Abstract
Purpose
Carriers of fragile X mental retardation 1 (FMR1) repeat expansions in the premutation range (55–200 CGG repeats) often develop a syndrome of kinetic tremor, cerebellar ataxia, and parkinsonism; designated the fragile X-associated tremor ataxia syndrome (FXTAS). Neurological signs have not been reported in carriers of gray zone (45–54 CGG repeats) expansions.
Methods/Results
We describe three patients with FMR1 gray zone alleles who meet diagnostic criteria for FXTAS.
Conclusions
Our cases suggest that the definition of the FXTAS may need to be broadened to include individuals with FMR1 repeat expansions in the gray zone. These neurological signs may be due to elevated levels of expanded CGG repeat FMR1 mRNA in the gray zone carriers, similar to the changes seen in premutation carriers with FXTAS.
Keywords: FMR1, FXTAS, genetics, ataxia, gray zone
Introduction
The fragile X-associated tremor/ataxia syndrome (FXTAS) occurs in individuals with a premutation range (55–200 CGG) expansion in the 5′ untranslated region of the fragile X mental retardation (FMR1) gene. It manifests clinically as kinetic tremor, cerebellar gait ataxia, parkinsonism, and executive dysfunction; typically in males over age 55.1 Premature ovarian failure, clinically diagnosed as menopause earlier than the age of 40, is also associated with a premutation size expansion in FMR1.2 Larger expansions in the gene (>200 CGG repeats) result in fragile X syndrome, the most common cause of inherited intellectual disability in boys.
Smaller expansions in the FMR1 gene, variably defined as 41–54 or 45–54 CGG repeats, have been termed as gray zone or intermediate alleles based on the lower likelihood of the CGG repeat increasing and causing fragile X syndrome in later generations. The prevalence of FMR1 gray zone alleles is 0.3% (40–54 CGG) to 2.6% (40–59 CGG) in the general population.3–5 Recently, gray zone alleles have been associated with special needs in children, primary ovarian insufficiency, and men with parkinsonism; similar to phenotypes seen in the premutation range.6–7 This paper describes individuals that meet diagnostic criteria for FXTAS that have FMR1 gray zone alleles.
Case Reports
Case 1
A 63 year old man presented with a ten year history of difficulty walking, memory problems, and tremor. His initial symptoms were decreased arm swing, dragging of the right foot, and short-term memory difficulties. Two years later, he was diagnosed with parkinsonism, but was not treated. He then developed bilateral kinetic tremor, which caused difficulty with cutting food and getting dressed. He had problems getting out of a car, softer speech, depression, apathy, urinary frequency, and hypoosmia. He was started on carbidopa/levodopa, which helped his tremors but did not improve his walking. He developed wearing off of the medication nine years after symptom onset and was given pramipexole, which caused hallucinations, and then rasagiline, which reduced his tremors. On examination at age 63, he scored 25/30 on the Mini Mental Status Examination8. He had mild dysarthria, saccadic pursuits, impaired vertical optokinetic nystagmus, and masked facial expression (Video 1). He had increased tone on the right, a jerky irregular tremor in the right arm at rest and with action, especially with handwriting and pouring water (Figure 1a), and an irregular bilateral postural tremor. He had freezing on gait initiation and slowed shuffling gait, with decreased stride length and dragging of the right foot. He had retropulsion on the pull test, and was unable to complete tandem walking. MRI of the brain showed mild scattered white matter disease and global volume loss. There was no middle cerebellar peduncle (MCP) sign. Six months after he was seen, he developed orthostasis and rapidly progressive dementia with delusions and severe hallucinations, and died after having a fall. Autopsy was not performed. He had a FMR1 allele of 48 CGG repeats.
Figure 1.
