Abstract
We recently reported that a combination of dietary grape polyphenols resveratrol, quercetin, and catechin (RQC), at low concentrations, was effective at inhibiting metastatic cancer progression. Herein, we investigate the molecular mechanisms of RQC in breast cancer and explore the potential of RQC as a potentiation agent for the epidermal growth factor receptor (EGFR) therapeutic gefitinib. Our in vitro experiments showed RQC induced apoptosis in gefitinib-resistant breast cancer cells via regulation of a myriad of pro-apoptotic proteins. Since the Akt/mammalian target of rapamycin (mTOR) signaling pathway is often elevated during development of anti-EGFR therapy resistance, the effect of RQC on the mTOR upstream effector Akt and the negative regulator AMP kinase (AMPK) was investigated. RQC was found to reduce Akt activity, induce the activation of AMPK and inhibit mTOR signaling in breast cancer cells. Combined RQC and gefitinib decreased gefitinib resistant breast cancer cell viability to a greater extent than RQC or gefitinib alone. Moreover, RQC inhibited Akt and mTOR, and activated AMPK even in the presence of gefitinib. Our in vivo experiments showed combined RQC and gefitinib was more effective than the individual treatments at inhibiting mammary tumor growth and metastasis in nude mice. Therefore, RQC treatment inhibits breast cancer progression and may potentiate anti-EGFR therapy by inhibition of Akt/mTOR signaling.
Keywords: grape polyphenols, gefitinib, Akt/mTOR signaling, AMPK, potentiation
INTRODUCTION
Breast cancer is the most commonly diagnosed form of cancer in women 40-55 years of age and is the second cause of cancer deaths (1). Recent clinical advances have increased cancer survival rates; however, de novo and acquired resistance to therapy is common and contributes to disease relapse (2). Combination therapies that target several pathways are effective but can have devastating side effects (3;4). Therefore, developing new strategies for prevention of breast cancer progression and overcoming drug resistance represents a major challenge.
Grape polyphenols are desirable cancer therapeutics with potential for combination therapy. Individual grape polyphenols at high concentrations act as cancer preventives and have antiproliferative, antioxidant, antiangiogenic, antiinvasive, and pro-apoptotic properties (5). Moreover, grape polyphenols specifically inhibit the growth of breast cancer cells with low cytotoxicity towards normal mammary epithelial cells (6). These compounds are of particular relevance for gynecological cancers such as breast cancer, since they have been shown to act as selective estrogen receptor (ER) modulators and induce differential gene expression via ERα and ERβ (7). Grape juice constituents and grape seed extract (GSE) inhibit breast cancer initiation, reduce cancer in rodent models (8-10), and have been associated with regulation of Akt and extracellular regulated kinase (ERK) activities (11;12). However, the molecular mechanisms of grape polyphenols, or their effects on metastasis, are not fully understood.
A growing debate on the cancer-preventive properties of natural compounds is that dietary consumption is insufficient to achieve cancer inhibitory concentrations at target tissues (13-17). However, resveratrol, quercetin, and catechins are all considered viable chemopreventives because they are absorbed and metabolized rapidly in vivo and can be detected in plasma and urine samples in the intact form in humans and rodent models (13-18).
Resveratrol, quercetin and catechin are usually conjugated to glucoronic acid and sulfate during first-pass metabolism in the intestinal wall or the liver, while a portion of these metabolites can also be methylated (19-21). Glucorono-conjugates are hydrolyzed, yielding the aglycone, which is more lipophilic and can remain trapped in the tissues (19;22). Therefore, although the aglycone forms of resveratrol, quercetin and catechins are rapidly cleared from plasma, they can still be found in considerable amounts in tissues, together with conjugated metabolites (22). Accordingly, following oral consumption, these polyphenols are found mainly in the serum and urine as glucuronide and sulfate conjugates. In a human study, free polyphenols in the serum accounted for 1.7–1.9% (resveratrol), 1.1 to 6.5% (catechin), and 17.2 to 26.9% (quercetin) while more than 80% was absorbed (23). Therefore, studies that quantified plasma levels of polyphenols may be underestimating the actual amounts that reach the tissues, and more specifically the site of action. Although, more epidemiological data on the effects of grape polyphenols in humans need to be collected, a study reported that grape consumption was significantly inversely associated with breast cancer risk (24). However, few studies have documented the effect of grape polyphenols on metastatic breast cancer.
Overexpression of EGFR family members contributes to cancer progression and metastasis, including breast cancer, where 45% of patients have been shown to be EGFR positive (25). Clinical trials have demonstrated the utility of gefitinib, an EGFR-specific tyrosine kinase inhibitor (26). However, de novo and acquired resistance to gefitinib treatment is a recurrent problem (27;28). Some of the early trials on the efficacy of gefitinib in breast cancer were negative or discontinued due to adverse effects or lack of response probably via resistance mechanisms (29-32). Therefore, multiple studies have investigated the potential of combination therapies to overcome gefitinib resistance (33). A number of recent trials have reported the efficacy of gefitinib in combination with other therapeutics for both human epidermal growth factor receptor 2 (HER2) positive and ER positive breast cancers (34-38). Grape polyphenols are attractive candidates for potentiation to EGFR therapy because they have been shown to individually inhibit EGFR 1 and 2 (HER2) activities (39;40) and decrease HER2 expression, and to regulate pathways that confer therapy resistance (41).
In breast cancer, the EGFR targets phosphoinositide 3-kinase (PI3-K)/Akt and mTOR pathways have been shown to be central for malignancy and evasion to gefitinib treatment (42)}. Akt activates mTOR complex 1 (mTORC1), which contains mTOR and regulatory associated protein of mTOR (Raptor), via inhibition of tuberous sclerosis protein (TSC) 2. TSC2 is a GTPase activating protein (GAP) and thus, a negative regulator of Rheb GTPase that activates mTOR (43). AMPK, a key metabolic sensor of low nutrients and stress, acts disparate to Akt to inhibit mTORC1 via an activating phosphorylation of TSC2 and by direct inhibition of Raptor (44). Therefore, dual PI3-K/mTOR inhibitors have potential in aggressive cancer therapy (45). Since combination therapy can have devastating side effects, a safer alternative is the use of dietary compounds, such as grape polyphenols, with low toxicity that can inhibit both pathways (46);20551291;22574221;21300025;21168265}.
We recently reported that a combination of the major polyphenols in grape and red wine, resveratrol, quercetin, and catechin (RQC), at low concentrations, can inhibit proliferation, cell cycle progression, migration, and survival in breast cancer cells; and reduce tumor growth and site-specific metastasis (47;48). Herein, we show that RQC induces apoptosis and reduces mTOR signaling probably via inhibition of Akt and activation of AMPK in breast cancer and, in combination with gefitinib, can reduce cell proliferation, tumor growth, and metastasis of a gefitinib resistant breast cancer cell line.
MATERIALS AND METHODS
Cell culture
The human metastatic breast cancer cell line MDA-MB-231 (ERα(−), ERβ(+)) expressing green fluorescent protein (GFP) (kind gift of Dr. Danny Welch, The University of Alabama at Birmingham, AL, 2009) (49) was cultured, as described in (47).
Treatments
Resveratrol, quercetin, and catechin were purchased as 99% pure compounds (LKT Laboratories, St. Paul, MN) and stock solutions made in dimethyl sulfoxide (DMSO) or ethanol. Stock solutions for gefitinib (LC Laboratories, Woburn, MA) were prepared in DMSO.
