Abstract
Purpose of review
Synesthesia is an extraordinary perceptual phenomenon, in which individuals experience unusual percepts elicited by the activation of an unrelated sensory modality or by a cognitive process. Emotional reactions are commonly associated. The condition prompted philosophical debates on the nature of perception and impacted the course of art history. It recently generated a considerable interest among neuroscientists, but its clinical significance apparently remains underevaluated. This review focuses on the recent studies regarding variants of color synesthesia, the commonest form of the condition.
Recent findings
Synesthesia is commonly classified as developmental and acquired. Developmental forms predispose to changes in primary sensory processing and cognitive functions, usually with better performances in certain aspects and worse in others, and to heightened creativity. Acquired forms of synesthesia commonly arise from drug ingestion or neurological disorders, including thalamic lesions and sensory deprivation (e.g., blindness). Cerebral exploration using structural and functional imaging has demonstrated distinct patterns in cortical activation and brain connectivity for controls and synesthetes. Artworks of affected painters are most illustrative of the nature of synesthetic experiences.
Summary
Results of the recent investigations on synesthesia offered a remarkable insight into the mechanisms of perception, emotion and consciousness, and deserve attention both from neuroscientists and from clinicians.
Keywords: cerebral disorders, color, consciousness, emotion, perception, synesthesia, vision
INTRODUCTION
Synesthesia is an extraordinary perceptual phenomenon, in which the world is experienced in unusual ways. In this condition, a particular stimulation in a given sensory modality (e.g., touch) or cognitive process (e.g., computing) automatically triggers additional experiences in one or several other unstimulated domains (e.g., vision, emotion) [1]. An illustrative presentation of the condition would be that of a given person in whom hearing the sound of a trumpet consistently elicits the vision of brightly colored triangles dancing in front of his eyes, in association with a sensation of pressure on his arms, letting him feel uncomfortable to sit still. Stimuli generating additional unusual experiences are termed ‘inducers’, whereas internally produced synesthetic percepts are termed ‘concurrents’ [2].
Synesthetic experiences have had over the centuries far-reaching sociocultural implications. They prompted philosophical debates on the nature of perception, consciousness and even talent and creativity, and significantly impacted the course of art history, notably at the turn of the 20th Century [3–6]. Moreover, favored by the emergence of sophisticated tools for functional brain exploration, they have generated a considerable interest among neuroscientists [6,7]. Clinical significance of synesthesia, however, is still largely underevaluated.
Although some synesthetic phenomena express the presence of a disease, developmental synesthesia as a rule is considered an individual cognitive variant in the normal population [8]. Unfortunately, the astonishing features of these percepts have too often led the entourage of affected persons, including physicians, to wrongfully consider them as confabulators, drug users, or schizophrenics [7]. In this regard, the following history reported by Vincent Van Gogh is representative. While in 1885 the painter was taking piano lessons, his teacher noticed that he was continually relating the sounds of the piano keys with specific colors; considering then that his pupil was insane, the teacher sent him away [9]. It is therefore understandable that synesthetes (i.e., persons affected by synesthesia) commonly avoid mentioning their percepts and even tend to close on themselves in psychological distress [10–12]. For that very reason, scientific studies probably underestimate synesthesia prevalence in the general population.
Box 1.
no caption available
SYNESTHESIA VARIANTS
Synesthesia is commonly classified as developmental and acquired. Developmental synesthesia appears to be the most frequent type of this condition, with a 4.4% estimated prevalence rate [13]. It can run in families and demonstrate Mendelian transmission [14]. Different forms of synesthesia can be observed in the same person or in the same family [15]. The condition is occasionally associated with autism spectrum disorders, like Asperger syndrome [16].
The following criteria have been proposed to help establishing a diagnosis of developmental synesthesia: induced percepts should be elicited by a specific stimulus, they should be automatically generated, and typically have percept-like qualities [8,17,18]. Usually, pairings of inducers and concurrents are specific (i.e., a particular stimulus consistently triggers the same synesthetic percept). They tend to be stable over time in a given individual, although this has recently been challenged by the finding that synesthetic ability can disappear over time [19▪].
Acquired forms of synesthesia have also been reported, essentially associated with neurologic disorders or following psychotropic drug ingestion [20–23]. In contrast to its developmental counterpart, acquired synesthesia does not demonstrate either idiosyncrasy or automaticity or stability [3,24▪].
So far, over 60 types of synesthetic phenomena have been described. The apparently most common form (with a 64.4% prevalence among synesthetes) is grapheme–color synesthesia, in which achromatic letters or digits automatically trigger an idiosyncratic color perceptual experience (e.g., the letter ‘m’ induces blue color percepts) [25,26] (Fig. 1[27]). The second most prevalent form is time unit (e.g., Monday, January)–color synesthesia (22.4%), followed by musical sound–color synesthesia (18.50%) [26,28] (Figs 2 and 3[29,30]). Inducers and concurrents also include smells, tastes, temperatures, personalities and emotions [26], and can be multiple during a single synesthetic experience. Thus, percepts induced by grapheme–color synesthesia are occasionally accompanied by shape, texture, movement features, and even nonvisual percepts such as smells and tastes, particularly emotions [31,32]. Synesthetic colors generated in grapheme–color synesthesia are determined by systematic rules rather than randomly occurring, and based on the psycholinguistic mechanisms of language processing. The same occurs with both Latin characters and Chinese ideograms [33,34▪].
FIGURE 1.
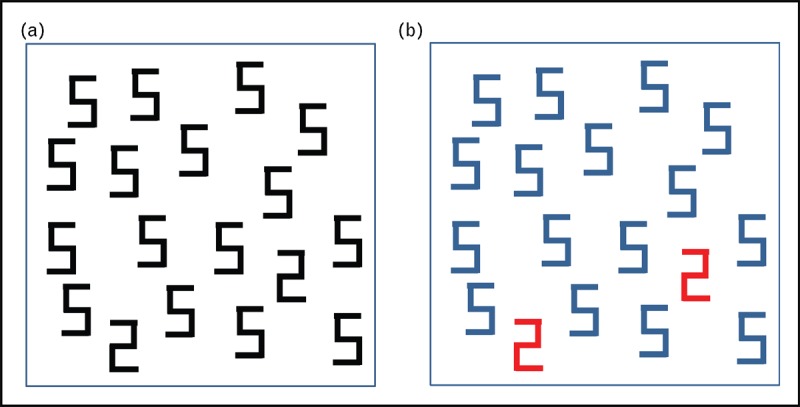
Visual segregation test demonstrating improved digit identification performances by grapheme–color synesthetes. (a) Pattern presented to the tested individuals and (b) same pattern as perceived by a grapheme–color synesthete. To identify digits ‘2’, a person with regular visual perception must perfom a systematic search; in contrast, for a grapheme–color synesthete, who links a specific color with a given number, digits ‘2’ instantly pop-out. Adapted with permission [27].