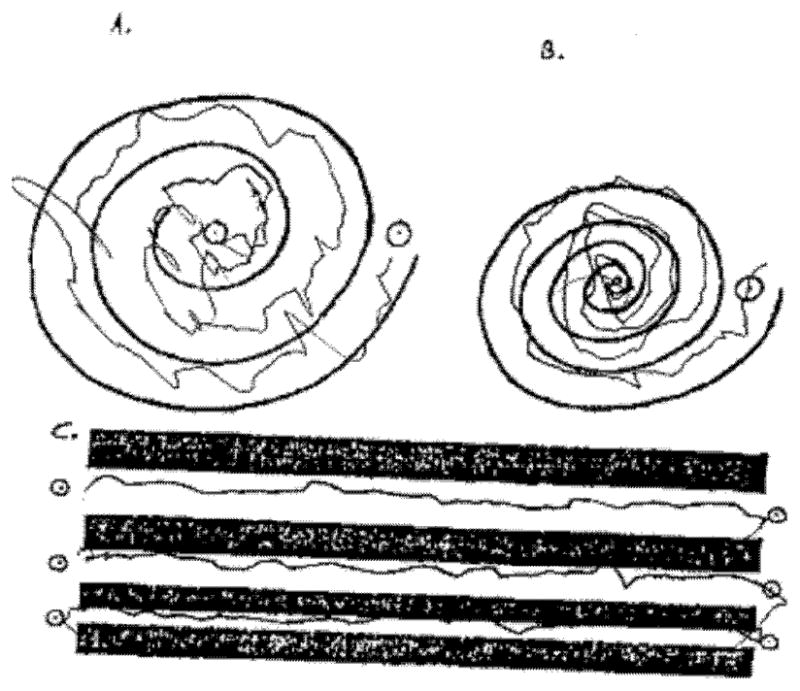
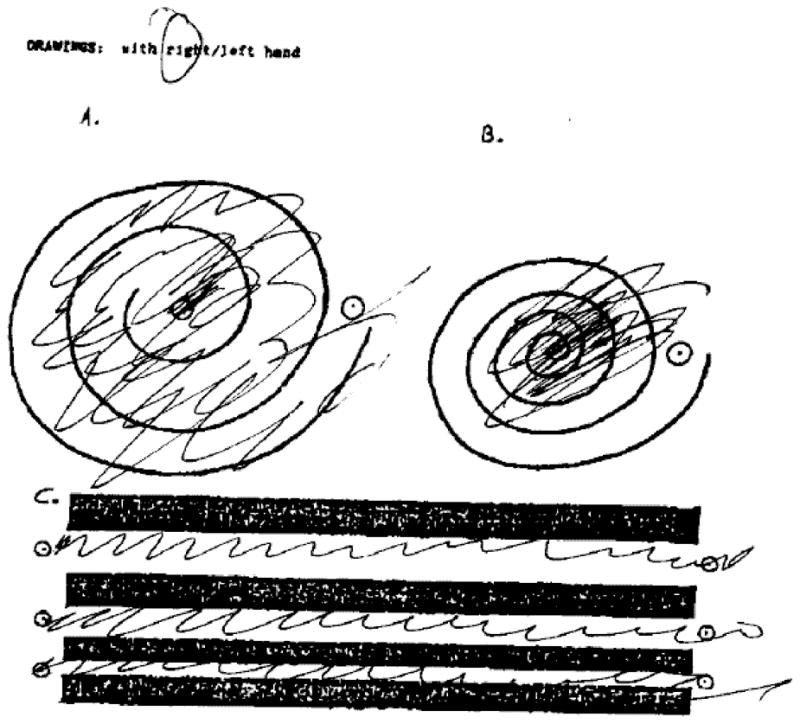
Spirals from the Clinical Rating Scale for Tremor: a. Case 1 b. Case 2.
