Abstract
Association of weight loss achieved through various decongestive strategies with clinical outcomes in acute decompensated heart failure (HF) patients is not well described. Our goal was to determine the relationship between weight change during hospitalization and subsequent clinical events in decompensated HF patients. We evaluated data on 433 patients hospitalized with advanced HF enrolled in the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial. The influence of change in weight during hospitalization to clinical outcomes (days alive out of hospital in the first 6 months; death; death or rehospitalization; and death, rehospitalization or cardiac transplantation) was evaluated. On average patients lost approximately 3.6 Kg during hospitalization. When categorized into 3 weight loss tertiles, those in highest tertile were more likely to be older, females, smokers, with higher body weight, prior percutaneous coronary intervention(s), baseline heart rate, and BNP and blood urea nitrogen values, but lower ejection fraction and peak oxygen consumptions. No significant differences were observed between weight change and any in-hospital or follow-up events (days well HR 0.995 [95% CI 0.975–1.016]; 180 days death HR 1.012 [95% CI 0.969–1.057]; death/rehospitalization-180 days HR 1.014 [95% CI 0.990–1.038]). In conclusions, weight loss in patients with acute decompensated HF during hospitalization was not related to clinical end-points. This data challenges the merit of using weight as a surrogate endpoint for more important clinical events i.e. death and/or rehospitalization in patients with heart failure in the design of treatment strategies for novel therapeutic agents in randomized controlled clinical trials.
Keywords: heart failure, weight, outcomes
Whether the ubiquitous use of weight change as a surrogate end-point in clinical HF trials is associated with important clinical adverse events (i.e. death and/or rehospitalization) remains uncertain. The relationship between weight loss and subsequent clinical outcomes in the EVEREST and UNLOAD trials was divergent [1–3]. The EVEREST investigators demonstrated that a 1 kg difference in weight loss at time of discharge was not associated with a reduction in rehospitalization for HF or cardiovascular death [1,2]. In contrast, the UNLOAD investigators in showed that the greater decrease in weight in the ultrafiltration group was associated with a greater reduction in rehospitalization days in the ultrafiltration group compared with the diuretic treated cohort (1.4 days versus 4.2 days) [3]. Accordingly, the purpose of this investigation was to determine the relationship between weight change during the index hospitalization and subsequent clinical events, specifically death and/or rehospitalization in patients with acute decompensate HF patients who were enrolled in the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial [4]. We hypothesized that weight loss would significantly and positively influence subsequent clinical outcomes including re-hospitalization following discharge.
METHODS
The ESCAPE trial enrolled 433 patients hospitalized with advanced HF at 26 sites in North America between January 18, 2000 and November 17, 2003. The design, primary endpoints, and results of the ESCAPE trial have been previously published [4]. Briefly, patients hospitalized with severe symptomatic HF despite recommended therapies were randomly assigned to receive clinical assessment or pulmonary artery catheter -guided therapy. Patients met the following inclusion criteria within the prior year: 1) an urgent visit to the emergency department, 2) treatment during the proceeding month with more than 160 mg of furosemide daily, 3) therapy with an angiotensin-converting enzyme (ACE) inhibitor and diuretic for at least 3 months, 4) left ventricular ejection fraction of ≤0.30, 5) systolic blood pressure of ≤125 mm Hg, and 6) at least 1 sign and 1 symptom of congestion. The exclusion criteria included: 1) serum creatinine >3.5 mg/dL, 2) prior use of dobutamine or dopamine >3 μg/kg/min, or 3) any prior use of milrinone during the current hospitalization. The target in both groups was resolution of clinical symptoms and signs of congestion (orthopnea, edema, and jugular venous pressure elevation) with the additional therapeutic goals in the pulmonary artery catheter group of achieving a pulmonary capillary wedge pressure of ≤15 mm Hg and a right atrial pressure of ≤8 mm Hg. Medication use was not specified, but intravenous inotropic agents were discouraged. ACE inhibitor and beta-blocker doses were titrated in the outpatient HF programs at these selected centers during the 6 months after randomization according to patient tolerability and current guidelines [5]. Diuretics were adjusted both during and after hospitalization to optimize fluid balance without progressive deterioration in renal function. For the purpose of this analysis, we included all patients in whom hospitalization weight change data was available.