Caspase 3 activity assay
Apoptosis was determined by analyzing the caspase 3 activity of MDA-MB-231 cell lysates following vehicle (0.2% DMSO) or RQC at 5 μM each for 48 h or 96 h using a Caspase-3 Colorimetric Assay Kit as per manufacturer’s instructions (Sigma-Aldrich, St. Louis, MO). Briefly, the p-nitroaniline moiety resulting from caspase 3-mediated hydrolysis of acetyl-Asp-Glu-Val-Asp p-nitroaniline was detected at 405 nm (εmM=10.5) after incubating the reaction mixture at 37°C for 22 h.
Antibody arrays
Total protein extracts from MDA-MB-231 cells treated for 48 h or 96 h with vehicle, or 5 μM RQC were incubated overnight with a human apoptosis antibody array (R&D Systems, Minneapolis, MN), as per manufacturer’s instructions. Unbound proteins were washed away and the array incubated with a cocktail of biotinylated antibodies that were detected using streptavidin-Horseradish peroxidase chemiluminescent reagents.
Data was analyzed using average integrated density signal (calculated using Image J software) for the two replicates of each antibody. Then, the two sets of data were normalized using a normalization factor calculated as the average signal for positive controls in one data set vs. the other. Fold changes compared to vehicle controls were calculated from averages of 3 biological replicates.
Western blotting
Quiescent MDA-MB-231 cells were treated with vehicle, or 5 μM resveratrol, quercetin, or catechin, or a combination (RQC) at 5 μM each; or 5 μM RQC, 30 μM gefitinib, or a combination of 5 μM RQC and 30 μM gefitinib, for 15 or 30 min. Cells were immediately lysed as in (48) and total protein was quantified using the Precision Red protein assay kit (Cytoskeleton, Inc., Denver, CO). Equal total protein amounts were western blotted using anti-Akt, anti-phospho AktS473, anti-AMPKα, anti-phospho AMPKαThr172, anti-ribosomal p70 S6 kinase (p70S6K), or anti-phospho p70S6KThr389 (Cell Signaling Technology, Inc., Danvers, MA) antibodies. The integrated density of positive bands was quantified using Image J software.
Cell viability
MDA-MB-231 (5×104) cells in 6 well plates were treated for 48 h (in culture media with 5% fetal bovine serum) with vehicle, or RQC (0.167, 0.5, 1.67, 5, or 15 μM), gefitinib (0.1, 1, 10, 20, 30, or 40 μM), or RQC and gefitinib (0.5, 1.67, or 5 μM RQC and 10, 20, or 30 μM gefitinib). Cells were fixed, nuclei stained with propidium iodide (PI), and the viable cells quantified.
For the generation of dose response curves and the determination of IC50 values, data from three independent experiments was pooled and four parameter dose–response curves were fitted using the non-linear regression function of GraphPad Prism®.
Animals
Hairless severe combined immunodeficiency (SCID) female mice, 5 to 6 wk old (Charles River Laboratories, Inc., Wilmington, MA) were maintained under pathogen-free conditions in Hepa-filtered cages (5 mice per cage) under controlled light (12 h light and dark cycle), temperature (22-24°C), and humidity (25%). The animals received autoclaved AIN 76-A phytoestrogen-free diet (Tek Global, Harlan Teklad, Madison, WI) with 14% protein and 3.5% fat and water ad libitum. All the animal procedures were conducted in a room dedicated to immunocompromised mice at the institution’s animal facility. Anesthetized animals were euthanized by cervical dislocation. This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Tumor establishment
GFP-MDA-MB-231 cells (~ 1 × 106) in Matrigel (BD Biosciences, San Jose, CA) were injected at the fourth right mammary fat pad under isofluorane inhalation (1-3% in air using inhalation chamber or nose cone, at 2 L/min) to produce orthotopic primary tumors as described in (48). Isofluorane was chosen as the anesthetic agent as it requires minimal animal handling and has a large margin of safety and quick recovery times. After tumor establishment (1wk post-inoculation), the animals from the same litter with similar weight and tumor size were randomly divided into experimental treatment groups (n=10-12 per treatment group).
Diet administration
Mice were orally gavaged either with vehicle for RQC treatment (90% neobee oil, 10% ethanol), vehicle for gefitinib (1% Tween-80 in 1X PBS, pH 7.4), a combination of 5 mg/kg body weight (BW) resveratrol, 5 mg/kg BW quercetin, and 5 mg/kg BW catechin (RQC), or 200 mg/kg BW gefitinib, in a 100 μL volume every other day, 7 days a week. The group receiving both treatments was gavaged one day with 5 mg/kg BW RQC, and the next day with 200 mg/kg BW gefitinib in a 100 μL volume, 7 days a week. Treatments continued until sacrifice at day 84.
Whole body fluorescence image analysis
Mammary tumor growth was quantified as changes in the integrated density of GFP fluorescence, using methods developed by Hoffman and co-workers (50). Mice were imaged one week following breast cancer cell inoculation (on day 1 of diet administration) and once a week thereafter. A 300 Watt power source with two optical delivery systems fitted with excitation filters (470/40 nm) was used for whole body imaging of GFP fluorescence (LT99D2, Lightools Research, Encinitas, CA). Images were captured with a Spot II charge-coupled device (CCD) camera (Diagnostic Instruments, Sterling Heights, MI) mounted with a 530/25 nm emission filter (Chroma Technology, Rockingham, VT).
Tumor fluorescence intensities were analyzed using Image J software (National Institutes of Health, Bethesda, MD). The final images were acquired on day 84. Relative tumor growth was calculated as the integrated density of fluorescence of each tumor on each day of imaging relative to the integrated density of fluorescence of the same tumor on day 1 of diet administration, as described in (47;48). Tumor growth in vehicle for RQC group was not statistically different than tumor growth in vehicle for gefitinib group. Therefore, the average of both groups was used for data analysis.
Analysis of metastases
Following sacrifice, lungs, kidneys, and livers were excised and immediately stored in liquid N2. Stored organs were thawed and analyzed using an Olympus MV10 fluorescence macro zoom system microscope and images acquired with an Olympus DP71 digital camera, as described in (47;48). Each organ was imaged on both sides. The fluorescent lesions (green component of RGB images) were quantified for integrated density of fluorescent pixels using Image J software.
Statistical analysis
Data are expressed as the mean ± SEM. Statistical analyses were done using Microsoft Excel and GraphPad Prism®. Differences between means were determined using Student’s t-Test or one-way ANOVA with Dunnett’s Multiple Comparison Test. The Kruskal-Wallis test with Dunn’s Multiple Comparison was used for groups that did meet the requirements of normality and/or equal variance for one-way ANOVA. Differences between groups are considered to be statistically significant at p≤0.05.
RESULTS
Effects of combined grape polyphenols on apoptosis and apoptotic signaling proteins
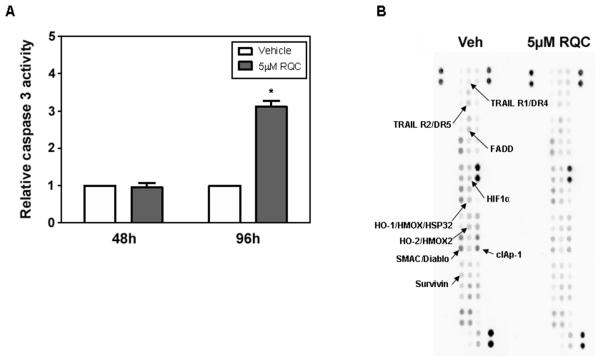
We previously reported that the RQC combination of grape polyphenols was more effective than resveratrol, quercetin, or catechin at inhibiting cancer cell proliferation, cell cycle progression and cell migration using ERα(−), ERβ(+) MDA-MB-231 low metastatic breast cancer cells and ER(−) MDA-MB-435 highly metastatic cancer cells (47;48). In addition, RQC induced apoptosis of MDA-MB-435 cells (48). Since RQC treatment at 5 μM each, for 96 h, decreased MDA-MB-231 cell viability by ~80% compared to vehicle controls and caused S phase cell cycle arrest (48), the effect of 5 μM RQC treatment on apoptosis of MDA-MB-231 cells was determined via caspase 3 activity. RQC at 5 μM did not induce apoptosis in 48 h, while treatment with 5 μM RQC for 96 h increased caspase 3 activity by three-fold with a p≤0.05 thus, implicating RQC treatment in apoptosis regulation (Fig. 1A).