FIGURE 2.
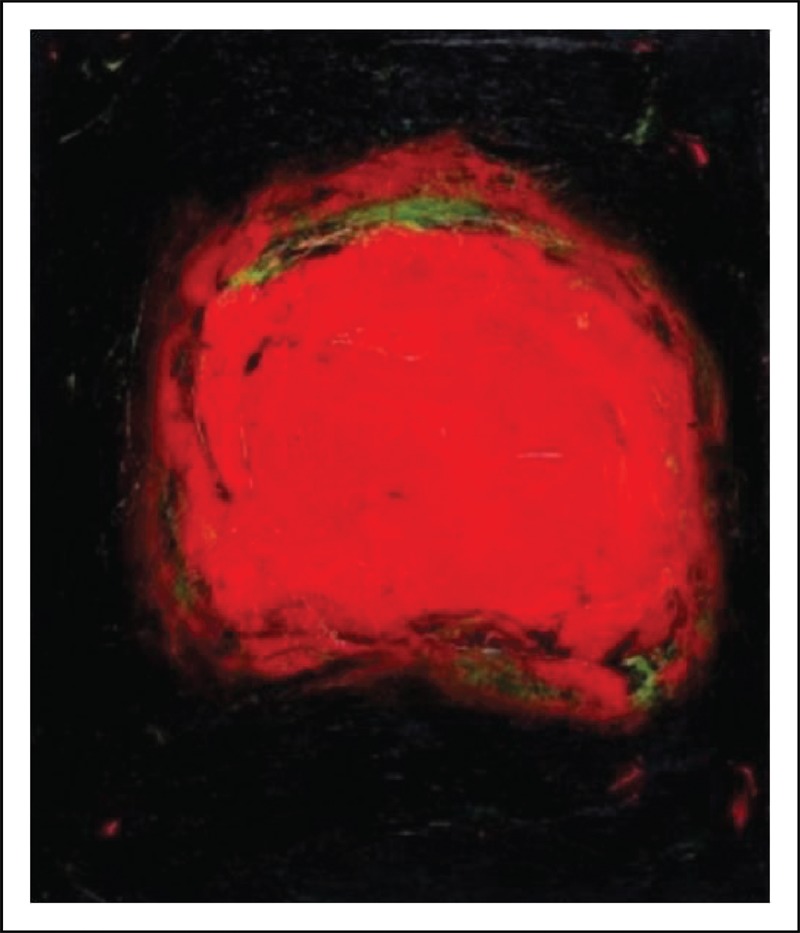
‘Vision’ 1996, by Carol Steen, private collection, represents a synesthetic visual experience elicited in this synesthetic painter by a needle puncture during an acupuncture session [29]. Reproduced with permission.
FIGURE 3.

‘Kondo's Trumpet’ 2010, by Carol Steen, private collection, depicts the synesthetic visual experience elicited by the timbre of that trumpet [30]. Reproduced with permission.
An additional type of colored synesthetic experience was recently described and termed ‘swimming-style color synesthesia’. It is characterized by the generation of specific colored percepts upon conceptual representation of swimming in a particular style (i.e., breast, backstroke, crawl, and butterfly) [35,36]. This phenomenon could be triggered by either presenting a picture of a swimming individual or asking the tested individual to think about a given swimming style. It was speculated that this synesthetic type was caused by overactivity in the mirror neuron system responding to the specific representation [37].
Synesthetic experiences are labeled ‘lower’ when triggered by elementary perceptual processes (e.g., texture) or ‘higher’ when involving a higher cognitive process (e.g., semantic, computing) [7,38,39]. Synesthetes who experience the atypical percepts in an internal space (‘in the mind's eye’, as they sometimes describe it) have been categorized into ‘associators’, whereas those for whom the additional, atypical percept appears to be ‘out there’, overlaying the actual, external surrounding, are designated as ‘projectors’ [40,41].
PAINTERS’ COMPREHENSION OF SYNESTHESIA
Painters commonly demonstrate unique skills in the observation of visual phenomena, in which depiction offers an invaluable source of information for neuroscientists investigating visual function in health and disease [42–46]. Regarding our understanding of synesthesia, painters’ contribution is particularly precious. Among this population, the prevalence of synesthesia was found higher than in general population; in addition, their percepts are frequently represented in their artworks [47]. Indeed, synesthete prevalence among fine art students was estimated to be 23% [48]. Among art students, prevalence of grapheme–color synesthesia alone was reported to be 7%, compared to 2% in controls [49].
Interestingly, synesthetes’ personality profile favors their involvement in creative, artistic activities [50]. Recently evaluated by a structured measure of personality (the ‘Big Five Inventory’) and by questionnaires assessing empathy, synesthetes exhibited higher levels of ‘Openness to Experience’, considered to be related to imagination and artistic tendencies, and higher levels of ‘Fantasizing’, conceptually related to ‘Openness’ [51▪]. Moreover, grapheme–color synesthetes show a distinct cognitive style, with a preference for processing information in both verbal and vivid imagery styles [52▪].
The peculiar world perception decisively impacted the artistic work of numerous ‘synesthetic’ artists. Thus, Kandinsky's nonfigurative paintings and theory of synesthesia [53], prompted by his experience of extraordinary visions of lines and colors elicited by the sound of musical instruments, paved the way to abstract art and thus marked a turning point in history of art [47]. A recent analysis of Kandinsky's works using the Implicit Association Test found no implicit association between the original color–form combinations, and authors concluded that these are probably not a universal property of the visual system [44].
