Abstract
Objective
Because altered serotonin (5-HT) function appears to persist after recovery from bulimia nervosa (RBN), we investigated the 5-HT1A receptor, which could contribute to regulation of appetite, mood, impulse control, or the response to antidepressants.
Method
Thirteen RBN individuals were compared to 21 healthy control women (CW) using positron emission tomography and [carbonyl-11C]WAY100635 ([11C]WAY).
Results
RBN had a 23–34% elevation of [11C]WAY binding potential (BP)P in subgenual cingulate, mesial temporal, and parietal regions after adjustments for multiple comparisons. For CW, [11C]WAY BPP was related negatively to novelty seeking, whereas for RBN, [11C]WAY BPP was related positively to harm avoidance and negatively related to sensation seeking.
Discussion
Alterations of 5-HT1A receptor function may provide new insight into efficacy of 5-HT medication in BN, as well as symptoms such as the ability to inhibit or self-control the expression of behaviors related to stimulus seeking, aggression, and impulsivity.
Keywords: bulimia nervosa, 5-HT1A receptor, positron emission tomography, behavioral inhibition, subgenual cingulate, mesial temporal cortex
Introduction
Bulimia nervosa (BN) is a disorder of unknown etiology that tends to occur in adolescent and young adult women.1 Individuals with this illness suffer from cycles of binge eating, usually followed by self-induced vomiting or other purging behaviors, as well as disturbances of mood and impulse control.
Considerable physiologic and pharmacologic data show that disturbances of serotonin (5-HT) function occur in individuals with eating disorders.2,3 The 5-HT1A receptor is of interest in eating disorders because it has been implicated in the modulation4,5 of mood, impulse control, and appetite as well as the response to antidepressant medication.6 Positron emission tomography (PET) and the ligand [carbonyl-11C]WAY100635 ([11C]WAY) can be used to investigate the binding potential (BP) of this receptor. The 5-HT1A autoreceptor is located presynaptically on 5-HT somatodendritic cell bodies in the raphe nuclei, where it functions to decrease 5-HT neurotransmission.4 High densities of postsynaptic 5-HT1A exist in the hippocampus, septum, amygdala, and entorhinal and frontal cortices, where they serve to mediate the effects of released 5-HT.
Despite differences in BP measurements [for a definition of BPs see consensus nomenclature for in vivo imaging for reversibly binding radioligands7] and radioligands used, studies have tended to show elevated binding of the 5-HT1A receptor2,3 in individuals with eating disorders, and some relationship between 5-HT1A receptor binding and measures of harm avoidance (HA). Specifically, individuals ill with BN had elevated [11C]WAY BPP,8 previous studies from our group found9 that women ill with anorexia nervosa (AN) had a highly significant (30–70%) increase in [11C]WAY BPP, whether they were restrictive or bulimic-type AN. Finally, our group10 found that women recovered from bulimic-type AN had a persistent 22% to 43% increase in [11C]WAY BPP. While women recovered from restrictive-type AN had normal [11C]WAY BPP,10 [11C]WAY BPP values were markedly elevated in some participants and were most recently found to be significantly increased in lean and recovered restricting-type AN individuals (using the radioligand [18F]MPPF and BPND)11
This is the first study to investigate 5-HT1A receptor binding in recovered BN (RBN) individuals who have never had AN. It is not known whether extremes of dietary intake, or other factors related to the ill state, are a cause or consequence of abnormal 5-HT function. To avoid these possible confounding effects, we studied RBN, and compared them to age- and weight-matched control women (CW). More than 50% of individuals who have BN recover (i.e., their binge and purge symptoms disappear). Nonetheless, these individuals often continue to have persistent dysphoric mood, obsessional thoughts, and body image concerns that are modest compared to the ill state.12 Such behavioral symptoms are present in childhood, before the onset of BN. Thus, they may reflect traits that contribute to a vulnerability to develop BN. It should be emphasized that other 5-HT system abnormalities in recovered BN are profound, including approximately a 50% elevation of cerebrospinal fluid hydroxyindoleacetic acid (CSF 5-HIAA) (the major metabolite of brain 5-HT),13 reduced 5-HT2A receptor binding in frontal regions,14 altered mood response to 5-HT agents,13,15 and evidence of reduced 5-HT transporter function in RBN16 and ILL BN.17 Together, these findings support the hypothesis that a substantial dysregulation of serotonergic neuronal circuits occurs in BN.
Method
Participants
We studied 13 RBN and 21 healthy CW recruited through local advertisements. None of the BN subjects had a history of AN. To be considered “recovered,” individuals had to have met the following criteria for the previous year: (1) maintain a weight above 90% of average body weight; (2) have regular menstrual cycles; (3) have not binged, purged, restricted food intake or exercised excessively; (4) not used psychoactive medications such as antidepressants; (5) no current alcohol or drug abuse/dependence. CW had no history of any psychiatric, serious medical or neurological illness. This study was conducted according to local institutional review board regulations and all subjects gave written informed consent. The PET imaging was performed during the first 10 days of the follicular phase of the menstrual cycle for all participants as the potential effect of different phases of the menstrual cycle on 5-HT1A binding in some regions, e.g. the dorsal raphe, cannot be fully excluded.18 Methods are described in detail elsewhere.10 Mean BPP values for 21 CW were previously reported.9,10 Data on the 13 RBN participants have not been reported previously.
Behavioral Assessments
All participants underwent a face-to-face interview with a psychiatrist using the Structured Clinical Interview for DSM-IV Axis I Disorders19 to assess lifetime prevalence of Axis I psychiatric disorders. Current psychopathology and personality traits (Table 1) were assessed with a battery of standardized instruments designed to characterize temperament (Temperament and Character Inventory, TCI),20 mood (Beck Depression Inventory, BDI),21 anxiety (Spielberger State-Trait Anxiety Inventory, STAI),22 and impulse self-control (Barratt Impulsiveness Scale, BIS).23 The Novelty Seeking, Harm Avoidance, and Self-Transcendence scales were used from the TCI. The value for one RBN who had a BIS self-control score over two standard deviations (SDs) below the mean was removed from the correlations.
TABLE 1.
