Abstract
A new drug-free nanotherapeutic approach for B-cell malignancies was developed. Exposure of B-cells to an anti-CD20 Fab′-morpholino oligonucleotide1 (MORF1) conjugate decorated the cell surface with MORF1; further exposure of the decorated cells to multivalent polymer-oligonucleotide2 conjugates (P-MORF2) resulted in CD20 clustering at the cell surface with induction of apoptosis. We evaluated this concept in chronic lymphocytic leukemia (CLL) cells isolated from 10 patients. Apoptosis and cytotoxicity were observed in eight samples, including 2 samples with the 17p13 deletion, which suggested a p53-independent mechanism of apoptosis induction. When compared to an anti-CD20 monoclonal antibody (mAb), the nanotherapeutic showed significantly more potent apoptosis-inducing activity and cytotoxicity. This was due to the multivalency effect (8 binding sites per polymer chain) of our design in comparison to the divalent mAb. In conclusion, we have developed a novel and potent therapeutic system against CLL and other B-cell malignancies with significant advantages over conventional chemo-immunotherapy.
Keywords: CLL, B-cell, CD20, apoptosis, HPMA copolymer, nanomedicine
Introduction
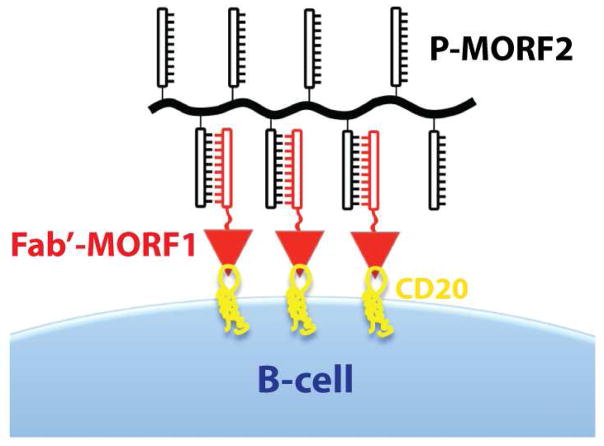
B-cell malignancies are prevalent cancers worldwide [1]. Crosslinking of CD20 receptors at the surface of B-cells initiates apoptosis [2–4]. Based on this mechanism, we designed a therapeutic system (Figure 1) composed of two hybrid macromolecules: (1) a conjugate of the Fab′ fragment of anti-CD20 1F5 monoclonal antibody (mAb) with a morpholino oligonucleotide MORF1; (2) a linear N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer grafted with multiple copies of the complementary oligonucleotide MORF2 (P-MORF2) [5]. Exposure of a B-cell non-Hodgkin lymphoma line (Raji) to the anti-CD20 Fab′-MORF1 conjugate decorated the cell surface with MORF1. Further exposure of the decorated cells to P-MORF2 resulted in MORF1/MORF2 hybridization, which initiated CD20 crosslinking and triggered apoptosis in vitro and in vivo [5]. In this design, the morpholino oligonucleotide pair serves as a physical crosslinker to cluster CD20 antigens at the cell surface. Extracellular hybridization of MORF1/MORF2 translates into innate biological responses, i.e., apoptosis. This “drug-free macromolecular therapeutic” involves no cytotoxic drug, and the individual parts of the system do not have apoptosis-inducing activity. The two nanoscale conjugates (Fab′-MORF1 and P-MORF2) can be administered consecutively (for pre-targeting approaches) or as a premixture.
Figure 1. Drug-free macromolecular therapeutics for apoptosis induction.
Apoptosis of B-cells is triggered by crosslinking of the CD20 antigens due to MORF1/MORF2 hybridization at the cell surface. Schematic representation is not drawn to scale.
Here, we evaluated the efficacy of drug-free macromolecular therapeutics on primary chronic lymphocytic leukemia (CLL) cells isolated from patients. We hypothesized that this CD20 receptor crosslinking system is cytotoxic to CLL cells and could constitute a new nanomedicine-based therapy for CLL and other B-cell malignancies.