FIG. 1.
Effect of RQC on apoptosis of MDA-MB-231 cells. Apoptosis of MDA-MB-231 cells was detected by caspase 3 activity assays following 48 and 96 h incubation with vehicle (Veh) or combined resveratrol (Res), quercetin (Quer), and catechin (Cat) at 5 μM each (RQC). A: Caspase 3 activity relative to Veh (n=3 ± SEM) as quantified from absorbance at 405 nm of p-nitroaniline, a product released by caspase 3 activity. An asterisk indicates statistical significance (p≤0.05) when compared to vehicle. B: Apoptotic signaling proteins regulated in MDA-MB-231 cells at 48 h following Veh or 5 μM RQC treatment were studied using antibody arrays. Normalized data was analyzed using average integrated density signal for the two replicates of each antibody. A representative image of 3 biological replicates is presented. Proteins that were significantly regulated by RQC are highlighted.
The molecular mechanisms of RQC-induced apoptosis were studied using human apoptotic antibody arrays. Treatment of MDA-MB-231 cells with combined grape polyphenols at 5 μM for 48 h and 96 h resulted in the regulation of a myriad of proteins in the apoptotic signaling cascade (Fig. 1B, Table 1). Table 1 summarizes results from western arrays of apoptotic signaling proteins that were significantly regulated by RQC treatment for 48 h and 96 h in three independent experiments. At 48 h, pro-survival proteins such as cellular inhibitor of apoptosis protein 1 (cIAP-1) and survivin were decreased with no change in caspase 3 activity, indicating that regulation of expression of apoptosis inhibitors precedes induction of apoptosis by RQC. At 96 h, a significant increase in cleaved caspase 3 and FAS ligand and its receptor was observed, correlative to RQC-increased caspase 3 activity (Fig. 1, Table 1). These results indicate a potential death receptor-mediated regulation of apoptosis by RQC. The observed decrease in Hemeoxygenase 1 (HO-1) levels by RQC is in contrast to reports of quercetin upregulating HO-1 in response to oxidant stress in normal cells (51). However, since our study was conducted with aggressive cancer cells in an oxidant stress-free environment, this result may reflect a cancer cell-specific effect or a combination effect of RQC.
TABLE 1. Apoptotic signaling proteins regulated by 5 μM RQC as evidenced from western arrays (n=3).
| 48 h | 96 h | ||
|---|---|---|---|
|
| |||
| Protein | FC | Protein | FC |
| cIAP-1 | −1.75 | Trail R2/DR5 | −2.5 |
| Trail R2/DR5 | −3.07 | Fas/TNSF6 | 1.8 |
| HIF-1 alfa | −2.03 | Cleaved caspase 3 | 5.0 |
| HO-1/HMO/HSP32 | −1.89 | TNF R1/TNFRSF1A | 2.5 |
| HO-2/HMOX2 | −1.64 | ||
| SMAC/Diablo | −1.85 | ||
| Survivin | −1.81 | ||
| Trail R1/DR4 | −2.87 | ||
| FADD | −1.80 | ||
Effects of RQC on regulation of the mTOR pathway
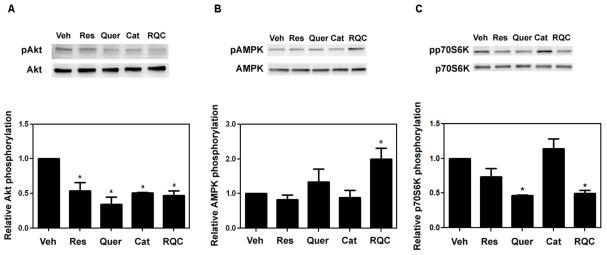
To further investigate the molecular mechanisms by which RQC reduces breast cancer cell survival, we determined the effect of RQC on PI3-K/Akt signaling by determining the activation status, i.e. phosphorylation levels, of Akt. Akt is phosphorylated and activated by phosphoinositide-dependent kinase when phosphatidylinositol 3,4,5-trisphosphate (PIP3) levels are increased due to PI3-K activity. In MDA-MB-231 cells, 5 μM treatment with individual quercetin or RQC for 15 min resulted in a ~50% inhibition of Akt activity without affecting total Akt expression (Fig. 2A). Treatment with 5 μM RQC for 15 min also decreased Akt activity significantly in MDA-MB-435 cells (data not shown).
FIG 2.
Effect of individual and combined resveratrol, quercetin, and catechin (RQC) on Akt, AMPK, and p70S6K phosphorylation in MDA-MB-231 cells. Confluent MDA-MB-231 cells were serum-starved for 24 h, treated with Veh or 5 μM Res, Quer, Cat, or combined Res, Quer, and Cat at 5 μM each (RQC) for 15 min, lysed immediately, and western blotted for active and total proteins: phospho-AktSer473 and Akt, phospho-AMPKThr172 and AMPK, or phospho-p70S6KThr389 and p70S6K. A: Upper panel, representative western blots (from 4 separate experiments); lower panel, Akt activity (phospho-Akt/Akt) as quantified from Image J analysis of integrated density of positive bands. B: Upper panel, representative western blots (from 4 separate experiments); lower panel, AMPK activity (phospho-AMPK/AMPK) as quantified from Image J analysis of integrated density of positive bands. C: Upper panel, representative western blots (from 4 separate experiments); lower panel, p70S6K activity (phospho-p70S6K/p70S6K) as quantified from Image J analysis of integrated density of positive bands. An asterisk indicates statistical significance (p≤0.05) when compared to vehicle.
We previously reported that combined RQC was more efficient than individual compounds at inhibiting breast cancer cell proliferation and cell cycle progression (47;48). Therefore, RQC is expected to inhibit breast cancer progression via additional molecular mechanisms. The activities of AMPK, a negative regulator of mTOR, and mTOR were determined by western blot analysis of total and phosphorylated AMPK and p70S6K, a direct downstream effector of mTOR. Treatment with 5 μM each resveratrol, quercetin, or catechin did not significantly affect AMPK activity of MDA-MB-231 cells. However, combined RQC treatment significantly increased AMPK activity by ~1.75-fold (Fig. 2B). Finally, treatment of MDA-MB-231 cells with 5 μM quercetin or RQC resulted in a ~50% significant decrease in p70S6K phosphorylation, while resveratrol or catechin alone did not affect p70S6K phosphorylation (Fig. 2C).
