Abstract
Rapidly emerging evidence continues to describe an intimate and causal relationship between sleep and emotional brain function. These findings are mirrored by longstanding clinical observations demonstrating that nearly all mood and anxiety disorders co-occur with one or more sleep abnormalities. This review aims to (1) provide a synthesis of recent findings describing the emotional brain and behavioral benefits triggered by sleep, and conversely, the detrimental impairments following a lack of sleep, (2) outline a proposed framework in which sleep, and specifically rapid-eye movement (REM) sleep, supports a process of affective brain homeostasis, optimally preparing the organism for next-day social and emotional functioning, and (3) describe how this hypothesized framework can explain the prevalent relationships between sleep and psychiatric disorders, with a particular focus on post-traumatic stress disorder and major depression.
Keywords: Sleep, rapid-eye movement (REM), Emotion, Memory, Major Depression, post-traumatic stress disorder (PTSD)
Introduction
The ability of the human brain to generate, regulate and be guided by emotions represents a fundamental process governing our personal lives, our mental health as well as our societal structure. Advances in cognitive neuroscience over the past two decades have helped characterize the mechanisms underlying affective brain processes (Critchley 2005, Delgado et al 2006, Hartley & Phelps 2010, Ochsner et al 2009), translationally bridging animal models of emotion regulation and relevant clinical disorders (Davidson 2002, Delgado et al 2006, Drevets et al 2008, Etkin 2010). In parallel, an exciting collection of recent human neuroscience findings has established a causal role for sleep in the optimal regulation of affective brain function. Moreover, these reports offer tentative neural explanations for the pervasive co-occurrence of sleep abnormalities in psychiatric disorders (Armitage 2007, Buysse 2004, Franzen & Buysse 2008, Gottesmann & Gottesman 2007, Harvey et al 2003, Tsuno et al 2005).
Here, we first review basic experimental data in humans that establish an obligate symbiosis between sleep and affect; both maladaptive consequences caused by the absence of sleep, and adaptive benefits following the presence of sleep, with an emphasis on rapid eye movement sleep (REM). Building on these findings, we next propose a REM sleep neurobiological framework that may account for the observed interactions between sleep and affective brain function. We conclude by discussing how basic experimental evidence, and our hypothesized REM sleep framework, may provide mechanistic and therapeutic understanding of the prominent co-occurrence of sleep disruption and affective disorders, with a specific emphasis on posttraumatic stress disorder (PTSD) and major depression. It should be noted that this review targets the relationship between sleep and emotional processing. In this necessarily focused capacity, it does not consider the nonetheless fascinating, but currently less well characterized, interaction between sleep and mood states; the latter which we and others considered distinct from emotions (emotions being short-lived events, often in response to external stimuli, while mood states are more sustained events, often internally generated (Mendl et al 2010)).
Neurobiology of the Sleeping Brain
Before considering the impact of sleep on emotional brain function, we first outline respective neurobiological features that will importantly link these two processes in subsequent sections. In humans (and other mammals), sleep is separated into two main types: rapid eye movement (REM) sleep and non-rapid eye movement (NREM) sleep, the latter being further subdivided into 4 stages (corresponding to increasing depth of sleep). These sleep stages are associated with dramatic alterations in functional brain activity and brain neurochemistry, with changes in REM sleep being most relevant to the current review. Neuroimaging studies reveal significant activity increases during REM sleep in emotion-related regions both subcortically, in the amygdala, striatum and hippocampus, and cortically, in the insula and medial prefrontal cortex (mPFC) (Dang-Vu et al 2010, Miyauchi et al 2009, Nofzinger 2005). These changes in functional brain activity are paralleled (and likely governed) by striking alterations in neurochemistry (Kametani & Kawamura 1990, Marrosu et al 1995, McGinty & Harper 1976). Perhaps most remarkable is a substantial reduction in levels of noradrenaline (norepinephrine) during REM sleep, falling to concentrations below that of either NREM sleep or wake (Kametani & Kawamura 1990, Marrosu et al 1995, Ouyang et al 2004, Park 2002, Shouse et al 2000); the lowest of any time during the 24hr period. This REM sleep reduction is pertinent to emotion processing, since noradrenaline is associated with numerous arousal-related emotion processes within the brain (and body), and in dysfunctional ranges, is associated with specific psychopathologies including PTSD and major depression.
Therefore, the neuroanatomical and neurochemical changes that dominate REM sleep show a strong convergence with waking brain mechanisms of emotion reactivity, regulation and consequential action (Dolcos et al 2005); themes that we will return to throughout the remaining sections.
Impact of sleep loss on emotional brain function: Reactivity and recognition
Emotional Reactivity
Together with impairments of attention, alertness and memory, sleep loss has consistently been associated with subjective reports of irritability and emotional volatility (Horne 1985). Restricting sleep to only 5hr a night across a 1-week period leads to a progressive increase in emotional disturbance in participants on the basis of questionnaire mood scales, together with diary documentation of increasing subjective emotional difficulties (Dinges et al 1997). Moreover, accumulated sleep loss leads to an amplification of negative emotions in response to disruptive daytime experiences, while blunting the affective benefit associated with goal-enhancing activities (Zohar et al 2005). Congruently, one night of experimentally controlled sleep loss increases subjective reports of stress, anxiety and anger in response to low-stress situations (Minkel et al 2012), and increases impulsivity towards negative stimuli (Anderson & Platten 2011). This is of particular clinical interest considering that impulsivity is significantly correlated with aggressive behavior and suicidality (Plutchik 1995), both of which are associated with sleep disruption (Bernert & Joiner 2007, Kamphuis et al 2012).
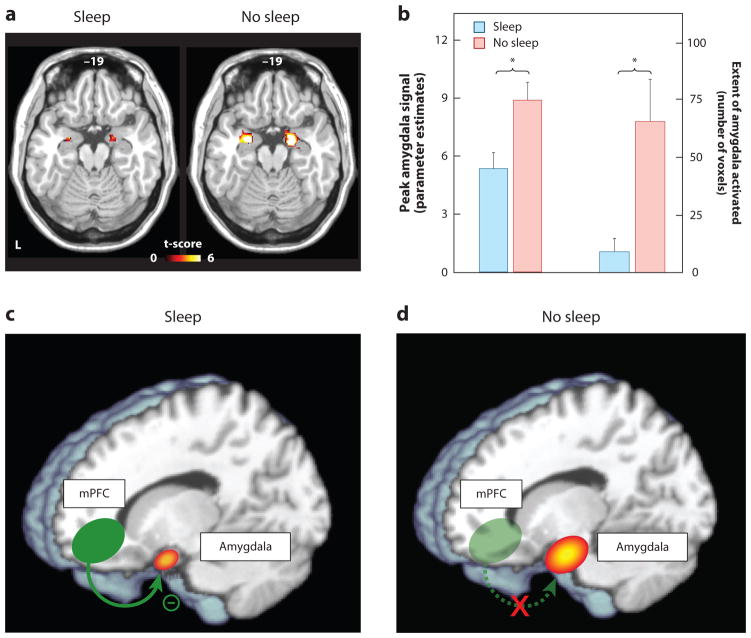
Studies assessing objective physiological and neural measures of affect have provided additional verification of, and explanatory mechanisms for, emotional dysregulation following sleep deprivation. Assessed using functional MRI (fMRI), one night of sleep deprivation triggers a 60% amplification in reactivity of the amygdala in response to emotionally negative pictures, relative to a normal night of sleep (Figure 1a&b) (Yoo et al 2007). Moreover, this increase in amygdala reactivity is paired with a reduction in functional connectivity with regions of the mPFC that exert top-down regulatory control of the amygdala (Figure 1c&d), yet increased coupling with the fight/flight adrenergic-activating brainstem center of the locus coeruleus. A similar profile of exaggerated amygdala reactivity and reduced prefrontal connectivity occurs after 5 nights of 4hr sleep restriction; arguably a more ecologically relevant paradigm in the context of societal sleep behavior and clinical disorders (Motomura et al 2013). Moreover, habitual subjective sleep quality outside of the laboratory is also significantly related to amygdala reactivity, as well as features of negative affect and stress (Prather et al 2013). Additionally, inter-individual differences in the change in connectivity between the amygdala and mPFC caused by sleep deprivation accurately predicts the concurrent increase in subjective anxiety (Motomura et al 2013).
Figure 1. The impact of sleep deprivation on emotional brain reactivity and functional connectivity.
(a) Amygdala response to increasingly negative emotional stimuli in the Sleep deprivation and Sleep rested (control) conditions, and (b) Corresponding differences in intensity and volumetric extent of amygdala activation between the two groups (average ± s.e.m. of left and right amygdala), (c) Changes in functional connectivity between the medial prefrontal cortex (mPFC) and the amygdala. With sleep, the prefrontal lobe was significantly connected to the amygdala, regulating and exerting and inhibitory top-down control, (d) Without sleep, however, amygdala-mPFC connectivity was decreased, potentially negating top-down control and resulting in an overactive amygdala. *p < 0.01; error bars indicate s.e.m. Modified from (Yoo et al 2007).