Experimental Methods
Patient samples and treatments
Peripheral blood mononuclear cells were isolated from 10 untreated CLL patients (clinical details summarized in Table 1). All samples expressed dim CD20, a typical phenotype of CLL. Specimens were collected after informed consent under a protocol approved by the University of Utah Institutional Review Board. Cells were suspended in RPMI-1640 medium (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT). Incubation was carried out at 37 °C in a humidified atmosphere with 5% CO2. Cells were treated with Fab′-MORF1 and P-MORF2 (at equimolar MORF1/MORF2 concentrations), either consecutively (1 h interval) or as a premixture (1 h, 37 °C). Fab′-MORF1 and P-MORF2 were prepared as previously described [5].
Table 1.
Clinical characteristics of CLL patients.
| Patient # | Age (y) | a Sex | Rai Stage | b IgVH Mutation | CD38 | ZAP70 | FISH Cytogenetics |
|---|---|---|---|---|---|---|---|
| P1 | 83 | F | I | n/a | neg | n/a | n/a |
| P2 | 76 | M | I | M | neg | n/a | 13q- |
| P3 | 52 | M | I | U | neg | pos | normal |
| P4 | 80 | F | II | n/a | pos | n/a | ts12 |
| P5 | 67 | M | I | n/a | neg | pos | 13q- |
| P6 | 59 | M | II | U | neg | pos | 13q- |
| P7 | 72 | M | I | U | n/a | pos | 17p-/11q-/ts12 |
| P8 | 60 | M | I | U | neg | pos | 17p-/13q- |
| P9 | 49 | F | 0 | n/a | pos | neg | 13q- |
| P10 | 72 | F | 0 | U | neg | int | 13q- |
IgVH, immunoglobulin heavy variable chain; ZAP70, ζ-chain-associated protein kinase 70; FISH, fluorescence in situ hybridization; n/a, not available; neg, negative; pos, positive; int, intermediate; ts12, trisomy 12.
M, male; F, female.
U, unmutated; M, mutated.
Apoptosis and cytotoxicity assays
In all experiments, an anti-CD20 mAb (1F5 [6]) hypercrosslinked with a goat anti-mouse (GAM) secondary antibody (KPL, Gaithersburg, MD) was used as a positive control (molar ratio 1F5:GAM=2:1). Untreated cells were used as negative controls. All experiments were conducted in duplicate or triplicate wells.
Cytotoxicity
Viability of cells was determined by Trypan blue staining at the indicated time points. Alternatively, cells were stained with propidium idodie (PI) and the percentage of PI-positive cells was quantified by flow cytometry.
Annexin V/propidium iodide (PI) binding
Two hundred thousand cells were suspended in 0.4 mL medium for different treatments. Cells were harvested at the indicated time intervals, washed twice with PBS prior to staining by propidium iodide and FITC-conjugated annexin V. Staining was performed following the RAPID™ protocol provided by the manufacturer (Oncogene Research Products, Boston, MA).
Caspase-3 activity
Two hundred thousand cells were suspended in 0.4 mL medium for different treatments. Cells were harvested at the indicated time intervals and washed twice with PBS. A PhiPhiLux® kit was used to assay Caspase-3 activity using the manufacturer’s protocol (OncoImmunin, Gaithersburg, MD).
Terminal deoxynucleotide mediated-dUTP nick-end labeling (TUNEL) assay
One million cells were suspended in 0.5 mL medium for different treatments. At the indicated time points, cells were fixed with 1% paraformaldehyde in PBS (1 h, 4 °C) and permeabilized in 70% ethanol overnight at −20 °C. An Apo Direct TUNEL kit was used to assay apoptosis using the manufacturer’s protocol (Phoenix Flow Systems, San Diego, CA).