Most informative indications on the character of percepts commonly observed by synesthetic painters, as well as on the compulsive manner they depict their visions, were provided by Carol Steen, a remarkable synesthetic painter [54]. She emphasized that synesthetes’ internal world differs tremendously from what is commonly perceived by others. For instance, colors can be perceived intensely bright, ‘similar to sunlight streaming through a stained glass window’. Noteworthy, she felt that the ‘overwhelming beauty of what she has seen’ powerfully compelled her to capture and reproduce her visions, and that ‘urgency to paint needed to be expressed’. To depict the brightness of colors perceived, synesthetic painters reportedly often apply with speed, pure, unmixed oil paint, or watercolor straight from the tube. Faithfully representing their perceptions may require breaking some long-standing rules, a feature that – as underlined by Carol Steen – characterizes modern art. The artist also specified that her visions were never representational nor figurative. This is apparently typical of many synesthetes’ experiences, and probably explains why synesthetic artwork commonly looks abstract, even though it is a ‘ realistic’ depiction of the artist's perceptions [54].
EMOTIONAL DIMENSION OF SYNESTHESIA
Emotional reactions play a prominent role in synesthetic processes. They are commonly experienced in such conditions [32,55], acting as either inducer, concurrent, or modulator [32,56]. A conflict between the actual color of a stimulus and synesthetically induced percepts can generate discomfort, whereas ‘pleasantness’ is experienced when synesthetic and actual stimulus features match. Some synesthetes indicate that all disagreeable events generate same color, specific for the given individual. Saturation of evoked colors is susceptible to be altered by mood [32]. In some personality–color type of synesthesia, viewing known faces elicits emotionally mediated color percepts, presenting either as colored faces or colored auras around heads [1,32,57] (Figs 4 and 5[58]), conceivably as a result of cross-activation between right, face recognition area and neighboring V4 color cortex [7,59]. In this regard, the following delightful dedication by Julia Simner, a prominent expert in synesthesia, is illustrative: ‘For my two children: the blue one (Indigo) and the brown one (Tommy Bruno)’ [60]. In the so-called ‘ordinal linguistic personification’ synesthesia, letters have emotional valences, as well as a sex and personality [61,62]. Sexual arousal also triggers synesthetic experiences in some 2% of individuals [26]. These perceptual phenomena mainly consist of colored shapes, less commonly of flavors, smells, sounds, or temperatures, and are associated with a higher degree of trance and loss of environmental boundaries [63].
FIGURE 4.

‘I and the Village’ 1911, by Marc Chagall. Museum of Modern Art, New York, USA. Reproduced with permission, © Adagp, Paris 2014. Over decades, Chagall repeatedly depicted using intense green or blue colors, the faces of central characters in his paintings [58]. This most probably reflected a variant of personality–color synesthesia.
FIGURE 5.

‘Half-Past Three’ (‘The Poet’) 1911, by Marc Chagall. Philadelphia Museum of Art, The Louise and Walter Arensberg Collection, Philadelphia, PA, USA. Reproduced with permission, © Adagp, Paris 2014. For comments, see the legend of Fig. 4.
Cerebral structures processing emotion are altered in developmental synesthestes. MRI exploration of associator grapheme–color synesthetes recently brought further evidence of structural changes in emotional areas both at cortical and at subcortical levels [64▪▪]. Acquired cerebral disorders are also susceptible to cause emotional synesthetic percepts. Thus, a patient who had sustained a posterolateral thalamus hemorrhage [24▪] experienced blue photisms, intense extracorporeal sensation, and ‘orgasmic’ ecstasy when hearing brass instruments, or severe disgust sensations when reading words printed in blue characters. Occurrence, expression, and the underlying mechanisms of affect-related forms of synesthesia have recently been reconsidered [56].
IMPACT OF SYNESTHESIA ON COGNITIVE FUNCTIONS
Synesthetic experiences impact the cognitive functions to a larger extent than believed in the past [65,66▪▪,67,68]. Constitutional synesthesia predisposes to better performances in certain aspects and worse in others. Although having better color perception compared with nonsynesthetes [69,70], synesthetes present impaired motion [66▪▪,67] and speech perception [71]. Speech perception deficit could be a consequence of the impaired motion perception, namely the biological movement of lips or of a much wider deficit in multisensory integration [71]. In grapheme–color and tone–color synesthetes, increased gray matter volume in the left posterior fusiform gyrus and decreased gray matter volume of the anterior part of the same gyrus and in the left MT/V5 support these hypotheses [72]. Improved perception can occur within both inducing stimulus and concurrent domains [68]. Memory was also found enhanced when using synesthetic percepts [25,73].
Improved performances depend partly on preconscious mechanisms, operating early in sensory processing [74]. Thus, a recent investigation using pictures containing hidden letters found that grapheme–color projectors recognized the letters faster than nonsynesthetes; interestingly, tested individuals noted that concurrent colors were generated before conscious letter recognition [75▪]. Grapheme–color synesthesia even allows computing via synesthetically perceived colors [68] and as expected, emotional experience modulates synesthetes’ performances [55].
CEREBRAL DISORDERS CAUSING SYNESTHESIA
Acquired forms of synesthesia have been related to a variety of neurological conditions, including migraine [76,77], multiple sclerosis – radiologically isolated syndrome [34▪], posthypnotic suggestion [78], and drug ingestion [20,79]. In recent years, secondary synesthesia has been reported following thalamic stroke [24▪,80–83]: two of these affected individuals experienced colored synesthetic percepts [24▪,83]. Thalamic insult may induce large-scale reorganization of the brain, modify the balance between excitatory and inhibitory connections in high-order visual areas, and favor the development of synesthesia [80].
Sensory deprivation favors the occurrence of synesthetic phenomena. With blind people, nonvisual stimuli tend to elicit various percepts in the suppressed sensory modality, including colored photisms [84,85] presumably by cross-modal activation of the deafferented cortex [86]. Sound-induced photisms in visually affected people are a well recognized phenomenon [87]. Six late-blind individuals were recently reported experiencing colored phenomena when hearing or thinking about letters, numbers, and time-related terms [88,89]. In one of these individuals, touching Braille characters induced colored photisms. A patient of ours, blinded by bilateral arteritic anterior ischemic optic neuropathy, reported perceiving colored photisms when brushing his teeth or hearing a hand clap (personal observation). We also recently observed an unusual case of a late-blind individual suffering from retinitis pigmentosa who volunteered consistently ‘seeing’ his limbs when moving them, a phenomenon presumably related to cross-modal activation of his visual cortex by proprioceptive inputs [90▪].
