Abstract
Objective
Neuroanatomical and functional imaging studies have identified the cerebellum as an integral component of motor and language control. Few studies, however, have investigated the role of the cerebellum in Tourette syndrome (TS), a condition defined by the presence of semi-involuntary movements and sounds.
Methods
Magnetic resonance imaging was conducted in 163 persons with TS and 147 control participants. Multivariate linear regression models were used to explore effects on cerebellar surface morphology and underlying volumes for the main diagnosis effects of TS as well as comorbid obsessive-compulsive disorder (OCD) and attention-deficit/hyperactivity disorder. Additionally, the correlations of symptom severity with cerebellar morphology were also assessed.
Results
The TS group demonstrated reduced volumes of the cerebellar hemispheres bilaterally that derived primarily from reduced gray matter in crus I and lobules VI, VIIB, and VIIIA. These decreased regional volumes accompanied increasing tic symptom severity and motoric disinhibition as demonstrated by a finger tapping test. Males had reduced volumes of these same regions compared with females, irrespective of diagnosis. Comorbid OCD was associated with relative enlargement of these regions in proportion to the increasing severity of OCD symptoms.
Interpretation
The cerebellum is involved in the pathogenesis of TS and tic-related OCD. Baseline gender differences in cerebellar morphology may in part account for the more prevalent expression of TS in males.
Tourette syndrome (TS) is defined by the presence of motor and vocal tics. Symptom severity varies over time but usually increases during grade school years and attenuates during adolescence. TS is 3–4× more common in males.1,2 Obsessive-compulsive disorder (OCD) and attention-deficit/hyperactivity disorder (ADHD) are common co-occurring conditions.3
Disturbances of corticostriatothalamocortical (CSTC) circuits are thought to produce TS and comorbid OCD.4 Caudate nucleus volumes are smaller in persons with TS,5,6 and cortical thinning is present in primary motor, somatosensory, and premotor cortices in direct proportion to tic severity.7 Neurophysiological studies demonstrate increased excitability of motor cortices in persons with TS with and without OCD,8 which is believed to be a consequence of reduced inhibitory activity of GABAergic interneurons.9,10
Human and animal lesion studies have shown that the cerebellum participates in the control of motor functions.11 Virus tracing studies have demonstrated numerous closed-circuit loops linking motor cortical areas to cerebellar lobules VI, VIIB, and VIII and to the cerebellar dentate nucleus,12,13 a region integrally involved in the planning and execution of movements.14
The cerebellum also supports various language functions. Functional magnetic resonance imaging (fMRI) demonstrates activation of right crus I and lobule VI during verb generation.15,16 fMRI studies suggest participation of 2 corticocerebellar networks in verbal working memory. One functional network involves crus I and lobule VI of the cerebellum, as well as Broca’s area and premotor cortex (BA 44/6).17,18 Another involves lobules VIIB and VIIIA together with the inferior parietal lobe (BA 40).19 Right cerebellar lesions impair language development in children20 and verbal working memory and linguistic error detection in adults.21,22
Cerebellar involvement in motor and language functions has motivated cerebellar imaging studies in TS. A volumetric study reported no differences in cerebellar volumes of 20 TS boys compared with matched controls.23 Functional studies have reported cerebellar activation during tic generation,24,25 specifically in crus 1 and lobules VI, VIIB, and VIIIA.26 We report herein a high-resolution MRI study of cerebellar morphology in 310 persons, 163 with TS and 147 healthy controls. We hypothesize that cerebellar morphology in participants with TS differs from controls, and that these differences correlate significantly with tic severity.
Subjects and Methods
Further details regarding subject recruitment and characterization, pulse sequences, morphometric procedures, and statistical analyses are provided in the Supplemental Materials.
Subjects
We acquired magnetic resonance images in 310 individuals aged 6 to 60 years (Table 1). Patients with TS were recruited from a clinic at the Yale Child Study Center. Healthy controls were recruited at random from a telemarketing list of 10,000 households.
TABLE 1.