Cell cycle analysis
Cells were washed twice with PBS after the indicated treatments and permeabilized in 70% ethanol overnight at −20 °C. Cells were stained with an excess of PI in PBS, and DNA content was determined by flow cytometry.
Statistical analysis
All experiments were at least duplicated. Data are presented as mean ± standard deviation (SD). Differences were considered significant for p values < 0.05 using the Student’s t test.
Results and Discussion
To evaluate the potential of drug-free macromolecular therapeutics for the treatment of chronic lymphocytic leukemia (CLL), cells from 10 patients were obtained (Table 1). As shown in Table 1, these patients fell into different prognostic categories, including 2 with the 17p13 deletion.
The isolated cells were treated with conjugates Fab′-MORF1 (58.5 kDa; equimolar MORF1/Fab′) and P-MORF2 (P: 136 kDa; 8 MORF2 per chain), either consecutively or as a premixture. The experimental conditions and results of apoptosis and cytotoxicity assays are summarized in Tables 2 and 3, respectively. Results showed that both treatment regimens (consecutive and premixed) effectively induced apoptosis of CLL cells. Data from 8 patient samples (P1, P2, P4, P6-P10) showed significantly higher apoptotic and/or cytotoxic indices in the nanomedicine groups when compared to the non-treated cells. A trend of apoptosis induction was also observed in P3 and P5; however, the changes with the latter samples were not statistically significant. Interestingly, the treatment showed activity against the 2 samples with the 17p13 deletion (P7 and P8). Deletions of 17p are associated with the loss of one allele of p53 and portend a worse prognosis [7]. Our results suggest that apoptosis induction by drug-free macromolecular therapeutics is p53-independent. It has been reported that the CD20-crosslinking-mediated B-cell death is a distinct pathway that can bypass mitochondria and caspase activation, which could be an advantage in the treatment of chemoresistant malignancies [8]. This indicates that what we propose has significant advantages over conventional chemo- and radiotherapy approaches, especially for high-risk patients with the 17p deletion whose disease is particularly difficult to treat. More mechanistic studies are needed to further elucidate the pathway(s) leading to apoptosis induction by drug-free macromolecular therapeutics.
Table 2.
Experimental conditions and results of apoptosis assays.
| Patient # | Treatment Time | Dose | Assay | Apoptotic Index (%) | |||
|---|---|---|---|---|---|---|---|
| Non-treated | mAb + 2° Ab | Consecutive | Premixed | ||||
| P1 | 20 h | 0.5 μM | Annexin V | 30 ± 0.6 | 27 ± 2.1 | 48 ± 9.6 | ‡49 ± 3.3 |
| P2 | 20 h | 0.5 μM | Annexin V | 27 ± 1.4 | 37 ± 4.4 | ‡52 ± 0.8 | ‡58 ± 0.6 |
| P3 | 20 h | 0.5 μM | Annexin V | 28 ± 4.6 | 25 ± 3.6 | 34 ± 6.8 | 32 ± 7.9 |
| P4 | 20 h | 0.5 μM | Annexin V | 34 ± 4.6 | †54 ± 5.4 | †54 ± 5.9 | †59 ± 2.8 |
| P5 | 20 h | 0.5 μM 1 μM |
Annexin V | 25 ± 8.9 | 40 ± 3.0 39 ± 0.2 |
38 ± 0.1 43 ± 1.5 |
38 ± 0.1 40 ± 2.3 |
| P6 | 20 h 30 h |
0.5 μM | Annexin V | 61 ± 2.8 59 ± 3.9 |
64 ± 0.3 †76 ± 0.3 |
‡69 ± 1.6 ‡78 ± 0.8 |
‡72 ± 2.6 †77 ± 0.7 |
| 20 h 30 h |
0.5 μM | TUNEL | 24 ± 2.3 39 ± 11.0 |
†31 ± 1.5 †58 ± 1.3 |
†36 ± 9.9 ‡71 ± 1.6 |
‡42 ± 4.5 †66 ± 6.2 |
|
| P7 | 30 h | 0.5 μM | Annexin V | 41 ± 1.7 | †52 ± 1.4 | ‡66 ± 0.3 | ‡62 ± 1.1 |
| P8 | 30 h | 0.5 μM 1 μM |
Annexin V | 15 ± 1.4 | 17 ± 0.8 16 ± 7.5 |
23 ± 6.0 †30 ± 7.4 |
22 ± 3.3 †34 ± 4.7 |
| P9 | 48 h | 0.5 μM | Annexin V | 46 ± 7.2 | †77 ± 1.0 | ‡82 ± 0.1 | †78 ± 2.8 |
| P10 | 6 h | 0.5 μM | Caspase-3 | 10 ± 0.1 | 17 ± 4.2 | †23 ± 3.5 | †21 ± 4.6 |
Significantly higher than non-treated (p < 0.05; Student’s t test).