Potential of RQC as a potentiation agent for gefitinib in vitro
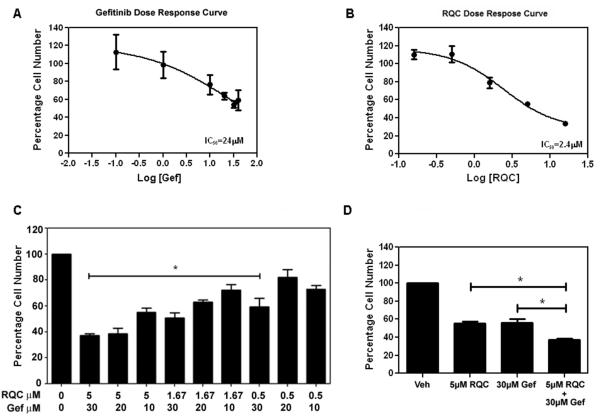
Since RQC inhibited Akt/mTOR signaling, a common pathway upregulated during therapy resistance, the potential of RQC to act as a potentiation agent for gefitinib, an EGFR inhibitor, was characterized. The MDA-MB-231 breast cancer cell line overexpresses EGFR and is intrinsically resistant to gefitinib, with a >10 μM IC50 (52). As shown in Fig. 3, the number of viable MDA-MB-231 cells in response to various concentrations of RQC, gefitinib, or RQC and gefitinib in combination was determined following 48 h treatment. The IC50 for inhibition of cell number by gefitinib was 24 μM and 2.4 μM for RQC (Fig. 3A, B). The gefitinib response plateaued at 50% up to 50 μM gefitinib. Neither treatment resulted in 100% cell death, which is expected for cancer cells that have multiple mechanisms for evading cell death.
FIG. 3.
Effect of individual and combined RQC and gefitinib on MDA-MB-231 cell viability. MDA-MB-231 cells in 5% serum and phenol red-free media were treated for 48 h with Veh or combined Res, Quer, and Cat at 0.167, 0.5, 1.67, 5, or 15 μM each (RQC) for the generation of RQC dose response curve. For the generation of gefitinib (Gef) dose response curve, MDA-MB-231 cells were treated for 48 h with Veh or Gef at 0.1, 1, 10, 20, 30, or 40 μM. Cell number was quantified from PI-stained intact (non-apoptotic) nuclei. Percentage of viable MDA-MB-231 cells ± SEM for 25 microscopic fields/triplicate treatments is shown. Dose response curve for A: RQC and B: Gef are presented. IC50 values for RQC and gefitinib were obtained from dose response curve fittings using the non-linear regression function of GraphPad Prism®. C: Effect of combined RQC and gefitinib on MDA-MB-231 cell viability. MDA-MB-231 cells in 5% serum and phenol red-free media were treated for 48 h with Veh, or different combinations of RQC at 0.5, 1.67, or 5 μM and Gef at 10, 20, or 30 μM. Cell number was quantified from PI-stained intact (non-apoptotic) nuclei. Percentage of viable MDA-MB-231 cells ± SEM for 25 microscopic fields/triplicate treatments is shown. D: Cell number following Veh, 5 μM RQC, 30 μM Gef, or combined RQC and Gef. An asterisk indicates statistical significance (p≤0.05) when compared to vehicle (C) or when comparing combined treatment with either RQC or Gef individual treatments (D).
In combination, the concentrations of gefitinib and RQC required to give a ~50% inhibition was 30 μM and 1.67 μM respectively (Fig. 3C). A similar effect was observed by the combination of 10 μM gefitinib and 5 μM RQC. The combination of RQC and gefitinib that exerted a maximum effect on cell viability was 5 μM RQC and 30 μM gefitinib (Fig. 3C). At these concentrations, combined RQC and gefitinib treatments resulted in a 65% significant decrease in cell number, while individual 5 μM RQC or 30 μM gefitinib demonstrated a 45% significant decrease in cell viability (Fig. 3D). These results suggest that a combination of RQC and gefitinib confers an advantage over individual RQC or gefitinib.
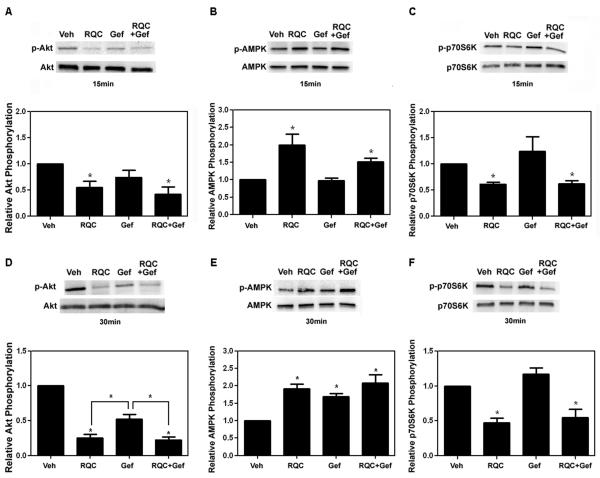
To further evaluate the potential of RQC as a potentiation agent for gefitinib, the activation of Akt, AMPK, and p70S6K were determined in MDA-MB-231 cells treated with individual or combined 5 μM RQC and 30 μM gefitinib (Fig. 4). Gefitinib (30 μM) alone reduced Akt activity following 15 min or 30 min by ~40 and ~50% respectively (Fig. 4A, D). However, this change was not statistically significant. RQC alone or combined 5 μM RQC and 30 μM gefitinib for 15 or 30 min decreased Akt activity significantly by ~50-75%. As expected, 5 μM RQC alone or in combination with gefitinib at 15 and 30 min increased AMPK activity of MDA-MB-231 cells by ~1.75-2-fold in a statistically significant manner (Fig. 4B, E). Although 30 μM gefitinib for 15 min did not affect AMPK activity; at 30 min, gefitinib showed a moderate increase in AMPK activity. Finally, the phosphorylation status of mTOR’s downstream effector p70S6K was determined by western blot analysis following individual or combined RQC and gefitinib treatment. Treatment with RQC alone or in combination with gefitinib for 15 or 30 min resulted in a significant decrease of ~50% in p70S6K activity, while gefitinib alone had no effect (Fig. 4C, F).
FIG. 4.
Effect of individual and combined RQC and gefitinib on Akt, AMPK, and p70S6K phosphorylation in MDA-MB-231 cells. Confluent MDA-MB-231 cells were serum starved for 24 h, treated with Veh, combined Res, Quer, and Cat at 5 μM each (RQC), 30μM Gef or a combination of both for 15 or 30 min, lysed immediately, and western blotted for active and total proteins: phospho-AktSer473 and Akt, phospho-AMPKThr172 and AMPK, or phospho-p70S6KThr389 and p70S6K. A,D: Upper panel, representative western blots (from 3 separate experiments); lower panel, Akt activity (phospho-Akt/Akt) as quantified from Image J analysis of integrated density of positive bands. B,E: Upper panel, representative western blots (from 4 separate experiments); lower panel, AMPK activity (phospho-AMPK/AMPK) as quantified from Image J analysis of integrated density of positive bands. C,F: Upper panel, representative western blots (from 4 separate experiments (15 min treatment) or 6 separate experiments (30 min treatment)); lower panel, p70S6K activity (phospho-p70S6K/p70S6K) as quantified from Image J analysis of integrated density of positive bands. An asterisk indicates statistical significance (p≤0.05) when compared to vehicle unless otherwise specified.
