Abstract
We investigated whether Helicobacter pylori (H. pylori) CagA contributes to the DNA copy change and mRNA transcript expression of the HER-2 gene and, consequently, affects HER-2 protein expression to evaluate the significance of CagA and HER-2 amplification in gastric cancer. We used the AGS andMKN1 gastric cancer and HFE-145 immortalized non-neoplastic gastric mucosa cell lines. We also confirmed the effects of CagA on HER-2 expression in human gastric cancer tissues and gastric mucosal tissues of H. pylori infected C57BL/6 mice. Ectopic CagA expression in AGS, MKN1 and HFE-145 cells showed a significant increase in HER-2 gene copy number and expression. The gastric mucosae of H. pylori infected C57BL/6 mice also showed increased HER-2 DNA copy number and protein expression. In addition, CagA expression was detected in 17 (56.7%) of 30 gastric cancer tissues, and eight (47%) of them showed HER-2 DNA amplification of more than two-fold. In immunohistochemistry, HER-2 overexpression was detected in 12 (40%) of 30 gastric cancers and a positive correlation was observed among DNA copy number, the mRNA transcript, and protein expression of the HER-2 gene in gastric cancer (P < 0.05). These results suggest that H. pylori CagA may induce overexpression of the HER-2 protein by increasing HER-2 DNA and mRNA copy number.
Keywords: H. pylori, CagA, HER-2/neu, DNA amplification, Gastric cancer
1. Introduction
Gastric cancer (GC) is one of the most common cancers and remains the second leading cause of cancer mortality worldwide (Ferlay et al., 2010). Helicobacter pylori (H. pylori) has been identified as the causative agent of chronic gastric inflammation, such as atrophic gastritis and metaplastic gastritis, which can progress to mucosa-associated lymphoid tissue lymphoma or even gastric cancer (Atherton, 2006; Cover and Blaser, 2009; Peek and Blaser, 2002). The H. pylori Cag pathogenicity island is a strain-specific locus that encodes a type IV secretion system mediating the translocation of bacterial virulence factor cytotoxin-associated gene A (CagA) into host epithelial cells (Amieva et al., 2004; Atherton, 2006; Backert and Meyer, 2006; Covacci and Rappuoli, 2000; Hatakeyama, 2006; Peek and Blaser, 2002). H. pylori induces the transcription of thousands of host genes, while repressing another set of genes (Guillemin et al., 2002). CagA-expressing cells showed chromosomal instability by perturbing the microtubule-based mitotic spindle (Umeda et al., 2009) and ectopically expressing activation-induced cytidine deaminase (Matsumoto et al., 2007). In addition, H. pylori infection of cultured gastric epithelial cells downregulates the components of the mismatch repair (MMR) and base excision repair machineries at the RNA and protein levels and impairs the efficiency of DNA repair as judged by an MMR activity assay (Kim et al., 2002; Machado et al., 2009).
Recent research on cancer biology has provided molecular target therapies that block specific cell differentiation, proliferation and apoptosis signaling pathways. The HER-2 oncogene (also referred to as HER-2/neu or ERBB2) encodes a 185-kD transmembrane tyrosine kinase receptor that belongs to the epidermal growth factor receptor family (Hung and Lau, 1999). Activation of HER-2 plays a pivotal role in cell proliferation and survival mediated through the RAS-mitogen activated protein kinase pathway, and it inhibits cell death through the phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway (Miller et al., 2009). In GC, HER-2 overexpression rates were7–34% by immunohistochemistry with a monoclonal antibody (HerceptTest) and/or gene amplification with fluorescence in situ hybridization (FISH)/or chromogenic in situ hybridization (Gravalos and Jimeno, 2008; Hofmann et al., 2008; Tanner et al., 2005). Recently, Bang et al. (2010) reported that adding trastuzumab (Herceptin), which is a humanized monoclonal antibody that selectively targets HER-2, to chemotherapy improves survival in patients with advanced GC or gastroesophageal junction cancer compared with chemotherapy alone; this survival advantage is mainly conferred to subgroups with high HER-2 protein expression. However, little is known about the molecular mechanisms underlying the HER-2 overexpression in GC and the contribution of CagA to gastric carcinogenesis.
In this study, we investigated the underlying molecular mechanism of HER-2 overexpression and the relationship between HER-2 DNA copy number change and expression of mRNA transcripts and protein in GC tissue samples. Overall, we found that H. pylori CagA may induce overexpression of the HER-2 protein by increasing HER-2 DNA and mRNA copy number.
2. Materials and methods
2.1. Cell culture and CagA transfection
The AGS and MKN1 gastric cancer cell lines and HFE-145 immortalized non-neoplastic gastric mucosa cell line were obtained from the American Type Culture Collection (Manassas, VA, USA) and Dr. Hassan (Washington, DC, USA), respectively. These cell lines were cultured in 5% CO2 at 37 °C in an RPMI-1640 medium (Lonza, Basel, Switzerland) with 10% heat-inactivated fetal bovine serum. The H. pylori CagA gene was cloned into the pSP65SRalpha vector containing a hemagglutinin tag. The CagA formulation was provided by Dr. Hatakeyama (University of Tokyo, Tokyo, Japan). AGS,MKN1 and HFE-145 cells were transfected in 60 mm-diameter dishes with expression plasmids (2 µg total DNA) using a Lipofectamine Plus transfection reagent (Invitrogen) according to the manufacturer's recommendations.
2.2. Measurement of cell growth
For cell viability assay, MTT [3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was performed at 24, 48, and 72 h after transient transfection of CagA. Absorbance was measured using spectrophotometer at 540 nm and growth was expressed relative to mock (empty vector + Lipofectamine).