While the majority of studies to date have focused on changes within the central nervous system (and specifically brain), it is of note that such central alterations are paralleled by changes in peripheral nervous system physiology. One night of sleep deprivation amplifies pupil diameter responses—an index of peripheral autonomic nervous system reactivity—during the passive viewing of negative emotional picture stimuli (Franzen & Buysse 2008). Congruently, sleep deprivation also increases sympathetic dominance of the autonomic nervous system, indexed by changes in heart rate variability (Sauvet et al 2010, Zhong et al 2005). The latter is important, since this sympathetic bias is associated with a lack of flexibility and capacity to respond to emotional challenges, and has been positively associated with psychopathology (Appelhans 2006).
Growing evidence suggests that sleep loss imposes a bi-directional nature of affective imbalance, additionally triggering excessive reactivity to positive, reward-relevant stimuli. One night of sleep loss enhances reactivity throughout regions of the dopaminergic mesolimbic systems in response to pleasure-evoking emotional picture stimuli (Gujar et al 2011). As with aversive reactivity, this enhanced mesolimbic reward sensitivity is further associated with decreased functional connectivity in regions of the medial and orbital prefrontal cortex. Similar enhanced mesolimbic reactivity following sleep deprivation can occur using monetary reward incentive paradigms (Libedinsky et al 2011, McKenna et al 2007, Venkatraman et al 2007). Beyond more abstract reward stimuli, such as money, this impact of sleep loss extends to more primary reward-motivated behaviors – that of appetitive food desire. Both acute and chronic sleep loss are associated with elevated reactivity to food stimuli in salience and hedonic regions of the striatum and amygdala, together with blunted activity in appetitive choice and decision making regions of the frontal cortex (Benedict et al 2012, Greer et al 2013, Killgore et al 2013, St-Onge et al 2012). Moreover, these neural changes are accompanied by an increased preference for higher calorie foods (Greer et al 2013) and greater tendencies to overeat (Killgore et al 2013).
In addition to changes in neural reactivity at the time of experiencing an emotional event, sleep loss further alters the preemptive neural anticipation of impending emotional experiences in both the amygdala and anterior insula cortex (Goldstein et al 2013). Of special clinical relevance, trait anxiety levels predict inter-individual differences in the extent of this exaggerated sleep deprived anticipation response (Goldstein et al 2013). Specifically, high trait anxious participants express the most severe increase in anticipatory reactivity under conditions of sleep loss; concerning considering these are already the individuals at greatest risk for developing an anxiety disorder.
Amplified anticipatory responding following sleep deprivation is also observed for reward-relevant cues. One night of sleep deprivation elevates anticipatory activity within the reward-sensitive region of the striatum to monetary decisions that can lead to either future reward payoffs or losses – especially when those gambles are risky (Venkatraman et al 2007). Consistent with studies discussed earlier regarding appetitive food stimuli, the increases in subcortical striatal reactivity following sleep deprivation co-occurs with blunted activity in anterior insula cortex and orbitofrontal cortex, specifically to monetary losses. Thus, sleep deprivation appears to trigger a state were rewards are overvalued (increased striatal sensitivity), yet losses (through punishment) are undervalued.
Taken as a whole, these data establish that insufficient sleep exaggerates subcortical limbic and striatal responses to both negative and positive affective stimuli, commonly associated with impoverished prefrontal cortex activity and/or connectivity. The consequence appears to be a pendulum like, bi-directional state of emotion imbalance at both ends of the valence spectrum, fitting early anecdotal reports of affective liability following a lack of sleep (Dahl 1996). Furthermore, this exaggerated reactivity can if cued, be observed preemptively, before the emotional stimulus itself.
Such a model of altered emotion reactivity following sleep loss is of translational relevance for at least three clinical areas. First, remarkably similar patterns of altered mesolimbic system emotion reactivity, as well as limbic-prefrontal cortex connectivity, have been reported in several affective psychopathologies, including major depression, bipolar disorder, generalized anxiety disorder and PTSD (Davidson 2002, Drevets et al 2008, Etkin 2010, Etkin & Wager 2007, Nitschke et al 2009, Paulus & Stein 2006, Rauch et al 2000, Shin et al 2006, Siegle et al 2007). Crucially, every one of these clinical conditions expresses highly comorbid sleep disruption (Harvey 2011, Peterson & Benca 2006), and in some of these disorders, sleep abnormalities form part of their diagnostic criteria. Considering the overlap between the neural correlates of such conditions that demonstrate co-occurring sleep disruption, and the patterns of neural dysfunction that can be experimentally induced by sleep deprivation, it raises the important issue of whether sleep loss plays a causal role in the etiology of these conditions. Moreover, should sleep be a contributing factor, it would represent a novel treatment intervention target (Harvey et al 2011).
Second, in the context of reward sensitivity, sleep disturbance is a recognized hallmark of addiction (Arnedt et al 2007, Brower & Perron 2010, Dimsdale et al 2007, Pace-Schott et al 2005), leading to the recent proposal that sleep loss represents a common and reliable predictor of relapse in numerous addiction disorders (Brower & Perron 2010). Additionally, a prospective study has demonstrated that sleep problems assessed during childhood significantly predict early onset of drug and alcohol use years later in adolescence, even when controlling for effects of anxiety and attention deficits (Volkow et al 2009). As such, the mesolimbic dopaminergic system appears to represent one common pathway through which the effects of sleep loss and heightened addiction sensitivity can be understood. The experimental evidence discussed earlier, demonstrating an interaction between a lack of sleep and enhanced mesolimbic reward-reactivity, implicates sleep loss as a predisposing and causal (rather than co-occurring) risk factor in heightened responsivity and hence acquired addiction potential to reward-stimulating drugs. Moreover, beyond acquisition, they further indicate a possible role for sleep disruption in the maintenance of addiction habits, especially during attempted withdrawal, leading to higher relapse rates.
Third, while anticipation is generally an adaptive process, aiding preparatory responses to potentially threatening or rewarding events, exaggerated expectancy activity, such as that observed following sleep deprivation, can be maladaptive. In the context of aversive events, increased anticipatory limbic activity positively predicts clinical features of anxiety disorders, such as worry and rumination (Etkin & Wager 2007, Nitschke et al 2009, Paulus & Stein 2006), many of which express co-occurring impairments in the quantity and quality of sleep (Papadimitriou & Linkowski 2005). Perhaps more concerning is that individuals with higher levels of trait anxiety and are therefore already at higher risk for developing an anxiety disorder, appear to be the most vulnerable to these anxiogenic effects of insufficient sleep (Goldstein et al 2013).
Emotion recognition and expression
Intriguingly, a number of studies have reported what may at first be considered a paradoxical blunting, rather than over-estimation, in the subjective rating of emotions in others by sleep-deprived participants. For example, sleep deprivation decreases the subjective intensity ratings of threat-relevant (angry) and reward-relevant (happy) static facial expressions (van der Helm et al 2010). Sleep loss additionally decreases the outward expression of emotion by sleep-deprived individual themselves, as judged by expert raters (Minkel et al 2011). Similarly, decreases in the vocal expression of positive emotion by deprived participants is observed after a single night of sleep loss, suggesting that multiple routes of emotional expression (e.g. facial muscles, vocalizations) are compromised by insufficient sleep (McGlinchey et al 2011). In addition to diminishing outward emotive expression, sleep deprivation also slows the generation of facial reactions in response to visual presentation of faces (Schwarz et al 2013). Of concern, insufficient sleep appears to trigger as much, if not more, of an impact on emotional expression in young children. Recent evidence demonstrates that three-year-olds who do not obtain an afternoon nap show dysregulation of both positive and negative emotional expression in response to emotional stimuli, relative to those who have obtained a nap (Berger et al 2012).
The potential disparity between these impairments and the objective neural data described earlier, which have reported amplifications (rather than impairments) in limbic brain reactivity following sleep deprivation can be reconciled when considering the concomitant neural impairments in the prefrontal cortex. Not only are prefrontal regions implicated in top-down regulatory control of subcortical limbic networks, they critically integrate primary affective signals arising from these subcortical systems (such as the brainstem, limbic system and basal ganglia) into second-order maps of the internal state of the organism (Craig 2010, Craig 2011, Critchley 2005, Critchley 2009, Harrison et al 2010). It has been argued that only through such mapping and hence appreciation of the current state of the body in the frontal lobe, can the brain select appropriate behavioural actions for the organism (actions that include emotion expression) (Craig 2010, Craig 2011, Critchley 2005, Critchley 2009, Harrison et al 2010). Set against this evidence, the above disparate findings may be resolved. Specifically, the sleep-deprived brain may suffer a mismatch between excessive subcortical reactivity yet impaired higher-order prefrontal functioning, the latter preventing optimal integration and hence use of the former, as well as control over the former. As a consequence, there can be a failure of affectively guided judgments, decisions and, down-stream, behavioural emotive (re)actions.