Case 2
A 75 year woman presented with a ten year history of kinetic tremor in her hands. Propranolol was initially effective, but stopped due to side effects. Trials of primidone, topiramate, neurontin, and clonazepam were not effective. After five years, the tremor became much worse (Figure 1b) and she developed difficulty with balance and falls. Eight years into her course, she had a deep brain stimulator placed into the right thalamus, with resultant improvement in her tremor per objective testing, but not according to the patient. Post-surgery, she developed worsened balance and cognition, as well as increasing lack of insight, anxiety, and agitation. Family history was relevant for a grandfather that had the same type of tremor and two aunts had voice tremor. Examination at age 75 showed mild bradykinesia in the left extremities, moderate kinetic tremor in the hands symmetrically, mild symmetric postural tremor, mild head tremor, and intention tremor of the right foot (Video 2). Stance was wide, gait was slow, and she was unable to complete tandem gait. Brain MRI showed moderate volume loss and mild white matter changes consistent with ischemia (Figure 2a). T2 hypointensity in the basal ganglia on MRI was felt to be secondary to normal aging. DNA testing revealed the presence of FMR1 alleles of 30 and 47 CGG repeats. FMR1 mRNA level was within the normal range [1.31 (±0.06 standard error of the mean)].
Figure 2.
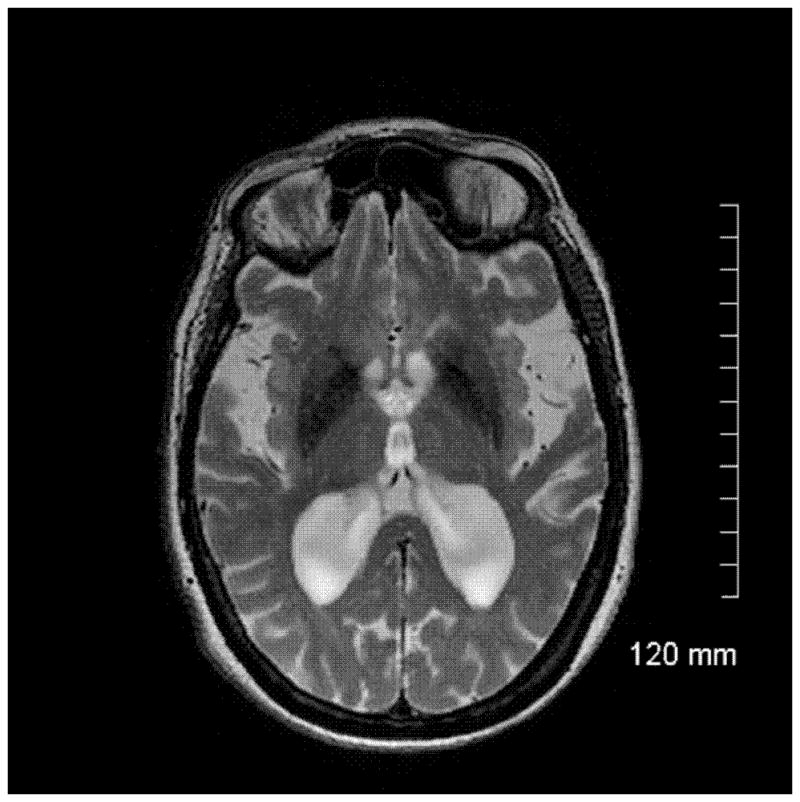
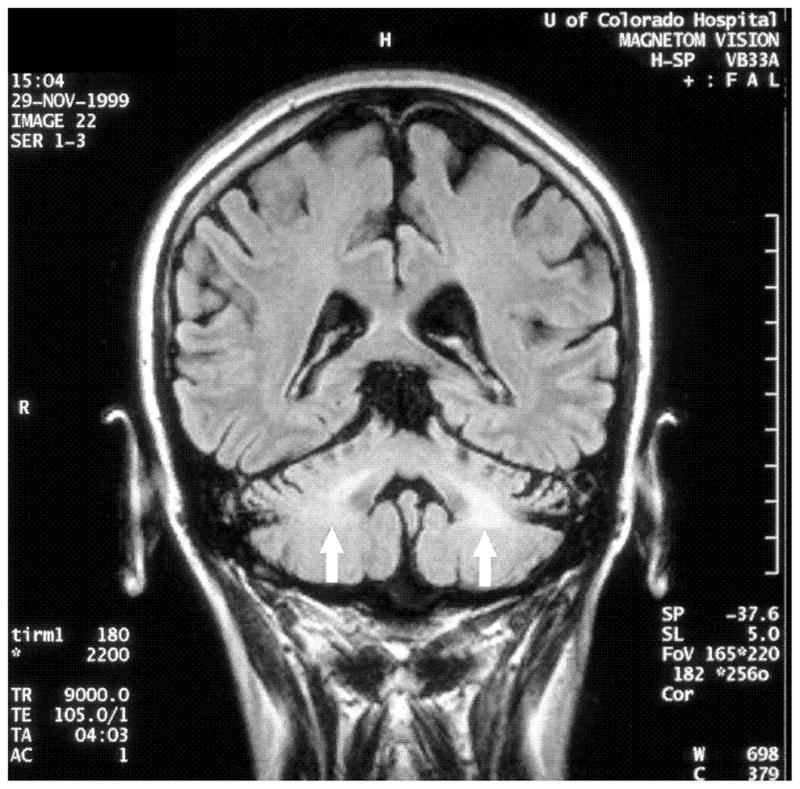
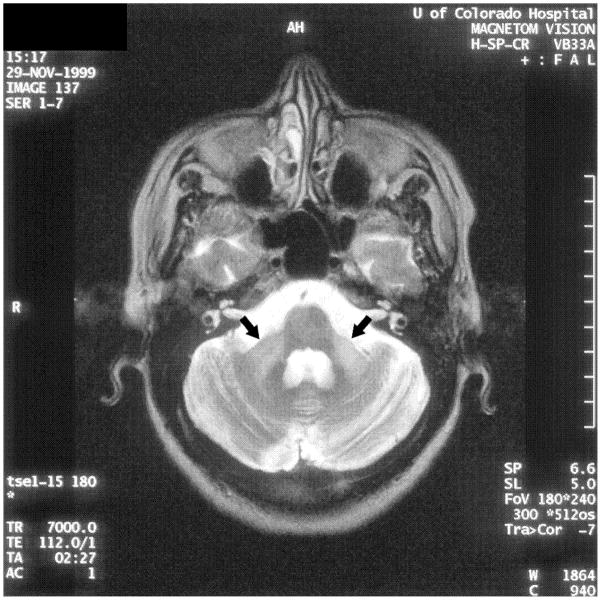
Case 2: a. Axial T2-weighted fast spin ECHO MRI showing basal ganglia hypodensities and volume loss Case 3: b. Axial T2-weighted MRI showing hyperintensities in the middle cerebellar peduncle (‘MCP sign’) c. Coronal FLAIR MRI showing hyperintensities in the middle cerebellar peduncle.