2.3. Effect of CagA on HER-2 expression
To determine whether CagA is involved in the regulation of the HER-2 gene, DNA copy number and expression of the HER-2 were examined in AGS, MKN1 and HFE-145 cells 24 h after transfection with CagA. After quantifying the genomic DNA extracted from the CagA transfected cells, real-time SYBR Green qPCR was performed on a Stratagene Mx 3000P qPCR system. The specific primers for detecting the HER-2 DNA copy number were designed according to the genomic sequence of GenBank accession no. NC-000017.11. All samples were subjected to PCR amplification with oligonucleotide primers specific for the constitutively expressed glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene and normalized. Primers for SYBR Green analysis were designed based on gene-specific non-homologous DNA sequences. The HER-2 gene primer sequences of human were forward; 5′-CCTCTGACGTCCATCGTC TC-3′, and reverse; 5′-CGGATCTTCTGCTGCCGTCG-3′. The GAPDH primers for human were as follows: forward primer, 5′-ACCCAGAAGACTGTGGAT GG-3′ and reverse primer, 5′-TTCTAGACGGCAGGTCAGGT-3′. The HER-2 gene primer sequences for mice were forward; 5′-CCGCAGTGATCATCAT GGA-3′, and reverse; 5′-CAAGCCAAGACCCACCTTG-3′. The GAPDH primers for mice were as follows: forward primer, 5′-CAACCTGGTTAA GTACAAAT-3′ and reverse primer, 5′-GCAGACCTCCTAAATCTC-3′.
Proteins extracted from gastric cell lines and gastric mucosa tissues of H. pylori infected C57BL/6 mice were separated on a 10% polyacrylamide gel and transferred onto a Hybond PVDF membrane (Amersham Pharmacia Biotech, Piscataway, NJ, USA) for HER-2 protein expression analysis. After blocking, the membrane was subsequently probed with HER-2 antibody. Protein bands were detected using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
2.4. Reactive oxygen species (ROS) analysis
Because DNA structural modifications primarily occur due to exposure to ROS (Jena, 2012), we measured ROS production after treatment with CagA, H2O2, or 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPOL, Sigma-Aldrich) in AGS, MKN1 and HFE-145 cells using 2′-7′-dichlorodihydrofluorescein diacetate (DCF-DA), as described previously (Yoon et al., 2011a).
We also examined the variation in HER-2 copy number after treatment with H. pylori CagA, H2O2, or TEMPOL in AGS cells by real-time PCR. H. pylori CagA and HER-2 protein expression was also examined by Western blot analysis.
2.5. H. pylori infection in mice
H. pylori 26695 (reference strain, CagA+, vacA+) was used (Tomb et al., 1997). H. pylori was cultured at 37 °C in a standard microaerobic atmosphere (5% O2, 10% CO2, and 85% N2) in brain–heart infusion medium (Difco, Detroit, MI, USA) with 7% laked horse blood (Oxoid, Cambridge, UK), 0.4% IsoVitalex™ (BBL, Sparks, MD, USA), vancomycin (6 µg/ml), amphotericin B (8 µg/ml), and trimethoprim (5 µg/ml). Five C57BL/6 female mice aged 5 weeks were purchased from Qu-BEST (Seongnam, Korea). Three mice were then inoculated 3 times by oral gavage with 0.4 ml of suspension containing H. pylori SS1 (2 × 109 c.f.u. ml−1). Four weeks post-inoculation, 2 control and 3 infected mice were sacrificed and their gastric mucosal tissues were used for molecular studies and determination of colonization.
2.6. Tissue samples
Thirty patients with gastric adenocarcinoma who underwent a gas-trectomy between 2010 and 2011 at the Department of Surgery, Seoul St. Mary's Hospital, The Catholic University, were included. All patients provided informed consent for additional molecular analyses at the time of their original operation. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital (IRB approval No. KC12SISE0315).
2.7. Measurement of H. pylori CagA in non-neoplastic gastric mucosa and cancer tissues
Genomic DNAs from each cancer tissues and the corresponding noncancerous mucosal tissues were amplified with primers covering the CagA gene coding region. The primer sequences of the CagA gene were forward; 5′-GATAACAGGCAAGCTTTTGAGG-3′, and reverse; 5′-CTGC AAAAGATTGTTTGGCA-3′. Each PCR procedure was performed under standard conditions. The reaction mixture was denatured at 94 °C for 12 min and then incubated for 35 cycles (denaturing at 94 °C for 40 s, annealing at 50–54 °C for 40 s and extension at 72 °C for 40 s). A final extension step was performed at 72 °C for 5 min. Each PCR product was loaded directly onto 2% agarose gels, stained with ethidium bromide, and visualized under UV illumination.
2.8. Measurement of HER-2 DNA, mRNA and protein status in GC
After quantification of genomic DNA and the mRNA extracted from 30 frozen human gastric cancer samples, real-time SYBR Green qPCR was performed on a Stratagene Mx 3000P QPCR system. The real-time qPCR to detect HER-2 DNA copy number was performed as described above (Yoon et al., 2011b). Data are expressed as means± standard deviations from at least two independent experiments.
cDNA was synthesized using the reverse transcription kit from Roche Molecular Systems (Roche, Mannheim, Germany) according to the manufacturer's protocol. For qPCR, 50 ng cDNA was amplified using the Fullvelocity SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA, USA) and 20 pmol/µl each of forward and reverse primers on the Stratagene Mx 3000P QPCR system with techniques published previously (Yoon et al., 2011a). The specific mRNA oligonucleotide primers were synthesized according to published information on the HER-2 gene as follows: forward primer, 5′-ATCTGCCTGACATC CACG-3′ and reverse primer, 5′-GCAATCTGCATACACCAGTTC-3′. To ensure the fidelity of DNA and mRNA extraction and reverse transcription, all samples were subjected to PCR amplification with oligonucleotide primers specific for the constitutively expressed GAPDH gene and normalized. The GAPDH primers were: forward primer, 5′-AAATCAAGTGGGGCGATGCTG-3′ and reverse primer, 5′-GCAGAGATGATGACCCTTTTG-3′. Primers for SYBR Green analysis were designed based on gene-specific non-homologous DNA sequences. The standard curve method was used to quantify the relative amounts of gene expression products. This method provides unit-less normalized expression values that can be used for a direct comparison of the relative amounts of target DNA and mRNA in different samples. All samples were tested in duplicate, and average values were used for quantification. HER-2 DNA and mRNA levels in GC were compared to those of corresponding gastric mucosae, and DNA amplification and mRNA overexpression were defined as a mean test (cancer)/reference (mucosa) ratio above 2 fold change.