Selected demographics, baseline characteristics, laboratory values, quality of life indices, and physiologic parameters were collected at baseline and throughout the hospitalization as well as at several time periods during follow-up using standard data collection forms. Weights were carefully measured throughout the hospitalization as were electrolytes, renal function, and biological markers. In hospital weight change was used as a surrogate for fluid loss. It was defined as the difference in baseline weight (in kg) and discharge weight. If the patient’s discharge weight was missing, then the hospital day 7 weight, day 5 weight, or day 3 weight was used in the calculation in that order. If the weight data was missing at all three time points, the patient was excluded from the analysis. Patients were followed for a total of 180 days. Data on quality of life, recurrent hospitalizations, and death were carefully collected. The all-cause mortality and recurrent hospitalization data were ascertained by the site investigators.
For descriptive purpose, patients were categorized in to 3 tertiles of their weight loss. Continuous variables were described using median (interquartile) values and categorical variables as percentages. The Kruskal Wallis test was used to test for differences in continuous variables, and the chi-square test was used to detect global differences in categorical variables. Multiple linear regression analysis was used to identify baseline predictors of observed weight loss. Baseline weight, age, presence of diabetes, blood urea nitrogen, serum creatinine, gender, length of initial hospitalization (from randomization), brain natriuretic peptide, sodium, baseline diuretic use, left ventricular ejection fraction, diuretic dose, V02, systolic blood pressure, treatment, and ischemic etiology were used as candidate variables. The relationship of observed weight loss to diuretic dose was also examined using the Pearson’s coefficient correlation.
Next we looked at the ability of weight loss to predict endpoints, including the primary endpoint (days well), death, and death/rehospitalization using Cox proportional hazard models to adjust for baseline confounders. A p-value of <0.05 was considered to indicate a significant difference. All analyses were performed using SAS software (version 8.2, SAS Institute, Cary, NC).
RESULTS
Of 433 patients enrolled in ESCAPE, information on change in weight during hospitalization was available in 383 (88.5%). The distribution of weight loss during the hospitalization is shown in Figure 1. On average there was a 3.6 Kg loss of weight during hospitalization with patients managed with a PAC having more weight loss than those in the clinical assessment arm (3.39 vs. 3.89; p=0.3327). Table 1 outlines the clinical features, laboratory data and medication use at baseline among patients in the 3 weight change categories. Compared with patients in the lowest weight change tertile, those in the highest tertile were more likely to be older, female, smokers, have higher baseline body weight and a history of prior percutaneous coronary intervention(s). Patients in the highest tertile of weight change also had the highest baseline heart rate and diastolic blood pressure, whereas left ventricular ejection fraction was lowest in this cohort. Laboratory values of blood urea nitrogen and brain natriuretic peptide were highest and that for peak oxygen consumption lowest among this cohort. At discharge, the orthopnea scale and jugular venous distention was significantly lower among patients in the highest weight change tertile (Table 2). Discharge serum sodium was lowest and blood urea nitrogen and serum creatinine highest in this cohort. Use of beta-blockers and ACE inhibitors or angiotensin receptor blockers decreased marginally (4.8% and 4.5%, respectively) whereas the use of digoxin and spironolactone increased (4.4% and 10%, respectively) from admission to discharge.
Figure 1.
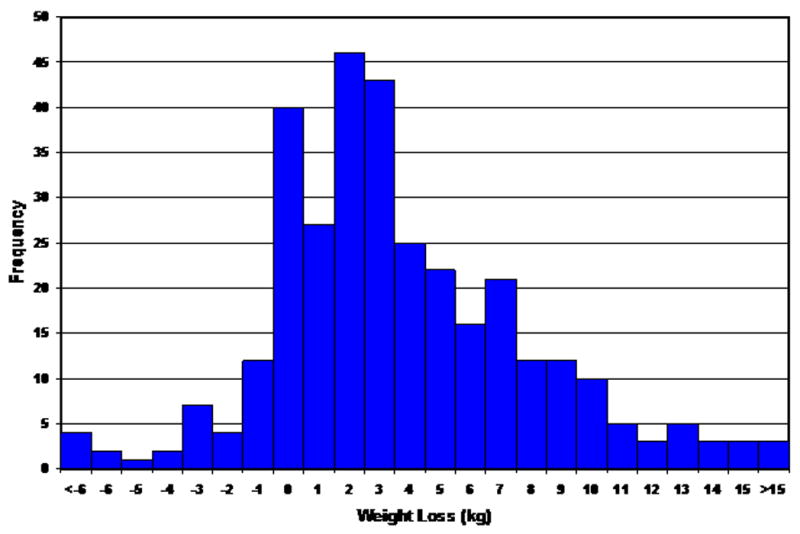
Distribution of Weight Changes during initial hospitalization
Table 1.