Case 3
A 54 year old woman from Saudi Arabia had onset of left arm stiffness and pain at age 50. Within six months she developed action tremor in the hand, slurring of her speech, and word finding difficulty. Her symptoms progressed over the next year, interfering with most activities of daily living and forcing her to stop working. One and a half years after onset of symptoms, she started feeling “off balance,” developed intermittent falls, occasionally drag her left leg and became dependent on a cane. Additionally, she complained of fatigue, memory difficulties, constipation, urinary urgency and incontinence. Neurological examination was notable for depressed affect, mild cogwheel rigidity, action induced dystonic posturing, and a mild fast amplitude action tremor of the left hand (Video 3). Fine motor and rapid alternating movements had markedly reduced amplitude with decrement in the upper extremities. Reflexes were increased in the left extremities and she had clonus at both ankles. Her gait showed mild left arm dystonic flexor posturing with minimal swing and a subtle left leg limp. At age 52, she was treated with carbidopa/levodopa 112.5/450 per day, which offered marked symptomatic improvement in her speed of walking, falls, tremor and stiffness. Four months later, she noted wearing off and one year later, she had left facial dyskinesia. At age 53, her family reported that she thrashed about when dreaming. By age 54, her balance had worsened so that she adopted a walker. The MCP sign was present on her MRI (Figure 2b–c). FMR1 allele sizes were 50 and 30. FMR1 mRNA was elevated at 3.38 ± 0.21 (SEM) times normal.