After quantification of the proteins extracted from 30 frozen human gastric cancer samples, a Western blot analysis was also done for HER-2 protein expression and HER-2 levels in gastric cancer tissues were compared with those in corresponding non-cancerous gastric mucosa.
2.9. HER-2 immunohistochemistry
Thirty formalin-fixed paraffin embedded gastric cancer tissue samples that were used for the above macromolecule analysis were also used for immunohistochemistry (IHC). Two micrometer sections were cut the day before use and stained according to standard protocols. Two strategies were used to maximize the IHC signal: antigen retrieval in citrate buffer, and signal amplification with biotinylated tyramide, as described previously (Yoon et al., 2011b). The sections were incubated overnight at 4 °C with HER-2 antibody (1/100; Sigma, St. Louis, MO, USA). Detection was carried out using biotinylated goat anti-mouse antibody (Sigma), followed by incubation with a peroxidase-linked avidin–biotin complex. Diaminobenzidine was used as the chromogen, and the slides were counterstained with Mayer's hematoxylin. Two experienced pathologists assessed HER-2 immunostaining using the HercepTest (Dako, Carpentaria, CA, USA). Modified HER-2 scoring criteria for gastric cancer were used; 0, no staining or <10% tumor cells with membrane staining; 1+, >10% tumor cells with faint staining in partial membrane; 2+, >10% tumor cells with weak to moderate staining in partial membrane; 3+, >10% tumor cells with strong staining in partial membrane (Hofmann et al., 2008). Themanufacturer provided the positive and the negative control.
2.10. Statistical analysis
The χ2, Pearson's and Spearman's correlation tests were used to examine the relationships among HER-2 DNA copy number, mRNA transcripts, and protein expression. Data are expressed as mean ± standard deviation from at least two independent experiments. To further evaluate the effect ofCagAontheHER-2, we considered receiver operating characteristic (ROC) curve analysis. A ROC curve is a plot of the true-positive fraction versus the false-positive fraction, evaluated for all possible cutoff point values. A P value less than 0.05 was considered to be of statistical significance.
3. Results
3.1. H. pylori CagA induces HER-2/neu overexpression
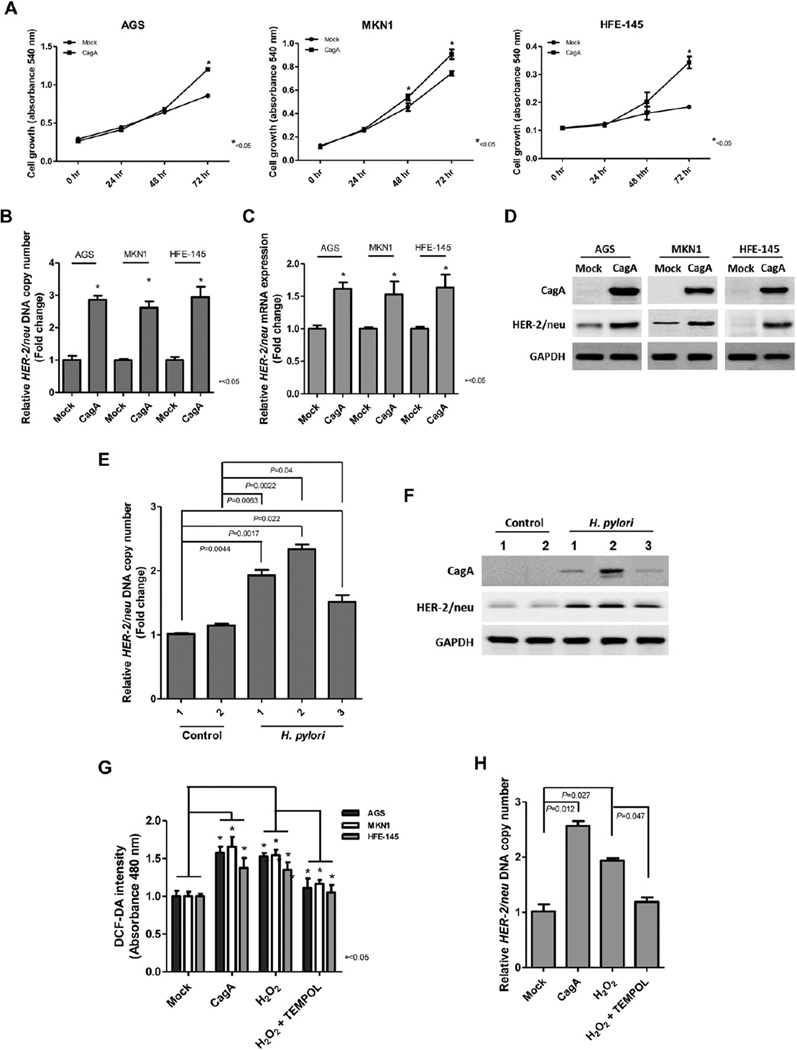
To investigate the effects of CagA on cell growth, MTT assay was performed in AGS, MKN1 and HFE-145 cells after CagA transfection. A significant increase in cell growth in CagA transfected cells was observed compared to that in mock transfected cells (Fig. 1A).
Fig. 1.