Baseline Clinical Characteristics
| Weight Change | |||||
|---|---|---|---|---|---|
|
| |||||
| Variable | Lowest Tertile (n=128) | Middle Tertile (n=128) | Highest tertile (n=127) | P value | |
| Age, (yrs), (median IQR) | 56 (46–67) | 55 (47–64) | 58 (46–66) | 0.645 | |
| Women | 38% | 25% | 19% | 0.003 | |
| Nonwhite | 41% | 37% | 44% | 0.486 | |
| Baseline weight, (kg) (median, IQR) | 78 (62–96) | 85 (73–97) | 87 (76–106) | <0.001 | |
| Etiology of the heart failure | |||||
| Ischemic | 43% | 50% | 54% | 0.209 | |
| Non-ischemic | 56% | 50% | 46% | ||
| Hypertension | 49% | 43% | 48% | 0.654 | |
| Diabetes mellitus | 30% | 30% | 37% | 0.386 | |
| Current smoker | 7.1% | 10% | 21% | 0.047 | |
| Prior stroke | 0% | 0.8% | 0% | 1.000 | |
| Prior percutaneous coronary intervention | 16% | 23% | 29% | 0.035 | |
| Prior coronary artery bypass graft surgery | 27 | 32 | 31 | 0.608 | |
| Heart rate, (bpm) (median, IQR) | 80 (70–89) | 80 (70–93) | 84 (72–96) | 0.029 | |
| Blood pressure, (mm Hg) (median, IQR) | |||||
| Systolic | 102 (90–116) | 105 (94–117) | 106 (95–117) | 0.389 | |
| Diastolic | 63 (58–70) | 68 (60–73) | 70 (60–76) | 0.001 | |
| Orthopnea scale (0–4) | 3 (3–4) | 3 (3–4) | 3 (3–4) | 0.207 | |
| Jugular venous pressure >/=12 mm Hg | 46% | 46% | 67% | 0.001 | |
| Symptom score (global) | 42 (30–60) | 40 (30–60) | 40 (20–60) | 0.468 | |
| Left ventricular ejection fraction, (%) (median, IQR) | 20 (15–25) | 20 (15–23) | 17 (15–20) | 0.017 | |
| Serum sodium, (mEq/L) (median, IQR) | 137 (135–139) | 137 (136–140) | 137 (134–140) | 0.100 | |
| Blood Urea Nitrogen, (mg/dL) (median, IQR) | 29 (20–49) | 26 (17–35) | 29 (19–41) | 0.034 | |
| Serum creatinine, (mg/dL) (median, IQR) | 1.4 | 1.3 | 1.3 | 0.107 | |
| Baseline Brain Natriuretic Peptide, (pg/mmol) (median, IQR) | 428 (131–869) | 463 (168–1132) | 890 (389–1500) | <0.001 | |
| Peak VO2 (median, IQR) | 9.9 | 10 | 7.9 | <0.001 | |
| Six-minute walk, (ft) (median, IQR) | 360 (0–700) | 343 (0–830) | 300 (0–632) | 0.770 | |
| Beta-blocker prior to admission | 70% | 66% | 51% | 0.005 | |
| Angiotensin Converting Enzyme inhibitors or Angiotensin Receptor Blockers prior to admission | 97% | 98% | 94% | 0.183 | |
| Digoxin prior to admission | 75% | 77% | 70% | 0.401 | |
| Spironolactone prior to admission | 48% | 46% | 40% | 0.446 | |
Table 2.