Discussion
These three patients have kinetic tremor and other signs typically seen in FXTAS, but have FMR1 CGG repeat expansions in the gray zone range. Published diagnostic criteria for FXTAS requires the presence of kinetic tremor or cerebellar gait ataxia, white matter changes or the middle cerebellar peduncle sign on imaging, and a premutation allele in the FMR1 gene.1 Our first case started with signs of cognitive dysfunction and parkinsonism, developing kinetic tremor shortly after. His cognitive issues at the onset of his symptoms lead to a diagnosis of ‘parkinsonism’ rather than idiopathic Parkinson disease (PD). Although he developed more typical signs for PD, including fluctuations and hallucinations; his kinetic tremor, the jerky quality of the tremor, and his rapid cognitive decline before death are unusual for PD, Lewy body dementia, or other atypical parkinsonian syndromes. Upon presentation to our tertiary center, he was suspected to have FXTAS and a FMR1 test was sent. He would meet diagnostic criteria for possible FXTAS if he had a FMR1 premutation rather than gray zone expansion. Our diagnostic certainty is lowest with this patient and would be higher with a MCP sign on imaging or neuropathology. Our second case had gait ataxia out of proportion to what would be expected with essential tremor (ET). She also had lack of insight and anxiety, which are common in fragile X premutation carriers. If she had the premutation, then she would meet diagnostic criteria for probable FXTAS. The third case is classic for FXTAS, and would meet diagnostic criteria for definite FXTAS if she had the premutation rather than the gray zone expansion.
Prior to this report, three other phenotypes had been described in gray zone carriers. A population screening of 1013 boys with learning disabilities and their mothers (n=760) showed a significant excess of gray zone alleles (41–60 CGG) in the affected boys.7 However, a follow up study in boys with special education needs (n=5084) did not confirm this result.9 Primary ovarian insufficiency has been shown to be increased in women with 41–58 CGG repeats.6,10 Lastly, Australian men with parkinsonism are two-fold more likely to carry an FMR1 gray zone allele (41–60 CGG repeats) than controls.11
The reason that clinical signs are being found in gray zone carriers is likely because molecular changes in FMR1 gray zone are similar to that seen in the premutation. As the repeat size increases from the normal range (<41 CGG repeats), there is an increase in expanded (the CGG repeat is transcribed) FMR1 mRNA levels and a mild decrease in fragile X mental retardation protein (FMRP) levels.12–14 FMRP is a RNA shuttling protein that works in functional opposition to metabotropic glutamate receptors.15–16 Lack of this protein leads to altered synaptic plasticity,17 but the effect when the protein is at slightly low levels in premutation carriers is unclear. A toxic gain of function of mRNA due to increased levels of the expanded FMR1 mRNA is proposed to account for neurological symptoms in the premutation carriers with FXTAS. This starts in the gray zone range and therefore may account for the observed phenotypes here described. Given that other phenotypes have been observed in FMR1 gray zone carriers and this plausible mechanism of pathology, our findings are not unexpected.
Gray zone alleles have been reported to expand over two generations to a full mutation range,18–20 but typically it takes at least three generations. The American College of Medical Genetics practice guidelines define the intermediate zone or gray zone alleles as 41–60 CGG repeats.21 However, 45–54 CGG repeats have been designated the intermediate or gray zone by the laboratory practice committee of the American College of Medical Genetics.22 This discrepancy is addressed in a paper that defines the gray zone as 45–54 based on the lack of widespread fragile X-associated disorders in individuals with the smaller 41–44 CGG repeats,23 despite studies showing phenotypes in this range.6–7, 10–11 Now that phenotypes have been associated with gray zone alleles, it may be important to redefine the range based on molecular findings, which suggest that transcript levels of FMR1 mRNA increase starting at just 39 CGG repeats.12
As noted, a major criterion for the current diagnosis of FXTAS is the presence of a premutation allele. Our findings suggest that the lower boundary for pathology associated with the premutation may need to be changed to include the gray zone range. In fact, recent reports also suggest that the upper boundary for pathology (i.e., 200 CGG repeats) may also need to be changed. FXTAS has been reported in a man with a fully unmethylated full mutation (297–480 CGG repeats) in FMR1.23 Further, intranuclear inclusions characteristically seen in FXTAS have also been reported in men with a full mutation.24
This paper reports three cases of signs of FXTAS in individuals with a FMR1 allele within the gray zone. This suggests that FXTAS could develop in persons that are gray zone carriers. However, this idea needs validation by finding other cases and especially by neuropathological confirmation. Given our three cases and the recent full mutation carrier with FXTAS, a new definition of FXTAS may involve both the lower end (gray) and the upper end (full) of the FMR1 mutation. It may also have implications for physicians who diagnose and treat movement disorders and providers who are counseling families with fragile X associated disorders, in addition to the 0.3–2.6%% of the general population who carry FMR1 gray zone alleles.