Significantly higher than non-treated and mAb + 2° Ab (p < 0.05; Student’s t test).
Table 3.
Experimental conditions and results of cytotoxicity assays.
| Patient # | Treatment Time | Dose | Assay | Cytotoxicity Index (%) | |||
|---|---|---|---|---|---|---|---|
| Non-treated | mAb + 2° Ab | Consecutive | Premixed | ||||
| P1 | 20 h | 0.5 μM | Trypan Blue | 38 ± 0.3 | †60 ± 1.3 | ‡69 ± 1.9 | ‡71 ± 2.1 |
| P2 | 20 h | 0.5 μM | Trypan Blue | 59 ± 5.3 | †88 ± 5.0 | †82 ± 5.4 | †91 ± 0.9 |
| P4 | 20 h | 0.5 μM | Trypan Blue PI |
52 ± 6.0 49 ± 1.1 |
63 ± 4.6 53 ± 6.0 |
76 ± 7.1 †60 ± 2.8 |
†76 ± 3.9 †61 ± 1.3 |
| P6 | 20 h 30 h |
0.5 μM | PI | 60 ± 1.8 63 ± 3.1 |
60 ± 2.5 †75 ± 0.3 |
‡68 ± 0.6 ‡78 ± 0.4 |
‡70 ± 3.0 †78 ± 1.8 |
| P7 | 30 h | 0.5 μM | PI | 22 ± 6.8 | 43 ± 2.5 | ‡52 ± 1.1 | †45 ± 0.7 |
| P9 | 48 h | 0.5 μM | PI | 44 ± 0.2 | †67 ± 1.4 | †69 ± 0.3 | †66 ± 0.1 |
Significantly higher than non-treated (p < 0.05; Student’s t test).
Significantly higher than non-treated and mAb + 2° Ab (p < 0.05; Student’s t test).
In our experiments, an anti-CD20 mAb hypercrosslinked with a goat anti-mouse secondary Ab was used as a positive control in order to reproduce the function of immune effector cells [9]. This control partly reproduces the therapeutic efficacy of anti-CD20 mAbs that are used in the clinic (e.g., rituximab). Our drug-free macromolecular approach is more effective at apoptosis induction in CLL cells than the mAb control. As shown in Tables 2 and 3, five patient samples (P1, P2, P6, P7 and P9) showed significantly higher apoptotic and/or cytotoxic indices in the nanomedicine group when compared to the positive control (mAb + 2° Ab). This was likely due to multivalency of the P-MORF2 conjugate (8 binding sites per chain) in comparison to the divalent mAb. Our lab [10, 11] and others [12, 13] have previously shown that the multivalency of anti-CD20 constructs can increase binding affinity and apoptosis-inducing efficiency by several fold, when compared to their monovalent or divalent counterparts. We have also reported that, in addition to valence, the polymer length (i.e., MW) had a positive influence on CD20 clustering and apoptosis [4]. Therefore, it is possible to further improve the efficacy of this design by using a P-MORF2 conjugate with a higher valence and/or a larger polymer backbone. For instance, multiblock backbone degradable HPMA copolymers can be synthesized [14–16]. This approach can produce P-MORF2 with substantially increased MW and valence.