Discharge Status and Medications
| Characteristics | Weight Change | |||
|---|---|---|---|---|
| Lowest Tertile (n=128) | Middle Tertile (n=128) | Highest tertile (n=127) | P value | |
| Weight, Kg, (median, IQR) | 78 (62–97) | 83 (70–94) | 79 (68–94) | 0.379 |
| Systolic Blood Pressure, (mm Hg) | 100 (92–113) | 100 (90–110) | 99 (90–110) | 0.329 |
| Orthopnea (0–4 scale) | 2 (1–3) | 2 (1–3) | 1 (1–3) | 0.05 |
| Estimated Jugular Venous Pressure >=12 mm Hg | 9.% | 5% | 5% | 0.264 |
| Creatinine, mg/dl, (median, IQR) | 1.4 | 1.3 | 1.5 | 0.015 |
| Blood Urea Nitrogen, mg/dl, (median, IQR) | 28 (20–44) | 32 (20–43) | 34 (25–49) | 0.056 |
| Serum sodium, mEq/L, (median, IQR) | 136 (133–138) | 136 (134–138) | 135 (132–138) | 0.061 |
| Symptom score (global) | 70 (50–83) | 70 (50–80) | 70 (50–80) | 0.637 |
| Beta-blockers at discharge | 65% | 64% | 44% | 0.001 |
| Angiotensin Converting Enzyme inhibitors or Angiotensin Receptor Blockers at discharge | 88% | 95% | 88% | 0.002 |
| Digoxin at discharge | 77% | 81% | 79% | 0.747 |
| Spironolactone at discharge | 52% | 61% | 51% | 0.207 |
Results of multiple linear regression analysis to identify baseline predictors of observed in-hospital weight loss are shown in Table 3. This analysis identified baseline weight, age, baseline serum creatinine, and baseline brain natriuretic peptide as independently associated with weight loss. Diuretic dose was not a significant predictor after adjusting for other factors (Table 3). The relationship of weight loss to diuretic dose is shown in Figure 2. Although a statistically significant relationship was observed between weight loss and maximal diuretic dose (t=3.15, p=0.0018), this association was not clinically meaningful as suggested by a very low Pearson’s correlation coefficient value (R =0.028).
Table 3.
Predictors of Weight Loss
| Parameter | Estimate | Std Error | t Value | Pr > |t| |
|---|---|---|---|---|
| Treatment (Pulmonary artery catheter versus clinical assessment group) | 0.906 | 0.560 | 1.62 | 0.1070 |
| Baseline weight (per 10 kg) | 0.829 | 0.144 | 5.77 | <0.0001 |
| Age (per 10 years) | 0.686 | 0.254 | 2.71 | 0.0072 |
| Blood Urea Nitrogen (per 10 mg/dL) | 0.131 | 0.201 | 0.65 | 0.5134 |
| Creatinine (per mg/dL) | −1.826 | 0.775 | 2.35 | 0.0192 |
| Female | −1.131 | 0.695 | 1.63 | 0.1048 |
| Length initial hospitalization (days) | 0.019 | 0.028 | 0.68 | 0.4946 |
| Brain Natriuretic Peptide (logarithm) (pcg/mmol) | 0.901 | 0.212 | 4.25 | <0.0001 |
| Serum sodium (mEq/dl) | −0.099 | 0.069 | −1.43 | 0.1549 |
| Baseline diuretic use | 1.514 | 1.089 | 1.39 | 0.1658 |
| Left Ventricular Ejection Fraction (%) | −0.030 | 0.044 | −0.69 | 0.4905 |
| Diuretic dose (logarithm) (mg/day) | 0.037 | 0.228 | 0.16 | 0.8757 |
| Low systolic blood pressure (amount < 120 mm Hg) | −0.011 | 0.023 | −0.49 | 0.6214 |
| Ischemic etiology versus non-ishemic | −0.562 | 0.630 | −0.89 | 0.3732 |
Figure 2.