The effects of CagA on cell growth and HER-2 expression. (A) Cell growth as measured by the MTT assay in AGS, MKN1, and HFE-145 cells after transfection. CagA transfected cells showed significantly increased growth. (B & C) Effect of CagA on HER-2 DNA copy number and mRNA expression in AGS, MKN1, and HFE-145 cells. Ectopic CagA expression induced an increase of HER-2 DNA copy number and mRNA transcript expression. (D) HER-2 protein expression measured by Western blot in CagA transfected AGS, MKN1, and HFE-145 cells. Increased expression of the HER-2 protein was observed in CagA transfected cells compared to those in mock (vector + Lipofectamine) transfected cells. (E & F) The gastric mucosae of H. pylori infected C57BL/6 mice showed increased HER-2 DNA copy number and protein expression. (G) CagA or H2O2 treatment significantly increased ROS production, but treatment with the antioxidant TEMPOL reverted the CagA- and H2O2-induced ROS level in AGS,MKN1, and HFE-145 cells. (H) CagA or H2O2 treatment significantly increased HER-2 DNA copy number, whereas TEMPOL reverted the H2O2-induced HER-2 copy number change in AGS cells.
Next, we analyzed HER-2 DNA copy number and mRNA expression by real time qPCR and protein expression by Western blot analysis in CagA transfected AGS, MKN1 and HFE-145 cells. Interestingly, ectopic CagA expression induced increased HER-2 DNA copy number and overexpression of mRNA transcript and protein in these cell lines (Fig. 1B–D). In addition, the gastric mucosal tissues of H. pylori infected C57BL/6 mice also showed increased HER-2 DNA copy number and protein expression (Fig. 1E and F).
As shown in Fig. 1G and H, we also investigated whether CagA-induced ROS was involved in the HER-2 DNA copy number change. Ectopic expression of CagA and H2O2 treatment significantly increased ROS levels and HER-2 DNA copy number in AGS, MKN1 and HFE-145 cells (Fig. 1G and H). Treatment with 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPOL, Sigma-Aldrich) reverted the CagA-and H2O2-induced ROS production and the HER-2 copy number change (Fig. 1G and H), indicating that CagA-induced ROS may play a role in HER-2 gene amplification.
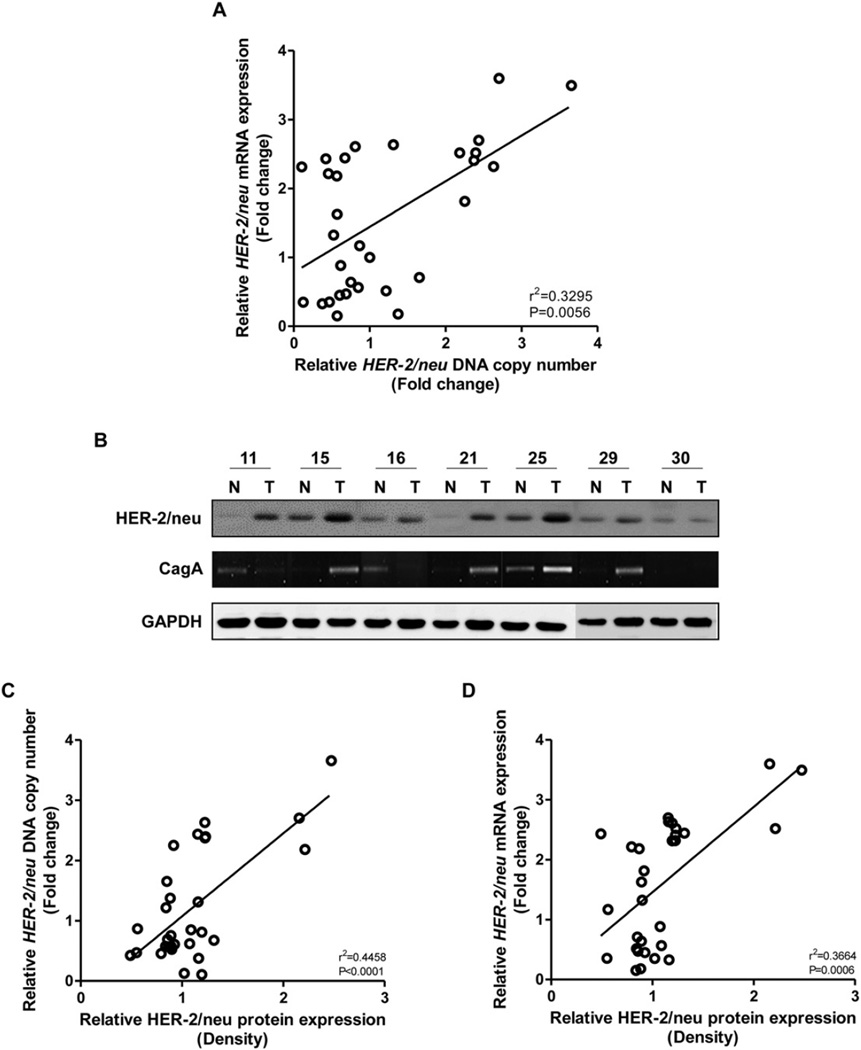
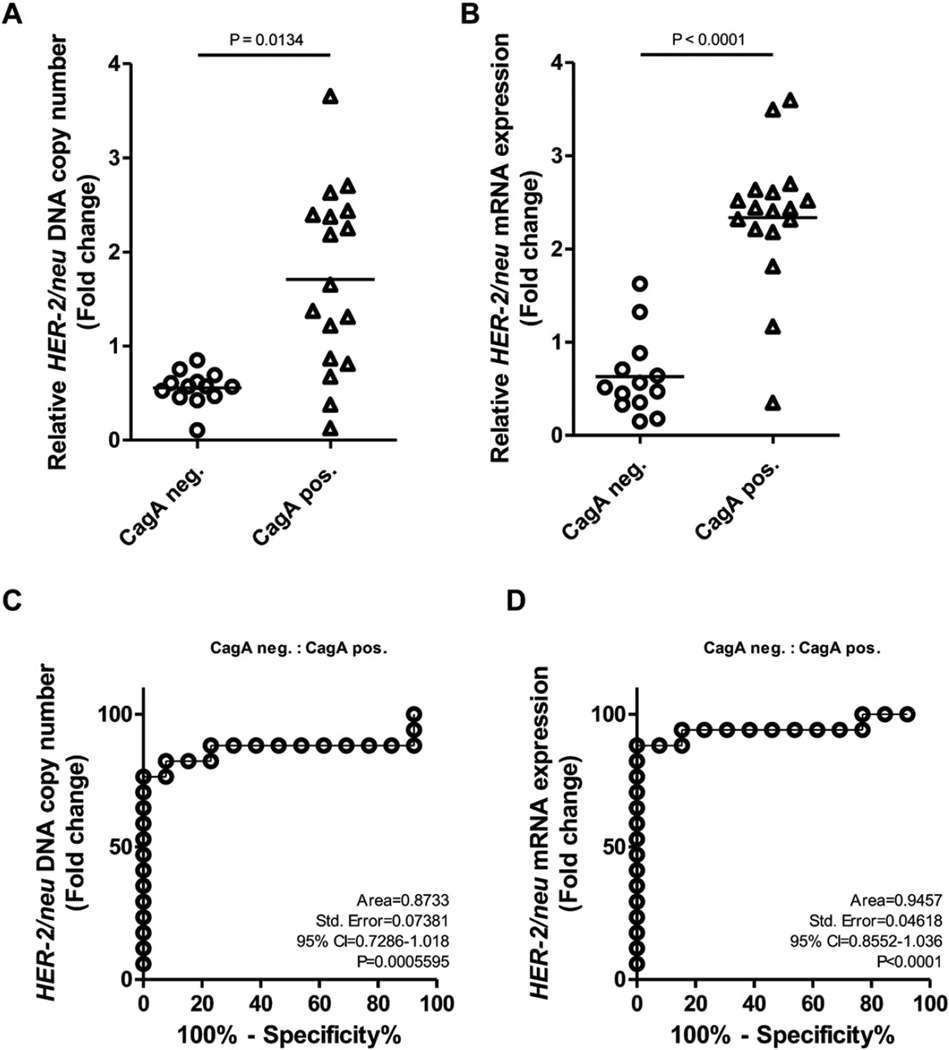
To further confirm the effect of CagA on the change in HER-2 DNA copy number, PCR for the CagA gene was performed in the 30 GC tissues and corresponding non-cancerous gastricmucosal tissues. A single CagA amplicon was detected in 17 (56.7%) GC and/or corresponding gastric mucosal tissues (Fig. 3B). When we compared the presence of CagA with the HER-2 DNA copy number change, HER-2 amplification was detected in eight (47.1%) of 17 cases with CagA positivity, and the presence of CagA was closely associated with the HER-2 DNA amplification and mRNA overexpression (Fig. 2A and B, P < 0.05).
Fig. 3.