Benefits of Sleep on Emotional Brain Function
Fear Conditioning: Acquisition, Generalization and Extinction
Beyond emotion reactivity, recognition and expression, sleep has further been demonstrated to play an influential role in modulating conditioned fear. In classical fear conditioning paradigms, neutral items (e.g. a tone) are repeatedly paired with a coinciding noxious event (unconditioned stimulus; e.g. electric shock), triggering fear reactions. After an association is formed between these two elements, the presentation of the previously neutral item alone (now referred to as the conditioned stimulus – the tone, in this example) is sufficient to elicit a conditioned fear response. However, if this conditioned stimulus is subsequently re-presented (the tone), but now in the absence of the coinciding unconditioned stimulus (the shock), the fear response to the conditioned stimulus (the tone) gradually dissipates—a process known as extinction. Importantly, extinction is not accomplished by “unlearning” the old fear association, but instead, by new learning of fear inhibition (Phelps et al 2004). Beyond learning such direct associations, contextual cues can also modify fear responses. After fear conditioning, even the testing environment itself—where the conditioned stimulus (the tone) is associated with the unconditioned stimulus (the shock) —can signal an unsafe context, and trigger a fear reaction. Conversely, a safe context that was not previously associated with the conditioned-unconditioned stimulus pairing, such as a novel or extinction environment, can promote inhibition of the fear response, even when the conditioned stimulus (tone) is presented. In the following sections, we describe the impact of sleep and sleep loss on 1) the consolidation of fear conditioned learning, 2) the extinction of conditioned fear, and 3) the appropriate maintenance or inhibition of fear responding depending on unsafe or safe contexts.
Focusing first on consolidation a night of sleep consolidates and strengthens conditioned fear responses (neural, physiological and behavioral) compared to the absence of intervening sleep, and as a consequence, results in superior discrimination of fear-related from non-fear-related cues (Menz et al 2013). Furthermore, the magnitude of this sleep-dependent beneficial fear discrimination was positively predicted by the amount of intervening REM sleep (Menz et al 2013). Together these findings suggest that sleep, and specifically REM sleep, consolidates conditioned fear memories, allowing for an improved next-day sensitivity to discriminate between threating and non-threating stimuli.
In addition to strengthening fear memories, sleep also adaptively facilitates the subsequent extinction of conditioned fear; a process that is know to be accomplished by way of top-down PFC inhibition of the amygdala. An intervening period of sleep, and specifically one containing REM sleep, subsequently promotes more rapid diminution of fear responses (here, skin conductance), and with it, the beneficial re-engagement of ventromedial PFC (vmPFC) involvement during post-sleep fear extinction recall (Spoormaker et al 2012) The latter finding is relevant considering the vmPFC is a region know to be necessary for successful development of lasting fear extinction (Phelps et al 2004). Combined with the findings described above, these data indicate that intervening sleep not only strengthens conditioned fear, but also primes the neural mechanisms to extinguish fear the next day, should experience dictate it. Moreover, that the extent of these benefits appear to correlate with the amount of REM sleep is perhaps no coincidence: the brain areas recognized to support fear acquisition and extinction (mPFC, amygdala, hippocampus) are all reactivated during REM sleep (Dang-Vu et al 2010, Miyauchi et al 2009, Nofzinger 2005, Phelps et al 2004), and through such REM sleep brain network restoration, may aide the appropriate return of top-down PFC action on the amygdala, governing these processes (van der Helm et al 2011).
Beyond simply strengthening or extinguishing fear responses, recent evidence demonstrates that sleep, relative to time spent awake, preferentially modifies the appropriate expression of fear, depending on whether the conditioned stimulus (the tone) is presented in an unsafe or a safe surrounding context (Menz et al 2013, Pace-Schott et al 2009). As a consequence, individuals who sleep demonstrate significantly more adaptive expressions of fear, either maintaining or inhibiting fear responses, depending on the presence of unsafe or safe contextual cues, respectively (Menz et al 2013, Pace-Schott et al 2009). Sleep therefore facilitates the most appropriate or “intelligent” expression of conditioned fear, based on environmental information signaling threat or safety.
As a whole, these data from fear conditioning experiments indicate that sleep, including REM sleep, supports adaptive fear responding across numerous levels. The benefits of such processes encourage proper generation and maintenance of fear responses in dangerous situations, while inhibiting fear responses to non-dangerous situations. In contrast, sleep deprivation impairs these same processes due, in part, to a loss of top-down prefrontal cortex control of subcortical limbic regions. This is particularly relevant in a clinical context since deficits in the extinction and ability to appropriately utilize surrounding contextual information underlie fear-related disorders such as specific phobia and PTSD (Phelps et al 2004), the latter associated with marked sleep disruption (Germain 2013). Early evidence already suggests treatment intervention promise: patients who slept immediately after exposure treatment—a form of fear extinction learning—showed greater subjective reductions in anxiety and negative cognition one week after treatment, relative to those who remain awake for some time after treatment (Kleim et al 2013).
Emotional Memory
REM sleep further plays an influential role in processing of affective information beyond basic fear conditioning, particularly in the “offline” consolidation of emotional episodic (autobiographical) experiences (for a review see (Payne & Kensinger 2010, Walker 2009, Walker & van der Helm 2009)). Early work demonstrated that such offline emotional memory consolidation could be attributed to late-night sleep, a time period rich in REM sleep (Wagner et al 2001). Moreover, this preferential emotional memory retention persists years later (Wagner et al 2006). Even individual emotional elements from a single affective experience can be selectively discriminated and retained during sleep. By experimentally varying the foreground and background elements of photographs, sleep targets the retention of emotional foreground objects within scenes, relative to either non-emotional foreground items or the peripheral background elements of the same scenes (Payne et al 2012). Thus, sleep (and not wake) can separate emotionally relevant from irrelevant components of a single memory experience for selective consolidation (Payne et al 2012).
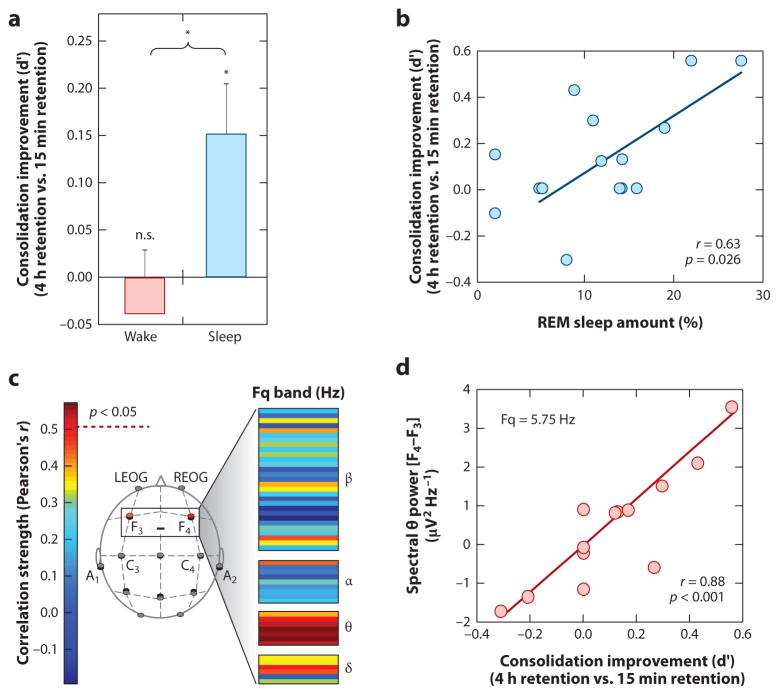
Mechanistically, not only does the amount of time and speed of entry into REM sleep predict the degree of subsequent strengthening and hence offline consolidation of emotional (and not neutral) memory (Figure 2a&b), but the amount of EEG theta activity (4 – 7 Hz) – a dominant electrical oscillation of REM sleep expressed over the prefrontal cortex – predicts the overnight emotional memory retention benefit (Figure 2c&d) (Nishida et al 2009). These findings have led to the proposal that REM sleep represents a neurobiological brain-state particularly amenable to selective emotional memory processing (Hu et al 2006, Pare et al 2002, Walker 2009, Walker & van der Helm 2009), with theta oscillations proposed as a carrier frequency that allows disparate brain regions that initially encode different aspects of the emotional experience (e.g. visual elements, auditory elements, olfactory elements etc.) to selectively interact offline. By doing so, REM sleep theta may afford the ability to discriminately select and strengthen distributed aspects of specific emotional memory representations across related but different anatomical networks, and/or promote their integration into pre-existing autobiographical memory networks (Cahill 2000, Jones & Wilson 2005).
Figure 2. REM sleep enhancement of negative emotional memories.
