central sympatholytic drugs.
Table 1.
Comparison of Baseline Conditions of Enrolled Patients (±S)
| Item | All Patients (N=77) | Procedure Group (n=39) | Control Group (n=38) | P Value |
|---|---|---|---|---|
| Male/female, No. | 44/33 | 24/15 | 20/18 | .494 |
| Age, y | 61.7±13.5 | 58.6±14.1 | 62.9±12.6 | .163 |
| Body weight index, kg/m2 | 30.7±0.7 | 29.9±1.8 | 30.1±0.9 | .541 |
| Heart rate, beats per min | 75.8±1.6 | 75.1±1.7 | 76.2±3.2 | .063 |
| Cases of type 2 diabetes, No. | 13 | 7 | 6 | .553 |
| Mean systolic pressure, mm Hg | 176.3±13.6 | 177.8±15.1 | 175±15.8 | .429 |
| Mean diastolic pressure, mm Hg | 97.9±5.2 | 98.3±5.6 | 97.3±4.9 | 408 |
| Hypertension duration, y | 10.4±4.2 | 10.9±4.4 | 10.2±4.8 | .507 |
| Antihypertensive drugs, No. | 5.1±0.5 | 5.3±1.3 | 4.9±1.6 | .114 |
| Serum creatinine, mg/dL | 91.8±39.7 | 89.6±31.4 | 92.6±34.5 | .691 |
| Cystatin C GFR, mL/min | 87.1±3.6 | 88.6±3.9 | 87.3±7.1 | .321 |
| Urinary protein content, mg/dL | 122.6±31.5 | 126.9±33.5 | 121.7±45.7 | .570 |
Abbreviation: GFR, glomerular filtration rate.
BP Changes in Patients
The mean systolic pressure in the RDN group at follow‐up decreased by 23.3±4.1 mm Hg, 25.3±4.4 mm Hg, 25.8±5.1 mm Hg, and 26.3±4.9 mm Hg after 1, 3, 6, and 12 months, respectively, while the mean diastolic pressure decreased by 6.8±1.5 mm Hg, 6.8±1.5 mm Hg, 6.8±1.5 mm Hg, and 6.8±1.5 mm Hg, respectively, compared with the values before RDN. These differences were significant (P<.001). The BP of the control group during the follow‐up period did not change significantly, but the differences between the two groups at the same time points were significant (P<.001) (Table 2). The decrease in systolic pressure at the end of follow‐up was <10 mm Hg in 8 cases (21%) in the RDB group; these patients were defined as nonresponders. Regarding the use of antihypertensive drugs, 2 patients in the RDN group had symptoms of hypotension after 3 months of follow‐up; therefore, their medication dosage was reduced.
Table 2.
Changes in Blood Pressure and Renal Function of Patients in the 2 Groups After RDN Procedure
| Group | Procedure Group (n=39) | Control Group (n=38) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 Month | 3 Months | 6 Months | 12 Months | 1 Month | 3 Months | 6 Months | 12 Months | |
| Mean systolic pressure, mm Hg | −21.3±4.1a , b | −23.3±4.4a , b | −25.8±5.1a , b | −27.3±4.9a , b | −6.3±4.8 | −4.9±4.3 | −4.3±34.1 | −5.3±2.6 |
| Mean diastolic pressure, mm Hg | −7.4±1.5a , b | −7.8±1.6a , b | −7.8±1.9a , b | −8.5±1.5a , b | −1.6±1.5 | −1.8±1.3 | −1.7±1.3 | −1.8±1.4 |
| Serum creatinine, mg/dL | −2.9±7.1 | −4.8±6.3 | −8.9±4.1 | −8.3±3.8 | 3.4±3.9 | 4.4±3.8 | 6.4±4.3 | 8.4±5.4 |
| Cystatin C GFR, mL/min | −1.2±2.9 | −2.6±2.3 | −1.9±2.8 | −2.1±2.1 | −5.2±2.5 | −6.1±3.8 | −7.1±2.8 | −9.2±2.2 |
| Urinary protein content, mg/dL | −4.9±1.9 | −8.9 ±7.9a , b | −14.9±6.4a , b | −16.9±5.8 | −1.1±7.6 | 0.9±6.9 | 1.5±5.8 | 4.5±6.3 |
Abbreviations: GFR, glomerular filtration rate; RDN, radiofrequency renal denervation. aCompared with baseline P<.001. bCompared with the control group at the same period P<.001.