Group comparisons of demographic variables and representational assessment data
| CW (N = 21)
|
RBN (N = 13)
|
CW vs. RBN
|
|||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Exact Sig. | |
| Age (years) | 26.2 | 6.7 | 24.7 | 5.8 | 0.60 |
| Current BMI (kg/m2) | 22.2 | 1.8 | 23.1 | 2.2 | 0.23 |
| BN onset (years) | — | — | 16.5 | 3.4 | — |
| Duration of recovery (months) | — | — | 24.2 | 17.8 | — |
| Estradiol (μmol/l) | 53.9 | 57.8 | 72.7 | 88.4 | 0.92 |
| Beta-hydroxy-butyrate (BHBA) (mmol/l) | 0.07 | 0.04 | 0.08 | 0.02 | 0.37 |
| Depression (BDI) | 1.1 | 1.4 | 6.2 | 7.3 | 0.0002 |
| Trait anxiety (STAI) | 26.9 | 4.8 | 44.4 | 10.1 | <0.0001 |
| Novelty seeking (TCI) | 21.4a | 4.8 | 22.7 | 7.4 | 0.39 |
| Harm avoidance (TCI) | 10.0 | 3.3 | 16.2 | 6.4 | 0.0008 |
| Self-transcendence (TCI) | 13.5 | 5.1 | 17.1 | 6.5 | 0.14 |
| Self-control (BIS) | 85.5 | 18.5 | 102.7 | 22.2 | 0.02 |
Notes: BN, bulimia nervosa; CW, healthy control women; BDI, Beck Depression Inventory; STAI, Spielberger State-Trait Anxiety Inventory; TCI, Temperament and Character Inventory; BIS, Barratt Impulsiveness Scale.
Indicates that one CW did not have a Novelty Seeking value available.
Image Acquisition
Magnetic resonance (MR) imaging and PET imaging were performed as previously described for arterial-based dynamic imaging of [11C]WAY binding to 5-HT1A receptors.24 [11C]WAY was synthesized according to established methods.25 A slow bolus intravenous injection of 13.9 ± 1.9 mCi (range: 9.2–15.9; RBN: 14.2 + 1.7; CW: 13.7 + 2.9; p = 0.5) high-specific activity [11C]WAY was administered and dynamic three-dimensional emission scanning with arterial blood sampling (34 sample input function) was performed over 60 min (a longer 90 min acquisition was collected in 9 of 13 RBN and 14 of 21 CW participants). Studies done earlier used 60 min acquisition. Later studies used 90 min acquisition to verify stability in the BPP measures in areas such as the raphe.26 A metabolite corrected input function was determined, as previously described.24 The temporal stability of the outcome measures was examined in the subset of participants for which a full 90 min emission data set was available. High correlations were observed between the 60-and 90-min datasets for both the Logan cerebellar distribution volume (VND) and regional Logan BPP measures, respectively (CW: r = .95–.99; RBN: r = .96–.99). The bias across regions of interest (ROIs) between the two measures was similar for CW (9.4% ± 4.1%) and RBN participants (8.4% ± 4.2%; p = .71). This observation supports the validity of the results for the 60 min interval.
PET Data Processing
The ROIs were hand-drawn on the coregistered MR images and applied to the dynamic PET data to generate time-activity curves. ROIs have been described previously.10,24 Briefly, ROIs included the cerebellum (left and right hemispheres) as a reference region, and prefrontal, lateral orbital frontal, medial orbital frontal, parietal, mesial temporal, subgenual cingulate cortical regions, and the dorsal raphe nucleus. Because the raphe nuclei cannot be delineated on MR, this ROI was directly identified on the PET image27 using circular fixed 6 mm radius ROIs (for all participants) placed over the area of highest radioactivity. The inferior border of the dorsal raphe nucleus was identified by the interpeduncular cistern. To reduce noise, right and left regions were combined.25
We denote here the outcome variables using the recently issued consensus nomenclature for in vivo imaging for reversibly binding radioligands.7 For the arterial-based kinetic analyses, regional [11C]WAY distribution volume (VT) was determined using both the Logan graphical method28 and three-compartment model (2-tissue compartments)26 that included a vascular volume term. A modified Logan analysis that applied generalized linear least squares smoothing to the data prior to analysis29 was used as this method effectively reduced noise-induced bias in the Logan VT as previously described for other PET radiotracers.28 The Logan analysis was performed using integrated PET data intervals that were each determined over 0 – Ti min after injection, where Ti ranged from 25 to 60 or 90 min, with 7 or 10 data points used for the analyses of the 60 and 90 min data sets, respectively. Our main outcome measure for this study was BP. The BP measure was determined as: BPP = VT – VND.26 This BPP is dependent on plasma protein binding (fP) rather than tissue-free fraction (fND).26 As a result, plasma protein binding was measured in all participants to determine the extent to which a group difference in [11C]WAY BPP could be influenced by this factor. For comparison purposes, we also determined BPND as: BPND = VT/VND − 1.
It is acknowledged that [11C]WAY is a radiotracer with low nondisplaceable tissue uptake and quantification of the cerebellar VND can be problematic. Complicating factors include technical issues related to PET imaging (i.e., scatter, spillover from occipital radioactivity), low levels of receptor binding in vermis, and variable sensitivity at the ends of the 3D PET field-of-view.26,30–32 In this work, and in our previous [11C]WAY studies, we carefully sought to minimize such factors through various means that included implementation of an improved scatter correction algorithm, standard subject positioning with the cerebellum within the central 7 cm of the field-of-view and cerebellar ROI placement that does not include vermis and ensures minimal contamination from occipital uptake. Parsey et al.33 utilized cerebellar white matter to approximate the [11C]WAY reference region kinetics. We also examined the use of cerebellar white matter as reference for [11C]WAY and other PET neuroreceptor binding radiotracers (data not shown). The cerebellar white matter was not used because it did not appear to exhibit in vivo kinetics consistent with nondisplaceable uptake in receptor-rich areas.
Statistical Analysis
Standard statistical software packages (SAS Version 8.2, StatExact 4.0, and SPSS 14.0) were used for all analyses except the multivariate profile analysis described later. Comparisons between RBN and CW were made using Wilcoxon rank-sum tests. Exact p-values were computed due to the small sample sizes. Standard regression diagnostics were used to assess the sensitivity of the model to outlying and highly influential observations in the data set. Pearson correlation coefficients were also computed and exact significance levels based on Monte Carlo methods are reported. All values are expressed as mean ± SD. We applied a Sidak correction to control for Type I errors in the analysis of group differences in ROIs for BPP and BPND (Table 2). This type of correction is more appropriate than other methods (e.g., Bonferroni) when the multiple tests performed are not independent.34 Otherwise, we assumed a p value of p < .05 for declaring significance.