Brain lesions disrupting canonical networks and sensory input to associative areas are also susceptible to induce synesthetic-like hallucinatory syndromes. A right monophtalm patient with right parosmia reported intricate visual and olfactory hallucinations following a right occipitotemporal stroke [91]. The patient described seeing people with strong odors. The presumed mechanism of these hallucinations was the desinhibition of the connections from the visual association areas to perirhinal and parahippocampal gyri [92].
ARTIFICIALLY ELICITED SYNESTHESIA
Sensory substitution devices (SSDs) have been developed to provide blind individuals with information on their visual surrounding. They convey visual information through another sensory modality, like audition [93]. Visual-to-auditory SSDs proceed by online translation of camera-captured views into sounds, which represent the visual features of the scene [93,94]. Users of such devices commonly claim to ‘see’ the objects figured by sounds, and therefore sensory substitution has been considered a kind of synthetic synesthesia [93]. Interestingly, functional magnetic resonance imaging (fMRI) investigations using a visual-to-auditory SSD, both in blindfolded healthy individuals [95] and in congenitally blind individuals [96▪], showed activation of visual areas. Whether – and to what extent – SSD users also perceive the auditory stimulus as a sound is debated [97,98].
Sensory substitution, however, differs in some respect from the naturally occurring synesthesia. Indeed, intended to reliably figure the visual surrounding, percepts elicited by SSDs are elaborated, whereas regular synesthetic phenomena exhibit essentially idiosyncratic features [8]. Further, in contrast to SSD-provoked synesthetic experiences, in developmental synesthesia, inducers do not conform to sensorimotor contingencies of the concurrent modality [98].
NEURAL FOUNDATIONS OF SYNESTHESIA
Assumptions have been made on the mechanisms underlying synesthesia, including hyperconnectivity between cortical areas [99], reduced level of feed-back from inhibitory cerebral structures [2], learned association in early life [100], and a normal perceptual mechanism incompletely suppressed in synesthetes [17]. Neurocognitive models have been elaborated [101–105].
In recent years, brain-imaging studies brought further evidence that synesthetes connect more inside and between sensory regions and less with remote areas, especially the frontal cortex. Indeed, these individuals exhibit increased intranetwork connectivity in medial visual, auditory and intraparietal networks, and internetworks connectivity between the medial and lateral visual networks, the right frontoparietal network and between the lateral visual and auditory networks. In contrast, nonsynesthetes have more intranetwork connections within frontoparietal network [106]. When presented with inducers, synesthetes exhibit a clustering pattern of activated brain areas uniting more visual regions, whereas nonsynesthetes activate particularly frontal and parietal regions [107▪▪] (Fig. 6).
FIGURE 6.
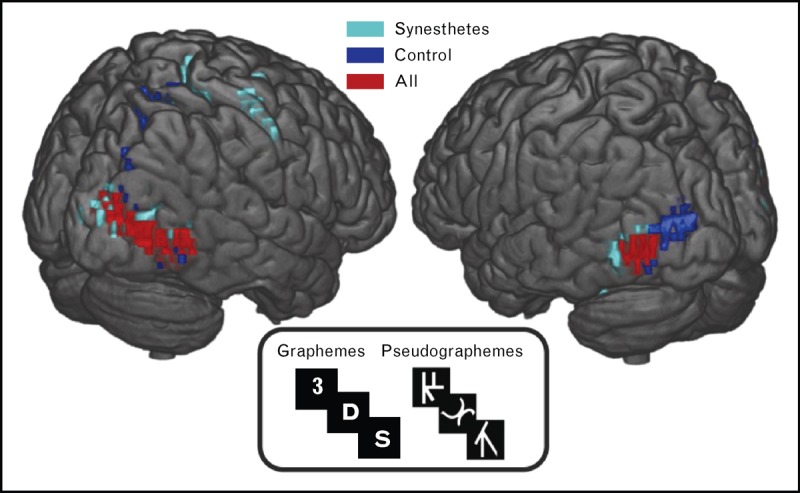
Cerebral activation revealing distinct activity patterns for controls and synesthetes during grapheme and pseudo-grapheme presentation. Synesthetes demonstrate the most significant activity in the bilateral posterior inferior temporal gyri. Reproduced with permission [107▪▪].
Involvement of the bottom-up and top-down mechanisms has further been considered [105,108–111]. The bottom-up model stipulates that the concurrent representation is prompted by the inducer representation via over represented and overactive horizontal connections, whereas the top-down model proposes that the inducer stimulates the concurrent percept via an input from a convergent, higher order integrator [2].
Using dynamic causal modeling, Van Leeuwen et al.[106] have shown that projectors exhibited effective connectivity patterns involving a bottom-up mechanism, whereas associators used a top-down mechanism. However, a recent electroencephalographic (EEG) study found evidence favoring the top-down disinhibited feedback model as the core of the synesthetic phenomenon [112▪▪]. Reduction of long-range couplings in the theta frequency band could facilitate the top-down feedback. An fMRI study demonstrated that, in contrast with projectors, associators’ synesthetic experience was related to areas linked to memory processes, including hippocampus and parahippocampal gyrus [113,114].
THALAMUS AND DEVELOPMENTAL SYNESTHESIA
It was suggested that congenital alterations in thalamic circuitry might be responsible for atypical cortical morphology and connections, found with different synesthetic phenotypes [64▪▪,115]. Cytoarchitectonic maturation of the primary sensory areas and the development of their specific connections are highly dependent on the thalamic input [116]. Enucleation in prenatal macaque drastically alters the equivalents of V1 and V2 visual cortices, and induces rich noncanonical connections with somatosensory, auditory, and frontal areas [117], resembling transient fetal connections [118]. Thus, the visual cortex ends up treating other types of information. Likewise, congenitally blind humans exhibit occipital cortex activation following auditory or somatosensory stimulation [96▪]. It is therefore conceivable that in developmental synesthesia, congenitally anomalous sensory input leads to abnormal synaptic pruning and differences in brain connectivity. In grapheme–color synesthetes, low white matter densities in pulvinar, medial and lateral ventral posterior nuclei, and low fractional anisotropy in medial dorsal and ventral anterior nuclei suggest a constitutional disconnection and hypoconnection between thalamus and cerebral cortex [64▪▪]. The concerned white matter tracts project to the left prefrontal cortex and bilateral temporal and posterior parietal cortex, regions that in synesthetes are distinct both in structure and function. Secondary synesthesia after thalamic stroke also support the involvement of thalamic output in synesthetic phenomena [24▪,80–83].