Supplementary Material
Video 1. The first video shows a patient with early cognitive decline with mixed rest, postural, (though not shown) kinetic tremor. He has parkinsonian signs in both extremities and a hesitant gait with small steps.
Video 2. The second videos shows a patient with mild postural tremor, intention tremor, and (though not shown) inability to tandem walk.
Video 3. The third video shows a patient with facial dystonia, mild dysarthria, dysmetria and intention tremor. She also has bradykinesia and a wide based, ataxic gait.
Acknowledgments
The authors also thank study subjects who participated in this study.
Footnotes
Author Roles: 1) Research project: A. Conception: DH, ML; B.Organization: DH; C. Execution: DH, OK, FT; 2) Statistical Analysis: A. Design: DH; Execution: DH; 3) Manuscript: A. First manuscript: DH; B. Review and critique: DH, ML, OK, FT
Full financial disclosure of all authors for the past year:
Grants: DH (NIH), FT (NIH), ML (NIH)
Foundations: DH (Anthony Krause Foundation, National Fragile X Foundation, Parkinson’s Disease Foundation), OK (Anthony Krause Foundation)
Contracts: ML (Schwartz Biosciences, Inc., Neurologix, Inc., Molecular Biometrics, Inc.)
Honoraria: OK (Teva)
Consultancies: OK (Medtronic)
Financial disclosures: This work was funded by NS052487 (D.H.), and a Parkinson Research Center grant from the Parkinson’s Disease Foundation. All authors have no further financial disclosures to make nor conflict of interests related to this study.
References
- 1.Jacquemont S, Hagerman RJ, Leehey MA, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allingham-Hawkins DJ, Babul-Hiriji R, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the international collaborative POF in fragile X study - preliminary data. Am J Med Genet. 1999;83:322–325. [PMC free article] [PubMed] [Google Scholar]
- 3.Dombrowski CLS, Morel ML, Rouillard P, Morgan K, Rousseau F. Premutation and intermediate-size FMR1 alleles in 10,572 males from the general population: loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Hum Mol Genet. 2002;11(4):371–378. doi: 10.1093/hmg/11.4.371. [DOI] [PubMed] [Google Scholar]
- 4.Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K. Prevalence of carriers of premutation-size alleles of the FMRI gene--and implications for the population genetics of the fragile X syndrome. Am J Hum Genet. 1995;57(5):1006–1018. [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson AJ, Chodirker BN, Chudley AE. Frequency of FMR1 premutations in a consecutive newborn population by PCR screening of Guthrie blood spots. Biochem Mol Med. 1995;56(1):63–69. doi: 10.1006/bmme.1995.1057. [DOI] [PubMed] [Google Scholar]
- 6.Bodega B, Bione S, Dalpra L, et al. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Repro. 2006;21(4):952–957. doi: 10.1093/humrep/dei432. [DOI] [PubMed] [Google Scholar]
- 7.Murray A, Youings SA, Dennis N, et al. Population screening at the FRAXA and FRAXE loci: molecular analyses of boyrs with learning difficulties and their mothers. Hum Mol Genet. 1996;5(6):727–735. doi: 10.1093/hmg/5.6.727. [DOI] [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Ennis S, Murray A, Youings S, et al. An investigation of FRAXA intermediate allele phenotype in a longitudinal sample. Ann Hum Genet. 2006;70(Pt 2):170–180. doi: 10.1111/j.1529-8817.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- 10.Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of the normal range are associated with premature ovarian failure. Hum Genet. 2005;117:376–382. doi: 10.1007/s00439-005-1326-8. [DOI] [PubMed] [Google Scholar]