TABLE 2.
Differences between groups using Logan graphical method and compartmental modeling for BPP (A) and BPND (B)
| Region of Interest | Logan [11C]WAY BPP
|
Compartmental Modeling [11C]WAY BPP
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CW (N = 21) | RBN (N = 13) | Exact. Sig | % Diff. | SIDAKa p-Value | CW (N = 20)b | RBN (N = 12)b | Exact. Sig | % Diff. | SIDAKa p-Value | |
| A | ||||||||||
| Prefrontal cortex | 4.16 (0.99) | 5.14 (1.03) | 0.018 | 24 | .119 | 4.08 (0.97) | 5.04 (1.11) | 0.024 | 24 | .156 |
| Lat. orbital front. | 3.83 (0.83) | 4.72 (1.19) | 0.030 | 23 | .192 | 3.68 (0.80) | 4.62 (1.34) | 0.020 | 26 | .132 |
| Med. orbit. frontal | 4.67 (1.25) | 6.07 (1.17) | 0.008 | 30 | .055 | 4.53 (1.32) | 5.87 (1.37) | 0.032 | 30 | .204 |
| Subgenual cingulate | 4.70 (1.22) | 6.00 (1.16) | 0.007 | 28 | .048 | 4.61 (1.27) | 5.82 (1.33) | 0.017 | 26 | .113 |
| Mesial temporal cortex | 7.05 (1.89) | 9.42 (1.73) | 0.001 | 34 | .007 | 6.88 (2.04) | 9.00 (2.02) | 0.012 | 31 | .081 |
| Parietal cortex | 3.91 (0.95) | 5.09 (1.27) | 0.007 | 30 | .048 | 3.83 (0.94) | 4.97 (1.37) | 0.024 | 30 | .156 |
| Dorsal raphe | 2.14 (0.56) | 2.67 (0.81) | 0.059 | 25 | .347 | 2.12 (0.69) | 2.63 (1.07) | 0.157 | 24 | .697 |
| Cerebellar VND | 0.72 (0.13) | 0.85 (0.17) | 0.030 | 18 | n/a | 0.53 (0.11) | 0.85 (0.19) | <0.001 | 60 | n/a |
| Logan [11C]WAY BPND
|
Compartmental Modeling [11C]WAY BPND
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CW (N = 21) | RBN (N = 13) | Exact. Sig | % Diff. | SIDAKa p-Value | CW (N = 20)b | RBN (N = 12)b | Exact. Sig | % Diff. | SIDAKa p-Value | |
| B | ||||||||||
| Prefrontal cortex | 5.73 (0.76) | 6.08 (0.59) | 0.104 | 6 | .536 | 7.65 (1.23) | 8.28 (1.16) | 0.182 | 8 | .755 |
| Lat. orbital front. | 5.13 (0.74) | 5.58 (0.89) | 0.246 | 9 | .861 | 6.69 (1.19) | 7.55 (1.30) | 0.116 | 13 | .578 |
| Med. orbit. frontal | 6.27 (1.16) | 7.23 (1.13) | 0.050 | 15 | .302 | 8.40 (1.82) | 9.68 (1.68) | 0.053 | 15 | .317 |
| Subgenual cingulate | 6.47 (1.12) | 7.19 (1.43) | 0.181 | 11 | .753 | 8.65 (1.91) | 9.64 (1.93) | 0.136 | 11 | .640 |
| Mesial temporal cortex | 9.73 (1.97) | 11.25 (1.92) | 0.039 | 17 | .243 | 12.81 (2.79) | 14.82 (2.64) | 0.053 | 16 | .317 |
| Parietal cortex | 5.39 (0.78) | 5.97 (0.74) | 0.060 | 11 | .352 | 7.17 (1.13) | 8.13 (1.56) | 0.070 | 13 | .398 |
| Dorsal raphe | 2.97 (0.59) | 3.10 (0.73) | 0.863 | 4 | .999 | 3.95 (0.94) | 4.27 (1.69) | 0.730 | 8 | .999 |
| Cerebellar VND | 0.72 (0.13) | 0.85 (0.17) | 0.030 | 18 | n/a | 0.53 (0.11) | 0.85 (0.19) | <0.001 | 60 | n/a |
Sidak post-hoc correction for multiple testing was applied within the context of each method.
Indicates deletion of a subject whose scan had a k4 for CER <0.
Multivariate distance matrix regression (MDMR)35 was used to examine the extent to which similarity in 5-HT1A receptor binding profiles was related to several predictor variables of interest. MDMR is an analytic method, which involves the construction of a dissimilarity or distance matrix that, in this case, reflects the correlation of study participants’ profiles with respect to BP values over all of the brain regions sampled. Predictor variables, including age, BMI, and measures of behavior were then tested for association with variation in the BPP distance matrix using the statistical program DISTLM forward.36 Independent variables were tested both individually and in a forward stepwise manner, with p-values computed via permutation analysis. The independent variables selected are based on the highest cumulative proportion of variance in BPP distance, explained by the inclusion of an additional variable in the regression model.
Finally, a repeated-measures ANOVA was used to explore potential group differences in radiolabeled metabolites of [11C]WAY. Because sphericity tests failed, p-values for the within-subject effects (time and the interaction of time X group) were adjusted using the conservative Lower Bound estimator. For the [11C]WAY metabolites, only 9 of the 13 RBN and 14 of the 21 CW participants had 90-min data, therefore, the model was run both with and without the 90-min measurement.
Results
Demographic and Clinical Variables
RBN and CW participants were similar in age, body mass index (BMI, kg/m2), plasma estradiol, and β-hydroxy-butyrate (BHBA) values. RBN women had elevated trait anxiety (STAI), depression (BDI), and Harm Avoidance (TCI) compared to CW (Table 1).
Group Comparison of [11C]WAY BPP
Using a Logan analysis (Table 2 and Fig. 1) the RBN women showed significant elevations of post-synaptic receptor BPP, and a trend for increased autoreceptor BPP compared to CW. After a conservative adjustment for multiple comparisons, findings persisted for the subgenual cingulate, mesial temporal, and parietal regions. Group differences for the compartmental analysis were similar, but less robust (Table 2). The Logan cerebellar VND values were higher in the RBN compared to the CW (0.85 ± 0.17 vs. 0.72 ± 0.13; p = .03). This difference was similar for the cerebellar VND from compartmental modeling (0.85 ± 0.19 vs. 0.53 ± 0.11; p < .001). There was no difference in regional [11C]WAY BPP values for RBN participants with a diagnosis of major depression (n = 8) or obsessive-compulsive disorder (n = 5).