Fold changes of DNA copy number, mRNA and protein expression of HER-2 inGC. (A) HER-2 DNA and mRNA levels were assessed by real time-qPCR, using the SYBR Green method in 30 gastric cancers and corresponding gastricmucosal tissues, and normalized to GAPDH DNA copy number and mRNA. A significant relationship was observed between HER-2 DNA copy number and mRNA transcript expression (Pearson's correlation test). (B) Representative results of HER-2 protein expression and the presence of CagA in gastricmucosal tissues. HER-2 protein expression was increased in GC tissues, compared to the corresponding gastricmucosal tissues. A single CagA amplicon was detected in GC and/or corresponding gastricmucosal tissues. (C & D) Significant correlations were observed between HER-2 protein expression and DNA copy number, and HER-2 mRNA and protein expression (Pearson's correlation test).
Fig. 2.
Association between the presence of CagA and HER-2 status in GC tissues. (A & B) The presence of CagA was closely associated with the HER-2 DNA amplification and mRNA overexpression. (C & D) CagA predicted HER-2 DNA amplification and mRNA overexpression with an area under the ROC curve (AUC) value of 0.8733 and 0.9457, respectively. Neg, negative; Pos, positive.
The ability of the CagA to discriminate the patients with HER-2 amplification and mRNA overexpression in GC from negative cases was also analyzed by using ROC curve. The CagA predicted HER-2 amplification and overexpression with an area under the ROC curve (AUC) value of 0.8733 and 0.9457, respectively (Fig. 2C and D), suggesting that CagA may be a potential diagnostic biomarker for HER-2 status in GC.
3.2. HER-2 DNA copy number change is closely correlated with mRNA and protein expression
Next, we examined whether the DNA copy change affects mRNA transcript and protein expression of the HER-2 gene in GC. Table 1 details the correlation between HER-2 DNA copy number and expression of mRNA transcript and protein in GC tissues. In the real time-qPCR analysis, HER-2 DNA copy number was increased in eight (26.6%) of 30 GCs compared to that in surrounding gastric mucosal tissue (Table 1). Increased HER-2 DNA copy number was detected in one (20%), five (31%), and two (22.2%) of five diffuse-, 16 intestinal-and nine mixed-type GCs, respectively. Interestingly, all corresponding gastric mucosal tissues expressed the HER-2 gene transcript and increased expression of the gene transcript was detected in 14 (46.6%) GC tissues. A significant relationship was observed between HER-2 DNA copy number and mRNA transcript expression (Fig. 3A, P = 0.0056). HER-2 protein expression by Western blot was increased in seven (23.3%) of 30 cancer tissues, compared to the corresponding gastric mucosal tissues (Table 1) (Fig. 3B). Interestingly, significant correlations were observed between HER-2 protein expression and DNA copy number (Fig. 3C, P < 0.0001), and HER-2 mRNA transcript and protein expression (Fig. 3D, P = 0.0006).
Table 1.
Correlation between HER-2 DNA, RNA, and protein expression in gastric cancers.
| CagA | P valuea | DNA fold change | P valueb | RNA fold change | P valueb | ||||
|---|---|---|---|---|---|---|---|---|---|
| − | + | <2 | >2 | <2 | >2 | ||||
| DNA fold change | 0.0134 | ||||||||
| <2 | 13 | 9 | |||||||
| >2 | 0 | 8 | |||||||
| RNA fold change | <0.0001 | 0.0056 | |||||||
| <2 | 13 | 3 | 15 | 1 | |||||
| >2 | 0 | 14 | 7 | 7 | |||||
| Protein overexpressionc | 0.0273 | <0.0001 | 0.0006 | ||||||
| − | 13 | 10 | 21 | 2 | 16 | 7 | |||
| + | 0 | 7 | 1 | 6 | 0 | 7 | |||
| Total | 13 | 17 | 22 | 8 | 16 | 14 | |||
Chi-square test.