Renal Function Changes in Patients Before and After RDN
The serum creatinine and GFR detected using cystatin C in the RDN group and the medication group did not exhibit any change from baseline. Regarding urinary protein secretion, the urinary protein content in the RDN group decreased significantly and exhibited significant differences from baseline after 6 and 12 months (P<.01), while the urinary protein content in the medication group increased slightly after 3 months but without statistical significance (P<.05) (Table 2).
Condition of the Renal Artery Structure of the Patients Before and After RDN
The diameter, length, and sectional area of the main renal artery, the number of cases of atherosclerosis, and the plaque burden between these two groups before RDN exhibited no differences (Table 3). No renal artery dissection or aneurysm was observed in the RDN group before the procedure or 12 months after the procedure, and the diameter, length, and cross‐sectional area of the main renal artery, the number of cases of atherosclerosis, and the plaque burden did not change compared with the values before RDN (P<.05). The re‐examination of the diameter, length, and L‐CSA of the main renal artery in the control group after 12 months revealed no change; the number of cases of atherosclerosis increased but did not have statistical significance; and the plaque burden was significantly higher than both baseline and the value in the RDN group (P<.05).
Table 3.
Changes in Renal Artery Structure in Patients After RDN (±S)
| Group | Procedure Group (n=39) | Control Group (n=38) | ||||
|---|---|---|---|---|---|---|
| Before Procedure | 12 Months After Procedure | P Value | Baseline | 12 Months | P Value | |
| Mean diameter of main left renal artery, mm | 6.1±1.4 | 6.0±1.5 | .762 | 5.7±2.3 | 5.5±1.8 | .674 |
| Mean diameter of main right renal artery, mm | 5.8±1.6 | 5.9±1.3 | .763 | 5.9±1.9 | 5.8±1.7 | .810 |
| Length of main left renal artery, mm | 34.3±2.8 | 33.8±3.1 | .400 | 36.3±2.5 | 36.1±1.9 | .370 |
| Length of main right renal artery, mm | 38.8±1.2 | 38.6±2.1 | .087 | 35.2±3.7 | 34.8±1.9 | .555 |
| Mean L‐CSA of left renal arteries, mm2 | 9.54±2.5 | 9.24±3.1 | .639 | 9.23±2.7 | 9.26±2.5 | .960 |
| Mean L‐CSA of right renal arteries, mm2 | 8.67±2.9 | 8.47±2.4 | .741 | 9.17±2.4 | 9.07±2.9 | .870 |
| Cases of renal atherosclerosis, No. (%) | 21/39 (53.8) | 22/39 (56.4) | 1.000 | 20/38 (52.6) | 26/38 (68.4) | .450 |
| Plaque burden, % | 35.6±12.2 | 37.4±11.4 | .503 | 34.6±14.1 | 48.6±12.8a , b | .001 |
Abbreviations: L‐CSA, cross‐sectional area of the lumen; RDN, radiofrequency renal denervation. Renal atherosclerosis referred to renal artery stenosis <50%. aCompared with baseline P<.05. bCompared with the procedure group at the same time point P<.05.