FIGURE 1.
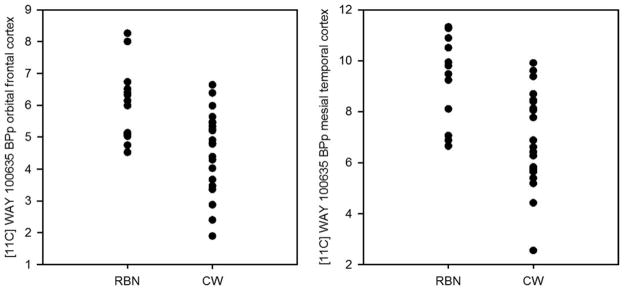
Representational scatter plots for logan [11C] WAY 100635 BPP in the medial orbital frontal cortex (left graph) and mesial temporal cortex (right graph) in RBN and CW.
Group Comparison of [11C]WAY BPND
Similar to BPP, the RBN women (Table 2) showed significant elevations of [11C]WAY BPND in the mesial temporal cortex and a trend for increased [11C]WAY BPND in prefrontal and parietal regions compared to CW (Logan analysis). However, after a conservative adjustment for multiple comparisons, findings did not persist. Group differences for the compartmental analysis were similar, but less robust (Table 2).
Relationship of [11C]WAY BPP to Behavior
RBN participants showed significant positive relationships between Logan regional [11C]WAY BPP and HA for the lateral orbital frontal (r = .76, p = .002), orbital frontal (r = .57, p = .04), parietal (r = .68, p =.01), and trends for the prefrontal regions (r = .53, p = .06). In addition, the BIS sensation seeking scale23 was negatively related to [11C]WAY BPP for the lateral orbital frontal (r = −.67, p = .02), prefrontal (r = −.61, p = .02), mesial temporal (r = −.63, p = .02) and parietal (r = −.58, p = .02) BPP, with trends for the orbital frontal BPP (r = −.51, p = .07). In comparison, for CW, Logan regional [11C]WAY BPP was significantly negatively related to novelty seeking20 for all ROIs surveyed (Fig. 2). These findings were the most robust for the pre-frontal cortex (r = −.73, p = .0003), orbital frontal cortex (r = −.61, p = .005), and parietal cortex (r = −.65, p = .002), but also were significant for the lateral orbital frontal cortex (r = −.55, p = .01), subgenual cortex (r = −.49, p = .03), mesial temporal cortex (r = −.52, p = .02), and dorsal raphe (r = −.55, p = .03). No relationship was found between regional [11C]WAY BPP or other clinical variables in Table 1 for either CW or RBN.
FIGURE 2.
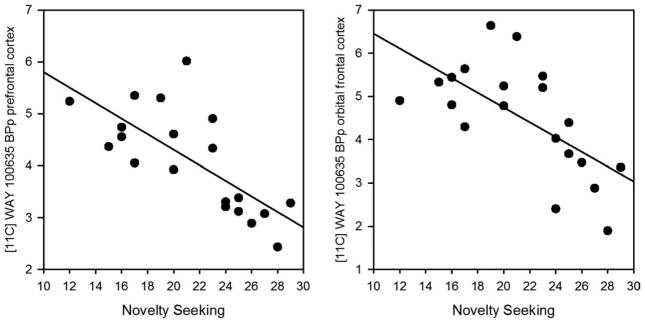
Correlation between Novelty Seeking (NS) and logan [11C] WAY 100635 BPP for prefrontal cortex (r = −.73, p = .0003, n = 20*) and orbital frontal cortex (r = −.61, p = .005, n = 20*) in CW; * indicates that one CW did not have NS value available.
MDMR analysis (Table 3) revealed that for the RBN group, HA was a significant predictor (p = .030) of similarity for Logan regional [11C]WAY BPP profiles across all of the brain regions sampled. Furthermore, HA accounted for 29.5% of the variance in BP profile similarity for this group. For the CW, novelty seeking was a significant predictor (p = .005) of similarity in [11C]WAY BPP profiles and accounted for 28.6% of the variance in profile similarity for this group.
TABLE 3.
| Variableb | SS (Trace)c | Pseudo-Fc | pd | PROPe | Cumulativef |
|---|---|---|---|---|---|
| BN | |||||
| Harm avoidance | 24.8015 | 4.6085 | .0300g | 0.2953 | 0.2953 |
| BMI | 11.2340 | 2.3421 | .1039 | 0.1337 | 0.4290 |
| STAI trait anxiety | 5.7743 | 1.2318 | .2847 | 0.0687 | 0.4977 |
| Sensation seeking | 3.0812 | 0.6303 | .5005 | 0.0367 | 0.5344 |
| Novelty seeking | 2.8643 | 0.5532 | .5624 | 0.0341 | 0.5685 |
| Self control | 5.0555 | 0.9725 | .3247 | 0.0602 | 0.6287 |
| Self transcendence | 2.3243 | 0.4026 | .6643 | 0.0277 | 0.6564 |
| Age | 0.7060 | 0.1003 | .9231 | 0.0084 | 0.6648 |
| Control women | |||||
| Novelty seeking | 39.9659 | 7.5909 | .0050g | 0.2855 | 0.2855 |
| Sensation seeking | 12.6512 | 2.6060 | .0969 | 0.0904 | 0.3758 |
| STAI trait anxiety | 9.6369 | 2.1072 | .1429 | 0.0688 | 0.4447 |
| Harm avoidance | 5.6214 | 1.2470 | .2597 | 0.0402 | 0.4848 |
| Age | 3.2416 | 0.7059 | .4775 | 0.2323 | 0.5080 |
| Self-transcendence | 3.0496 | 0.6485 | .4905 | 0.0218 | 0.5298 |
| Self-control | 2.5960 | 0.5337 | .5724 | 0.0185 | 0.5483 |
| BMI | 1.9940 | 0.3907 | .7173 | 0.0142 | 0.5625 |
Results should be interpreted as in multiple regression contexts whereby each variable in the model is effectively “accounted for” in the assessment of the others.
Each variable assessed in relation to the similarity matrix.
Test statistics relating each variable to variation in the similarity matrix.
The p-value derived from permutation tests.
The proportion of variation in the similarity matrix explained by a variable.