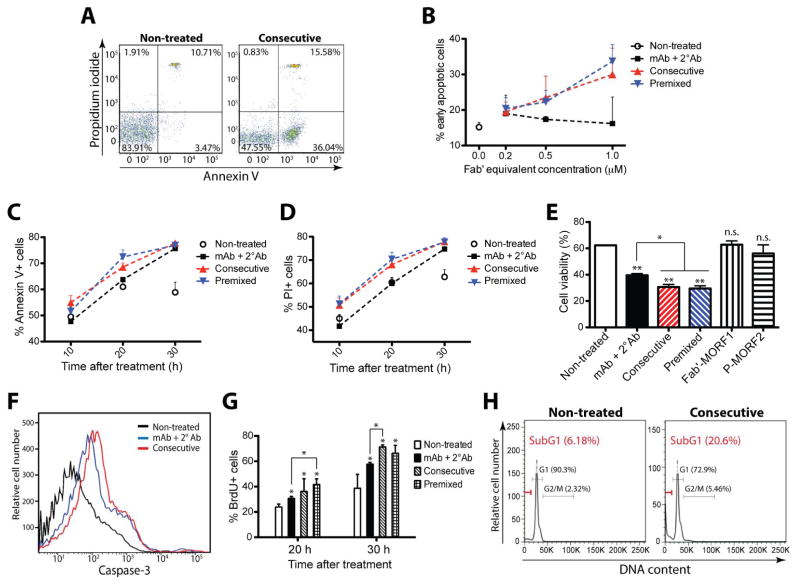
We evaluated apoptosis and cytotoxicity from multiple aspects: plasma membrane rupture (Figure 2A–E), caspase-3 activation (Figure 2F), and genomic DNA fragmentation (Figure 2G and 2H). Results consistently showed that the designed nanomedicine was effective at inducing apoptosis in CLL cells. In contrast, single-component treatments with either Fab′-MORF1 or P-MORF2 alone failed to induce apoptosis (Figure 2E), confirming the necessity to crosslink CD20 in order to trigger apoptosis. The effects of the nanomedicine were dose- (Figure 2B) and time-dependent (Figure 2C, 2D and 2G). In some cases, the mAb does not demonstrate apoptosis-inducing activity, e.g., in P1, P3, P6 and P8 (as shown in Table 2 and Figure 2B), whereas the nanomedicine is effective. This observation suggests a higher potency of drug-free macromolecular therapeutics when compared to mAb treatment.
Figure 2. Drug-free macromolecular therapeutics induce apoptosis of CLL cells from patients.
(A) Representative flow cytometric analysis of cells exposed to different treatments and stained by propidium iodide (PI) and FITC-labeled Annexin V. Non-treated: cells in culture medium; Consecutive: Fab′-MORF1 (0.5 μM) followed (1 h later) by P-MORF2 (0.5 μM; MORF2-eqv.). Incubation time was 20 h. Data from patient sample #2. (B) Dose-dependent apoptosis induction. Percentage of early apoptotic cells (Annexin V+/PI-) was quantified by flow cytometry. mAb + 2° Ab: 1F5 mAb followed (1 h later) by goat anti-mouse secondary Ab; Consecutive: Fab′-MORF1 followed (1 h later) by P-MORF2; Premixed: premixture of Fab′-MORF1 and P-MORF2. Incubation time was 30 h. Data from patient sample #8. (C) Time-dependent apoptosis induction. Fab′-equivalent concentration was 0.5 μM (applied to D-H). Data from patient sample #6. (D) Time-dependent cytotoxicity as determined by PI binding. Data from patient sample #6. (E) Cell viability as determined by Trypan blue staining. Incubation time was 20 h. Fab′-MORF1: single-component control at 0.5 μM; P-MORF2: single-component control at 0.5 μM (MORF2-eqv.). Data from patient sample #1. (F) Representative flow cytometric analysis of cells exposed to different treatments and stained by FITC-labeled anti-active caspase-3 Ab. Incubation time was 6 h. Data from patient sample #10. (G) Apoptosis as analyzed by TUNEL assay. FITC-labeled anti-bromodeoxyuridine (BrdU) Ab stains apoptotic cells. Data from patient sample #6. (H) Cell cycle analysis. Cells were permeabilized and stained by PI to measure DNA content. SubG1 phase represents apoptotic cells. Incubation time was 30 h. Data from patient sample #7. In B-E and G, data are presented as mean + SD (n = 2 or 3). Statistics in E and G, unless otherwise indicated, was performed by comparing each group with non-treated (**: p < 0.005, *: p < 0.05, n.s.: no significant difference; Student’s t test).