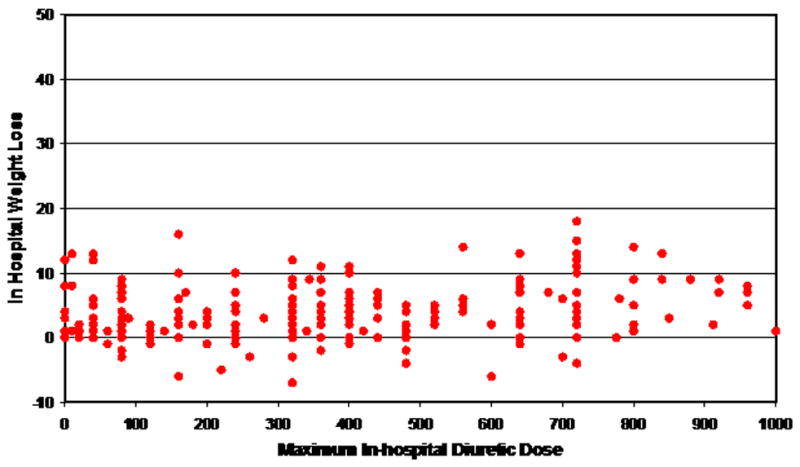
Diuretic dose (mg) and weight loss (kg)
Table 4 shows the clinical events in patients in various weight change tertiles. No significant differences were observed between weight change and any in-hospital or follow-up events. The primary end-point of the ESCAPE study, days alive out of hospital in the first 6 months, as well as other end-points (i.e. death; death or rehospitalization; and a combination of death, rehospitalization or cardiac transplantation) were all not significantly different amongst the 3 groups.
Table 4.
In-hospital and Follow-up Adverse Events
| Characteristics | Weight Change | |||
|---|---|---|---|---|
|
| ||||
| Lowest Tertile (n=128) | Middle Tertile (n=128) | Highest tertile (n=127) | P value | |
| Implantable cardioverter-defibrillator firing | 0% | 0% | 1.6% | 0.109 |
| Cardiogenic shock | 0.8% | 0.8% | 2.4% | 0.460 |
| Ischemia/angina | 2.3% | 2.1% | 2.4% | 1.000 |
| Pulmonary artery catheter infection | 1.4% | 2.4% | 0% | 0.197 |
| Stroke or transient ischemic attack | 0% | 0% | 0% | - |
| Cardiac arrest | 1.6% | 1.6% | 4.7% | 0.212 |
| Infection | 8.6% | 6.3% | 14% | 0.089 |
| Mortality and Hospitalization | ||||
| Days alive and well, median, IQR-Left ventricular assist device and transplant coded dead | 165 (120–174) | 167 (119–175) | 162 (68–172) | 0.140 |
| Days alive and well, median, IQR- Left ventricular assist device and transplant coded well | 166 (141–174) | 167 (143–175) | 163 (101–172) | 0.098 |
| 180-day mortality | 19% | 14% | 21% | 0.316 |
| Total days-Randomization to discharge, median, IQR | 5% | 5% | 6% | <0.001 |
| Composite endpoint (Death/rehospitalization/cardiac transplant (%) | 67% | 62% | 66% | 0.623 |
Cox Proportional Hazard analysis was performed to evaluate whether weight loss was significantly linked to the endpoints of days alive out of hospital in the first 6 months; death; and death or rehospitalization (table 5). This analysis failed to demonstrate a significant relationship between weight loss and any of these endpoints. We also modeled effective weight loss on the measures of quality of life, but were unable to demonstrate a relationship at one month, three months, and six months (data not shown).
Table 5.
Effect of Weight Loss on Endpoints
| Endpoint | Hazard Ratio | 95% Confidence Interval | Chi-square | P-Value |
|---|---|---|---|---|
| Primary (days well) | 0.995 | 0.975, 1.016 | 0.21 | 0.6461 |
| Death-180 days | 1.012 | 0.969, 1.057 | 0.28 | 0.5942 |
| Death/rehospitalization-180 days | 1.014 | 0.990, 1.038 | 1.26 | 0.2613 |
DISCUSSION
The most relevant observation from this analysis is that therapies used to reduce volume overload during a HF hospitalization result in significant weight loss, but that weight reduction between admission and discharge is not associated with a reduction in clinical events including days alive out of hospital, death and rehospitalization, even after adjustment for confounders. Furthermore, quality of life at 6 months was not significantly influenced by weight loss. In fact, an average of approximately 3.6 kg reduction in weight was achieved with therapies targeted to reduce volume overload and further enhanced by the use of pulmonary artery catheter in severe acute decompensated HF patients. But this decrease in weight from admission to discharge failed to influence clinical events of rehospitalization or death when adjusted for other confounders.