The cumulative variation explained.
Denotes p <0.05.
Plasma Data
The repeated-measures analysis of radiolabeled metabolites of [11C]WAY showed significantly higher values in RBN relative to CW at time points 1 min (0.94 ± 0.02 vs. 0.92 + 0.04; p = .021), 2.25 min (0.65 ± 0.14 vs. 0.54 ± 0.13; p = .021), and 5 min (0.17 ± 0.05 vs. 0.14 ± 0.03; p = .011), but similar values were found at time points 10, 30, 45, and 60 min. Both the group (F [1, 32] = 5.88, p = .021) and the group × time interaction (F [1, 32] = 5.58, p = .024) effects were significant in this model. When the data were analyzed for the subset of 9 RBN and 14 CW for whom there were 90-min data available, trends in the data remained similar, but the group (F [1, 21] = 2.80, p = .109) and the group × time interaction (F [1, 21] = 2.24, p = .149) effects no longer reached significance, which is likely due to decreased statistical power. Significant differences in plasma free fraction (fP) were found between RBN (fP = 0.14 ± 0.05, n = 10) and CW (fP = 0.09 ± 0.03, n = 17) (p = .008) in which these data were available.
Discussion
RBN individuals showed a significant 23–34% elevation of pre- and post-synaptic receptor [11C]WAY BPP compared to CW for subgenual cingulate, mesial temporal, and parietal cortices. Regional binding values for the RBN participants were also higher than in CW using the BPND measure (although not statistically significant). For CW, [11C]WAY BPP was related negatively to novelty seeking, whereas for RBN, [11C]WAY BPP was related positively to HA and negatively to sensation seeking. Moreover, novelty seeking and HA accounted for approximately 30% of the variance for [11C]WAY BPP in CW and RBN, respectively.
This is the first study to show that increased [11C]WAY BPP also occurs in women recovered from BN with no history of AN. These results supplement previous evidence showing that elevated binding of the 5-HT1A receptor occurs in individuals with eating disorders. Several interpretations are possible, which will require further testing to confirm. First, in recovered state, increased binding of the 5-HT1A receptor may be associated specifically with RBN, whether or not they have had a history of AN. RBN have been shown to have elevated cerebrospinal fluid concentrations of hydroxyindoleacetic acid (CSF 5-HIAA)13 and evidence of reduced 5-HT transporter function,16 consistent with increased extracellular 5-HT concentrations. Theoretically, increased postsynaptic 5-HT1A receptor activity could be compensatory means of counteracting increased extracellular 5-HT.37,38 Second, elevated 5-HT1A receptor binding may be further exaggerated in the ill state of both AN and BN individuals, suggesting a possible trait phenomenon that is exacerbated by nutritional abnormalities.
These data may provide insight into pharmaceutical treatments for BN. Although numerous controlled trials have shown some efficacy for a variety of antidepressant medications in BN, relatively few individuals achieve abstinence on medication, as most continue to binge and purge. For example, a large-scale controlled trial of fluoxetine, which showed that a relatively high dose of 60 mg/day was superior to 20 mg/day for BN,39 had a 1-year remission rate of only 17.7%. Many participants remained symptomatic on medication and there was a worsening on all measures of efficacy over time. This result is consistent with other clinical observations40 that suggest limited improvement and considerable relapse with long-term antidepressant treatment in BN. An important mechanism thought to contribute to the action of SSRIs is the desensitization of the somatodendritic 5-HT1A autoreceptor on the raphe neurons.6 Highly elevated 5-HT1A receptor activity in BN raises the question of whether BN individuals have difficulty in achieving SSRI-induced 5-HT1A autoreceptor desensitization. Such a difficulty could explain the need for higher doses of fluoxetine as well as partial response to drugs. Perhaps higher doses of SSRIs or the addition of 5-HT1A specific agents may prove useful in BN.
The RBN individuals continued to have mild to moderate levels of depressive and anxiety symptoms. However, while individuals with eating disorders tend to have elevated [11C]WAY BPP, reduced binding of 5-HT1A receptor ligands has been found in most [for review see Refs. 41 and 42], but not all studies of major depression (BPF).43 In addition, reduced binding of 5-HT1A receptor ligands has been found in social phobia (BPND)44 and panic disorder ([18F]FCWAY VT and BPND45 and [11C]WAY BPND46). Thus, it can be argued that these disorders may differ in etiology.
For CW, [11C]WAY BPP was diminished in those who were high in novelty seeking. For RBN, [11C]WAY BPP increased in relationship to HA and diminished in those who were sensation seeking. Moreover, novelty seeking and HA accounted for approximately 30% of the variance for the [11C]WAY BPP in CW and RBN, respectively. The instruments used to assess behavior in humans tend to assess complex phenomena that are likely to be a composite of many traits, therefore confounding the understanding of how behaviors might be associated with a 5-HT receptor. For example, HA measures anxiety and behavioral inhibition, whereas novelty seeking measures exploration and impulsivity.20 Similarly, assessment of behavior in animals is complex. Thus, while considerable studies in animals associate 5-HT1A receptor function with anxiety, most tests of anxiety in rodents are based in part on the approach/avoidance conflict between the innate tendency of an animal to explore a novel place and the tendency to avoid novel stimuli or environments.5
Studies of male and female healthy controls using PET and [11C]WAY BP have found negative relationships in frontal, temporal, and cingulate regions with self-transcendence (BPND),47 and negative (BPF)48 as well as positive (BPND)49 correlations with aggression. In individuals with major depression, [11C]WAY BPF was correlated negatively with somatic anxiety and positively with psychic anxiety in cingulate and frontal regions.50 The BPND measure in 5-HT1A receptor binding studies has been associated negatively with the neuroticism facet of anxiety on the NEO17 in healthy controls. Similar to RBN, [11C]WAY BPP was associated with HA in recovered restrictive-type AN.10
There is an extensive literature associating the serotonergic system and 5-HT1A receptor activity with fundamental aspects of behavioral inhibition.51 Reduced CSF 5-HIAA levels are associated with increased impulsivity and aggression in humans and non-human primates, whereas increased CSF 5-HIAA levels are related to behavioral inhibition.52 Activation of brainstem 5-HT1A receptors inhibits stress-induced sympathetic activity and fight-or-flight behavioral responses.53 5-HT1A receptors modulate impulse control through effects on catecholamine systems54 and blunted 5-HT1A receptor number or function is associated with increased aggression.55 Taken together, these data raise the possibility that 5-HT1A receptor may contribute to the emergent ability to inhibit or self-control the expression of a number of behaviors related to stimulus seeking, anxiety, aggression and impulsivity. Within the context of eating behavior, it is important to note that both AN and BN tend to restrict their eating and lose normal meal patterns56 and show high harm avoidance, a measure of anxiety and inhibition. However, AN can maintain this inhibition continuously, whereas BN have periodic disinhibition and loss of self-control. 5-HT1A functional activity reflects one part of a complex system of 14 or more receptors and many other components that modulate metabolism, firing rate, neuronal cascades, etc. For example, we find recovered restrictive-type and bulimic-type AN have differences in 5-HTT function57 which might explain why these subtypes have differences in impulse control or inhibition. Thus, an understanding of the complexities of 5-HT function will likely be needed to truly understand the relationship of this system to behavior.