Discussion
Many studies in both human and animal models have confirmed that the sympathetic nervous system plays an important role in the pathophysiological process of hypertension. The increase in sympathetic nerve activity not only is an initiating factor of hypertension but also plays an important role in the maintenance of hypertension; in particular, it plays an even more important role in the pathogenesis of resistant hypertension. The renal sympathetic nerves mainly emerge from the lateral horns of the spinal cord around T12‐L2 and are mainly distributed in the renal vascular adventitia.4 Grassi and colleagues5 used micro‐nerve technology to show that the amount of sympathetic output was directly correlated with the degree of increase in BP. When the renal sympathetic nerves are activated, they can regulate BP by releasing catecholamine substances (epinephrine, norepinephrine, and dopamine) to bind to their receptors α1, β1, and β2. The sympathetic nervous system can also activate the renin‐angiotensin system to increase BP.6, 7 Therefore, the partial transection or destruction of the renal sympathetic nerves can theoretically produce a certain antihypertensive effect; thus, the reduction of sympathetic nerve activity may be an important target for treating hypertension.8, 9
In the 1940s and 1950s, some researchers selectively resected the chest, abdominal, and pelvic nerves to treat resistant hypertension and obtained a certain effect. However, due to adverse effects such as surgical trauma, dysfunction of the small intestine and bladder, and orthostatic hypotension, this method could not be further clinically applied.10, 11 Studies in recent years have shown that RDN could ablate the afferent and efferent fibers of sympathetic nerves between the renal artery endothelia by using a radiofrequency catheter to block the pathway of renal sympathetic nerve activation and reduce the release of renin and angiotensin.12 In particular, the multicenter, larger clinical studies Symplicity HTN‐1 and Symplicity HTN‐2 both confirmed that after 6 months of RDN treatment, the BP of patients with resistant hypertension decreased significantly with no serious complications, while the BP of the control group did not change.1, 2 Furthermore, Krum and colleagues3 recently confirmed that after 3 years of follow‐up in Symplicity HTN‐1, RDN was still safe and effective.
Similar to the results in international studies, our study results also confirmed the effectiveness of treating resistant hypertension by RDN. After 1 year of follow‐up, the mean systolic pressure and mean diastolic pressure of patients after RDN both remained significantly reduced and there was no increase in BP during the observation period. After 1 year, the BP level could still be maintained at a lower level; this result confirmed the effectiveness of RDN after a longer observation time. However, our study also found approximately 8 (21%) patients without response (decrease of systolic pressure <10 mm Hg), suggesting that the mechanism of RDN still requires further investigation. RDN should work in its sensitive population, which is helpful to select patients with highly effective indications. This study found no occurrence of perioperative‐related serious complications such as renal artery dissection, thrombosis, and stenosis, which confirmed the immediate safety of RDN and was also similar to the conclusion of international studies. During the 1‐year follow‐up, no procedure‐related complications occurred. In addition, MSCTA of the renal artery performed after 1 year also showed no adverse changes in the renal artery such as stenosis and dissection, which also confirmed the long‐term safety of RDN. We used a standard radiofrequency catheter; in fact, the catheter material, radiofrequency energy, and ablation method were not substantially different from those of the Symplicity catheter (Medtronic, Minneapolis, MN). The major differences were the ablation electrode length on the top end of the catheter and its distal shape. The ablation electrode length at the top end of the catheter of a standard radiofrequency catheter is 4 mm, which can increase its contact area with the renal arteries, is more conducive to the control of the ablation temperature, reduces renal artery intima damage, and increases surgical safety. Its safety and effectiveness have been confirmed by various domestic and international studies.12, 13