(a) Offline benefit (change in memory recall for 4 hr versus 15 min old memories) across the day (wake, grey bar) or following a 90 min nap (sleep, filled bar); (b) Correlation between the amount of offline emotional memory improvement in the nap group (i.e. the offline benefit expressed in filled bar of figure a), and the amount of REM sleep obtained within the nap; (c) Correlation (Pearson’s r-value) between offline benefit for emotional memory in the sleep group (expressed in filled bar of figure a) and the relative right versus left prefrontal spectral-band power ([electrode F4 – electrode F3]) within the delta, alpha, theta and beta spectral bands, expressed in average 0.5 Hz bin increments. Correlation strength is represented by the color range, demonstrating significant correlations within the theta frequency band (hot colors), and (d) exhibiting a maximum significance at the 5.75 Hz bin. *p < 0.05; error bars indicate s.e.m. Modified from (Nishida et al 2009).
It is also possible that emotional memory consolidation during sleep receives an additional contribution from (although is not necessary for) the well-known changes in glucocorticoids (cortisol in humans). In humans, cortisol secretion and REM sleep correlate in their incidence during the night, with both dominating early in the morning close to the time of awakening. Cortisol is also released in response to stressful events (Sapolsky et al 2000), and can independently modulate emotional memory through direct and indirect effects on the hippocampus and amygdala (McIntyre et al 2012, Roozendaal et al 2009, Sapolsky et al 2000). Moreover, experimentally increasing and decreasing cortisol concentrations prior to sleep can bi-directionally alter the magnitude of emotional memory consolidation during sleep (van Marle et al 2013, Wagner et al 2005). However, it should also be noted that the impact of REM sleep on memory is not dependent on circadian regulation of cortisol: emotional memory consolidation associated with REM sleep also occurs following an afternoon nap that is decoupled from circadian timing of cortisol (Nishida et al 2009).
Together, these results demonstrate that REM sleep preferentially governs the long-term consolidation of emotional episodic memory experiences; an effect that may further be facilitated by (although can nevertheless act independently of) variations in glucocorticoids that coincide with nocturnal REM sleep. However, strengthening the memory of emotional experiences appears to be only one of at least two benefits that REM sleep offers, as we next describe.
REM Sleep Homeostasis of Affective Brain Function
Building on this collection of empirical evidence, combined with the well-characterized neurobiology of sleep and emotional processing in the brain, in the following sections we set forth a REM sleep emotional homeostasis hypothesis. From this framework emerge two unique, non-mutually exclusive, brain benefits of REM sleep that promote adaptive emotional next-day functioning. The first describes a function of REM sleep after an emotional experience, helping resolve strong emotions associated with those challenging memories. Specifically, REM sleep is proposed to strip away the visceral charge (the emotion) from affective experiences of the prior day(s), depotentiating their emotional strength while still consolidating the information (the memory) contained within that experience – a form of “overnight therapy”. The second outlines a role for REM sleep before an emotional experience, recalibrating the sensitivity and specificity of the brain’s response to initial emotional events. As a consequence REM sleep primes key brain regions to appropriately react to affective experiences, allowing accurate and adaptive next-day discrimination of one emotional experience from another by faithfully registering their respective salience values.
Emotional memory resolution: Sleeping to Forget and Sleeping to Remember (SFSR)
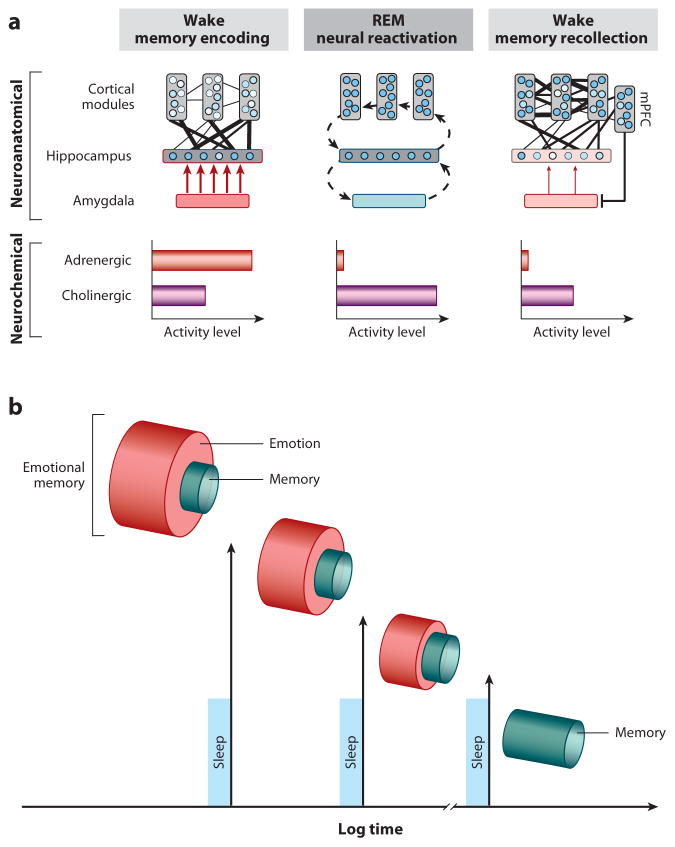
Our first proposed function of REM sleep takes place after the occurrence of emotional events, and involves aiding the reprocessing of prior affective experiences, including those most traumatic. Although there is abundant evidence to suggest that emotional experiences persist in our autobiographies over time (strengthening of the memory) (Dolcos et al 2005), an equally remarkable but less noted change is a reduction in the affective tone associated with their recall (depotentiation of emotion). Affective experiences appear to be remembered more robustly than neutral memories due to well characterized set of adrenergic and peripheral autonomic reactions elicited at the time of the experience, orchestrated, in part, by the locus coeruleus (McGaugh 2004). These adrenergic bursts at the time of affectively charged experiences are believed to adaptively prioritize the formation (and hence long-term retention) of such salient information within the brain, creating what is commonly termed an “emotional-memory” (Figure 3a). However, the later recall of these memories tends not to be associated with the same magnitude of autonomic (re)activation as that elicited at the moment of experience – suggesting that, overtime, the affective “blanket” (the emotion) that originally tagged the memory at the time of learning has been removed, whereas the information of the experience (the memory) remains (Figure 3b). We propose here (and elsewhere (Van der Helm 2012, Walker 2009, Walker & van der Helm 2009)) that the unique neurobiological state of REM sleep supports decoupling of emotion from memory such that we sleep to forget the emotional tone, yet sleep to remember the memory of that experience. This model further posits that if this process is not achieved, the magnitude of affective “charge” would persist, resulting in a condition of chronic anxiety within autobiographical memory networks.
Figure 3. The sleep to forget and sleep to remember (SFSR) model;
(a) Neural dynamics. Emotional memory formation involves the encoding of hippocampal-bound cortical information, facilitated by the amygdala and high concentrations of aminergic activity. During REM sleep, these neural structures are reactivated, supporting the reprocessing of emotional memories. However, this occurs in a brain-state with dramatically reduced adrenergic activity, allowing for both cortical strengthening (consolidation), dissipation of previously associated emotion (visceral tone), and reestablished mPFC-amygdala regulatory control. Cross-connectivity between structures is represented by number and thickness of lines. Circles within cortical and hippocampal structures represent information nodes; shade strength reflects extent of connectivity. Fill of amygdala and arrow thickness represents influence upon the hippocampus. (b) Conceptual outcome. Through multiple iterations of this REM-mechanism across one or multiple nights, such sleep-dependent reprocessing results in long-term strengthening of salient memories, yet a dissipation of the emotional charge. Thus, sleep transforms an emotional memory into a memory of an emotional event, that itself is no longer emotional.
Three specific biological features of REM sleep are proposed to provide an optimal biological milieu within which this form of “overnight therapy” can be achieved: neuroanatomical, neurophysiological and neurochemical (Figure 3a). First, the prominent increase in activity within limbic and paralimbic structures during REM sleep (Nofzinger 2005) supports the ability for reactivation and hence (re)processing of previously acquired affective memories. Second, the neurophysiological signature of REM sleep involving dominant theta oscillations within subcortical as well as cortical nodes offers large-scale network cooperation during REM sleep for the strengthening of distributed aspects of the emotional memory representation (e.g. perceptual, contextual), across such related but different anatomical networks. This then results in enhanced consolidation and integration of that memory. Third, these interactions during REM sleep (and perhaps through the conscious process of dreaming) critically and perhaps most importantly take place within a brain that is low in aminergic neurochemical concentration (Pace-Schott & Hobson 2002), particularly the suppressed noradrenergic input from the locus coeruleus (associated with stress and anxiety responses when highly active) (Itoi & Sugimoto 2010, Ramos & Arnsten 2007, Sullivan et al 1999, Valentino & Van Bockstaele 2008). Therefore, REM sleep is proposed to offer a unique biological condition in which to achieve, on one hand, strengthening and consolidation of the informational core of emotional experiences (the memory), yet additionally depotentiate and ultimately ameliorate the autonomic arousing charge originally acquired at the time of learning (the emotion). Through the process of developing stronger cortico-cortical connections, integration and assimilation of the affective event(s) in the context of past knowledge is supported. As a result, emotional experiences are preferentially retained long-term, but importantly the emotion, which was initially critical to signify salience and priority at the time of learning, has been dissipated. The brain therefore preserves a memory of an emotional event, but which itself is no longer emotional.