CONCLUSION
Over the last few years, substantial advances have been made in the understanding of synesthesia, and hence more globally in the comprehension of perception and consciousness. Fortunately, awareness of this condition in the societal environment also significantly improved, finally allowing synesthetes to feel relieved by the so badly needed recognition of their particular situation. In a near future, in addition to the expected deepening of the explorations undertaken, elaborating a more comprehensive definition of synesthesia would be welcomed. Currently used criteria are rather restrictive for a condition that is quite polymorphic in nature. This process, however, is customary in the history of medicine, which consists of initially establishing a restricted definition to encapsulate the core of the condition and then broadening it, taking into account the numerous subtle presentations encountered.
Acknowledgements
The authors thank Katia Marazova, MD, PhD, for editorial assistance.
Financial support and sponsorship
Funding: This study was supported in part by grants from LABEX and Humanis.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Hochel M, Milán EG, Martín JLM, et al. Congruence or coherence? Emotional and physiological responses to colours in synaesthesia. Eur J Cogn Psychol 2009; 21:703–723. [Google Scholar]
- 2.Grossenbacher PG, Lovelace CT. Mechanisms of synesthesia: cognitive and physiological constraints. Trends Cogn Sci 2001; 5:36–41. [DOI] [PubMed] [Google Scholar]
- 3.Sinke C, Neufeld J. Synaesthesia: a conceptualization (‘synthesis’-) phenomenon. Philosophical and neurobiological aspects. Theor Hist Sci 2013; 10:37–54. [Google Scholar]
- 4.Auvray M, Deroy O. How do synesthetes experience the world? In: Matthen M, editor. Oxford handbook of the philosophy of perception. New York: Oxford University Press, Inc; in press. [Google Scholar]
- 5.Sagiv N, Frith C. Synesthesia and consciousness. In: Simner J, Hubbard EM, editors. Oxford handbook of synesthesia. Oxford: Oxford University Press; 2013. 924–940. [Google Scholar]
- 6.Hubbard EM. Synesthesia and functional imaging. In: Simner J, Hubbard E, editors. Oxford handbook of synesthesia. Oxford: Oxford University Press; 2013. 475–499. [Google Scholar]
- 7.Ramachandran VS, Hubbard EM. Synaesthesia – a window into perception, thought and language. J Conscious Stud 2001; 8:3–34. [Google Scholar]
- 8.Ward J. Synesthesia. Annu Rev Psychol 2013; 64:49–75. [DOI] [PubMed] [Google Scholar]
- 9.Voskuil PHA. Van Gogh's disease in the light of his correspondence. In: Bogousslavsky J, Dieguez S, editors. Frontiers of neurology and neuroscience. Basel: Karger AG; 2013. pp. 116–125. [DOI] [PubMed] [Google Scholar]
- 10.Zedler M, Rehme M. Synesthesia: a psychosocial approach. In: Simner J, Hubbard E, editors. Oxford handbook of synesthesia. Oxford: Oxford University Press; 2013. 459–472. [Google Scholar]
- 11.Van Campen C. The discovery of synesthesia in childhood. Theor Hist Sci 2013; 10:195–206. [Google Scholar]
- 12.Day S. Synesthesia: a first-person perspective. In: Simner J, Hubbard EM, editors. Oxford handbook of synesthesia. Oxford: Oxford University Press; 2013. 903–923. [Google Scholar]
- 13.Simner J, Mulvenna C, Sagiv N, et al. Synaesthesia: the prevalence of atypical cross-modal experiences. Perception 2006; 35:1024–1033. [DOI] [PubMed] [Google Scholar]
- 14.Brang D, Ramachandran VS. Survival of the synesthesia gene: why do people hear colors and taste words? PLoS Biol 2011; 9:e1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson TE, Sandramouli S. Auditory–olfactory synesthesia coexisting with auditory–visual synesthesia. J Neuroophthalmol 2012; 32:221–223. [DOI] [PubMed] [Google Scholar]
- 16.Neufeld J, Roy M, Zapf A, et al. Is synesthesia more common in patients with Asperger syndrome? Front Hum Neurosci 2013; 7:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cytowic RE. Synesthesia: a union of the senses – second edition. Cambridge, MA: A Bradford Book; 2002. [Google Scholar]
- 18.Mattingley JB. Attention, automaticity, and awareness in synesthesia. Ann N Y Acad Sci 2009; 1156:141–167. [DOI] [PubMed] [Google Scholar]
- 19▪.Simner J, Bain AE. A longitudinal study of grapheme–color synesthesia in childhood: 6/7 years to 10/11 years. Front Hum Neurosci 2013; 7:603. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors observed the development of child synesthetes over 4 years and found that in some individuals, synesthesia apparently died out over time or developed more slowly in some individuals over others. This demonstrated that synesthetic phenomena are not as consistent over time as previously thought.
- 20.Brogaard B. Serotonergic hyperactivity as a potential factor in developmental, acquired and drug-induced synesthesia. Front Hum Neurosci 2013; 7:657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luke DP, Terhune DB. The induction of synaesthesia with chemical agents: a systematic review. Front Psychol 2013; 4:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terhune DB, Song SM, Duta MD, Cohen Kadosh R. Probing the neurochemical basis of synaesthesia using psychophysics. Front Hum Neurosci 2014; 8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Hout MC. Nod and wave: an Internet study of the codeine intoxication phenomenon. Int J Drug Policy 2014; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 24▪.Schweizer TA, Li Z, Fischer CE, et al. From the thalamus with love: a rare window into the locus of emotional synesthesia. Neurology 2013; 81:509–510. [DOI] [PubMed] [Google Scholar]; The authors for the first time report a case of acquired emotional synesthesia after focal thalamic lesion.