The kidney is both one of the target organs damaged by hypertension and an important cause of hypertension; renal sympathetic nerve activation is the bridge between these two factors. Renal sympathetic nerve excitation increases renin release, enhances the contraction of the renal blood vessels, and increases the reabsorption of water and sodium ions in the distal convoluted tubules, thus maintaining high BP or even causing resistant hypertension. Resistant hypertension increases incidence of renal events. Reduced GFR and increased urinary protein secretion predict damage to the kidney from hypertension. RDN is a local manipulation; it is minimally invasive, and the surgical time is relatively short. However, it is important to determine whether the potential tissue damage and changes in renal sympathetic nerve activity after ablation would result in changes in the renal function and renal artery structure. Animal studies showed that after RDN, the renal blood flow velocity, renal index, and renal blood flow peak did not change. In addition, RDN did cause acute or chronic adverse effects on renal blood flow.14 The clinical study by Mahfoud and colleagues15 showed that RDN could improve the resistance index of the renal artery and reduce the urinary protein secretion rate without affecting renal function. Our study indicated that the serum creatinine and GFR values did not change after 12 months of follow‐up and GFR even slightly increased, suggesting that this procedure did not damage the renal tubule function; rather, the procedure might even improve renal function by controlling BP. In addition, the 24‐hour urinary protein levels also exhibited a decreasing trend after RDN, and a large number of protein levels exhibited significant decreases. This result suggests that RDN could improve the filtration function of the renal microtubules. These results were consistent with the results of international reports and may be associated with the improvement in renal parenchyma perfusion caused by the reduction of renal sympathetic nerve activity.16
Hypertension is a common cause of structural changes in the renal artery, such as renal atherosclerosis and stenosis. Renal atherosclerosis can be confirmed by color Doppler ultrasound, MSCTA, and digital subtraction angiography (DSA). Color Doppler ultrasound has lower sensitivity to renal atherosclerosis; its diagnostic accuracy is influenced by a variety of subjective and objective factors, including the degree of renal artery stenosis, obesity of patients, intestinal gas interference, respiratory cooperation, experience of operators, and instrument quality. The limitations of DSA are its invasiveness and high cost, while MSCTA presents great advantages, particularly noninvasiveness, high accuracy, and low cost. CTA offers a higher sensitivity and specificity in the diagnosis of renal atherosclerosis. In particular, MSCTA represents a powerful tool for the post‐treatment 3‐dimensional reconstruction of the renal artery, unaffected by the overlap of the front and rear structures. Moreover, MSCTA can detect the renal artery morphology and its anatomical relationship at different angles; can evaluate the lumen structure, luminal wall structure, structure outside the luminal wall, and the properties of plaque inside the lumen; can 3‐dimensionally display the spatial relationship between the renal artery and its surrounding tissues and organs; and can display the existence and degree of calcification of the vascular wall, thrombosis, and stenosis.15, 17, 18 Currently, scholars generally consider that when the degree of localized luminal stenosis of the renal artery ≥50%, it is renal artery stenosis with clinical meaning and contraindicates RDN.19
We used MSCTA to observe structural changes in the renal arteries, specifically changes in the diameter, length, and L‐CSA of the main renal artery, the degree of atherosclerosis, and the plaque burden before RDN and 12 months after RDN. Our study showed that more than 50% of the patients in both groups had renal atherosclerosis, suggesting that hypertension is an important reason for renal atherosclerosis. In the RDN group, the diameter, length, L‐CSA, number of cases of atherosclerosis, and plaque burden did not exhibit significant changes 1 year after RDN. In the control group, renal atherosclerosis showed an aggravating trend: not only did the number of cases of renal atherosclerosis increase from 52.6% to 68.4%, but the plaque burden was also significantly higher compared with baseline and the procedure group (P<.05). These results suggest that RDN did not have adverse effects on the renal artery structure during long‐term follow‐up. In addition, it is possible that the control of BP and the improvement in vascular endothelial function may even inhibit the progress of renal artery atherosclerosis. Animal studies suggested that RDN could cause fibrosis of the renal arteries;20 however, whether the effect of fibrosis on the diameter of the lumen and the cross‐sectional area of blood vessels can lead to renal atherosclerosis is still unclear.
Limitations
Although the results in this study support the effectiveness and safety of RDN, there are still some limitations of this study, including not designing randomized samples and small sample size.
Conclusions
RDN significantly and persistently reduced BP and decreased urinary protein excretion rate in patients with resistant hypertension and did not exhibit any adverse effect on renal function and renal artery structure. Further studies with a larger sample size and extended follow‐up are needed.
Disclosure
This study was funded by the Key Science and Technology Project of the Science and Technology Department of Hunan Province of China (2012WK2002). The authors have no conflicts of interest to declare.
J Clin Hypertens (Greenwich). 2014;16:599–605. © 2014 The Authors Journal of Clinical Hypertension Published by Wiley Periodicals, Inc.