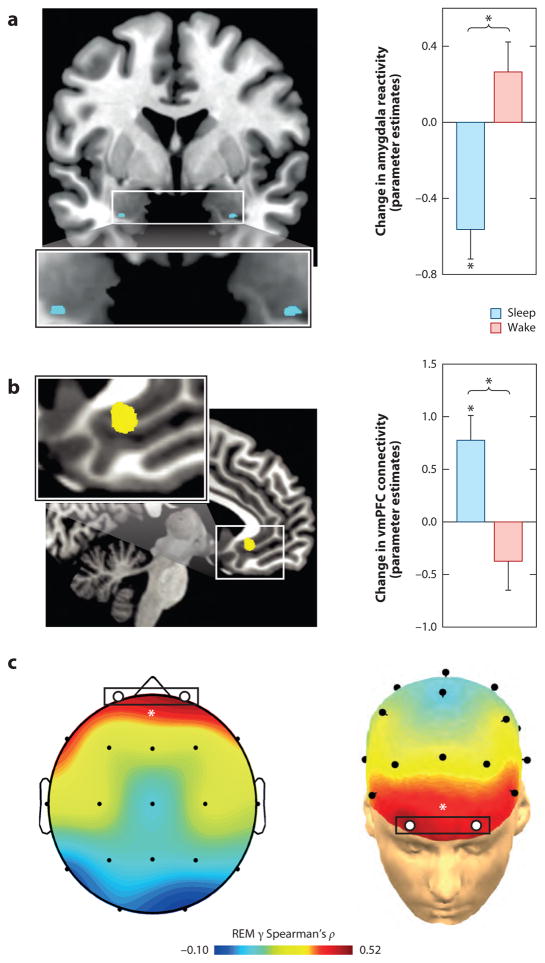
Specific predictions emerge from this model. As partially demonstrated (see Emotional Memory section), the first prediction is that the degree to which the information of those emotional experiences are retained, long-term, would be proportional to the amount of post-encoding REM sleep obtained, how quickly it is achieved (REM latency), as well as the power of theta oscillations during REM. Evidence for all three of these predictions exists (Nishida et al 2009, Pare et al 2002). Second, the inverse REM-relationship would hold for the magnitude of emotional depotentiation after sleep. This too appears to be the case; compared to those that remained awake, individuals who slept prior to re-exposure displayed a significant overnight decrease in amygdala response to previously seen emotional images (Figure 4a), together with a concomitant increase in amygdala-mPFC connectivity (Figure 4b). Furthermore, sleep also results in a significant dissipation of subjective emotional intensity ratings, relative to the equivalent period of wake. Additionally, the success of overnight emotion depotentiation at both a brain (amygdala) and behavioral (intensity ratings) level is predicted by gamma EEG activity – a validated but indirect measure of central adrenergic activity (Berridge & Foote 1991, Cape & Jones 1998, Keane et al 1976). Specifically, those participants that express the lowest REM gamma, show the greatest beneficial overnight reduction in emotion intensity (Figure 4c). Similarly, a daytime nap dissipates the intensity ratings of previously exposed negative emotional face expressions (Gujar et al 2010). However, not all participants who slept demonstrated this resetting of emotional reactivity. Instead, only those who obtained REM sleep during the nap displayed such a change in emotional sensitivity to the re-experience of the same previous face stimuli (Figure 5). Further fitting with a role for sleep in emotional depotentiation, if participants are not allowed to sleep the first night after being exposed to aversive emotional stimuli, there is a failure of subsequent amygdala depotentiation to the same emotional stimulus during a memory test, even following two nights of recovery sleep (Sterpenich et al 2007). The latter finding is relevant clinically, and argues against some proposals for the use of sleep deprivation after trauma experience to facilitate forgetting. Our model would argue the opposite; sleep deprivation would prevent the beneficial removing of emotion from the memory. Furthermore, while the emotion of the trauma can be clinically problematic, the information from the experience (the memory) is nevertheless useful, helping guide future actions based on past experience that may prevent experiencing the same negative experience. Thus, it is the emotion that needs to be targeted, not the memory.
Figure 4. REM sleep depotentiates amygdala reactivity to prior emotional experiences.
(a) Change in emotion reactivity: group x test session interaction in bilateral amygdala (blue), demonstrating a significant decrease in activity across a night of sleep in the sleep group, yet an increase in the wake group across a day of wake. (b) Change in functional connectivity: group x test session interaction in amygdala-ventromedial prefrontal cortex (vmPFC) connectivity (yellow), demonstrating increased connectivity from after a night of sleep yet decreased coupling after an equivalent time of wake. (c) Topographical Spearman’s correlation (ρ) plot of the relationship between electroencephalographic (EEG) gamma power during rapid-eye movement (REM) sleep and the extent of overnight emotional reactivity decrease across a night of sleep, with lower levels of prefrontal gamma activity (marked by white circles) predicting a larger overnight decrease in emotional reactivity. * p < 0.05. Modified from (van der Helm et al 2011).
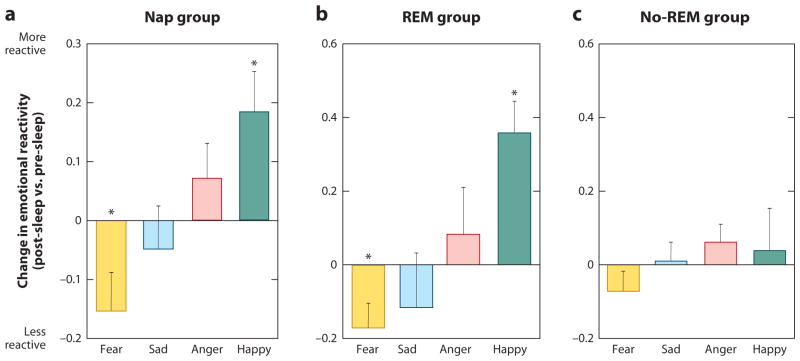
Figure 5. Differential impact of REM sleep on emotional reactivity.
Difference in mean ratings between the pre-sleep and post-sleep test sessions across 4 emotion categories (fear, sad, anger, and happy) for (a) the Nap group overall performance, (b) only for those in the Nap group who obtained REM sleep, and (c) only for those in the Nap group who did not obtain REM sleep. Within-group comparisons (symbol above individual bars) reflect paired t-test significance (relative to null) at *<0.05 and **<0.01. Error bars represent s.e.m. Modified from (Gujar et al 2010).
In summary, these studies provide support to the proposed neurobiological model of sleep-dependent emotional memory depotentiation, one in which REM sleep is capable of divorcing the visceral affective charge from prior emotional experiences, while preserving the information of those episodes.
REM Sleep Emotion Recalibration
Our second proposed function of REM sleep takes place before the occurrence of next-day emotional events, restoring optimal emotional reactivity. Adaptive emotional responding requires accurate discrimination between salient stimuli (a potential threat to be avoided, or reward to be approached) and non-salient stimuli within the environment. Failures in the ability to differentiate salient from non-salience information can lead to maladaptive behaviors, such as generating excessive fear responses to non-dangerous situations (e.g. a car backfiring). Based on marked changes in adrenergic neurochemistry described earlier, we propose that REM sleep offers an overnight resetting function within key brainstem, limbic and prefrontal brain regions. The functional consequence of this nightly recalibration is the restoration of next-day emotional brain sensitivity and specificity necessary to guide appropriate decisions and actions.
Three lines of evidence support the hypothesized capacity of REM sleep, which we describe in detail below: (1) the active role of REM sleep in maintaining proper waking noradrenaline tone, (2) that a lack of sleep, including REM sleep, alters brainstem noradrenergic cell firing, producing a profile of signal-to-noise activity that prevents accurate registration and discrimination of emotional salience, and (3) that altered noradrenergic brainstem activity caused by sleep loss, and restored by sleep, directly and indirectly modulate the responsivity profiles of the amygdala and mPFC; two regions critically involved in detecting emotional salience.