Demographic Characteristics of the 310 Study Participants
| Characteristic | Patients with TS (n = 163)a | Controls (n = 147) | p |
|---|---|---|---|
| Adults (aged >18 years), No. | 41 | 81 | <0.001 |
| Children (aged <13 years), No. | 98 | 58 | <0.001 |
| Age, mean ± SD, yr | 17.7 ± 12.5 | 22.5 ± 13.5 | 0.0012 |
| Height, mean ± SD, cm | 60.1 ± 6.9 | 61.9 ± 7.6 | 0.036 |
| SES at birth, mean ± SDb | 46.1 ± 11.4 | 45.9 ± 11.3 | 0.902 |
| Full-scale IQ, mean ± SD | 113.3 ± 16.5 | 119.7 ± 16.8 | 0.003 |
| Male sex, No. | 124 | 79 | <0.001 |
| Minority race, No. | 8 | 17 | 0.095 |
| Right-handed, No. | 134 | 130 | 0.026 |
In the TS group, 43 (26.4%) had a comorbid lifetime diagnosis of obsessive-compulsive disorder, 34 (20.9%) had attention-deficit/hyperactivity disorder, and 14 (8.6%) had both. At the time of imaging, 85 persons with TS (52.1%) were taking psychotropic medications, including typical neuroleptic agents (n = 18), atypical neuroleptic agents (n = 6), stimulants (n = 5), alpha-agonists (n = 26), selective serotonin reuptake inhibitors (n = 23), and tricyclic antidepressants (n = 13).
Estimated at the time of the participant’s birth to avoid bias attributable to downward drift in adults with TS, whose educational and occupational opportunities are often compromised by their persistent neuropsychiatric illness.
TS = Tourette syndrome; SD = standard deviation; SES = socioeconomic status.
MRI Scanning
High-resolution anatomical images were obtained using a single 1.5T scanner (GE Signa, Milwaukee, WI).
Morphometry
Brain regions were manually delineated on Sun Ultra 10 workstations using ANALYZE 7.5 software (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN) while blind to participant characteristics and hemisphere.
SURFACE MORPHOLOGIES
Following isolation of the cerebellum, the cerebellum of each participant was nonrigidly warped to a representative template cerebellum to label corresponding points across the surfaces of each cerebellum. The nonrigid warping was reversed to generate distances of each point on the surface of each cerebellum from the corresponding point on the surface of the template cerebellum. Those distances were then subjected to statistical analyses.27
VOLUME PRESERVED WARPING
We used volume preserved warping (VPW)28,29 to assess local expansion or reduction of tissue volumes at the cerebellar surface.
Statistical Analysis
ANALYSIS OF CONVENTIONAL VOLUMES
Statistical procedures were performed in SAS version 9.0 (SAS Institute, Cary, NC) using a mixed models repeated measures analysis (PROC MIXED) that included the within-subjects factors of hemisphere with 2 levels (left, right), diagnosis (TS, normal control) as a between-subject factor, and the covariates of age, sex, lifetime diagnoses of ADHD or OCD, and whole brain volume (WBV) to control for scaling effects. In addition to main effects, we considered for inclusion in the model all 2-and 3-way interactions of TS, sex, hemisphere, and age and the 2-way interaction of WBV with hemisphere. Nonsignificant terms were eliminated via backward stepwise regression, with the constraint that all lower-order component terms were included in the model, regardless of statistical significance. We assessed the main effect of diagnosis and the interactions of diagnosis with age and sex. All p values were 2-sided.
SURFACE MORPHOMETRY AND VPW
The distances from points on the cerebellar surface of each participant to corresponding points on the surface of the template cerebellum were compared statistically between groups using linear regression models that covaried for age, sex, and lifetime diagnoses of OCD and ADHD. Interactions were hierarchically modeled as described above. We applied identical statistical models for VPW. We corrected for the number of statistical comparisons in each model using the theory of Gaussian random fields (GRFs) for both surface analyses30 and for VPW. Probability values <0.05 were color-coded at each voxel and displayed across the template.
ASSOCIATIONS WITH SYMPTOM SEVERITY
In the TS group, we correlated cerebellar surface and VPW measures with symptom severity using a general linear model that covaried for age, sex, the age-by-sex interaction, and lifetime diagnoses of OCD and ADHD.
CORRELATIONS WITH PERFORMANCE ON A FINGER TAPPING TASK
Given a role for the cerebellum in controlling the timing and rhythm of motor behaviors,31 together with the presence of dysregulated motoric rhythms and timing in persons with TS, a simple finger tapping task was selected to assess the correlations of surface morphological measures with finger tapping performance.
MEDICATION AND COMORBIDITY EFFECTS
The effects of comorbid illnesses and medication use were assessed in 2 complementary ways: (1) by assessing their effects as statistical covariates in our final model for hypothesis testing, and (2) by assessing the stability of findings in analyses of participants who had either pure TS (ie, without OCD or ADHD) or who were not taking any medication.