The field of CLL therapy has entered a period of significant change with the recent approval of 2 agents, ibrutinib and idelasilib, targeting the Bruton tyrosine kinase and phosphatidylinositide 3-kinase-δ, respectively [17]. Mechanistically, both targets are related to the B-cell receptor signaling pathway. The drug-free nanotherapeutic approach constitutes yet another novel therapeutic approach. It induces apoptosis in the malignant cells without the need for cytotoxic compounds or immune system activation. It will be interesting to determine whether this strategy synergizes with the new, targeted agents. Furthermore, the proposed two-step (consecutive) approach allows the use of pre-targeting [18, 19]. For example, the timing of administration of P-MORF2 can be optimized in individual patients based on pharmacokinetics and biodistribution of Fab′-MORF1, in order to enable more efficient treatment while limiting potential side effects associated with off-target binding. We have currently validated this concept in mice bearing B-cell lymphoma xenografts [20]. With the use of an optimized time lag, a two-step pretargeting approach was employed to achieve favorable tumor-to-tissue accumulation, which resulted in significantly improved in vivo anticancer efficacy.
In conclusion, we have developed a novel and potent drug-free nanotherapeutic approach for the treatment of CLL and other B-cell malignancies. This approach has significant advantages over current cytotoxic therapies and immunotherapies. The clinical development of our strategy will likely contribute to the ongoing revolution in the treatment of these diseases.
Acknowledgments
This work was supported in part by NIH grant GM95606 (to J.K.) from the National Institute of General Medical Sciences and the University of Utah Research Foundation. The authors thank Dr. Ruozhen Hu for assisting with cell cycle analysis, and Dr. Jiyuan Yang and Dr. Rui Zhang for helpful discussions.
Footnotes
Conflict of Interest:
J.K. and T.-W.C. are inventors on a pending US patent application (PCT/US2014/023784; assigned to the University of Utah) related to this work. J.K. is Chief Scientific Advisor and P.J.S. Chief Medical Advisor for Bastion Biologics. Otherwise, the authors declare no relevant financial interests.