First, the defining neurochemical feature of REM sleep compared to NREM or wake is the lack of locus coeruleus firing and thus release of it’s principal neurochemical, noradrenaline (Ouyang et al 2004, Park 2002, Shouse et al 2000). As a result, the near absence of noradrenergic concentration permeating the brain during REM sleep is potentially capable of restoring post-sleep waking levels of noradrenergic tone within the brain (Kametani & Kawamura 1990, Mallick & Singh 2011, Marrosu et al 1995, Siegel & Rogawski 1988). Conversely, a lack of REM sleep, by way of selective deprivation, increases central noradrenaline concentrations to levels that exceed those of rested waking brain function (Mallick & Singh 2011, Siegel & Rogawski 1988). Thus, a dose dependent relationship may exist between the quantity/quality of REM sleep and the decrease of noradrenaline across the night. Moreover, an increase in REM sleep quantity/quality at night, either through pharmacological intervention (Stern & Morgane 1974, Taylor et al 2008) or through psychopathology states such as major depression (Blier & Briley 2011, El Mansari et al 2010, Hamon & Blier 2013, Ordway et al 2003), would predict a proportional decrease of noradrenergic tone the next day, and with it, alterations in emotional brain function. Therefore, REM sleep may serve an noradrenergic “housekeeping” function, one that reduces and thus restores concentrations of noradrenaline to baseline each day (in humans), allowing for optimal waking functioning (Mallick & Singh 2011, Siegel & Rogawski 1988).
Second, experimental manipulations that evoke elevated noradrenaline levels, similar to those induced by sleep loss, impair the salience sensitivity and specificity of locus coeruleus responding. During wake, noradrenaline neurons within the locus coeruleus display two distinct modes of overall activity. In one of these modes, the locus coeruleus responds in a predominantly phasic manner i.e. selectively, to salient stimuli within the environment (i.e. salience “signal”), while maintaining a low level of baseline (tonic) ongoing activity (i.e. background “noise”) (Aston-Jones & Cohen 2005, Mallick & Singh 2011, Valentino & Van Bockstaele 2008). Thus, the overall threshold of reactivity to external events is optimal along a gradient of potential emotional strengths (Figure 6a). However, the second mode of locus coeruleus activity, one that can occur under specific conditions (e.g. stress), is characterized by a shift to high levels of persistent baseline tonic firing and elevated noradrenaline levels. As a consequence of this high tonic background activity, the phasic signals in response to external emotional stimuli result in poor signal-to-noise within the system, and thus reduced specificity (Figure 6b) (Aston-Jones & Cohen 2005, Mallick & Singh 2011, Valentino & Van Bockstaele 2008). It is precisely this pattern of locus coeruleus activity and associated increased concentrations of noradrenaline that develop under conditions of sleep deprivation (Mallick & Singh 2011). It is therefore possible that the changes in the noradrenergic system caused by sleep deprivation produce a hypervigilant brain state far less able to discriminate salient from non-salient stimuli (Figure 6b). Conversely, the reduced next-day noradrenergic concentrations resulting from a recalibration during prior REM sleep (Mallick & Singh 2011) may advantageously restore this system, promoting the return of low tonic and strong phasic activity necessary for accurate emotional sensitive and specificity.
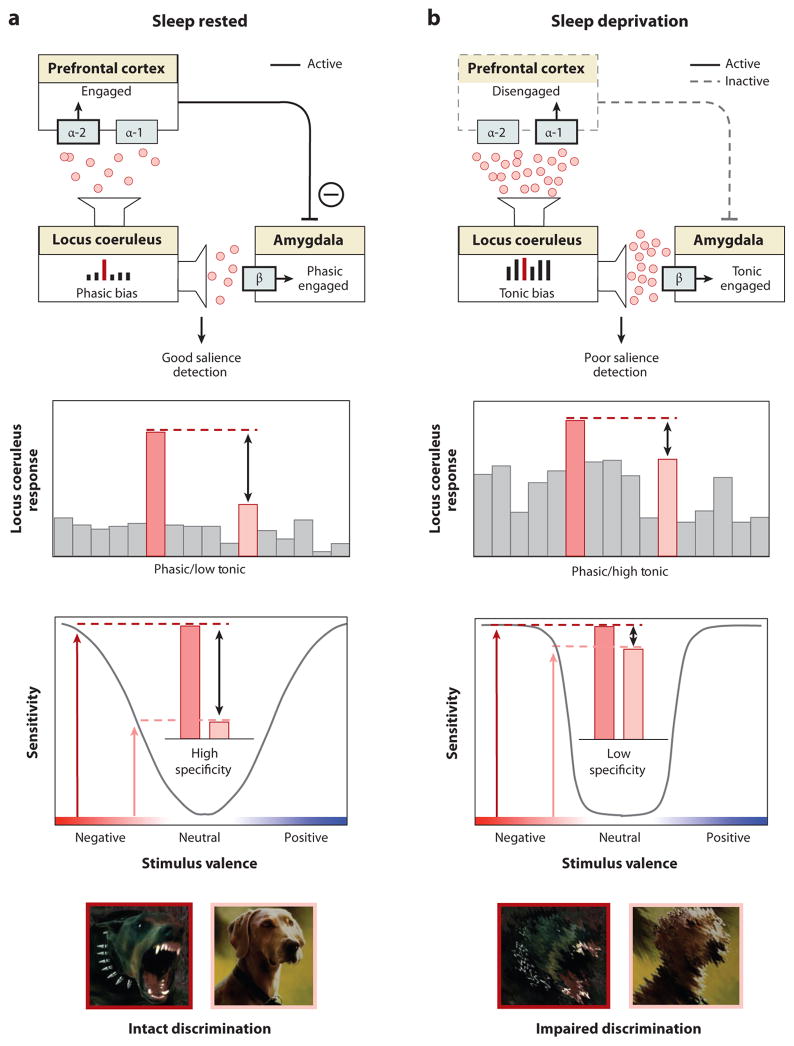
Figure 6. REM sleep emotion recalibration model.
(a) Under normal sleep rested conditions, the overnight reduction of noradrenaline levels promoted by REM sleep restores noradrenergic tone, resulting in a low tonic, high phasic locus coeruleus activity. This consequently primes downstream amygdala and PFC structures that, together with the locus coeruleus, form part of an emotional salience detection network (upper panel). Moderate next-day levels of noradrenaline, restored by REM sleep, induce PFC engagement through activation of the α-2 receptor, which in turn enables top-down PFC inhibition of the amygdala, reducing the likelihood of a non-specific amygdala response. Further, low tonic activity in locus coeruleus enhances the fidelity of phasic responses to external emotional stimuli, relative to the low baseline firing, promoting sensitivity to detect truly emotional stimuli, while also retaining the ability to discriminate between degrees of emotional salience (specificity) (middle panel). The functional outcome of this REM sleep recalibrated salience network state is the ability to discriminate between, and appropriately react to, emotionally salient events (e.g., threatening, aggressive dog) from non-salient stimuli (e.g., non-threatening dog) (lower panel). b) In contrast, sleep deprivation, results in high tonic locus coeruleus firing mode and increased noradrenaline concentrations, consequently impairing salience detection. Specifically, elevated noradrenaline levels increase binding to the inhibitory α-1 receptor in the PFC (upper panel). Unlike binding to α-2 receptors, α-1 receptor binding impairs PFC functioning and so reduces top-down inhibition of the amygdala. This disinhibition results in a non-specific, generalized amygdala responding to both emotionally salient and non-salient stimuli. Such impairments are further exacerbated by high tonic baseline locus coeruleus activity, which reduces the ability to differentiate phasic signals from high baseline activity (i.e. reduced signal-to-noise). In this mode of activity, the locus coeruleus responds non-specifically to both emotionally salient and non-emotionally salient information (middle panel). Together, these alterations to the emotional salience network caused by sleep loss impair the accurate discrimination of threatening from non-threatening stimuli, resulting in persistent sensitivity and in impaired emotional specificity (lower panel). Noradrenaline alpha-1 receptor (α-1), noradrenaline alpha-2 receptor (α-2) and noradrenaline beta receptor (β).
This model compliments a hypothesis posed by Siegel & Rogawski, which proposed a beneficial role for REM sleep in promoting optimal next-day noradrenergic signaling (Siegel & Rogawski 1988). However, the mechanism underlying this prior proposal of noradrenergic modulation by REM sleep is different to the current model. The former proposes a REM sleep action that alters noradrenaline receptor density, and by way of this structural change, adjusts noradrenergic action. In contrast, the current theory proposes a role for REM sleep in directly regulating the concentrations of noradrenaline itself, and through this functional (rather than structural) alteration, governs optimal next-day performance of the system.