Results
Overall Volumes
The main effect of diagnosis for overall volumes of the cerebellum was not statistically significant (F303 = 0.45, p = 0.50) (Table 2). Total age-and sex-adjusted cerebellar volume in TS participants was 130.5 ± 9.4cm3 and in controls was 131.1 ± 12.2cm3. Cerebellar volume in both groups decreased with increasing age (F303 = 5.4, p = 0.02) and with decreasing WBV (F303 = 202, p < 0.0001). WBV did not differ significantly across groups (TS = 1316.8 ± 126.0cm3; controls = 1315.1 ± 126.1 cm3; t280 = 0.12, p = 0.91).
TABLE 2.
Final Model for Conventional Volumesa
| Variable | df | F Score | p |
|---|---|---|---|
| TS | 303 | 0.45 | 0.50 |
| OCD | 303 | 0.12 | 0.73 |
| ADHD | 303 | 0.46 | 0.50 |
| Age | 303 | 5.42 | 0.02 |
| Sex | 303 | 2.57 | 0.11 |
| Hemisphere | 309 | 248.11 | <0.001 |
| WBV | 303 | 201.68 | <0.001 |
The model was determined by forcing all main effects into the model and removing higher-order terms via backward stepwise selection, with the constraint that the model was hierarchically well formulated at each step. The absence of a significant main effect of TS demonstrates no overall differences in cerebellar volume across diagnostic groups (TS vs controls). Additional significant terms demonstrated increasing cerebellar volume with decreasing age, left cerebellar hemisphere, and increasing WBV.
TS = Tourette syndrome; OCD = obsessive-compulsive disorder; ADHD = attention-deficit/hyperactivity disorder; WBV = whole brain volume.
Surface Morphology and VPW
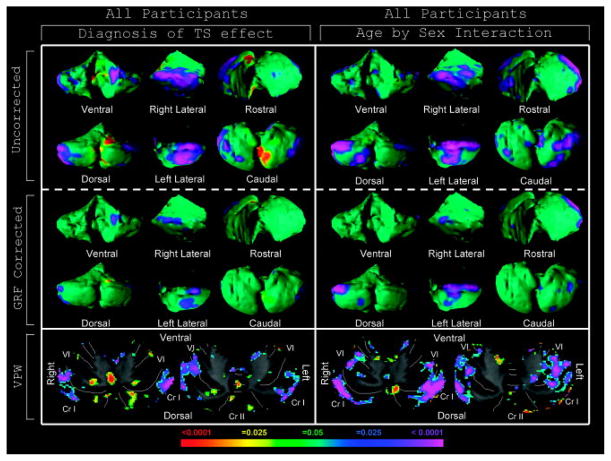
Analyses of the cerebellar surface demonstrated bilateral volume reductions that survived GRF correction over the lateral cerebellar hemispheres in the TS group compared with controls (Fig 1). Although this finding seemed to be strongest in children (Supplementary Fig 1), no significant diagnosis-by-age interaction was identified in this region (data not shown). VPW localized these regional volume reductions to gray matter portions of crus I and lobules VI and VIIB bilaterally (see Fig 1 and Supplementary Fig 1).
FIGURE 1.
Tourette syndrome (TS) effect and age-by-sex interaction effects in surface morphology and volume preserved warping (VPW). Rotational views and representative transverse slices (radiologic views, right=left) of the cerebellum are shown. Left: The statistical model included the main effect of diagnosis of TS and covaried for age, sex, obsessive-compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD), and an age-by-sex interaction. The color bar indicates the color coding for p values associated with the main effect of diagnosis. Warmer colors (red and yellow) indicate protrusion in surface morphology and volume expansion in VPW analyses, whereas cooler colors (purple and blue) indicate indentation in surface morphology and volume contraction in VPW analyses. The TS group exhibits local volume reductions in the lateral cerebellar hemispheres relative to healthy controls and is further localized to crus (Cr) I and lobules VI/VIIB/VIIIA on volumetric analysis. Right: The statistical model for the age-by-sex interaction included the main effect of a diagnosis of TS and covaried for age, sex, OCD, and ADHD. The color bar indicates the color coding for p values associated with significant interaction effects of age and sex (where males = 1 and females = 0 in statistical modeling), with warmer and cooler colors indicating different interaction effects on surface morphology and regional volumes. Significant age-by-sex interactions are present in the lateral cerebellar hemispheres in a territory that overlaps anatomically with that of the main effect of diagnosis. Volumetric analysis localizes this interaction effect to crus I and lobule VI. Gaussian random field (GRF)-corrected images indicate which voxels in the image survive rigorous correction for multiple comparisons. VPW images are not GRF-corrected.