- 25.Brang D, Rouw R, Ramachandran VS, Coulson S. Similarly shaped letters evoke similar colors in grapheme–color synesthesia. Neuropsychologia 2011; 49:1355–1358. [DOI] [PubMed] [Google Scholar]
- 26.Day S. Types of synesthesia. Synesthesia 2014. Available at http://www.daysyn.com [Accessed October 2014]. [Google Scholar]
- 27.Ramachandran VS, Hubbard EM. Hearing colors, tasting shapes. Sci Am 2003; 288:52–59. [DOI] [PubMed] [Google Scholar]
- 28.Day S. Some demographic and socio-cultural aspects of synesthesia. In: Robertson LC, Sagiv N, editors. Synesthesia: perspectives from cognitive neuroscience. New York: Oxford University Press, Inc; 2005. 11–33. [Google Scholar]
- 29.Steen C. Visions shared. A firsthand look into synesthesia and art. Leonardo 2001; 34:203–208. [Google Scholar]
- 30.McDonald F. Synesthesia: bringing out the contours. Aust Art Rev 2006. Available at http://www.synesthesia.info/Steen-Australian_Art_Review.pdf [Accessed 12 October 2014] [Google Scholar]
- 31.Tyler C. Varieties of synesthetic experience. In: Robertson LC, Sagiv N, editors. Synesthesia: perspectives from cognitive neuroscience. New York: Oxford University Press, Inc; 2005. 34–44. [Google Scholar]
- 32.Ward J. Emotionally mediated synaesthesia. Cogn Neuropsychol 2004; 21:761–772. [DOI] [PubMed] [Google Scholar]
- 33.Hung W-Y, Simner J, Shillcock R, Eagleman DM. Synaesthesia in Chinese characters: the role of radical function, position. Conscious Cogn 2014; 24:38–48. [DOI] [PubMed] [Google Scholar]
- 34▪.Simner J, Carmichael DA, Hubbard EM, et al. Rates of white matter hyperintensities compatible with the radiological profile of multiple sclerosis within self-referred synesthete populations. Neurocase (in press). [DOI] [PubMed] [Google Scholar]; The study reported an apparent statistical link between synesthesia and multiple sclerosis – radiologically isolated syndrome.
- 35.Nikolić D, Jürgens UM, Rothen N, et al. Swimming-style synesthesia. Cortex 2011; 47:874–879. [DOI] [PubMed] [Google Scholar]
- 36.Rothen N, Nikolić D, Jürgens UM, et al. Psychophysiological evidence for the genuineness of swimming-style colour synaesthesia. Conscious Cogn 2013; 22:35–46. [DOI] [PubMed] [Google Scholar]
- 37.Mroczko-Wasowicz A, Werning M. Synesthesia, sensory–motor contingency, and semantic emulation: how swimming style–color synesthesia challenges the traditional view of synesthesia. Front Psychol 2012; 3:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meier B. Semantic representation of synaesthesia. Theor Hist Sci 2013; 10:125–134. [Google Scholar]
- 39.Mroczko-Wasowicz A, Nikolic D. Semantic mechanisms may be responsible for developing synesthesia. Front Hum Neurosci 2014; 8:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dixon MJ, Smilek D, Merikle PM. Not all synaesthetes are created equal: projector versus associator synaesthetes. Cogn Affect Behav Neurosci 2004; 4:335–343. [DOI] [PubMed] [Google Scholar]
- 41.Mohr C. Synesthesia in space versus in the ‘mind's eye’: how to ask the right questions. In: Simner J, Hubbard E, editors. Oxford handbook of synesthesia. Oxford: Oxford University Press; 2013. 440–458. [Google Scholar]
- 42.Cavanagh P. The artist as neuroscientist. Nature 2005; 434:301–307. [DOI] [PubMed] [Google Scholar]
- 43.Blanke O, Forcucci L, Dieguez S. Don’t forget the artists when studying perception of art. Nature 2009; 462:984. [DOI] [PubMed] [Google Scholar]
- 44.Makin ADJ, Wuerger SM. The IAT shows no evidence for Kandinsky's color–shape associations. Front Psychol 2013; 4:616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeki S. Neurobiology and the humanities. Neuron 2014; 84:12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Safran AB, Sanda N, Sahel J-A. A neurological disorder presumably underlies painter Francis Bacon distorted world depiction. Front Hum Neurosci 2014; 8:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Campen C. Synesthesia in the visual arts. In: Simner J, Hubbard E, editors. Oxford handbook of synesthesia. Oxford: Oxford University Press; 2013. 631–646. [Google Scholar]
- 48.Domino G. Synesthesia and creativity in fine arts students: an empirical look. Creat Res J 1989; 2:17–29. [Google Scholar]
- 49.Rothen N, Meier B. Higher prevalence of synaesthesia in art students. Perception 2010; 39:718–720. [DOI] [PubMed] [Google Scholar]
- 50.Ward J, Thompson-Lake D, Ely R, Kaminski F. Synaesthesia, creativity and art: what is the link? Br J Psychol 2008; 1953: 99:127–141. [DOI] [PubMed] [Google Scholar]
- 51▪.Banissy MJ, Holle H, Cassell J, et al. Personality traits in people with synaesthesia: do synaesthetes have an atypical personality profile? Personal Individ Differ 2013; 54:828–831. [Google Scholar]; The authors found that, relative to matched controls, synaesthetes reported higher levels of imagination, artistic tendencies, and ‘fantasizing’.
- 52▪.Meier B, Rothen N. Grapheme–color synaesthesia is associated with a distinct cognitive style. Front Psychol 2013; 4:632. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors reported that grapheme–color synesthetes showed higher ratings on verbal and vivid imagery style dimensions.
- 53.Kandinsky W. Concerning the spiritual in art. London: Constable and Co Ltd; 1914. [Google Scholar]
- 54.Steen C, Berman G. Synesthesia and the artistic process. In: Simner J, Hubbard E, editors. Oxford handbook of synesthesia. Oxford: Oxford University Press; 2013. 671–691. [Google Scholar]
- 55.Perry A, Henik A. The emotional valence of a conflict: implications from synesthesia. Front Psychol 2013; 4:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dael N, Sierro G, Mohr C. Affect-related synesthesias: a prospective view on their existence, expression and underlying mechanisms. Front Psychol 2013; 4:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cytowic RE, Eagleman D. Wednesday is indigo blue: discovering the brain of synesthesia. Cambridge, MA: MIT Press; 2009. [Google Scholar]
- 58.Salcman M. The fiancée with a blue face by Marc Chagall (1887–1985). Neurosurgery 2007; 61:1322–1324. [DOI] [PubMed] [Google Scholar]
- 59.Hubbard EM, Brang D, Ramachandran VS. The cross-activation theory at 10. J Neuropsychol 2011; 5:152–177. [DOI] [PubMed] [Google Scholar]
- 60.Simner J, Hubbard E. Oxford handbook of synesthesia. Oxford: Oxford University Press; 2013. [Google Scholar]
- 61.Simner J, Holenstein E. Ordinal linguistic personification as a variant of synesthesia. J Cogn Neurosci 2007; 19:694–703. [DOI] [PubMed] [Google Scholar]
- 62.Smilek D, Malcolmson KA, Carriere JSA, et al. When ‘3’ is a jerk, ‘E’ is a king: personifying inanimate objects in synesthesia. J Cogn Neurosci 2007; 19:981–992. [DOI] [PubMed] [Google Scholar]
- 63.Nielsen J, Kruger THC, Hartmann U, et al. Synaesthesia and sexuality: the influence of synaesthetic perceptions on sexual experience. Front Psychol 2013; 4:751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64▪▪.Melero H, Peña-Melián Á, Ríos-Lago M, et al. Grapheme–color synesthetes show peculiarities in their emotional brain: cortical and subcortical evidence from VBM analysis of 3D-T1 DTI data. Exp Brain Res 2013; 227:343–353. [DOI] [PubMed] [Google Scholar]; This study demonstrates morphological differences in thalamocortical connections in synesthetes as well as increased gray matter volume in brain regions involved in emotional processing. It provides morphological evidence about the importance of emotion in synesthetic phenomena.