The present model can provide mechanistic insight into how REM sleep, and conversely sleep deprivation, may influence salience detection in emotion brain regions beyond the locus coeruleus, specifically the amygdala and PFC. The locus coeruleus modulates amygdala primarily through the “beta” adrenergic receptor, while influencing the PFC through two types of alpha receptors (alpha1/alpha2; (Ramos & Arnsten 2007). Under conditions of low noradrenergic activity and low tonic/high phasic signaling promoted by REM sleep, the locus coeruleus facilitates selective amygdala responsivity to salient stimuli, in a phasic manner (through beta receptor control) (Figure 6a) (Hermans et al 2011, van Marle et al 2009). This profile of amygdala responsivity is further enhanced by the effects of the locus coeruleus on mPFC functioning, by acting on the alpha-2 PFC receptor (Figure 6a). In this mode of activity, the locus coeruleus increases mPFC engagement that enables top-down control of the amygdala, preventing the amygdala from responding non-specifically i.e. to non-salient stimuli (Aston-Jones & Cohen 2005, Ramos & Arnsten 2007). Thus, REM sleep optimally restores the emotional salience sensitivity and specificity of this adrenergic locus coeruleus-mPFC-amygdala functional network. In contrast, the state of sleep deprivation promotes high background level of tonic locus coeruleus firing and increased noradrenaline concentrations. This results in a similar tonic firing profile in the amygdala (Figure 6b) (again through the beta receptor type) (Mallick & Singh 2011, van Marle et al 2009, van Marle et al 2011), resulting in low emotional signal-to-noise reactivity, decreasing specificity (the ability to discriminate stimuli along a gradient of emotional strength) while maintaining or even elevating sensitivity (magnitude of reactivity) to salient events. Moreover, this situation is further exacerbated by high noradrenaline concentrations that now impair PFC function due to a shift in binding to the (inhibitory) alpha-1 receptor. As a result, there is diminished top-down control from the mPFC to the amygdala, releasing amygdala inhibition (Figure 6a) and resulting in further generalized amygdala responding. In summary, the quiescence of locus coeruleus activity during REM sleep throughout the night restores the appropriate next-day tonic/phasic response specificity within this emotional salience network (locus coeruleus, amygdala, PFC). The functional outcome of this recalibration is the precise capacity for responding to, and discriminating between, signals of varying affective importance, resulting in a balanced degree of emotion sensitivity as well as specificity (Figure 6a).
Several specific predictions arise from this framework. First, REM sleep should promote increased top-down PFC control of the amygdala while sleep deprivation should be associated with decreased connectivity between the PFC and amygdala, and exaggerated amygdala reactivity. Early evidence supports these predictions. Sleep deprivation has been demonstrated to decrease amygdala-PFC connectivity, relative to a full night of sleep (Gujar et al 2011, Yoo et al 2007). Moreover, a night of sleep restores amygdala-mPFC connectivity, the extent of which is selectively and specifically predicted by physiological quality of prior REM sleep (van der Helm et al 2011).
Second, sleep loss, including that of REM sleep, should impair the ability for emotional discrimination, while still resulting in as much (or more) outright sensitivity. It is just such a profile of heightened sensitivity (neural) to strongly emotional stimuli that has been observed at a neural level (Gujar et al 2011, Yoo et al 2007), together with decrease discrimination specificity at a behavioral level (van der Helm et al 2010, Yoo et al). It should be noted, however, that these data employ total sleep deprivation, and confirmation for the involvement of REM sleep (either through selective deprivation, or demonstrating that qualitative features of REM sleep reflective of reduced adrenergic tone, such as EEG activity) has yet to be published.
Third, achieving optimal noradrenergic recalibration by way of REM sleep should facilitate the appropriate down-stream physiological and behavioral responses to salient relative to non-salient stimuli. Indeed, in fear conditioning and extinction paradigms REM sleep has been associated with more nuanced fear responding (measured by subjective shock expectancy as well as skin conductance) capable of appropriately generating or inhibiting fear responses, depending on environmental cues signaling threatening or non-threatening circumstances. In contrast, sleep deprivation is associated with increased fear responding to non-threating cues (Menz et al 2013, Pace-Schott et al 2009, Spoormaker et al 2012, Spoormaker et al 2010), indicative of a failure to appropriately discriminate between threatening and non-threatening information.
Implications for psychiatric conditions
The empirical evidence reviewed in this manuscript, combined with these two proposed REM sleep functional models, suggest that pathological disruption to sleep, including REM sleep, should lead to maladaptive neural and behavioral emotional responding. From a clinical standpoint, this is of particularly relevance considering that numerous psychiatric disorders demonstrate (1) comorbid abnormalities in REM sleep, (2) alterations noradrenaline activity, and (3) corresponding disruptions in limbic-prefrontal functioning. Below, we focus discussion on two disorders where both these models may be of causal importance: (i) Post-traumatic stress disorder (PTSD), in which there is diminished and disrupted REM sleep leading to excessive noradrenergic tone, and (ii) Major depression, in which there may be excess REM sleep, and consequently diminutive noradrenergic tone.
Post-traumatic stress disorder
Building on emerging empirical evidence (Germain et al 2008, Spoormaker & Montgomery 2008), both the above-proposed REM sleep models have relevance to the etiology and pathophysiology of PTSD, as well as understanding recent pharmacological treatment interventions.
PTSD has been associated not only with decreases in the total time spent in REM sleep (Germain 2013, Lavie et al 1979, Mellman et al 1997), but also qualitative REM sleep abnormalities. These included marked fragmentation of REM sleep (Breslau et al 2004, Habukawa et al 2007, Mellman et al 2002) (indicative of arousal-related awakenings from REM sleep linked to adrenergic surges), and increases sympathetic tone of the autonomic nervous system (Harvey et al 2003, Mellman & Hipolito 2006) (reflective of heightening adrenergic activity). Of further relevance, both objective sleep disturbances occurring in the immediate aftermath of trauma exposure, as well as heightened sympathetic vagal tone during REM sleep, are associated with an increased risk of PTSD (Koren et al 2002, Mellman et al 2002). Congruently, the presence of insomnia in war veterans during the 4 months after deployment is a significant predictor of PTSD symptom development (Wright et al 2011).
These sleep abnormalities are also paralleled by significant alterations in the noradrenergic system in PTSD. Based on recent neuroimaging data, patients with PTSD express exaggerated locus coeruleus activity during REM sleep compared to controls, suggesting a persistence (rather than normal quiescence) of the noradrenergic system in REM sleep (Germain et al 2013). Consistent with this finding, PTSD patients do not show the normal overnight reduction in noradrenaline concentrations (as indexed by noradrenaline metabolite concentrations in urine), relative to daytime concentrations (Mellman et al 1995). Furthermore, the degree of the nocturnal noradrenaline alterations are negatively correlated with measures of sleep quality in both control and PTSD groups, such that greater noradrenaline concentrations across the night are associated with worse sleep quality (Mellman et al 1995). PTSD patients additionally experience elevated post-sleep noradrenaline concentrations in cerebrospinal fluid, the extent of which positively predicts the severity of PTSD symptoms (Mellman et al 1995). Given recent findings suggesting a similar hyper-noradrenaline profile following REM sleep deprivation (Mallick & Singh 2011), it is possible that the elevated noradrenergic activity in PTSD is a direct consequence of REM sleep disruptions.
These reported PTSD deficits in REM sleep amount, together with adrenergic activity during REM have significant implications regarding the two above proposed functional models of REM sleep. Regarding the first emotional memory depotentiation hypothesis, if the decoupling emotion from memory is not achieved across the first night following a trauma experience, as may occur in PTSD, the model predicts a repeat attempt of the affective demodulation on subsequent nights as the strength of the emotional “tag” associated with the memory would remain high. If this process fails a second time, the same events will continue to repeat across ensuing nights. It is just such a cycle of REM sleep dreaming (nightmares) that represents a diagnostic key feature of PTSD (Lavie 2001). It may not be coincidental that these patients additionally continue to display hyperarousal reactions to associated trauma cues (Harvey et al 2003, Pole 2007). It further suggests that the process of separating the affective tone from the emotional experience has not been accomplished, and is hence consistently re-lived during subsequent cued or deliberate waking recollection (Figure 7a). We offer the thesis that the PTSD pathological persistence of central brain adrenergic activity during REM sleep in particular, reflected in hyperarousal signals (Harvey et al 2003, Pole 2007, Strawn & Geracioti 2008) prevents the capacity of REM sleep to palliatively decrease the emotion from the traumatic memory, leaving some patients unable to depotentiate the emotion of this stored experience. Moreover, the consequential next-day persistent amygdala hyper-reactivity may further prevent the capacity for a return of adaptive amygdala-PFC connectivity and hence regulation.
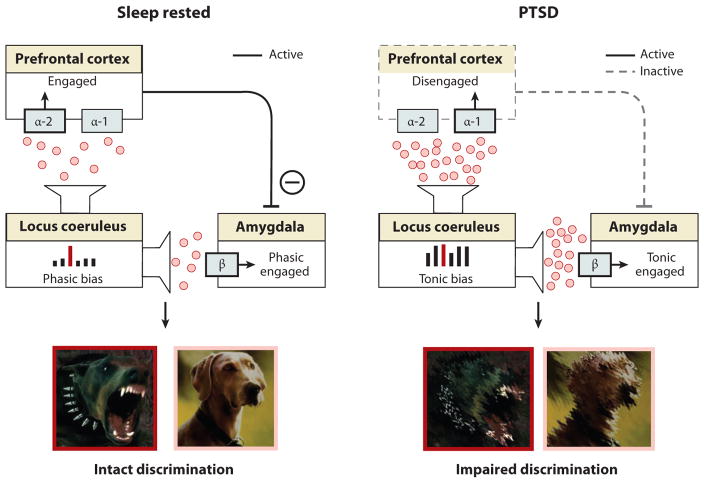
Figure 7. REM Sleep to forget sleep to remember model applied to PTSD.