A strong age-by-sex interaction was identified bilaterally in gray matter portions of crus I and lobule VI (see Fig 1). No significant age-by-sex-by-diagnosis interaction was detected in these regions (Supplementary Fig 2B), indicating that the age-by-sex interaction did not differ significantly across diagnostic groups. Post hoc analyses of age effects in males and females separately demonstrated that morphology in this region did not vary significantly with age in females, whereas local volumes declined progressively with age in males (see Supplementary Fig 2C–D).
Significant OCD effects were detected bilaterally over the lateral cerebellar hemispheres (Fig 2 and Supplementary Fig 3A), in the lateral gray matter regions of crus I and lobule VI, and in lobules VIIB and VIIIA (Supplementary Fig 4). These effects were located in similar regions as those of the effects of volume reduction in the TS group, but they were opposite in direction, with OCD in the TS group being associated with local hypertrophy relative to persons with TS who did not have comorbid OCD. Indeed, post hoc comparisons demonstrated that morphological abnormality was minimal in persons with TS and comorbid OCD, compared with healthy controls (Supplementary Fig 5B), whereas prominent volume reduction was noted in TS subjects without OCD (Supplementary Fig 5C). No significant OCD-by-age interaction was detected in this TS subgroup (data not shown). We detected no effects of comorbid ADHD on cerebellar surface morphology in the TS group that survived GRF correction (see Supplementary Fig 3B).
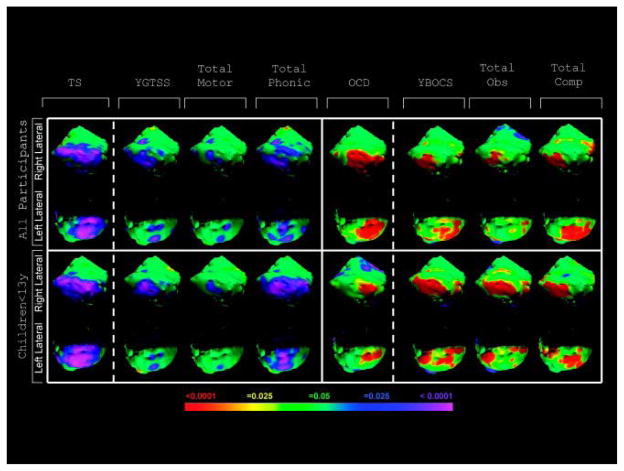
FIGURE 2.
Symptom severity effects on surface morphology. The main effect of diagnosis of Tourette syndrome (TS) (see Fig 1) is shown in the left-most column for comparison. The statistical model for obsessive-compulsive disorder (OCD) effects in the TS group covaried for age, sex, age by sex, and attention-deficit/hyperactivity disorder (ADHD). A diagnosis of comorbid OCD imparts local volume increases over the lateral cerebellar hemispheres relative to a diagnosis of TS without OCD. Warmer colors (red and yellow) indicate protrusion in surface morphology, and cooler colors (purple and blue) indicate indentation in surface morphology. The statistical models assessing correlations with TS symptom severity were conducted in only the TS group and covaried for age, sex, OCD, ADHD, and the age-by-sex interaction term. Yale Global Tic Severity Scale (YGTSS) scores (n = 289) correlated inversely with local volumes in regions of the lateral cerebellar hemispheres, where the local volume reductions were located in the TS group. This correlation with severity was most prominent for vocal tics, particularly in the right cerebellar hemisphere. Yale Brown Obsessive Compulsive Scale (YBOCS) scores (n = 291) correlated positively with local volumes in regions of the lateral cerebellar hemispheres where the local increases in volume were located for those who had a diagnosis of comorbid OCD. For all correlation analyses, the color bar indicates the color coding for p values associated with the symptom severity effect, with warmer colors (red and yellow) indicating increasing symptom severity scores with protruding surfaces in surface morphology images and cooler colors (purple and blue) indicating increasing symptom severity scores with indented surfaces in surface morphology images. These images are not Gaussian random field-corrected. Obs = obsession; Comp = compulsion.
Correlations with Symptom Severity
Progressively more severe tic symptoms accompanied progressively greater volume contraction of the lateral cerebellar hemispheres. This correlation was strongest for vocal tic severity and was most prominent in the right cerebellar hemisphere of children (see Fig 2), particularly in lateral gray matter portions of crus I. Much of this region overlays the main effect of diagnosis in children with TS (see Supplementary Fig 4). Progressively more severe OCD symptoms accompanied progressively greater protrusion of these same cerebellar regions, and they seemed to derive equally from obsessions and compulsions, in both children and adults (see Fig 2). The correlations were localized predominantly to portions of crus I with some involvement of lobules VI, VIIB, and VIIIA. Many of these regions overlay the effect of comorbid OCD and the main effect of TS (see Supplementary Fig 4). We ascertained no correlative effects of ADHD symptom severity with any cerebellar region (data not shown).