- 65.Mottron L, Bouvet L, Bonnel A, et al. Veridical mapping in the development of exceptional autistic abilities. Neurosci Biobehav Rev 2013; 37:209–228. [DOI] [PubMed] [Google Scholar]
- 66▪▪.Banissy MJ, Tester V, Muggleton NG, et al. Synesthesia for color is linked to improved color perception but reduced motion perception. Psychol Sci 2013; 24:2390–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that synesthesia for color is linked to facilitated color sensitivity, but decreased motion sensitivity.
- 67.McCarthy JD, Caplovitz GP. Color synesthesia improves color but impairs motion perception. Trends Cogn Sci 2014; 18:224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCarthy JD, Barnes LN, Alvarez BD, Caplovitz GP. Two plus blue equals green: grapheme–color synesthesia allows cognitive access to numerical information via color. Conscious Cogn 2013; 22:1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen Kadosh R, Gertner L, Terhune DB. Exceptional abilities in the spatial representation of numbers and time: insights from synesthesia. Neuroscientist 2012; 18:208–215. [DOI] [PubMed] [Google Scholar]
- 70.Brang D, Miller LE, McQuire M, et al. Enhanced mental rotation ability in time-space synesthesia. Cogn Process 2013; 14:429–434. [DOI] [PubMed] [Google Scholar]
- 71.Sinke C, Neufeld J, Zedler M, et al. Reduced audiovisual integration in synesthesia – evidence from bimodal speech perception. J Neuropsychol 2014; 8:94–106. [DOI] [PubMed] [Google Scholar]
- 72.Banissy MJ, Stewart L, Muggleton NG, et al. Grapheme–color and tone–color synesthesia is associated with structural brain changes in visual regions implicated in color, form, and motion. Cogn Neurosci 2012; 3:29–35. [DOI] [PubMed] [Google Scholar]
- 73.Witthoft N, Winawer J. Learning, memory, and synesthesia. Psychol Sci 2013; 24:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rothen N, Scott RB, Mealor AD, et al. Synesthetic experiences enhance unconscious learning. Cogn Neurosci 2013; 4:231–238. [DOI] [PubMed] [Google Scholar]
- 75▪.Ramachandran VS, Seckel E. Synesthetic colors induced by graphemes that have not been consciously perceived. Neurocase (in press). [DOI] [PubMed] [Google Scholar]; In pictures that contained hidden letters, grapheme–color projector synesthetes recognized the letters faster and reported that the colors were evoked before conscious letter recognition, suggesting that in some synesthetes colors are evoked preconsciously early in sensory processing.
- 76.Podoll K, Robinson D. Auditory–visual synaesthesia in a patient with basilar migraine. J Neurol 2002; 249:476–477. [DOI] [PubMed] [Google Scholar]
- 77.Alstadhaug KB, Benjaminsen E. Synesthesia and migraine: case report. BMC Neurol 2010; 10:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Terhune DB, Cardeña E, Lindgren M. Disruption of synaesthesia by posthypnotic suggestion: an ERP study. Neuropsychologia 2010; 48:3360–3364. [DOI] [PubMed] [Google Scholar]
- 79.Sinke C, Halpern JH, Zedler M, et al. Genuine and drug-induced synesthesia: a comparison. Conscious Cogn 2012; 21:1419–1434. [DOI] [PubMed] [Google Scholar]
- 80.Ro T, Farnè A, Johnson RM, et al. Feeling sounds after a thalamic lesion. Ann Neurol 2007; 62:433–441. [DOI] [PubMed] [Google Scholar]
- 81.Beauchamp MS, Ro T. Neural substrates of sound–touch synesthesia after a thalamic lesion. J Neurosci 2008; 28:13696–13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naumer MJ, van den Bosch JJF. Touching sounds: thalamocortical plasticity and the neural basis of multisensory integration. J Neurophysiol 2009; 102:7–8. [DOI] [PubMed] [Google Scholar]
- 83.Fornazzari L, Fischer CE, Ringer L, Schweizer TA. ‘Blue is music to my ears’: multimodal synesthesias after a thalamic stroke. Neurocase 2012; 18:318–322. [DOI] [PubMed] [Google Scholar]
- 84.Niccolai V, van Leeuwen TM, Blakemore C, Stoerig P. Synaesthetic perception of colour and visual space in a blind subject: an fMRI case study. Conscious Cogn 2012; 21:889–899. [DOI] [PubMed] [Google Scholar]
- 85.Armel K, Ramachandran VS. Acquired synesthesia in retinitis pigmentosa. Neurocase 1999; 5:293–296. [Google Scholar]
- 86.Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci 2010; 11:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobs L, Karpik A, Bozian D, Gøthgen S. Auditory–visual synesthesia: sound-induced photisms. Arch Neurol 1981; 38:211–216. [DOI] [PubMed] [Google Scholar]
- 88.Steven MS, Hansen PC, Blakemore C. Activation of color-selective areas of the visual cortex in a blind synesthete. Cortex 2006; 42:304–308. [DOI] [PubMed] [Google Scholar]
- 89.Steven MS, Blakemore C. Visual synaesthesia in the blind. Perception 2004; 33:855–868. [DOI] [PubMed] [Google Scholar]
- 90▪.Safran AB, Sabbah N, Sanda N, Sahel JA. The blind man who saw his hands. Cross-modal plasticity revisited. In: The Association for the Research in Vision and Ophthalmology (ARVO); 2014 Annual Meeting; Poster no: 4147. [Google Scholar]; A patient blinded as a result of retinitis pigmentosa volunteered that he visually perceived the shape of his hands when waving them; the visual synesthetic percept was presumably elicited by proprioceptive inputs.