Two prior studies have reported the impact of weight change during a HF hospitalization and subsequent clinical outcomes. The EVEREST investigators randomly assigned patients admitted with heart failure to receive 30 mg daily of oral tolvaptan (n=2072) or placebo for 60 days on the background of standard therapies [1,2]. Tolvaptan resulted in significant reduction in mean body weight compared with placebo (1.76±1.91 kg vs. 0.97±1.84 kg; p<0.001). However, the end points of death and cardiovascular death or rehospitalization did not differ between the 2 groups (death HR 0.98, 95% CI 0.87–1.11; p=0.68; and cardiovascular death or rehospitalization HR 1.04, 95% CI 0.95–1.14 [referent placebo group]). In contrast, the UNLOAD investigators randomized patients with decompensated HF to veno-venous ultrafiltration (n=100) or standard intravenous diuretic therapy (n=100) [3]. At 48 h, ultrafiltration was associated with a greater reduction of weight (5.0 ±3.1 kg vs. 3.1±3.5 kg; p <0.001) and net fluid (4.6±2.6 liters vs. 3.3±2.6 liters; p = 0.001). However, at 90 days the ultrafiltration group had fewer patients rehospitalized for HF (18% versus 32%; p=0.037), HF rehospitalizations (0.22±0.54 vs. 0.46+0.76; p=0.022), rehospitalization days per patient (1.4±4.2 versus 3.8±8.5; p=0.022), and unscheduled office and emergency room visits (21% versus 44%; p=0.009). Mortality was similar in the 2 groups (9.6% versus 11.6%). Thus, our study findings concur with that of the much larger EVEREST trial [1,2], but contradicts the results of the smaller UNLOAD study [3]. While it is possible that the difference in findings observed in the above studies including ours may be related to differences in the sample size, the possibility that different strategies used to achieve the volume loss in above studies may have accounted for this disparate results cannot be excluded. The UNLOAD investigators hypothesized that the removal of isotonic rather than hypotonic fluid may account for the prolonged impact of ultrafiltration on recurrent hospitalizations. Preliminary evidence also suggests that the effects of hemofiltration are superior to the effect of diuretic therapy [6–9]. Particularly, following similar amount of fluid loss achieved with ultrafiltration and diuretics, there was a greater decline in norepinephrine, renin and aldosterone levels with the former strategy compared with diuretics [8].
Clinicians use daily weights as a way to evaluate therapeutic response of strategies to reduce volume overload during the hospital course for decompensated HF. It is assumed that reducing weight in these patients improves not only patients’ symptoms of congestion, but also post-discharge clinical outcomes. In fact, clinical trials have argued to use weight change as a component of composite endpoints or as an isolated primary endpoint to evaluate the advantage of novel therapeutic agents. Weight loss was the primary end-point of the UNLOAD trial. However, in this analysis, we are unable to demonstrate that a change in weight reflected improved clinical outcomes in the subsequent six months, a finding consistent with the larger EVEREST study. These results were contrary to our hypothesis and suggest that weight change as a surrogate of alteration of the volume status and prolonged improvement in clinical outcomes is inappropriate.