References
- 1. Krum H, Schlaich M, Whitboum R, et al. Catheter‐based renal denervation for resistant hypertension: a multicentre safety and proof‐of‐principle cohort study. Lancet. 2009;373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 2. Symplicity HTN‐2 Investigators , Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal denervation in patients with treatment‐resistant hypertension (The Symplicity HTN‐2 Trial): a randomised controlled trial. Lancet. 2010; 376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 3. Krum H, Schlaich M, Schlaich P, et al. Percutaneous renal denervation in patients with treatment‐resistant hypertension: final 3‐year report of the Symplicity HTN‐1 study. Lancet. 2014;383:622–629. [DOI] [PubMed] [Google Scholar]
- 4. Schlaich MP, Sobotka PA, Krum H, et al. Renal denervation as a therapeutic approach for hypertension: novel implications for an old concept. Hypertension. 2009;54:1195–1201. [DOI] [PubMed] [Google Scholar]
- 5. Grassi G, Cattaneo BM, Seravalle G, et al. Baroreflex control of sympathetic nerve activity in essential and secondary hypertension. Hypertension. 1998;3l:68–71. [DOI] [PubMed] [Google Scholar]
- 6. Campese VM, Ye S, Zhong H. Downregulation of neuronal nitric oxide synthese and interleukin 1 beta mediates angiotensin II dependent stimulation of sympathetic nerve activity. Hypertension. 2002;39:519–524. [DOI] [PubMed] [Google Scholar]
- 7. Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin‐angiotension‐system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. [DOI] [PubMed] [Google Scholar]
- 8. Martin EA, Victor RG. Premise, promise, and potential limitations of invasive devices to treat hypertension. Curr Cardiol Rep. 2011;13:86–92. [DOI] [PubMed] [Google Scholar]
- 9. Katholi RE, Rocha‐Singh KJ. The role of renal sympathetic nerves in hypertension: has percutaneous renal denervation refocused attention their clinical significance. Prog Cardiovasc Dis. 2009;52:243–248. [DOI] [PubMed] [Google Scholar]
- 10. Grimson KS. Total thoracic and partial to total lumbar sympathectomy and celiac ganglionectomy in the treatment of hypertension. Ann Surg. 1941;114:753–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knepper PA, Neudorff LG. Hypertension: surgical treatment by transthoracic thoracolumbar sympathectomy. Mo Med. 1949;46:855–860. [PubMed] [Google Scholar]
- 12. Lu CZ, Liu J, Xia DV, et al. Efficacy of catheter‐based renal denervation in mongrel neurogenic hypertensive dogs. Zhonghua Xin Xue Guan Bing Za Zhi. 2012;40:14–17. [PubMed] [Google Scholar]
- 13. Prochnau D, Figulla HR, Surber R. Efficacy of renal denervation with a standard EP catheter in the 24‐h ambulatory blood pressure monitoring—long‐term follow‐up. Int J Cardiol. 2012;157:447–448. [DOI] [PubMed] [Google Scholar]
- 14. Yaduvanshi A, Patra S, Gupta S, Nair M. Renal denervation using standard 5F radiofrequency ablation catheter in patients with end stage renal disease: a 6 month follow‐up. J Am Coll Cardiol Intv. 2014;7:S3–S4. [Google Scholar]
- 15. Mahfoud F, Cremers B, Janker J, et al. Renal hemodynamics and renal function after catheter‐based renal denervation in patients with resistant hypertension. Hypertension. 2012;60:419–424. [DOI] [PubMed] [Google Scholar]
- 16. Tsioufis C, Papademetriou V, Dimitriadis K, et al. Catheter‐based renal denervation exerts acute and chronic effects on renal hemodynamics in swine. Int J Cardiol. 2013;168:987–992. [DOI] [PubMed] [Google Scholar]
- 17. Papadopoulou SL, Neefjes LA, Schaap M, et al. Detection and quantification of coronary atherosclerotic plaque by 64‐slice multidetector CT: a systematic head‐to‐head comparison with intravascular ultrasound. Atherosclerosis. 2011;219:163–170. [DOI] [PubMed] [Google Scholar]
- 18. Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64‐multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. [DOI] [PubMed] [Google Scholar]
- 19. Regine G, Stasolla A, Miele V. Multidetector computed tomography of the renal arteries in vascular emergencies. Eur J Radiol. 2007;64:83–91. [DOI] [PubMed] [Google Scholar]
- 20. Widimský P, Filipovský J, Branny M. Expert consensus statement of the Czech Society of Cardiology and the Czech Society of Hypertension on catheter‐based sympathetic renal denervation procedures (RDN) in the Czech Republic. Cor et Vasa. 2012;54:108–112. [Google Scholar]