- 91.Gubernick D, Ameli P, Teng Q, et al. Visual–olfactory hallucinatory synesthesia: the Charles Bonnet Syndrome with olfactory hallucinations. Cortex 2014; 50:204–207. [DOI] [PubMed] [Google Scholar]
- 92.Ninomiya T, Sawamura H, Inoue K-I, Takada M. Multisynaptic inputs from the medial temporal lobe to V4 in macaques. PLoS One 2012; 7:e52115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suslick KS. Synesthesia in science and technology: more than making the unseen visible. Curr Opin Chem Biol 2012; 16:557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Proulx MJ. Synthetic synaesthesia and sensory substitution. Conscious Cogn 2010; 19:501–503. [DOI] [PubMed] [Google Scholar]
- 95.Striem-Amit E, Cohen L, Dehaene S, Amedi A. Reading with sounds: sensory substitution selectively activates the visual word form area in the blind. Neuron 2012; 76:640–652. [DOI] [PubMed] [Google Scholar]
- 96▪.Striem-Amit E, Amedi A. Visual cortex extrastriate body-selective area activation in congenitally blind people ‘seeing’ by using sounds. Curr Biol 2014; 24:687–692. [DOI] [PubMed] [Google Scholar]; This study demonstrates the presence of selective activation of extrastriate body area in congenitally blind while detecting body shapes with a sensory substitution device, supporting the view that brain has a sensory independent, task-selective organization.
- 97.Ortiz T, Poch J, Santos JM, et al. Recruitment of occipital cortex during sensory substitution training linked to subjective experience of seeing in people with blindness. PLoS One 2011; 6:e23264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ward J, Wright T. Sensory substitution as an artificially acquired synaesthesia. Neurosci Biobehav Rev 2014; 41:26–35. [DOI] [PubMed] [Google Scholar]
- 99.Ramachandran VS, Hubbard EM. Psychophysical investigations into the neural basis of synaesthesia. Proc Biol Sci 2001; 268:979–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Calkins M. Synesthesia. J Psychol 1895; 7:90–107. [Google Scholar]
- 101.Harvey JP. Sensory perception: lessons from synesthesia: using synesthesia to inform the understanding of sensory perception. Yale J Biol Med 2013; 86:203–216. [PMC free article] [PubMed] [Google Scholar]
- 102.Froese T. Steps toward an inactive account of synesthesia. Cogn Neurosci 2014; 5:126–127. [DOI] [PubMed] [Google Scholar]
- 103.O’Regan JK, Degenaar J. Predictive processing, perceptual presence, sensorimotor theory. Cogn Neurosci 2014; 5:130–131. [DOI] [PubMed] [Google Scholar]
- 104.Rouw R, Ridderinkhof KR. The most intriguing question in synesthesia research. Cogn Neurosci 2014; 5:128–130. [DOI] [PubMed] [Google Scholar]
- 105.Van Leeuwen TM. Constructing priors in synesthesia. Cogn Neurosci 2014; 5:124–126. [DOI] [PubMed] [Google Scholar]
- 106.Van Leeuwen TM, den Ouden HEM, Hagoort P. Effective connectivity determines the nature of subjective experience in grapheme–color synesthesia. J Neurosci 2011; 31:9879–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107▪▪.Tomson SN, Narayan M, Allen GI, Eagleman DM. Neural networks of colored sequence synesthesia. J Neurosci 2013; 33:14098–14106. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates hodological differences between synesthetes and nonsynesthetes. Synesthetes cluster more in visual areas and nonsynesthetes in frontal and parietal areas. These clustering patters may provide an explanation for the different cognitive performances associated with synesthetic phenotype.
- 108.Hubbard EM. Neurophysiology of synesthesia. Curr Psychiatry Rep 2007; 9:193–199. [DOI] [PubMed] [Google Scholar]
- 109.Hubbard EM. A real red-letter day. Nat Neurosci 2007; 10:671–672. [DOI] [PubMed] [Google Scholar]
- 110.Brogaard B, Vanni S, Silvanto J. Seeing mathematics: perceptual experience and brain activity in acquired synesthesia. Neurocase 2013; 19:566–575. [DOI] [PubMed] [Google Scholar]
- 111.Brogaard B, Marlow K, Rice K. The long-term potentiation model for grapheme–color binding in synesthesia. In: Bennett D, Hill C, editors. Sensory integration and the unity of consciousness. Cambridge, MA: MIT Press; in press. [Google Scholar]
- 112▪▪.Volberg G, Karmann A, Birkner S, Greenlee MW. Short- and long-range neural synchrony in grapheme–color synesthesia. J Cogn Neurosci 2013; 25:1148–1162. [DOI] [PubMed] [Google Scholar]; This study provides electrophysiological arguments that favor the top-down disinhibited model as the core of synesthetic phenomena.
- 113.Rouw R, Scholte HS. Neural basis of individual differences in synesthetic experiences. J Neurosci 2010; 30:6205–6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rouw R, Scholte HS. Increased structural connectivity in grapheme–color synesthesia. Nat Neurosci 2007; 10:792–797. [DOI] [PubMed] [Google Scholar]
- 115.Mitchell KJ. Synesthesia and cortical connectivity – a neurodevelopmental perspective. In: Simner J, Hubbard EM, editors. Oxford handbook of synesthesia. Oxford: Oxford University Press; 2013. 530–550. [Google Scholar]
- 116.Dehay C, Kennedy H. Cell-cycle control and cortical development. Nat Rev Neurosci 2007; 8:438–450. [DOI] [PubMed] [Google Scholar]
- 117.Rakic P, Suñer I, Williams RW. A novel cytoarchitectonic area induced experimentally within the primate visual cortex. Proc Natl Acad Sci USA 1991; 88:2083–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Price DJ, Kennedy H, Dehay C, et al. The development of cortical connections. Eur J Neurosci 2006; 23:910–920. [DOI] [PubMed] [Google Scholar]