- 11.Loesch DZ, Khaniani MS, Slater HR, et al. Small CGG repeat expansion alleles of FMR1 gene are associated with parkinsonism. Clinical Genetics. 2009;76:471–476. doi: 10.1111/j.1399-0004.2009.01275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loesch DZ, Bui QM, Huggins RM, Mitchell RJ, Hagerman RJ, Tassone F. Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised and correlate with the number of CGG repeats. J Med Genet. 2007;44(3):200–204. doi: 10.1136/jmg.2006.043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tassone F, Hagerman RJ, Taylor AK, Gane L, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenneson A, Zhang F, Hagedorn CH, Warren ST. Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Hum Mol Genet. 2001;10(14):1449–54. doi: 10.1093/hmg/10.14.1449. [DOI] [PubMed] [Google Scholar]
- 15.Corbin F, Bouillon M, Fortin A, Morin S, Rousseau F, Khandijian EW. The fragile X mental retardation protein is associated with poly(A) + mRNA in actively translating polyribosomes. Hum Mol Genet. 1997;6:1465–1472. doi: 10.1093/hmg/6.9.1465. [DOI] [PubMed] [Google Scholar]
- 16.Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irwin SA, Galves R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- 18.Zuniga A, Juan J, Mila M, Guerrero A. Expansion of an intermediate allele of the FMR1 gene in only two generations. Clin Genet. 2005;68:471–473. doi: 10.1111/j.1399-0004.2005.00514.x. [DOI] [PubMed] [Google Scholar]
- 19.Terracciano A, Pomponi MG, Marino GM, et al. Expansion to full mutation of a FMR1 intermediate allele over two generations. Eur J Hum Genet. 2004;12(4):333–336. doi: 10.1038/sj.ejhg.5201154. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Carvajal I, Lopez Posadas B, Pan R, Raske C, Hagerman PJ, Tassone F. Expansion of an FMR1 grey-zone allele to a full mutation in two generations. J Molec Diagnostics. 11(4):306–10. doi: 10.2353/jmoldx.2009.080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherman S, Pletcher MA, Driscoll DA. Fragile X syndrome: diagnostic and carrier testing. Genet Med. 2005;7:548–587. doi: 10.1097/01.GIM.0000182468.22666.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddalena A, Richards CS, McGinniss MJ, et al. Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genet Med. 2001;3(3):200–205. doi: 10.1097/00125817-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kronquist KE, Sherman SL, Spector EB. Clinical significance of tri-nucleotide repeats in Fragile X testing: a clarification of American College of Medical Genetics guidelines. Genet Med. 2008;10(11):845–847. doi: 10.1097/GIM.0b013e31818b0c8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loesch DZ, Sherwell S, Kinella G, Tassone F, Taylor A, Amor D, Sung S, Evans A. Fragile X-associated tremor/ataxia phenotype in a male carriers of unmethylated full mutation in the FMR1 gene. Clin Genet. 2011 doi: 10.1111/j.1399-0004.2011.01675.x. in press. [DOI] [PubMed] [Google Scholar]
- 25.Hunsaker MR, Greco CM, Tassone F, Berman RF, Willemsen R, Hagerman RJ, Hagerman PJ. Rare intranuclear inclusions in the brains of 3 older adult males with fragile X syndrome: Implications for the spectrum of fragile X-associated disorders. J Neuropathol Exp Neurol. 2011;70(6):462–469. doi: 10.1097/NEN.0b013e31821d3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. The first video shows a patient with early cognitive decline with mixed rest, postural, (though not shown) kinetic tremor. He has parkinsonian signs in both extremities and a hesitant gait with small steps.
Video 2. The second videos shows a patient with mild postural tremor, intention tremor, and (though not shown) inability to tandem walk.
Video 3. The third video shows a patient with facial dystonia, mild dysarthria, dysmetria and intention tremor. She also has bradykinesia and a wide based, ataxic gait.