Limitation
The interpretation of the findings in this study is complicated because of observed group differences in nonspecific factors (e.g., cerebellar VND and plasma protein binding, 1/fP). The receptor binding measures reflect both the concentration of available receptors (Bavail) and the dissociation constant (KD), as well as nonspecific factors (i.e., BPP = VT – VND = fPBavail/KD and BPND = VT/VND − 1 = fNDBavail/KD). Therefore, we performed a secondary examination of the data to facilitate interpretation of the BP differences.
First, the cerebellar VND measure was greater for RBN participants than controls whether determined using a Logan analysis or two-tissue compartment model (i.e., VND = K1/k2 × (1 + k3/k4)). The greater RBN cerebellar VND resulted more from the (K1/k2 × k3/k4) term than the K1/k2 term that were respectively, 0.42 and 0.44, as compared to the values for control participants of 0.15 and 0.38, respectively. The greater difference observed for the compartmental VND—relative to the Logan VND—may result, in part, from variability in the kinetic parameter estimates determined in an area of low radioactivity concentration. Despite the greater RBN VND measures, significantly greater 5-HT1A receptor binding was observed in multiple brain areas whether BP was determined through VND subtraction or ratio (using Logan or compartmental analysis) and the greater RBN VND measures would serve to minimize increases in either BPP or BPND for the RBN group.
Second, the average fP value for CW (i.e., 0.09) was consistent with [11C]WAY fP values recently reported for controls,58,59 while the average fP value for RBN participants (i.e., 0.14) was about 56% greater (with inversely lower protein binding of 36%) relative to CW. This fP difference was statistically significant despite large measurement variation (~ 35%) for both groups. The regional VT values (Logan or compartmental analysis) for RBN participants were also significantly greater than those for CW across all regions, with the exception of the dorsal raphe. This observation could be consistent with greater radiotracer availability as a result of lower plasma protein binding in the RBN participants, but the magnitude of the VT increases (relative to CW) varied across regions (21–32%) rather than being on the order of 56% (i.e., the group difference in fP).
Correction of the BPP measure for fP yields BPF = Bavail/KD. A group comparison of the BPF measures yielded a reversal of the BPP difference, reflecting BPF values of CW greater than those of RBN (data not shown). This difference was not statistically significant when an individual fP correction was performed on a subject-by-subject basis, but was significant when the group fP average values were applied. Confidence in the correction was dampened by the level of variation in fP and the fact that fP values were not available for all participants. Potential associations between the fP values and use of birth control pills among participants and estradiol or BHBA levels were also examined but these results were unremarkable. Significantly lower fP has most recently been found in remitted depressed participants compared to controls,43 as well as nonsignificantly lower fP among currently depressed participants compared to controls,59 although the origin of these differences remain unclear. Paradoxical group differences for BPP and BPND relative to those for BPF have been noted in other [11C]WAY PET investigations of neuropsychiatric disorders,43 particularly when BPF was determined based on the use of cerebellar white matter to approximate nondisplaceable radiotracer uptake. In this work, the cerebellar reference uptake was determined in predominantly gray matter areas (low white matter contribution) using methods that were carefully defined to minimize well-known sources of error (see Methods section).
In conclusion, the BPP binding measure showed evidence of significantly greater 5-HT1A receptor binding in subgenual cingulate, mesial temporal and parietal cortices of participants recovered from bulimia nervosa, relative to healthy controls. This work also indicated group differences in nonspecific factors that raised concern about the direction of this group difference in 5-HT1A receptor binding. The BPP measure is determined using standard methods with minimal “nonspecific” corrections. This work further highlights the importance of careful measurement and evaluation of non-specific factors in neuroreceptor binding studies including the need to verify potential plasma protein binding differences between eating disorder groups, and to address the potential nature of such fP differences in RBN.
Acknowledgments
Supported by MH046001, MH004298, K05-MH001894, T32-MH018399 from National Institute of Mental Health (NIMH), by J2188, J2359-B02 from Austrian Science Fund (Erwin-Schrödinger Research Fellowship) and by Price Foundation.
We thank the UPMC PET Laboratory staff for their invaluable contribution to this study and Eva Gerardi for the manuscript preparation. We are indebted to the participating participants for their contribution of time and effort in support of this study.