Potential of RQC as a potentiation agent for gefitinib in vivo
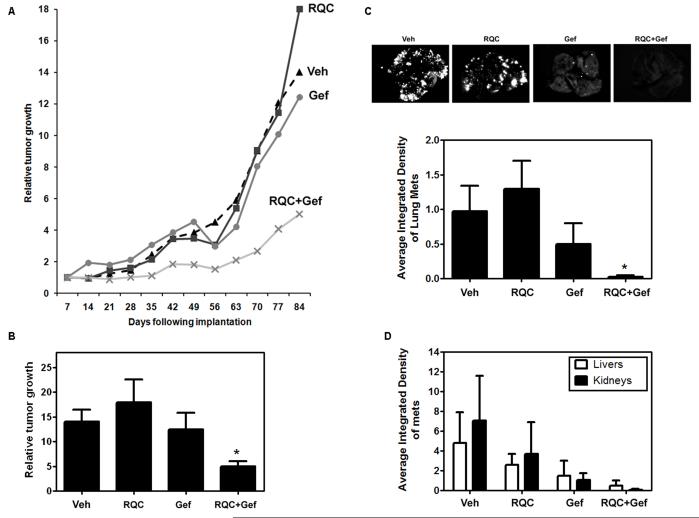
The efficacy of RQC to potentiate effects of gefitinib in vivo was tested in SCID mice. Mammary fat pad tumors from GFP-MDA-MB-231 breast cancer cells were established as previously described (48). One week following tumor establishment, mice (10-12 mice/group) were gavaged with vehicle, 5 mg/kg BW RQC, or 200 mg/kg BW gefitinib every other day, or with combined 5 mg/kg BW RQC and 200 mg/kg BW for 84 days. The RQC concentration was based on our previous studies where 5 mg/kg BW RQC caused significant inhibition of mammary tumor growth and metastasis to bone and liver in nude mice (47;48). The concentration of gefitinib was based on previous studies where 100-200 mg/kg BW gefitinib was orally gavaged to mice, up to 100 days with no toxic effects (53;54). In the present study, administration of RQC alone for 84 days did not significantly affect the average weight of mice compared to controls. However, gefitinib at 200 mg/kg BW demonstrated a 20% significant decrease and the combination treatment caused a 15% significant decrease in average weight (supplemental data, Fig. 1). As shown in Fig. 5A, B, tumor growth was significantly inhibited in mice treated with RQC and gefitinib, whereas treatment with individual RQC or gefitinib showed no reduction in tumor size.
FIG. 5.
Effect of individual and combined RQC and Gefitinib on the progression of MDA-MB-231 mammary fat pad tumors. GFP-tagged MDA-MB-231 cells (1 × 106) in Matrigel were inoculated at the mammary fat pad of female SCID mice, 5 to 6 wk old. One week following injection, mice (n=10-12) were treated with Veh, 5 mg/kg BW RQC (RQC), 200 mg/kg BW gefitinib (Gef) every other day, or a combination of 5 mg/kg BW RQC and 200 mg/kg BW Gef (RQC+Gef) by oral gavage. The group receiving the combination of RQC and Gef was treated one day with RQC and the following day with Gef until sacrifice at day 84. Whole body fluorescence images were acquired once a week. A: Average relative tumor growth as a function of days following injection. Relative tumor growth was calculated as the integrated density of fluorescence on each imaging day as a function of the integrated density of fluorescence of the same tumor on day 1. B: Relative tumor growth for each treatment group at day 84. C,D: Effect of grape polyphenols on metastases. Following necropsy, lungs, livers, and kidneys were excised from mice with GFP-MDA-MB-231 mammary tumors that received Veh, RQC, Gef, or RQC+Gef diets and analyzed for metastases by fluorescence microscopy followed by quantitative image analysis. C: Lung metastatic efficiency expressed as average integrated density of fluorescence from lungs ± SEM (n=10-12). D: Liver and kidney metastatic efficiency expressed as average integrated density of fluorescence ± SEM (n=10-12). An asterisk denotes statistical significance (p≤0.05) when compared to vehicle.
The effect of RQC and gefitinib on metastasis was investigated by fluorescence image analysis of excised organs. As was shown previously by us, in a nude mouse model of metastatic cancer (48), treatment with RQC did not affect the incidence of lung metastases but reduced liver and kidney metastases (Fig. 5C, D). Gefitinib alone reduced lung metastases by 50%; however, this reduction was not statistically significant compared to controls. Combined RQC and gefitinib inhibited metastases to the lung by 97% in a statistically significant manner (Fig. 5C). In combined RQC+geftinib treated mice, only 40% presented with lung metastases and the average integrated density of metastatic foci was 0.03 compared to ~1.0 for vehicle controls, where 80% of mice presented with lung metastases. The number and integrated density of liver and kidney metastases followed a similar trend but was not statistically significant compared to vehicle controls. RQC treatment alone resulted in a 50% reduction in liver metastases, while gefitinib inhibited metastases by 70% and the combined treatment demonstrated a 90% inhibition compared to controls. Similarly, although a majority of the kidneys from all groups did not present with metastases, metastases to this organ were reduced by 99% following the combined RQC and gefitinib treatment (Fig. 5D). Overall, these results suggest that RQC has the potential to sensitize EGFR therapy-resistant breast cancers to EGFR-targeted therapeutics.
DISCUSSION
Most studies on cancer preventive effects of grape polyphenols use individual compounds at high concentrations or combinations such as grape seed extract, or red wine extract in the proportions that exist naturally. Our studies are unique in using an equimolar combination of resveratrol, quercetin, and catechin (RQC) at low concentrations within the range of 0.1-10 μM that may be accumulated in the circulation following consumption of grape products (15;18;55;56). However, caution must be used with interpretation of in vitro data because the compounds that were added to the tissue culture cells are relatively stable in their aglycone forms compared to the dietary polyphenols that are conjugated to glucoronic acid and sulfate during metabolism (19-21). Nevertheless, although the unmodified forms of resveratrol, quercetin and catechins are found in minimal amounts in plasma, they are found in substantial amounts in tissues (22), and may be available at the sites of action in the aglycone form.
Herein, we have characterized the molecular mechanisms and efficacy of RQC to potentiate metastatic breast cancer cells to current anti-EGFR therapy. The inhibitory effects of RQC on breast cancer may be due to reduced cell survival and induction of cell cycle arrest and apoptosis. The RQC-mediated regulation of PI3-K/Akt/mTOR signaling was investigated because this pathway is central to multiple cellular processes of tumorigenesis and development of gefitinib resistance in breast cancer cells (42). For these experiments we studied the effects of short term RQC treatment (15 min) on important phosphorylation events within the PI3K/Akt/mTOR pathway. The 15 min phosphorylation times were selected to reflect rapid signaling events. Regulation of Akt and mTOR signaling by RQC is expected to eventually result in decreased cell survival, cell viability, and cell cycle progression at longer times.
Our results show that individual quercetin is as effective as RQC at reducing Akt activity. This observation suggests that only quercetin, which is a direct inhibitor of PI3-K that has been shown to inhibit Akt activity in breast cancer (57-59), is responsible for RQC effects on Akt activity.
We also investigated a role for AMPK, a negative regulator of the mTOR pathway, in RQC-mediated regulation of mTOR signaling. AMPK is a target of the anti-diabetic drug Metformin, which has been associated with decreased cancer incidence in epidemiological studies and with direct inhibition of cancer cell and tumor growth (60). Metformin has been implicated in chemosensitization to diverse anti-cancer agents through activation of AMPK and the subsequent inhibition of the mTOR pathway (61). Several studies have shown that induction of AMPK activity might be another mechanism through which resveratrol and quercetin inhibit cancer cell growth (62). Both Akt and AMPK regulate mTOR signaling via disparate regulation of TSC2, a negative regulator of mTOR (43;44). Although resveratrol or quercetin at high concentrations (50-200 μM) increase AMPK activity (63;64); in our studies, individual resveratrol, quercetin, or catechin at 5 μM did not affect phospho AMPKT172 levels significantly. Conversely, the RQC combination significantly increased AMPK phosphorylation by ~1.75 fold. This result supports our previous reports of RQC being more effective than individual polyphenols (47;48). AMPK is known to affect lipid metabolism by inhibiting acetyl-CoA carboxylase (ACC), which provides the malonyl-CoA substrate for fatty acid biosynthesis. Accordingly, we have found that RQC inhibits ACC by increased phosphorylation (supplemental data, Fig. 2), indicating that RQC activation of AMPK regulates mechanisms other than mTOR signaling. Other effects of AMPK on autophagy and protein synthesis inhibition via phosphorylation of eukaryotic elongation factor 2 kinase (65) may also contribute to RQC effects on cancer, and will be the subject of future studies.