Correlation with Finger Tapping Speed
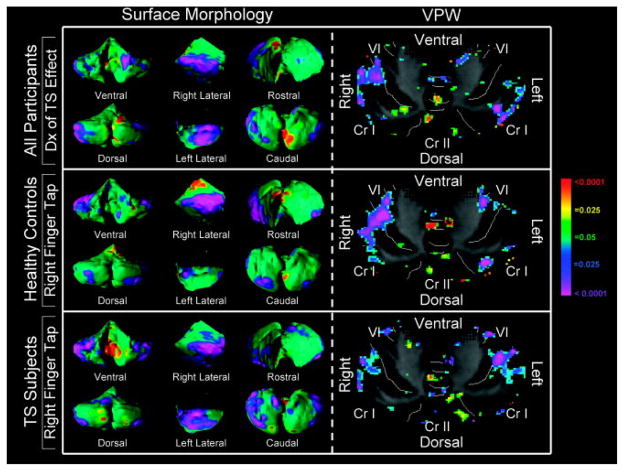
Improved performance on right-handed finger tapping in healthy controls and TS subjects correlated strongly and inversely with the magnitude of regional volume contraction in gray matter of the ventrolateral right crus I and lateral lobule VI subregions, closely approximating the location of the main effect of TS (Fig 3). We did not detect a significant performance-by-diagnosis interaction in an analysis of all participants (n = 165) (data not shown), indicating that this correlation of performance with morphological features of the cerebellum was comparable across diagnostic groups. We detected no statistically significant differences between TS (n = 96) and control (n = 69) groups in finger tapping speed (TS = 47.1 ± 7.2; controls = 49.1 ± 7.2; p = 0.09).
FIGURE 3.
Correlations of finger tapping speed with surface morphology and volume preserved warping (VPW). The main effect of a diagnosis of Tourette syndrome (TS) on surface morphology and VPW is presented in the top row for comparison (see Fig 1). The statistical model for the correlation of finger tapping speed with surface morphology was assessed in 69 control subjects and 96 TS subjects for whom this measure was available. Analysis for the control group included covariates for age, sex, and the age-by-sex interaction. Analysis for the TS group included covariates for ADHD, OCD, age, sex, and the age-by-sex interaction. The color bar indicates the color coding for p values associated with the effect of finger tapping speed, with warmer colors (red and yellow) indicating protruding surfaces in surface morphology images or local volume expansion with improved finger tapping performance, whereas cooler colors (purple and blue) indicate indented surfaces in surface morphology images or local volume contraction with greater finger tapping speed. These images are not Gaussian random field-corrected. Increasing performance of right finger tapping accompanies progressively more prominent volume reductions of the right lateral cerebellar hemisphere and less substantial volume reduction of the left lateral cerebellar hemisphere in both control and TS participants. This finding localizes to right crus (Cr) I and lobule VI, overlapping anatomic regions where a local volume reduction is present in those with a diagnosis of TS.
Medication Effects
We did not discern appreciable effects of medication use on any of our findings (data not shown).
Discussion
Three main conclusions can be drawn from this study. First, although overall cerebellar volumes do not differ significantly in TS patients compared with those in healthy controls,23 significant but highly localized reductions in cerebellar volumes are present in gray matter portions of lateral crus I and lobules VI, VIIB, and VIIIA bilaterally. Second, the degree of volume reduction in lateral crus I and lobule VI accompanies progressively more severe tic symptoms, particularly vocal tics. Finally, TS patients who have comorbid OCD have a relative hypertrophy of crus I and lobules VI, VIIB, and VIIIA compared with those who have TS without OCD; more prominent hypertrophy of this region accompanies more severe OCD symptoms.