Footnotes
Disclosure: Dr. Bailer has received honoraria for presentations from Wyeth. Dr. Kaye has received salary support from the University of Pittsburgh and the University of California, San Diego; Research funding for an investigator initiated treatment study from Astra-Zeneca and consulting fees from Lundbeck and Merck. In addition, there are honoraria for presentations from academic institutions and meetings, and compensation for grant review activities from the National Institutes of Health. Over the past 3 years, Dr. Geyer has received compensation from Acadia, Addex, Amgen, Amylin, AstraZeneca, Cenomed, Chakra, Medivation, Omeros, Organon, Sepracor, Takeda, Teva, and Wyeth-Ayerst and holds an equity interest in San Diego Instruments. Dr. Mathis has received royalty payments for licensed technology from GE Healthcare and Neuroptix as well as research grant support from Neuroptix. Dr. Mathis also served as a consultant for Janssen/Elan, Wyeth/Pfizer, and Novartis over the past 3 years. The remaining authors, CS Bloss, GK Frank, JC Price, CC Meltzer, A Wagner, CR Becker, and NJ Schork declare that, except for income received from their primary employers and the aforementioned funding, no further financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- 2.Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–35. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaye W, Fudge J, Paulus M. New insight into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–584. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 4.Rabiner EA, Messa C, Sargent PA, Husted-Kjaer K, Montgomery A, Lawrence AD, et al. A database of [11C]WAY-100635 binding to 5-HT1A receptors in normal male volunteers: Normative data and relationship to methodological, demographic, physiological, and behavioral variables. NeuroImage. 2002;15:620–632. doi: 10.1006/nimg.2001.0984. [DOI] [PubMed] [Google Scholar]
- 5.Groenink L, van Bogaert M, van der Gugten J, Oosting R. 5-HT1A receptor and 5-HT1B receptor knockout mice in stress and anxiety paradigms. Behav Pharmacol. 2003;14:369–383. doi: 10.1097/01.fbp.0000087737.21047.75. [DOI] [PubMed] [Google Scholar]
- 6.Blier P, de Montigny C. Possible serotonergic mechanisms underlying the antidepressant and anti-obsessive-compulsive disorder responses. Biol Psychiatry. 1998;44:313–323. doi: 10.1016/s0006-3223(98)00114-0. [DOI] [PubMed] [Google Scholar]
- 7.Innis R, Cunningham V, Delforge J, Fujita M, Gjedde A, Gunn R, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 8.Tiihonen J, Keski-Rahkonen A, Lopponen M, Muhonen M, Kajander J, Allonen T, et al. Brain serotonin 1A receptor binding in bulimia nervosa. Biol Psychiatry. 2004;55:871–873. doi: 10.1016/j.biopsych.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Bailer UF, Frank G, Henry S, Price J, Meltzer C, Mathis C, et al. Exaggerated 5-HT1A but normal 5-HT2A receptor activity in individuals ill with anorexia nervosa. Biol Psychiatry. 2007;61:1090–1099. doi: 10.1016/j.biopsych.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Weissfeld L, et al. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11C]WAY100635. Arch Gen Psychiatry. 2005;62:1032–1041. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]
- 11.Galusca B, Costes N, Zito N, Peyron R, Bossu C, Lang F, et al. Organic background of restrictive-type anorexia nervosa suggested by increased serotonin(1A) receptor binding in right frontotemporal cortex of both lean and recovered patients: [(18)F]MPPF PET scan study. Biol Psychiatry. 2008;64:1009–1013. doi: 10.1016/j.biopsych.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Wagner A, Barbarich N, Frank G, Bailer U, Weissfeld L, Henry S, et al. Personality traits after recovery from eating disorders: Do subtypes differ? Int J Eat Disord. 2006;39:276–284. doi: 10.1002/eat.20251. [DOI] [PubMed] [Google Scholar]
- 13.Kaye WH, Greeno CG, Moss H, Fernstrom J, Fernstrom M, Lilenfeld LR, et al. Alterations in serotonin activity and psychiatric symptomatology after recovery from bulimia nervosa. Arch Gen Psychiatry. 1998;55:927–935. doi: 10.1001/archpsyc.55.10.927. [DOI] [PubMed] [Google Scholar]
- 14.Kaye WH, Frank GK, Meltzer CC, Price JC, McConaha CW, Crossan PJ, et al. Altered serotonin 2A receptor activity in women who have recovered from bulimia nervosa. Am J Psychiatry. 2001;158:1152–1155. doi: 10.1176/appi.ajp.158.7.1152. [DOI] [PubMed] [Google Scholar]
- 15.Smith KA, Fairburn CG, Cowen PJ. Symptomatic relapse in bulimia nervosa following acute tryptophan depletion. Arch Gen Psychiatry. 1999;56:171–176. doi: 10.1001/archpsyc.56.2.171. [DOI] [PubMed] [Google Scholar]
- 16.Steiger H, Richardson J, Israel M, Ng Ying Kin N, Bruce K, Mansour S, et al. Reduced density of platelet-binding sites for [3H]paroxetine in remitted bulimic women. Neuropsychopharmacology. 2005;30:1028–1032. doi: 10.1038/sj.npp.1300693. [DOI] [PubMed] [Google Scholar]
- 17.Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5-HT1A receptor binding and anxiety: A [11C]WAY-100635 PET investigation in healthy volunteers. Am J Psychiatry. 2001;158:1326–1328. doi: 10.1176/appi.ajp.158.8.1326. [DOI] [PubMed] [Google Scholar]
- 18.Jovanovic H, Karlsson P, Cerin A, Halldin C, Nordstrom A. 5-HT(1A) receptor and 5-HTT binding during the menstrual cycle in healthy women examined with [(11)C] WAY100635 and [(11)C] MADAM PET. Psy Res. 2009;172:31–37. doi: 10.1016/j.pscychresns.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Gibbon M, Spitzer RL, Williams JBW. Users Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders –Research Version (SCID-I, version 2.0, February 1996 FINAL VERSION) New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 20.Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory (TCI): A Guide to its Development and Use. St. Louis, MO: Center for Psychobiology of Personality, Washington University; 1994. [Google Scholar]
- 21.Beck AT, Ward M, Mendelson M, Mock J, Erbaugh J. An Inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 22.Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 23.Barratt ES, Patton JH. Impulsivity: Cognitive, behavioral, and psychophysiological correlates. In: Zuckerman M, editor. Biological Bases of Sensation Seeking, Impulsivity, and Anxiety. Hillsdale, NJ: Lawrence Earlbaum Associates; 1983. p. 85. [Google Scholar]
- 24.Meltzer CC, Price J, Mathis C, Butters M, Ziolko S, Moses-Kolko E, et al. Serotonin 1A receptor binding and treatment responses in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- 25.Meltzer C, Drevets W, Price J, Mathis C, Lopresti B, Greer P, et al. Gender-specific aging effects on the serotonin 1A receptor. Brain Res. 2001;895:9–17. doi: 10.1016/s0006-8993(00)03211-x. [DOI] [PubMed] [Google Scholar]