Interestingly, a parallel pattern of Akt and p70S6K inhibition was observed for individual quercetin and combined RQC; while AMPK activation showed a different behavior. In the same breast cancer cell line, Akt and p70S6K were inhibited by quercetin and combined RQC, whereas AMPK was only activated by RQC. The decrease in Akt and p70S6K phosphorylation by RQC might be entirely due to the action of quercetin; suggesting quercetin alone is sufficient to inhibit the Akt/mTOR signaling axis. However, in our previous publications we have shown the RQC combination to be more effective than the individual polyphenols at inhibiting cell proliferation, cell cycle progression, and cell migration (47;48). These previous findings and the fact that combined RQC and not individual quercetin induced AMPK activity support an enhanced effect by RQC when compared to the individual polyphenols. Since p70S6K activity is directly under mTORC1 regulation, it is possible that quercetin is directly affecting mTORC1 kinase activity, while the RQC combination has multiple effects on other signaling nodes. Moreover, the effect of RQC on p70S6K activity may also be via inhibition of mitogen activated protein kinase (MAPK) activity since the Ras/ ERK pathway has been shown to affect p70S6K activity (66). Nevertheless, we found no significant effects of RQC on ERK, or the activities of other MAPKs p38 and c-Jun N-terminal kinase (JNK) (supplemental data, Fig. 3). Taken together, these results suggest a complex mode of regulation of mTOR signaling by RQC.
EGFR is a key mediator of cancer progression and metastasis, thus representing an excellent target for cancer therapy. Gefitinib is a low molecular weight tyrosine kinase inhibitor with high specificity towards EGFR and therapeutic promise for the treatment of numerous human cancers; however, resistance to gefitinib greatly impairs its effectiveness in the clinic. In breast cancer, ERK/mitogen activated protein kinase kinase (MEK), PI3-K/Akt, and mTOR pathways have been shown to be relevant for evasion to gefitinib treatment (42). Since the current approaches of combination therapies to overcome drug resistance often result in severe toxicity, the RQC formulation of natural non-toxic grape polyphenols was tested for its ability to potentiate the anticancer effects of gefitinib.
We show that RQC alone or in combination with gefitinib can reduce breast cancer cell viability and Akt/mTOR signaling. Gefitinib does not affect RQC-mediated regulation of Akt, AMPK, and p70S6K. Since up-regulation of PI3-K/Akt/mTOR is known to be involved in drug resistance, these results further implicate RQC as a potentiation agent for gefitinib. Interestingly, 30 min treatment with gefitinib alone did not affect p70S6K activity; however, at this time point, gefitinib moderately inhibits Akt and increases AMPK activity. This result suggests that resistance to gefitinib in MDA-MB-231 cells may be downstream of mTOR signaling at the p70S6K level, possibly via activation of MAPK or other gefitinib-insensitive p70S6K activators.
Herein, we tested the aglycone forms of resveratrol, quercetin, and catechin on cancer cells, and by oral administration to mice. A number of studies have reported low levels of resveratrol and quercetin in the serum and plasma of rodents following dietary administration (16;17;67), However, the in vivo concentrations of dietary grape polyphenols depend on their bioavailability at the site of action, which may not be reflected by the levels in plasma or urine (23).
Our in vivo study demonstrates that RQC can potentiate the effect of gefitinib in resistant breast cancers, as shown by the significant inhibition in tumor growth induced by RQC and gefitinib combined treatment. Although we have previously reported RQC at 5 mg/kg BW to significantly reduce tumor growth in nude mice bearing MDA-MB-231 and MDA-MB-435 tumor xenografts (47;48), RQC alone did not affect mammary fat pad tumor growth in the present study. This discrepancy may be accounted for by the differences in the experimental designs. In the previous study, MDA-MB-231 xenografts were implanted in nude mice as equal-size tumor slices from previously established tumors. In addition, the RQC treatments were administrated 1 day following xenograft implantation. Moreover, the RQC polyphenols were dissolved in 90% corn oil:10% ethanol. In the present study, MDA-MB-231 cells in 50% Matrigel were inoculated directly at the mammary fat pad of SCID mice, treatments were started after tumor establishment (one week after breast cancer cell inoculation), and polyphenols were dissolved in 90% neobee oil:10% ethanol. Therefore, the difference in mouse species (athymic nude mice vs. T- and B-cell deficient SCID mice), establishment of xenografts vs. cells (a xenograft represents a more established solid tumor compared to cells in Matrigel), time of compound administration, and relative solubility of the polyphenols in corn oil vs. neobee oil may account for these observed differences in RQC responses.
Regardless, in this study, we show the potential of RQC to inhibit breast cancer progression in the presence of gefitinib; and thus, potentiate anti-EGFR therapy. In mice with an average bodyweight of 20 g, the concentrations of grape polyphenols used in this study, 5 mg/kg BW of each compound in a 100 μL gavage volume, equates to 4.38 μM resveratrol, 3.3 μM quercetin, and 3.48 μM catechin. These concentrations are found in dietary components rich in grape polyphenols such as red wine (68;69). Therefore, the 5 mg/kg BW combined polyphenols that potentiated the effects of gefitinib and reduced mammary tumor growth in this study and our previous reports (47;48) should be effective at the cellular level at these low concentrations. Additionally, RQC treatment may have a slight protective effect on gefitinib toxicity because the combined treatment did not decrease mouse weights to the same extent as gefitinib alone.
Our data demonstrating that combined RQC and gefitinib therapy causes a dramatic and significant reduction in lung metastases is noteworthy because lung cancer is the leading cause of death from cancer in the United States and breast cancer mortality is usually due to metastasis to lung and other distant organs (1). We also found a trend in reduced kidney and liver metastases with combined RQC and gefitinib treatment. Interestingly, gefitinib alone at 200 mg/kg BW caused a reduction in metastasis. This result indicates the utility of continuing gefitinib therapy in patients whose primary tumors are no longer responsive.
Our results demonstrate that combined grape polyphenols resveratrol, quercetin, and catechin in equimolar concentrations inhibit mTOR signaling by a dual mechanism of PI3-K/Akt and AMPK regulation, and potentiate breast cancers to anti-EGFR therapy. The findings from this study and our previous publications on the potential of combined grape polyphenols as breast cancer therapeutics (47;48) suggest that the RQC mixture may have synergistic effects for certain functions. Since development of therapy resistance is a common failure in cancer therapy, this study highlights the potential of combined RQC and gefitinib as a therapeutic regimen to impede progression of gefitinib resistant metastatic breast cancers.
Supplementary Material
ACKNOWLEDGEMENTS
This study is sponsored by the Department of Defense/Breast Cancer Research Program awards W81XWH-07-1-0330 to SD and W81XWH-08-01-0258 to LCP, NIH/NIGMS SC3GM084824 to SD, NIH/NCRR grants 2G12RR003035 to UCC and G12-RR03051 to UPR MSC. We thank Dr. Aldo Pérez for his excellent technical assistance with animal protocols and Vanita Flanagan for assistance with imaging organs. We also acknowledge the services of the Animal Resources Center, Universidad Central del Caribe, Bayamón, Puerto Rico.