Crus I/Lobule VI and Lobules VIIB/VIIIA in TS
Reduced volumes in crus I and lobules VI, VIIB, and VIIIA were similar across children and adults in the TS group. Greater volume reduction in these regions accompanied more severe tic symptoms. These findings suggest that reduced volumes in these portions of the cerebellum are unlikely to be compensatory and may instead play a more central role in the pathogenesis of TS. This possibility is consistent with tracing studies demonstrating connectivity of Purkinje cells in these regions with primary motor cortices and other cortical regions.12 These known anatomical connections together with our findings prompt our speculation that disruption of corticocerebellar regulatory loops may increase excitability of motor circuits and thereby worsen tic symptoms. Furthermore, tracing studies have demonstrated connectivity of these regions to thalamic and striatal neurons, suggesting that the cerebellum may contribute to the regulation of CSTC circuits strongly implicated in TS pathogenesis.14,32
The significant inverse correlation of local volumes in these regions with finger tapping speed suggests that reduced local volumes increase motor excitation. Consistent with this, a prior fMRI study demonstrated that finger tapping activates ipsilateral lobules IV, V, and VI in healthy persons.33 As demonstrated in our sample and other studies, patients with TS do not differ from healthy controls in finger tapping speed, although they typically perform poorly on most motor tasks.34 In addition, males typically outperform females on this task.35,36 Our finding of reduced local volumes of crus I and lobule VI in males compared with women, irrespective of diagnosis, suggests that reduced local volumes of these regions in the TS group may have contributed even further to a more male-typed performance on the finger tapping task and to an increased excitability of motor cortices in the TS group.
The presence of a more male-typed morphology of the cerebellum in both males and females with TS, and the possibility that a more male-typed cerebellum increases the excitability of motor cortices, could explain at least in part the greater prevalence of TS in males compared with females. The presence of a greater, more male-typed excitation of the motor cortex would then be superimposed on a motor cortex that has previously been shown to be hypoplastic and inherently more excitable than in healthy controls.7–10 Therefore, this preexisting tendency to cortical hyperexcitability, combined with a more male-typed cerebellar excitation of motor cortices, could produce more severe symptoms. More severely affected individuals in turn would be more likely to come to diagnostic attention and increase the prevalence estimates of TS in males.37
Crus I/Lobule VI and Lobules VIIB/VIIIA in Vocal Tics
Crus I and lobule VI in the right cerebellar hemisphere seem to participate in storage of phonetic information, whereas lobules VIIB and VIIIA mediate subvocal rehearsal in verbal working memory.19 The stronger association of these regions with vocal compared with motor tic severity is consistent with a prior study showing increased activation of crus I and lobule VI during the generation of vocal relative to motor tics.25 The stronger association of vocal tics with right compared with left crus I and lobule VI is consistent with fMRI and lesion studies suggesting a stronger role for language functions in the right cerebellum.17 We suspect that the superior cerebellar hemispheres (right > left) are inhibitory regulators of language control. Although lesions of the right superior cerebellum are not known to cause vocal tics, they do commonly produce difficulty in the appropriate selection of words and the detection of linguistic errors. Perhaps persons with TS are impaired in their capacity to encode phonetic information through both impaired phonetic storage (crus I and lobule VI) and impaired subvocal rehearsal (lobules VIIB and VIIIA), which could in turn disrupt language processing, impair the inhibition of spontaneous language generation, and thereby produce or exacerbate vocal tics.
Cerebellum in TS with Comorbid OCD
The effects of comorbid OCD on cerebellar morphology overlapped spatially with the main effects of TS over the lateral cerebellar hemispheres, imparted relative hypertrophy compared with TS subjects without comorbid OCD, and were present in both children and adults. Greater hypertrophy accompanied progressively more severe OCD symptoms. Thus, persons with TS without comorbid OCD had more prominent volume reductions in the lateral cerebellum than did persons with tic-related OCD.
This finding has several possible explanations. First, if TS and OCD represent 2 variant manifestations of a single neuropsychiatric disorder, then those with greater reduction in lateral cerebellar volumes would manifest severe TS but relatively little OCD; those with minimal volume reductions would manifest severe OCD but relatively little TS; and those with intermediate volumes in this region would tend to manifest both disorders of intermediate severity. In this case, the lateral cerebellum would act as a rheostat modifying the gain of 2 distinct brain regions that more directly generate tics or OCD symptoms independently of each other. A second possibility is that different corticocerebellar circuits within the same anatomic regions of the cerebellum, but beyond a spatial resolution detectable with our images, could generate or modulate OCD and TS symptoms independently. TS, for instance, could result from loss of inhibitory projections from the cerebellum, whereas comorbid OCD could result from hypertrophy of excitatory projections, both of which would increase excitation of cortical target regions. Third, TS with and without comorbid OCD could represent neurobiological subtypes of TS that have differing or only partially overlapping morphological substrates, with persons who have comorbid OCD having minimal cerebellar abnormalities, and those without comorbid OCD having prominent hypoplasia in the lateral cerebellar hemispheres. Consequently, in this view, cerebellar morphology would represent a unique endophenotype that differentiates those with TS who are likely to develop comorbid OCD, although this possibility requires confirmation prospectively in a longitudinal study. A latent class analysis seems to lend further support to this final possibility.38 Unfortunately, our data are unable at present to definitively confirm or refute any of these possibilities.