Spearman's correlation test.
Protein expression byWestern blot analysis.
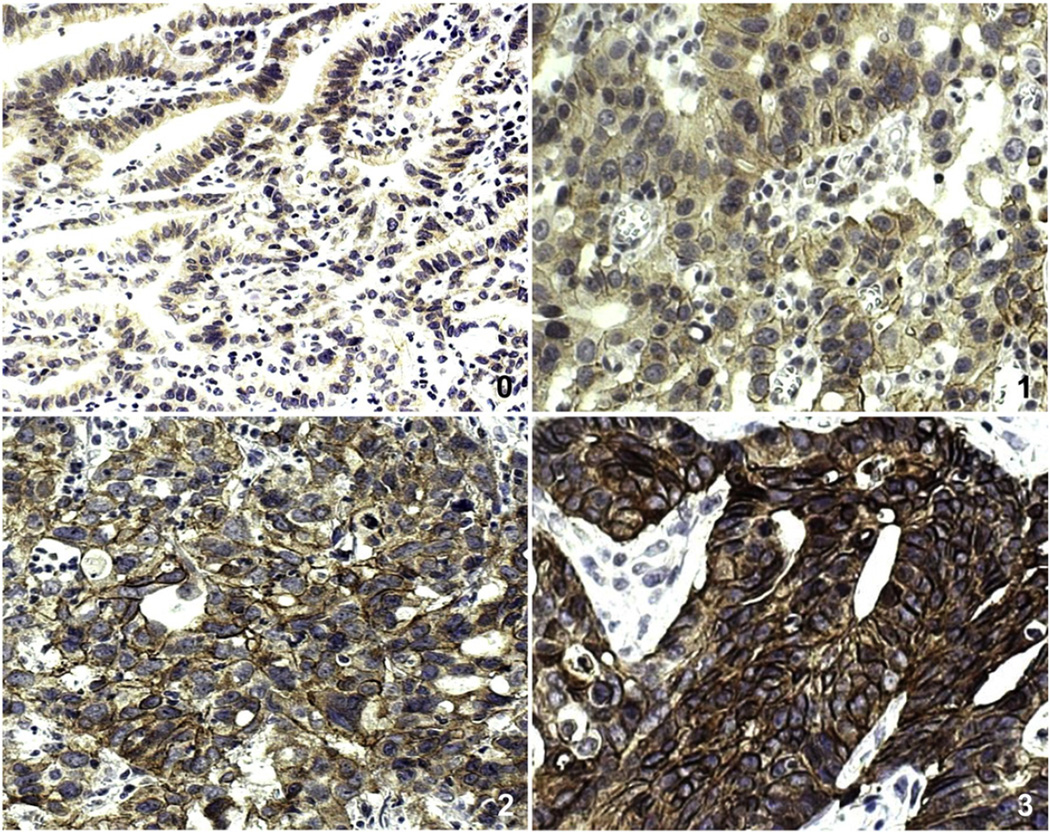
In IHC, faint to moderate HER-2 staining was clearly marked on the membranes of non-neoplastic gastric mucosal cells. However, surrounding stromal cells such as fibroblasts were negative for HER-2. HER-2 overexpression, which was graded by Hoffman's revised system, was detected in 12 (40%) of 30 GCs (Fig. 4) (Table 2). The details of the histopathological characteristics are as follows: one (20%), seven (43.7%), and four (44.4%) of five diffuse-, 16 intestinal-, and nine mixed-type GCs, respectively. Aberrant HER-2 expression was found in two (50%) and 10 (38.5%) of four early and 26 advanced GCs, respectively. Although borderline significance was observed between HER-2 overexpression and lymph node metastasis (Chi-square test, P = 0.0503), no significant correlation was observed between HER-2 overexpression and clinicopathological parameters, including age and gender (Table 2).
Fig. 4.
HER-2 immunostaining of GCs. HER-2 immunohistochemical staining showing 0 (A, ×200), 1+(B, ×200), 2+(C, ×200) and 3+immunostaining (D, ×200) in GCs. Both 2+and 3+ uniform immunostaining were considered as HER-2 protein overexpression.
Table 2.
Correlation between HER-2 overexpression by immunohistochemistry and clinicopathologic parameters in gastric cancers.
| Parameters | HER-2 overexpression |
P value | |
|---|---|---|---|
| + | − | ||
| Age (year) | 0.9203 | ||
| <60 | 5 | 9 | |
| >60 | 7 | 9 | |
| Gender | 0.7913 | ||
| Male | 9 | 14 | |
| Female | 3 | 4 | |
| Site | 0.5598 | ||
| Cardia | 0 | 1 | |
| Body | 8 | 12 | |
| Antrum & pylorus | 4 | 5 | |
| Size (cm) | 0.3711 | ||
| <5.7 | 10 | 11 | |
| >5.7 | 2 | 7 | |
| Depth of invasion | 0.9203 | ||
| Early | 2 | 2 | |
| Advanced | 10 | 16 | |
| Differentiation | 0.512 | ||
| Well | 1 | 0 | |
| Moderate | 8 | 8 | |
| Poor | 3 | 10 | |
| Lauren classification | 0.8625 | ||
| Intestinal | 7 | 9 | |
| Diffuse | 1 | 4 | |
| Mixed | 4 | 5 | |
| Lymph node metastasis | 0.0503 | ||
| + | 7 | 17 | |
| − | 5 | 1 | |
| Total | 12 | 18 | |
When we compared HER-2 overexpression by IHC with DNA, mRNA and protein level of the HER-2 gene, HER-2 overexpression was found in seven (87.5%) of 8 GCs, 11 (78.5%) of 14 GCs and 7 (100.0%) of 7 GCs with increased DNA copy number, and mRNA transcript and protein expression, respectively. HER-2 overexpression by IHC was closely associated with DNA, mRNA and protein status of the gene in the GCs (Table 3, P < 0.001).