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 3.Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Curr Opin Genet Dev. 2008;18:73–79. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Ricciardi S, Tomao S, de MF. Toxicity of targeted therapy in non-small-cell lung cancer management. Clin Lung Cancer. 2009;10:28–35. doi: 10.3816/CLC.2009.n.004. [DOI] [PubMed] [Google Scholar]
- 5.Kaur M, Agarwal C, Agarwal R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr. 2009;139:1806S–12S. doi: 10.3945/jn.109.106864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakimuddin F, Paliyath G, Meckling K. Selective cytotoxicity of a red grape wine flavonoid fraction against MCF-7 cells. Breast Cancer Res Treat. 2004;85:65–79. doi: 10.1023/B:BREA.0000021048.52430.c0. [DOI] [PubMed] [Google Scholar]
- 7.Harris DM, Besselink E, Henning SM, Go VL, Heber D. Phytoestrogens induce differential estrogen receptor alpha- or Beta-mediated responses in transfected breast cancer cells. Exp Biol Med (Maywood) 2005;230:558–68. doi: 10.1177/153537020523000807. [DOI] [PubMed] [Google Scholar]
- 8.Singletary KW, Stansbury MJ, Giusti M, van Breemen RB, Wallig M, et al. Inhibition of rat mammary tumorigenesis by concord grape juice constituents. J Agric Food Chem. 2003;51:7280–86. doi: 10.1021/jf030278l. [DOI] [PubMed] [Google Scholar]
- 9.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–40. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 10.Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in fischer 344 rats. Mol Carcinog. 2010;49:641–52. doi: 10.1002/mc.20643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaur M, Tyagi A, Singh RP, Sclafani RA, Agarwal R, Agarwal C. Grape seed extract upregulates p21 (Cip1) through redox-mediated activation of ERK1/2 and posttranscriptional regulation leading to cell cycle arrest in colon carcinoma HT29 cells. Mol Carcinog. 2011;50:553–62. doi: 10.1002/mc.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Zhang K, Chen S, Wen W. Grape seed extract inhibits VEGF expression via reducing HIF-1alpha protein expression. Carcinogenesis. 2009;30:636–44. doi: 10.1093/carcin/bgp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gescher AJ, Steward WP. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev. 2003;12:953–57. [PubMed] [Google Scholar]
- 14.Meng X, Maliakal P, Lu H, Lee MJ, Yang CS. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J Agric Food Chem. 2004;52:935–42. doi: 10.1021/jf030582e. [DOI] [PubMed] [Google Scholar]
- 15.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–42S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 16.Asensi M, Medina I, Ortega A, et al. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med. 2002;33:387–98. doi: 10.1016/s0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer P, Asensi M, Segarra R, et al. Association between pterostilbene and quercetin inhibits metastatic activity of B16 melanoma. Neoplasia. 2005;7:37–47. doi: 10.1593/neo.04337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soleas GJ, Grass L, Josephy PD, Goldberg DM, Diamandis EP. A comparison of the anticarcinogenic properties of four red wine polyphenols. Clin Biochem. 2002;35:119–24. doi: 10.1016/s0009-9120(02)00275-8. [DOI] [PubMed] [Google Scholar]
- 19.Terao J, Murota K, Kawai Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct. 2011;2:11–17. doi: 10.1039/c0fo00106f. [DOI] [PubMed] [Google Scholar]
- 20.ndres-Lacueva C, Macarulla MT, Rotches-Ribalta M, et al. Distribution of resveratrol metabolites in liver, adipose tissue, and skeletal muscle in rats fed different doses of this polyphenol. J Agric Food Chem. 2012;60:4833–40. doi: 10.1021/jf3001108. [DOI] [PubMed] [Google Scholar]
- 21.Lotito SB, Zhang WJ, Yang CS, Crozier A, Frei B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic Biol Med. 2011;51:454–63. doi: 10.1016/j.freeradbiomed.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Vizcaino F, Duarte J, Santos-Buelga C. The flavonoid paradox: conjugation and deconjugation as key steps for the biological activity of flavonoids. J Sci Food Agric. 2012;92:1822–25. doi: 10.1002/jsfa.5697. [DOI] [PubMed] [Google Scholar]
- 23.Cimino S, Sortino G, Favilla V, et al. Polyphenols: key issues involved in chemoprevention of prostate cancer. Oxid Med Cell Longev. 2012;2012:632959. doi: 10.1155/2012/632959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levi F, Pasche C, Lucchini F, Ghidoni R, Ferraroni M, La Vecchia C. Resveratrol and breast cancer risk. Eur J Cancer Prev. 2005;14:139–42. doi: 10.1097/00008469-200504000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Chan SK, Hill ME, Gullick WJ. The role of the epidermal growth factor receptor in breast cancer. J Mammary Gland Biol Neoplasia. 2006;11:3–11. doi: 10.1007/s10911-006-9008-2. [DOI] [PubMed] [Google Scholar]
- 26.Von PJ. Gefitinib (Iressa, ZD1839): a novel targeted approach for the treatment of solid tumors. Bull Cancer. 2004;91:E70–E76. [PubMed] [Google Scholar]
- 27.Kosaka T, Yamaki E, Mogi A, Kuwano H. Mechanisms of Resistance to EGFR TKIs and Development of a New Generation of Drugs in Non-Small-Cell Lung Cancer. J Biomed Biotechnol. 2011;2011:165214. doi: 10.1155/2011/165214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshikawa S, Kukimoto-Niino M, Parker L, et al. Structural basis for the altered drug sensitivities of non-small cell lung cancer-associated mutants of human epidermal growth factor receptor. Oncogene. 2012 doi: 10.1038/onc.2012.21. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 29.Baselga J, Albanell J, Ruiz A, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23:5323–33. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 30.Bernsdorf M, Balslev E, Lykkesfeldt AE, et al. Value of post-operative reassessment of estrogen receptor alpha expression following neoadjuvant chemotherapy with or without gefitinib for estrogen receptor negative breast cancer. Breast Cancer Res Treat. 2011;128:165–70. doi: 10.1007/s10549-011-1535-x. [DOI] [PubMed] [Google Scholar]
- 31.Osborne CK, Neven P, Dirix LY, et al. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011;17:1147–59. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernsdorf M, Ingvar C, Jorgensen L, et al. Effect of adding gefitinib to neoadjuvant chemotherapy in estrogen receptor negative early breast cancer in a randomized phase II trial. Breast Cancer Res Treat. 2011;126:463–70. doi: 10.1007/s10549-011-1352-2. [DOI] [PubMed] [Google Scholar]
- 33.Knight LA, Di NF, Whitehouse P, et al. The in vitro effect of gefitinib (‘Iressa’) alone and in combination with cytotoxic chemotherapy on human solid tumours. BMC Cancer. 2004;4:83. doi: 10.1186/1471-2407-4-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dragowska WH, Weppler SA, Qadir MA, et al. The combination of gefitinib and RAD001 inhibits growth of HER2 overexpressing breast cancer cells and tumors irrespective of trastuzumab sensitivity. BMC Cancer. 2011;11:420. doi: 10.1186/1471-2407-11-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somlo G, Martel CL, Lau SK, et al. A phase I/II prospective, single arm trial of gefitinib, trastuzumab, and docetaxel in patients with stage IV HER-2 positive metastatic breast cancer. Breast Cancer Res Treat. 2012;131:899–906. doi: 10.1007/s10549-011-1850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massarweh S, Tham YL, Huang J, et al. A phase II neoadjuvant trial of anastrozole, fulvestrant, and gefitinib in patients with newly diagnosed estrogen receptor positive breast cancer. Breast Cancer Res Treat. 2011;129:819–27. doi: 10.1007/s10549-011-1679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cristofanilli M, Valero V, Mangalik A, et al. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16:1904–14. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 38.Gutteridge E, Agrawal A, Nicholson R, Leung CK, Robertson J, Gee J. The effects of gefitinib in tamoxifen-resistant and hormone-insensitive breast cancer: a phase II study. Int J Cancer. 2010;126:1806–16. doi: 10.1002/ijc.24884. [DOI] [PubMed] [Google Scholar]