Cerebellum in TS with Comorbid ADHD
We detected nonsignificant statistical trends for reduced local volumes of the anterior and superior posterior vermal lobes in participants with a diagnosis of comorbid ADHD (see Supplementary Fig 3B). Previous studies of ADHD youth without comorbid tic disorders report reduced cerebellar volumes that accompany increasing symptom severity.39 These differences from our findings suggest that ADHD with and without tics may have differing neurobiological underpinnings, at least with respect to cerebellar morphology.
Limitations and Conclusions
The study’s cross-sectional design limits conclusions regarding the absence of age effects on our findings, which requires confirmation in future longitudinal studies.40 Although we controlled statistically for age and sex differences between control and TS groups, imperfect estimation of these effects could have influenced our findings. Nevertheless, similar results in analyses of children and adults separately suggest that age differences across groups did not substantially affect our findings. Similarly, current or past exposure to a variety of psychoactive medications could have influenced the morphometric data, but because our findings were confirmed in a medication-free subsample, medication effects were likely minimal. In addition, several findings were post hoc and should be interpreted with caution, as they were not hypothesis-driven. Nonetheless, we provide strong evidence for involvement of the cerebellum in the generation or modulation of TS symptoms and comorbid OCD. Additionally, we offer a plausible hypothesis that sex-specific differences in the prevalence of TS are mediated by neuroanatomical features of the cerebellum that produce cortical excitability and a speeded, more male-typed execution of simple motor behaviors both in healthy males and in persons with TS. Identifying regions that modulate the symptoms of TS has important implications for the development of novel therapeutic interventions, as those regions are potential targets for future electrophysiological or neurochemical manipulation.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Mental Health (grants MH59139, MH068318, and K02-74677 all to B.S.P.); National Institute of Biomedical Imaging and Bioengineering R03 (grant 1R03EB008235-01A1 to D.X.); Tourette Syndrome Association to B.S.P.; and Opening Project of Shanghai Key Laboratory of Magnetic Resonance (East China Normal University). We thank K. Durkin and K. Walsh for their technical assistance.
Footnotes
Potential Conflicts of Interest
Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Apter A, Pauls DL, Bleich A, et al. An epidemiologic study of Gilles de la Tourette’s syndrome in Israel. Arch Gen Psychiatry. 1993;50:734–738. doi: 10.1001/archpsyc.1993.01820210068008. [DOI] [PubMed] [Google Scholar]
- 2.Comings DE, Himes JA, Comings BG. An epidemiologic study of Tourette’s syndrome in a single school district. J Clin Psychiatry. 1990;51:463–469. [PubMed] [Google Scholar]
- 3.Spessot A, Peterson BS. Developmental psychopathology. 2. Vol. 3. John Wiley & Sons; 2006. [Google Scholar]
- 4.Graybiel AM, Canales JJ. The neurobiology of repetitive behaviors: clues to the neurobiology of Tourette syndrome. Adv Neurol. 2001;85:123–131. [PubMed] [Google Scholar]
- 5.Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson BS, Thomas P, Kane MJ, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 7.Sowell ER, Kan E, Yoshii J, et al. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci. 2008;11:637–639. doi: 10.1038/nn.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orth M, Rothwell JC. Motor cortex excitability and comorbidity in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry. 2009;80:29–34. doi: 10.1136/jnnp.2008.149484. [DOI] [PubMed] [Google Scholar]
- 9.Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette’s disorder: evidence from transcranial magnetic stimulation. Am J Psychiatry. 1997;154:1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
- 10.Rossi S, Bartalini S, Ulivelli M, et al. Hypofunctioning of sensory gating mechanisms in patients with obsessive-compulsive disorder. Biol Psychiatry. 2005;57:16–20. doi: 10.1016/j.biopsych.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Glickstein M, Doron K. Cerebellum: connections and functions. Cerebellum. 2008;7:589–594. doi: 10.1007/s12311-008-0074-4. [DOI] [PubMed] [Google Scholar]
- 12.Kelly RM, Strick PL. Cerebellar loops with motor cortex and pre-frontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clower DM, Dum RP, Strick PL. Basal ganglia and cerebellar inputs to ’AIP’. Cereb Cortex. 2005;15:913–920. doi: 10.1093/cercor/bhh190. [DOI] [PubMed] [Google Scholar]