Despite the results of this study, weight change does have a role in the management of patients hospitalized with HF. Clearly weight loss has been shown to correlate closely with changes in volume in patients with HF. Specifically, symptoms (orthopnea scale) and signs of congestion (i.e. jugular venous distention) improved in our patients at the time of discharge with maximum improvement in those with the greatest change in weight. Similarly, the EVEREST investigators showed that composite global clinical status and dyspnea improved in the tolvaptan group that had more weight loss [2]. Most clinicians would agree with the clinical observation that there is a correlation between improvement in congestion and exercise tolerance. Conversely, it is well recognized that gain in weight precedes manifestation of volume overload and predates rehospitalization for decompesated HF. Chaudhry et al, in a nested case-control study, demonstrated that increases in body weight are associated with hospitalization for HF and begin at least 1 week before admission [10]. Furthermore, they demonstrated that the rates of HF hospitalization progressively increased with greater weight gain relative to baseline (mean increases of >2 and ≤5 pounds, >5 and ≤10 pounds, and >10 pounds associated with adjusted ORs for HF hospitalization of 2.77 [95% CI 1.13–6.80], 4.46 [95% CI 1.45–13.75], and 7.65 [95% CI 2.22–26.39], respectively [referent ≤2 pounds]). Thus, our data when interpreted in conjunction with all information that relates weight to outcomes in patients with HF, suggests that change in weight more than likely reflects change in volume status and symptoms over short-term in these patients, but correlates poorly with short- and long-term clinical outcomes.
Our analysis was retrospective and is subject to missing information and other confounding due to lack of information collected. Specifically, we did not have any information on compliance with medications, diet, fluid restriction, modification of diuretic dose in accordance with changes in weight and other life style changes factors that are significantly linked to HF outcomes. Inference regarding causation should also be made with caution. Our study sample consisted of only class IV HF patients and its findings need to be validated among patients with lesser degree of HF. We also did not have information on the proportion of weight loss relative to the gain from dry body weight/baseline weight. A greater weight loss is targeted and/or expected for patients with highest weight gain relative to their baseline weight. Whether this parameter is a better surrogate than that used currently (weight at admission minus weight at discharge) for subsequent outcomes in patients with HF need to be evaluated in future studies.
Acknowledgments
The authors appreciate Dr. Lynne Stevenson’s critical review of the manuscript with regard to the limitations of weight change as a predictor of outcomes after heart failure hospitalization.
Source of funding: National Heart Lung and Blood Institute (NO1-HV-98177) and Duke Clinical Research Institute, Durham, NC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Konstam MA, Gheorghiade M, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Konstam MA, Burnett JC, Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C. Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA. 2007;297:1332–1343. doi: 10.1001/jama.297.12.1332. [DOI] [PubMed] [Google Scholar]
- 3.Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, Jaski BE, Fang JC, Feller ED, Haas GJ, Anderson AS, Schollmeyer MP, Sobotka PA UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49:675–683. doi: 10.1016/j.jacc.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 4.The ESCAPE Investigators and ESCAPE Study Coordinators. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 5.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) American College of Cardiology Web Site. [Accessed February 10, 2008]. Available at: http://www.acc.org/clinical/guidelines/failure//index.pdf.
- 6.Cipolla CM, Grazi S, Rimondini A, Susini G, Guazzi M, Della Bella P, Guazzi MD. Changes in circulating norepinephrine with hemofiltration in advanced congestive heart failure. Am J Cardiol. 1990;66:987–994. doi: 10.1016/0002-9149(90)90938-w. [DOI] [PubMed] [Google Scholar]
- 7.Agostino P, Marenzi G, Lauri G, Perego G, Schianni M, Sganzerla P, Gauzzi M. Sustained improvement in functional capacity after removal of body fluid with isolated ultrafiltration in chronic cardiac insufficiency: Failure of furosemide to provide the same result. Am J Med. 1994;96:191–199. doi: 10.1016/0002-9343(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 8.Agostino PG, Marenzi G, Pepi M, Doria E, Salvioni A, Perego G, Lauri G, Giraldi F, Grazi S, Guazzi MD. Isolated ultrafiltration in moderate congestive heart failure. J Am Coll Cardiol. 1993;21:424–431. doi: 10.1016/0735-1097(93)90685-t. [DOI] [PubMed] [Google Scholar]
- 9.Forslund T, Ridderwald F, Fauchald P, Torvik D, Fyhrquist F, Simons S. Hormonal changes in patients with severe chronic congestive heart failure treated by ultrafiltration. Nephrol Dial Transplant. 1992;7:306–310. doi: 10.1093/oxfordjournals.ndt.a092133. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116:1549–1554. doi: 10.1161/CIRCULATIONAHA.107.690768. [DOI] [PMC free article] [PubMed] [Google Scholar]