Table 3.
Association of HER-2 overexpression by IHC with HER-2 DNA, RNA, and protein expression in gastric cancers.
| HER-2 | Overexpression by IHCa | P value | |
|---|---|---|---|
| 0, 1+ | 2+, 3+ | ||
| DNA copy number | 0.0007 | ||
| <2fold | 17 | 5 | |
| >2fold | 1 | 7 | |
| mRNA transcript | <0.0001 | ||
| <2fold | 15 | 1 | |
| >2fold | 3 | 11 | |
| Protein overexpressionb | <0.0001 | ||
| − | 18 | 5 | |
| + | 0 | 7 | |
| Total | 18 | 12 | |
Immunohistochemistry.
Protein expression by Western blot analysis.
4. Discussion
The HER-2 oncogene is a member of the human epidermal growth factor receptor family (Hung and Lau, 1999). Gene amplification and overexpression of HER-2 occurs in many cancer types, including gastric cancers (Ishida et al., 1994; Tokunaga et al., 1995; Yonemura et al., 1991). However, the molecular mechanism of HER-2 overexpression in GCs has not been elucidated. H. pylori is the causative agent of chronicgastric inflammation, such as atrophic gastritis and metaplastic gastritis, which is even further related to GC (Atherton, 2006; Cover and Blaser, 2009; Peek and Blaser, 2002). H. pylori CagA is a crucial factor for apoptosis, cell proliferation, and cell mortality, and, consequently, induces gastric carcinogenesis (Chaturvedi et al., 2011; Handa et al., 2007). H. pylori also produces ROS, which affect gastric cells (Handa et al., 2007). Therefore, we examined whether H. pylori CagA could affect cell growth. In a transient transfection assay, ectopic CagA expression resulted in significant cell growth in AGS, MKN1 and HFE-145 cells (Fig. 1A). When we analyzed HER-2 DNA copy number, and mRNA and protein expression in CagA transfected AGS, MKN1 and HFE-145 cells and the gastricmucosal tissues of H. pylori infected C57BL/6mice, we successfully demonstrated that CagA and ROS increased DNA copy number and protein expression of the HER-2 gene (Fig. 1B–H). To further confirm these initial observations, we examined and compared the presence of CagA with HER-2 status in GC specimens. Interestingly, a positive correlation was observed between HER-2 amplification and CagA positivity (P < 0.001). We also found that HER-2 overexpression was closely associated with HER-2 DNA and mRNA status and H. pylori CagA positivity (Fig. 2A &B, P < 0.05).When the ability of CagA to discriminate the GC with HER-2 expression was analyzed using an ROC curve, CagA predicted HER-2 amplification and mRNA overexpression in GC with AUC values of 0.8733 and 0.9457, respectively (Fig. 2C and D). Finally, we conclude that CagA may be one of the candidate biomarkers for HER-2 amplification and contribute to the development of GC by inducing HER-2 amplification and overexpression.
The ToGA trial (Bang et al., 2010) reported improved overall survival of patients with advanced GC and strongly positive HER-2 status (IHC, 2+/FISH or IHC 3+). Based on that result, accurate testing for HER-2 expression status, the target of trastuzumab, is critical to determine treatment strategy in patients with advanced GC. Hoffman's criteria, which have been commonly used for testing HER-2 status in GC, have been adopted for immunohistochemical staining as a standard to detect the HER-2 protein (Hofmann et al., 2008). However, GC is heterogeneous, which can result in false positives or negatives of HER-2 expression status (Kim et al., 2011; Lee et al., 2011). Thus, unlike breast cancer, Hoffman's criteria may not be sufficient to assess HER-2 status in GC. The inhomogeneous tumor cell distribution in GC could affect the accuracy of HER-2 testing with IHC and FISH. Therefore, FISH and IHC testing for multiple site-biopsy samples might be an option to enhance the accuracy of the test results. However, FISH probes are expensive and a specialist is required to capture the most-amplified area to ensure accuracy of the results. Thus, inter-observer variation could be an important determinant in the FISH result and tissue biopsy sampling error could result in significant errors assessing HER-2 status in GC (Warneke et al., 2013).
We investigated DNA copy number and expression of the HER-2 gene mRNA transcript and protein in 30 GC tissues to partly overcome these problems and to diagnose HER-2 status in GC. Increased HER-2 DNA copy number and overexpression at the mRNA and protein were detected in 8 (26.6%), 14 (46.6%), and 7 (23.3%) of 30 GCs, respectively (Fig. 3). Significant correlations were observed between HER-2 protein expression, the change in DNA copy number, and mRNA transcript expression (Table 1, P < 0.01). Furthermore, HER-2 overexpression by IHC was detected in 12 (40%) of the 30 GCs (Fig. 4) and was closely associated with DNA and mRNA status of the gene in the GCs (Table 3, P < 0.001). All of these results suggest that testing DNA copy number might be more useful to assess genetic status of HER-2 in GC tissue. In particular, DNA copy number testing is much easier, cheaper, and objective than that of FISH. Thus, it is likely that a HER-2 DNA copy number analysis with DNAs extracted from multiple cancer tissue sites could be a better option to overcome GC heterogeneity. In addition, because DNA is easily obtainable from a formalin-fixed paraffin block and is relatively stable, it is also possible to use formalin fixed paraffin sections.
A previous report showed that HER-2 expression and/or gene amplification is significantly related with invasion and nodal metastasis in GC (Mizutani et al., 1993). However, Ozen et al. (2013) reported that HER-2 expression was not significantly correlated with histopathological parameters or overall survival. In our study, altered HER-2 protein expression was not associated with clinicopathological parameters including depth of invasion or lymph no demetastasis (Table 2). It is likely that HER-2 amplificationmay play an important role as an early event in the multi-step process of GC. Further studies on large sample sizes are necessary to clarify the association between HER-2 expression and clinicopathologic parameters.