- 14.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 15.Frings M, Dimitrova A, Schorn CF, et al. Cerebellar involvement in verb generation: an fMRI study. Neurosci Lett. 2006;409:19–23. doi: 10.1016/j.neulet.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 16.Petersen SE, Fox PT, Posner MI, et al. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 17.Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005;24:332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Desmond JE, Chen SH, Shieh PB. Cerebellar transcranial magnetic stimulation impairs verbal working memory. Ann Neurol. 2005;58:553–560. doi: 10.1002/ana.20604. [DOI] [PubMed] [Google Scholar]
- 19.Chen SH, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Scott RB, Stoodley CJ, Anslow P, et al. Lateralized cognitive deficits in children following cerebellar lesions. Dev Med Child Neurol. 2001;43:685–691. doi: 10.1017/s0012162201001232. [DOI] [PubMed] [Google Scholar]
- 21.Fiez JA, Petersen SE, Cheney MK, Raichle ME. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study Brain. 1992;115(pt 1):155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- 22.Hokkanen LS, Kauranen V, Roine RO, et al. Subtle cognitive deficits after cerebellar infarcts. Eur J Neurol. 2006;13:161–170. doi: 10.1111/j.1468-1331.2006.01157.x. [DOI] [PubMed] [Google Scholar]
- 23.Hong KE, Ock SM, Kang MH, et al. The segmented regional volumes of the cerebrum and cerebellum in boys with Tourette syndrome. J Korean Med Sci. 2002;17:530–536. doi: 10.3346/jkms.2002.17.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohlhalter S, Goldfine A, Matteson S, et al. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–2037. doi: 10.1093/brain/awl050. [DOI] [PubMed] [Google Scholar]
- 25.Stern E, Silbersweig DA, Chee KY, et al. A functional neuroanatomy of tics in Tourette syndrome. Arch Gen Psychiatry. 2000;57:741–748. doi: 10.1001/archpsyc.57.8.741. [DOI] [PubMed] [Google Scholar]
- 26.Lerner A, Bagic A, Boudreau EA, et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology. 2007;68:1979–1987. doi: 10.1212/01.wnl.0000264417.18604.12. [DOI] [PubMed] [Google Scholar]
- 27.Bansal R, Staib LH, Whiteman R, et al. ROC-based assessments of 3D cortical surface-matching algorithms. Neuroimage. 2005;24:150–162. doi: 10.1016/j.neuroimage.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 28.Xu D, Hao X, Bansal R, et al. Seamless warping of diffusion tensor fields. IEEE Trans Med Imaging. 2008;27:285–299. doi: 10.1109/TMI.2007.901428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu D, Hao X, Bansal R, et al. Unifying the analyses of anatomical and diffusion tensor images using volume-preserved warping. J Magn Reson Imaging. 2007;25:612–624. doi: 10.1002/jmri.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bansal R, Staib L, Xu D, et al. Statistical analyses of brain surfaces using Gaussian random fields on 2-D manifolds. IEEE Trans Med Imaging. 2007;26:46–57. doi: 10.1109/TMI.2006.884187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- 32.Middleton FA, Strick PL. Cerebellar output channels. Int Rev Neurobiol. 1997;41:61–82. doi: 10.1016/s0074-7742(08)60347-5. [DOI] [PubMed] [Google Scholar]
- 33.Desmond JE, Gabrieli JD, Wagner AD, et al. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17:9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz RT, Carter AS, Gladstone M, et al. Visual-motor integration functioning in children with Tourette syndrome. Neuropsychology. 1998;12:134–145. doi: 10.1037//0894-4105.12.1.134. [DOI] [PubMed] [Google Scholar]
- 35.Ruff RM, Parker SB. Gender-and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard tests. Percept Mot Skills. 1993;76:1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 36.Shimoyama I, Ninchoji T, Uemura K. The finger-tapping test. A quantitative analysis. Arch Neurol. 1990;47:681–684. doi: 10.1001/archneur.1990.00530060095025. [DOI] [PubMed] [Google Scholar]
- 37.Alexander GM, Peterson BS. Testing the prenatal hormone hypothesis of tic-related disorders: gender identity and gender role behavior. Dev Psychopathol. 2004;16:407–420. doi: 10.1017/s095457940404458x. [DOI] [PubMed] [Google Scholar]
- 38.Grados MA, Mathews CA. Latent class analysis of Gilles de la Tourette syndrome using comorbidities: clinical and genetic implications. Biol Psychiatry. 2008;64:219–225. doi: 10.1016/j.biopsych.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 40.Peterson BS. Conceptual, methodological, and statistical challenges in brain imaging studies of developmentally based psychopathologies. Dev Psychopathol. 2003;15:811–832. doi: 10.1017/s0954579403000385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.