Informed Consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Hofmeister JK, Cooney D, Coggeshall KM. Clustered CD20 induced apoptosis: src-family kinase, the proximal regulator of tyrosine phosphorylation, calcium influx, and caspase 3-dependent apoptosis. Blood Cells Mol Dis. 2000;26:133–43. doi: 10.1006/bcmd.2000.0287. [DOI] [PubMed] [Google Scholar]
- 3.Zhang N, Khawli LA, Hu P, Epstein AL. Generation of rituximab polymer may cause hyper-cross-linking–induced apoptosis in non-Hodgkin’s lymphomas. Clin Cancer Res. 2005;11:5971–80. doi: 10.1158/1078-0432.CCR-05-0554. [DOI] [PubMed] [Google Scholar]
- 4.Chu T-W, Yang J, Kopeček J. Anti-CD20 multivalent HPMA copolymer-Fab′ conjugates for the direct induction of apoptosis. Biomaterials. 2012;33:7174–81. doi: 10.1016/j.biomaterials.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu T-W, Yang J, Zhang R, Sima M, Kopeček J. Cell surface self-assembly of hybrid nanoconjugates via oligonucleotide hybridization induces apoptosis. ACS Nano. 2014;8:719–30. doi: 10.1021/nn4053827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Press OW, Appelbaum F, Ledbetter JA, et al. Monoclonal antibody 1F5 (anti-CD20) serotherapy of human B cell lymphomas. Blood. 1987;69:584–91. [PubMed] [Google Scholar]
- 7.Byrd JC, Jones JJ, Woyach JA, Johnson AJ, Flynn JM. Entering the era of targeted therapy for chronic lymphocytic leukemia: impact on the practicing clinician. J Clin Oncol. 2014;32:3039–47. doi: 10.1200/JCO.2014.55.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Kolk LE, Evers LM, Omene C, et al. CD20-induced B cell death can bypass mitochondria and caspase activation. Leukemia. 2002;16:1735–44. doi: 10.1038/sj.leu.2402559. [DOI] [PubMed] [Google Scholar]
- 9.Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood. 1998;91:1644–52. [PubMed] [Google Scholar]
- 10.Johnson RN, Kopečková P, Kopeček J. Synthesis and evaluation of multivalent branched HPMA copolymer-Fab′ conjugates targeted to the B-cell antigen CD20. Bioconjug Chem. 2009;20:129–37. doi: 10.1021/bc800351m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RN, Kopečková P, Kopeček J. Biological activity of anti-CD20 multivalent HPMA copolymer-Fab′ conjugates. Biomacromolecules. 2012;13:727–35. doi: 10.1021/bm201656k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aluri SR, Shi P, Gustafson JA, et al. A hybrid protein-polymer nanoworm potentiates apoptosis better than a monoclonal antibody. ACS Nano. 2014;8:2064–76. doi: 10.1021/nn403973g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Eckert MA, Ali MM, et al. DNA-scaffolded multivalent ligands to modulate cell function. ChemBioChem. 2014;15:1268–73. doi: 10.1002/cbic.201402100. [DOI] [PubMed] [Google Scholar]
- 14.Pan H, Yang J, Kopečková P, Kopeček J. Backbone degradable multiblock N-(2-hydroxypropyl)methacrylamide copolymer conjugates via reversible addition-fragmentation chain transfer polymerization and thiolene coupling reaction. Biomacromolecules. 2011;12:247–52. doi: 10.1021/bm101254e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Luo K, Pan H, Kopečková P, Kopeček J. Synthesis of biodegradable multiblock copolymers by click coupling of RAFT-generated heterotelechelic polyHPMA conjugates. React Funct Polym. 2011;71:294–302. doi: 10.1016/j.reactfunctpolym.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang R, Yang J, Sima M, Zhou Y, Kopeček J. Sequential combination therapy of ovarian cancer with degradable N-(2-hydroxypropyl)methacrylamide copolymer paclitaxel and gemcitabine conjugates. Proc Natl Acad Sci US A. 2014;111:12181–6. doi: 10.1073/pnas.1406233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danilov AV. Targeted therapy in chronic lymphocytic leukemia: past, present, and future. Clin Ther. 2013;35:1258–70. doi: 10.1016/j.clinthera.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodwin DA, Meares CF. Advances in pretargeting biotechnology. Biotechnol Adv. 2001;19:435–50. doi: 10.1016/s0734-9750(01)00065-9. [DOI] [PubMed] [Google Scholar]
- 19.Gunn J, Park SI, Veiseh O, Press OW, Zhang M. A pretargeted nanoparticle system for tumor cell labeling. Mol Biosyst. 2011;7:742–8. doi: 10.1039/c005154c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu T-W, Zhang R, Yang J, Chao MP, Shami PJ, Kopeček J. Unpublished material. doi: 10.7150/thno.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]