- 26.Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, et al. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: Comparison of arterial and reference tissue input functions. J Cereb Blood Flow Metab. 2000;20:1111–1133. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nuclear Med Biol. 2000;27:499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- 28.Logan J, Fowler J, Volkow N, Ding Y, Wang G, Alexoff D. A strategy for removing the bias in the graphical analysis method. J Cereb Blood Flow Metab. 2001;21:307–320. doi: 10.1097/00004647-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Price J, Xu L, Mazumdar S, Meltzer C, Drevets W, Mathis C, et al. Impact of graphical analysis bias on group comparisons of regional [carbonyl-11C]WAY binding potential measures. Neuroimage. 2002;16:S72. [Google Scholar]
- 30.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 31.Gunn RN, Sargent PA, Bench CJ, Rabiner EA, Osman S, Pike VW, et al. Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY- 100635 for PET. Neuroimage. 1998;8:426–440. doi: 10.1006/nimg.1998.0379. [DOI] [PubMed] [Google Scholar]
- 32.Hirvonen J, Kajander J, Allonen T, Oikonen V, Nagren K, Hietala J. Measurement of serotonin 5-HT1A receptor binding using positron emission tomograpy and [carbonyl-(11)C]WAY-100635-considerations on the validity of cerebellum as a reference region. J Cereb Blood Flow Metab. 2007;27:185–195. doi: 10.1038/sj.jcbfm.9600326. [DOI] [PubMed] [Google Scholar]
- 33.Parsey R, Arango V, Olvet D, Oquendo M, Van Heertum R, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomograpny. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- 34.Holland B, Copenhaver M. Improved Bonferroni-type multiple testing procedures. Psychol Bull. 1988;104:145–149. [Google Scholar]
- 35.Zapala M, Schork N. Multivariate regression analysis of distance matrices for testing associations between gene expression patterns and related variables. Proc Natl Acad Sci USA. 2006;103:19430–19435. doi: 10.1073/pnas.0609333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson M. DISTLM Forward: A FORTRAN Computer Program to Calculate a Distance-based Multivariate Analysis for a Linear Model Using Forward Selection. New Zealand: Department of Statistics, University of Auckland; 2003. [Google Scholar]
- 37.Hajos M, Gartside SE, Varga V, Sharp T. In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrainraphe nuclei: Role of 5-HT1A receptors. Neuropharmacology. 2003;45:72–81. doi: 10.1016/s0028-3908(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 38.Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of serotoinin1A and serotonin2A receptor in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- 39.Romano SJ, Halmi KA, Sarkar NP, Koke SC, Lee JS. A placebo-controlled study of fluoxetine in continued treatment of bulimia nervosa after successful acute fluoxetine treatment. Am J Psychiatry. 2002;159:96–102. doi: 10.1176/appi.ajp.159.1.96. [DOI] [PubMed] [Google Scholar]
- 40.Walsh BT, Hadigan CM, Devlin MJ, Gladis M, Roose SP. Long-term outcome of antidepressant treatment for bulimia nervosa. Am J Psychiatry. 1991;148:1206–1212. doi: 10.1176/ajp.148.9.1206. [DOI] [PubMed] [Google Scholar]
- 41.Drevets W, Thase M, Moses-Kolko E, Price J, Frank E, Kupfer D, et al. Serotonin-1A receptor imaging in recurrent depression: Replication and literature review. Nucl Med Biol. 2007;34:865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savitz J, Lucki I, Drevets W. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88:17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller JB, Ogden T, Oquendo M, Sullivan G, Mann J, Parsey R. Elevated serotonin 1A binding in remitted major depressive disorder: Evidence for a trait biological abnormality. Neuropsychopharmacology. 2009;34:2275–2284. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lanzenberger R, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien L, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61:1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Neumeister A, Bain E, Nugent A, Carson R, Bonne O, Luckenbaugh D, et al. Reduced serotinin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nash J, Sargent P, Rabiner E, Hood SA, Potokar J, Grasby PN, et al. Serotonin 5-HT1A receptor binding in people with panic disorder: Positron emission tomography study. Br J Psychiatry. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- 47.Borg J, Bengt A, Soderstrom H, Farde L. The serotonin system and spiritual experiences. Am J Psychiatry. 2003;160:1965–1969. doi: 10.1176/appi.ajp.160.11.1965. [DOI] [PubMed] [Google Scholar]
- 48.Parsey R, Oquendo M, Simpson N, Ogden R, Van Heertum R, Arango V, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954:173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 49.Witte A, Floel A, Stein P, Savli M, Mien L, Wadsak W, et al. Aggression is related to frontal serotonin-1A receptor distribution as revealed by PET in healthy subjects. Hum Brain Mapp. 2009;30:2558–2570. doi: 10.1002/hbm.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan G, Oquendo M, Simpson N, Van Heertum R, Mann JJ, Parsey RV. Depression is related to psychic and somatic anxiety. Biol Psychiatry. 2005;58:947–954. doi: 10.1016/j.biopsych.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Geyer MA. Serotonergic functions in arousal and motor activity. Behav Brain Res. 1996;73:31. doi: 10.1016/0166-4328(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 52.Fairbanks L, Melega W, Jorgensen M, Kaplan J, McGuire M. Social impulsivity inversely associated with CSF 5-HIAA and fluoxetine exposure in vervet monkeys. Neuropschopharmacology. 2001;24:370–378. doi: 10.1016/S0893-133X(00)00211-6. [DOI] [PubMed] [Google Scholar]
- 53.Johnson P, Lightman S, Lowry C. A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Ann NY Acad Sci. 2004;1018:58–64. doi: 10.1196/annals.1296.006. [DOI] [PubMed] [Google Scholar]
- 54.Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: Therapeutic implications for impulse control disorders. Neuropschopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- 55.Cleare A, Bond AJ. Ipsapirone challenge in aggressive men shows an inverse correlation between 5HT1A receptor function and aggression. Psychopharmacology (Berl) 2000;148:344–349. doi: 10.1007/s002130050061. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell J, Crow S, Peterson CW, Crosby R. Feeding laboratory studies in patients with eating disorders: A review. Int J Eat Disord. 1998;24:115–124. doi: 10.1002/(sici)1098-108x(199809)24:2<115::aid-eat1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 57.Bailer UF, Frank G, Henry S, Price J, Meltzer CC, Becker C, et al. Serotonin transporter binding after recovery from eating disorders. Psychopharmacology. 2007;195:315–234. doi: 10.1007/s00213-007-0896-7. [DOI] [PubMed] [Google Scholar]
- 58.Frankle W, Lombardo I, Kegeles L, Slifstein M, Martin J, Huang Y, et al. Serotonin 1A receptor availability in patients with schizophrenia and schizo-affective disorder: A positron emission tomography imaging study with [11C]WAY 100635. Psycho-pharmacology (Berl) 2006;189:155–164. doi: 10.1007/s00213-006-0543-8. [DOI] [PubMed] [Google Scholar]
- 59.Parsey RV, Oquendo MA, Ogden RT, Olvet D, Simpson N, Huang Y, et al. Altered serotonin 1A binding in major depression: A [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59:106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]