- 39.Fridrich D, Teller N, Esselen M, Pahlke G, Marko D. Comparison of delphinidin, quercetin and (−)-epigallocatechin-3-gallate as inhibitors of the EGFR and the ErbB2 receptor phosphorylation. Mol Nutr Food Res. 2008;52:815–22. doi: 10.1002/mnfr.200800026. [DOI] [PubMed] [Google Scholar]
- 40.Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia. 2005;7:128–40. doi: 10.1593/neo.04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeong JH, An JY, Kwon YT, Li LY, Lee YJ. Quercetin-induced ubiquitination and down-regulation of Her-2/neu. J Cell Biochem. 2008;105:585–95. doi: 10.1002/jcb.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Regan R, Hawk NN. mTOR inhibition in breast cancer: unraveling the complex mechanisms of mTOR signal transduction and its clinical implications in therapy. Expert Opin Ther Targets. 2011;15:859–72. doi: 10.1517/14728222.2011.575362. [DOI] [PubMed] [Google Scholar]
- 43.Dobashi Y, Watanabe Y, Miwa C, Suzuki S, Koyama S. Mammalian target of rapamycin: a central node of complex signaling cascades. Int J Clin Exp Pathol. 2011;4:476–95. [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Heijden MS, Bernards R. Inhibition of the PI3K pathway: hope we can believe in? Clin Cancer Res. 2010;16:3094–99. doi: 10.1158/1078-0432.CCR-09-3004. [DOI] [PubMed] [Google Scholar]
- 46.Garg AK, Buchholz TA, Aggarwal BB. Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal. 2005;7:1630–47. doi: 10.1089/ars.2005.7.1630. [DOI] [PubMed] [Google Scholar]
- 47.Schlachterman A, Valle F, Wall KM, et al. Combined resveratrol, quercetin, and catechin treatment reduces breast tumor growth in a nude mouse model. Transl Oncol. 2008;1:19–27. doi: 10.1593/tlo.07100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castillo-Pichardo L, Martinez-Montemayor MM, Martinez JE, Wall KM, Cubano LA, Dharmawardhane S. Inhibition of mammary tumor growth and metastases to bone and liver by dietary grape polyphenols. Clin Exp Metastasis. 2009;26:505–16. doi: 10.1007/s10585-009-9250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phadke PA, Vaidya KS, Nash KT, Hurst DR, Welch DR. BRMS1 suppresses breast cancer experimental metastasis to multiple organs by inhibiting several steps of the metastatic process. Am J Pathol. 2008;172:809–17. doi: 10.2353/ajpath.2008.070772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang M, Baranov E, Jiang P, et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc Natl Acad Sci U S A. 2000;97:1206–11. doi: 10.1073/pnas.97.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashi Y, Matsushima M, Nakamura T, et al. Quercetin protects against pulmonary oxidant stress via heme oxygenase-1 induction in lung epithelial cells. Biochem Biophys Res Commun. 2012;417:169–74. doi: 10.1016/j.bbrc.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 52.Ferrer-Soler L, Vazquez-Martin A, Brunet J, Menendez JA, De LR, Colomer R. An update of the mechanisms of resistance to EGFR-tyrosine kinase inhibitors in breast cancer: Gefitinib (Iressa) -induced changes in the expression and nucleo-cytoplasmic trafficking of HER-ligands (Review) Int J Mol Med. 2007;20:3–10. [PubMed] [Google Scholar]
- 53.Arpino G, Gutierrez C, Weiss H, et al. Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst. 2007;99:694–705. doi: 10.1093/jnci/djk151. [DOI] [PubMed] [Google Scholar]
- 54.Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–54. [PubMed] [Google Scholar]
- 55.Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochem Biophys. 2003;417:12–17. doi: 10.1016/s0003-9861(03)00284-4. [DOI] [PubMed] [Google Scholar]
- 56.Bottner M, Christoffel J, Jarry H, Wuttke W. Effects of long-term treatment with resveratrol and subcutaneous and oral estradiol administration on pituitary function in rats. J Endocrinol. 2006;189:77–88. doi: 10.1677/joe.1.06535. [DOI] [PubMed] [Google Scholar]
- 57.Saeed SA, Connor JD, Imran, et al. Inhibitors of phosphatidylinositide 3-kinase: effects on reactive oxygen species and platelet aggregation. Pharmacol Rep. 2007;59:238–43. [PubMed] [Google Scholar]
- 58.He X, Wang Y, Zhu J, Orloff M, Eng C. Resveratrol enhances the anti-tumor activity of the mTOR inhibitor rapamycin in multiple breast cancer cell lines mainly by suppressing rapamycin-induced AKT signaling. Cancer Lett. 2011;301:168–76. doi: 10.1016/j.canlet.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Gulati N, Laudet B, Zohrabian VM, Murali R, Jhanwar-Uniyal M. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006;26:1177–81. [PubMed] [Google Scholar]
- 60.Gonzalez-Angulo AM, Meric-Bernstam F. Metformin: a therapeutic opportunity in breast cancer. Clin Cancer Res. 2010;16:1695–700. doi: 10.1158/1078-0432.CCR-09-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berstein LM, Yue W, Wang JP, Santen RJ. Isolated and combined action of tamoxifen and metformin in wild-type, tamoxifen-resistant, and estrogen-deprived MCF-7 cells. Breast Cancer Res Treat. 2011;128:109–17. doi: 10.1007/s10549-010-1072-z. [DOI] [PubMed] [Google Scholar]
- 62.Hwang JT, Kwon DY, Yoon SH. AMP-activated protein kinase: a potential target for the diseases prevention by natural occurring polyphenols. N Biotechnol. 2009;26:17–22. doi: 10.1016/j.nbt.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 63.Puissant A, Robert G, Fenouille N, et al. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70:1042–52. doi: 10.1158/0008-5472.CAN-09-3537. [DOI] [PubMed] [Google Scholar]
- 64.Lee YK, Park SY, Kim YM, Lee WS, Park OJ. AMP kinase/cyclooxygenase-2 pathway regulates proliferation and apoptosis of cancer cells treated with quercetin. Exp Mol Med. 2009;41:201–07. doi: 10.3858/emm.2009.41.3.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong AK, Howie J, Petrie JR, Lang CC. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin Sci (Lond) 2009;116:607–20. doi: 10.1042/CS20080066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehman JA, Gomez-Cambronero J. Molecular crosstalk between p70S6k and MAPK cell signaling pathways. Biochem Biophys Res Commun. 2002;293:463–69. doi: 10.1016/S0006-291X(02)00238-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011;68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoo YJ, Prenzler PD, Saliba AJ, Ryan D. Assessment of some Australian red wines for price, phenolic content, antioxidant activity, and vintage in relation to functional food prospects. J Food Sci. 2011;76:C1355–64. doi: 10.1111/j.1750-3841.2011.02429.x. [DOI] [PubMed] [Google Scholar]
- 69.Faustino RS, Sobrattee S, Edel AL, Pierce GN. Comparative analysis of the phenolic content of selected Chilean, Canadian and American Merlot red wines. Mol Cell Biochem. 2003;249:11–19. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.