In conclusion, we found that H. pylori CagA induced HER-2 gene DNA amplification and overexpression of mRNA and protein, and that HER-2 DNA copy number was closely associated with protein expression. Here, we propose testing HER-2 DNA copy number changes with IHC, instead of FISH, to overcome the weakness of the current HER-2 assessment criteria.
Acknowledgments
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A2A2A01002531).
Abbreviations
- GC
gastric cancer
- FISH
fluorescence in situ hybridization
- CISH
chromogenic in situ hybridization
- ROS
reactive oxygen species
- H2O2
hydrogen peroxide
- TEMPOL
4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl
- PCR
polymerase chain reaction
- ROC
receiver operating characteristic
- AUC
area under the ROC curve
- cDNA
DNA complementary to RNA
- IHC
immunohistochemistry
Footnotes
Conflicts of interest
The authors have no conflicts to disclose.
References
- Amieva MR, et al. Disruption of the epithelial apical–junctional complex by Helicobacter pylori CagA. Science. 2004;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JV. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu. Rev. Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- Backert S, Meyer TF. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr. Opin. Microbiol. 2006;9:207–217. doi: 10.1016/j.mib.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology. 2011;141:1696–1708. doi: 10.1053/j.gastro.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covacci A, Rappuoli R. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell. J. Exp. Med. 2000;191:587–592. doi: 10.1084/jem.191.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann. Oncol. 2008;19:1523–1529. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- Guillemin K, et al. Cagpathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc. Natl. Acad. Sci. U. S. A. 2002;12:15136–15141. doi: 10.1073/pnas.182558799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa O, Naito Y, Yoshikawa T. CagA protein of Helicobacter pylori: a hijacker of gastric epithelial cell signaling. Biochem. Pharmacol. 2007;73:1697–1702. doi: 10.1016/j.bcp.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M. The role of Helicobacter pylori CagA in gastric carcinogenesis. Int. J. Hematol. 2006;84:301–308. doi: 10.1532/IJH97.06166. [DOI] [PubMed] [Google Scholar]
- Hofmann M, et al. Assessment of a HER-2 scoring system for gastric cancer: results form a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- Hung MC, Lau YK. Basic science of HER-2/neu: a review. Semin. Oncol. 1999;26:51–59. [PubMed] [Google Scholar]
- Ishida T, et al. Significance of erbB2 gene product as a target molecule for cancer therapy. Scand. J. Immunol. 1994;39:459–466. doi: 10.1111/j.1365-3083.1994.tb03401.x. [DOI] [PubMed] [Google Scholar]
- Jena NR. DNA damage by reactive species: mechanisms, mutation and repair. J. Biosci. 2012;37:503–517. doi: 10.1007/s12038-012-9218-2. [DOI] [PubMed] [Google Scholar]
- Kim JJ, et al. Helicobacter pylori impairs DNA mismatch repair in gastric epithelial cells. Gastroenterology. 2002;123:542–553. doi: 10.1053/gast.2002.34751. [DOI] [PubMed] [Google Scholar]
- Kim MA, Lee HJ, Yang HK, Bang YJ, Kim WH. Heterogenous amplification of ERBB2 in primary lesions in responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology. 2011;59:822–831. doi: 10.1111/j.1365-2559.2011.04012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epider-mal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resection. Histopathology. 2011;59:832–840. doi: 10.1111/j.1365-2559.2011.04017.x. [DOI] [PubMed] [Google Scholar]
- Machado AM, et al. Helicobacter pylori infection induces genetic instability of nuclear and mitochondrial DNA in gastric cells. Clin. Cancer Res. 2009;15:2995–3002. doi: 10.1158/1078-0432.CCR-08-2686. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat. Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- Miller WM, et al. Inhibition of mammalian target of rapamycin is required for optimal antitumor effect of HER2 inhibitors against HER2-overexpressing cancer cells. Clin. Cancer Res. 2009;15:7266–7276. doi: 10.1158/1078-0432.CCR-09-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T, Onda M, Tokunaga A, Yamanaka N, Sugisaki Y. Relationship of C-erbB-2 protein expression and gene amplification to invasion and metastasis in human gastric cancer. Cancer. 1993;72:2083–2088. doi: 10.1002/1097-0142(19931001)72:7<2083::aid-cncr2820720705>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ozen A, et al. The prognostic significance of p21 and HER-2 gene expression and mutation/polymorphism in patients with gastric adenocarcinoma. Med. Oncol. 2013;30:357. doi: 10.1007/s12032-012-0357-y. [DOI] [PubMed] [Google Scholar]
- Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- Tanner M, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase II alpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann. Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- Tokunaga A, et al. Clinical significance of epidermal growth factor (EGF),EGF receptor and c-erbB2 in human gastric cancer. Cancer. 1995;75:1418–1425. doi: 10.1002/1097-0142(19950315)75:6+<1418::aid-cncr2820751505>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Tomb JF, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- Umeda M, et al. Helicobacter pylori CagA causes mitotic impairment and induces chromosomal instability. J. Biol. Chem. 2009;14:22166–22172. doi: 10.1074/jbc.M109.035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warneke VS, et al. Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann. Oncol. 2013;24:725–733. doi: 10.1093/annonc/mds528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura Y, et al. Evaluation of immunoreactivity for erbB2 protein as a marker of poor short-term prognosis in gastric cancer. Cancer Res. 1991;51:1034–1058. [PubMed] [Google Scholar]
- Yoon JH, et al. Gastrokine 1 functions as a tumor suppressor by inhibition of epithelial–mesenchymal transition in gastric cancers. J. Cancer Res. Clin. Oncol. 2011a;137:1697–1704. doi: 10.1007/s00432-011-1051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, et al. Inactivation of the gastrokine 1 gene in gastric adenomas and carcinomas. J. Pathol. 2011b;223:618–625. doi: 10.1002/path.2838. [DOI] [PubMed] [Google Scholar]