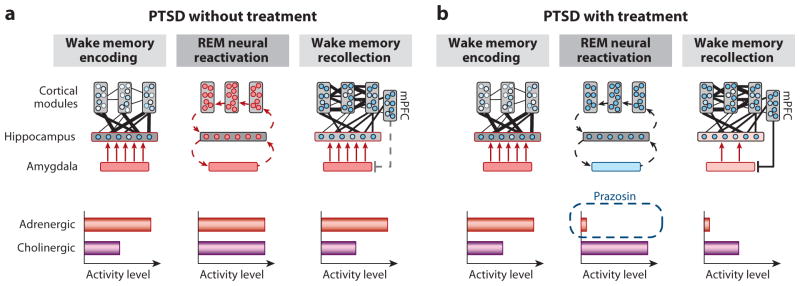
(a) REM sleep in PTSD without treatment, characterized by a pathological persistence of central brain adrenergic activity (note difference in middle panel to middle panel of Figure 3a). Elevated adrenergic activity during REM sleep prevents the depotentiation of the emotion tone associated with the salient, including traumatic, experiences. (b) REM sleep in PTSD with treatment by Prazosin, allowing for the reduction in adrenergic levels during REM sleep (note difference in middle panel between a and b). Consequently, there is the restored opportunity for the dissipation of emotion from the prior trauma experiences, preventing hyperarousal reactions during subsequent post-sleep memory recall.
The second model of REM sleep emotional recalibration would separately predict that the sleep disruption and related hyper-noradrenergic tone observed in PTSD should shift the locus coeruleus-amygdala-PFC network functioning into a mode of poor emotional stimulus specificity, yet persistent (or excessive) sensitivity (described in (Figure 8). Consistent with this prediction, common symptoms of PTSD not only include hyperarousal and an increased startle response, but also a generalization of the fear response to commonly non-threatening stimuli; the former two indicative of heightened sensitivity, the latter of decreased emotional specificity (Jovanovic et al 2012, Jovanovic et al 2009). Similarly, PTSD patients produce a fear response of equal magnitude to both threat and non-threat related stimuli, preventing emotion discrimination, while healthy controls show specificity only to threat cues (Grillon & Morgan 1999). Further supporting this theory, and potentially underlying the alterations in salience discrimination, PTSD patients express hyperactivity of the amygdala yet hypoactivity of the PFC; a similar profile to that observed in sleep-deprived healthy controls (Figure 8)(Motomura et al 2013, Yoo et al 2007). Thus, deficits of REM sleep in PTSD may prevent the normal overnight restoration of lowered adrenergic tone, and with it, result in an altered pattern of the locus coeruleus-amygdala-PFC activity, one that impairs emotional specificity and thus discrimination, leading to generalized and hyper-sensitive responding.
Figure 8. REM Sleep Emotion Recalibration model applied to PTSD.
Elevated noradrenergic activity during REM sleep in PTSD prevents the next day recalibration of the associated salience network, impairing optimal emotion discrimination, similar to the state if sleep deprivation (see also sleep deprivation profile in figure 6)
Finally, both models further predict that treatments dissipating adrenergic activity during REM sleep would have a therapeutic clinical outcome in PTSD. It is just such a benefit that appears to be represented by the recent pharmacological intervention success in PTSD patients using the drug Prazosin, which blocks the alpha-1 adrenergic receptor. Given nocturnally, patients with combat PTSD and civilian PTSD both demonstrate a restoration of REM sleep, together with decreases in trauma-dream symptomatology (Calohan et al 2010, Raskind et al 2007, Raskind et al 2003, Taylor et al 2008). The proposed REM sleep models offer putative underlying neurobiological mechanisms explaining this pharmacological treatment success (Figure 7b). Specifically, the nighttime blockade of central adrenergic activity during REM sleep in PTSD dissipates levels back to a potentially critical sub-threshold and normative level. As a consequence the pharmacologically imposed quiescence of the noradrenergic system at night would (1) allow the first stages of emotional (trauma) memory depotentiation during REM sleep to occur, and by doing so, improve clinical symptomatology associated with hyperarousal as well as trauma memory, and (2) additionally provide a recalibration of next-day noradrenaline levels that restore appropriate locus coeruleus-amygdala-PFC activity, allowing for appropriate sensitivity and specificity of emotion responding.
Major Depression
Major depression is associated with exaggerated REM sleep qualities, including (1) a faster entrance into REM sleep, (2) increased intensity of REM sleep (indexed in the number of rapid eye movements), and (3) longer duration of REM sleep periods (Armitage 2007, Buysse 2004, Franzen & Buysse 2008, Gottesmann & Gottesman 2007, Harvey et al 2003, Palagini et al 2013, Tsuno et al 2005). Major depression has also been linked to deficiencies in monoamine activity, including noradrenaline (Blier & Briley 2011, El Mansari et al 2010, Hamon & Blier 2013, Ordway et al 2003). In the context of the REM sleep recalibration model, the suppression of locus coeruleus activity occurring during REM sleep is predicted to cause a dose-dependent decrease in associated noradrenalin levels across the night. Therefore, the greater duration and/or intensity of REM sleep often reported in major depression may provide a mechanistic explanation for the abnormally low levels of noradrenaline commonly observed with this condition (Blier & Briley 2011, El Mansari et al 2010, Hamon & Blier 2013, Ordway et al 2003). Further, the resulting diminished noradrenaline concentrations caused by excess REM sleep would decrease binding to the alpha-2 receptor in the mPFC (Figure 9). As a consequence, there would be impaired mPFC engagement and top-down control of the amygdala (Aston-Jones & Cohen 2005, Ramos & Arnsten 2007). Due to this reduced noradrenaline availability and associated decreased mPFC activation, the REM sleep recalibration model would further predict increases in amygdala sensitivity to non-salient information in major depression, as well as impairments in emotional salience discrimination. Emerging evidence suggests exactly this profile: patients with major depression express non-specific amygdala reactivity that does not discriminate between negative, neutral and positive images (Ritchey et al 2011). Thus, the profile of generalized amygdala responsivity observed in major depression may, in part, be driven by the blunting of noradrenaline activity caused by excess amounts of REM sleep, altering PFC-amygdala emotional sensitivity and specificity.
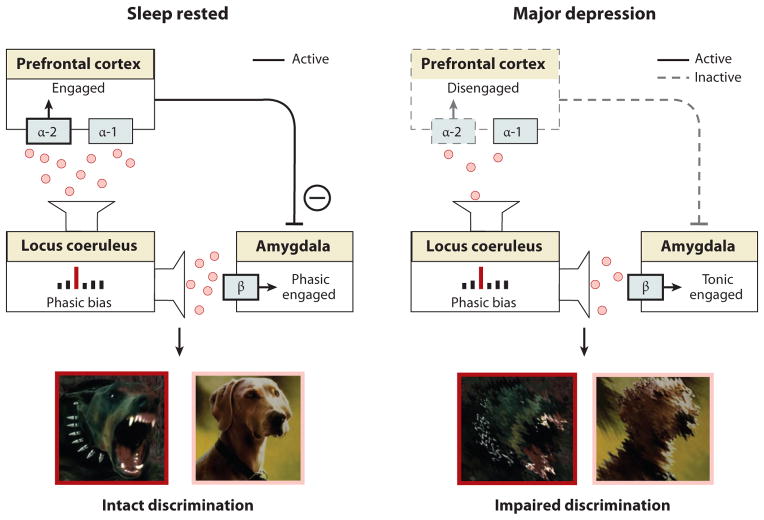
Figure 9. REM Sleep Emotion Recalibration model applied to major depression.
Exaggerated REM sleep qualities in major depression produce an excessive blunting in next-day noradrenergic activity, resulting in too little noradrenaline for appropriate emotional salience detection.
If correct, pharmacologically increasing noradrenaline concentrations and/or decreasing REM sleep should result in an amelioration of depressive symptoms, including restoration of appropriate salience discrimination. Consistent with the model, pharmacologically increasing concentrations of noradrenaline improves emotional signal discrimination of affective face stimuli (Harmer, 2009; Harmer, 2004). Moreover, noradrenaline reuptake inhibitors as well as dual noradrenaline/serotonin reuptake inhibitors prescribed for treating major depression—both of which increase noradrenaline concentrations (Hamon & Blier 2013, Holshoe 2009, Tribl et al 2013, Trivedi & Daly 2008)—restore REM sleep features in major depression, prolonging the time it takes to enter REM sleep and decreasing the total duration of REM sleep (Holshoe 2009, Tribl et al 2013). Furthermore, total and REM specific sleep deprivation alone, without additional pharmacological intervention, can lead to a rapid antidepressant effect in major depression (reviewed in (Benedetti & Colombo 2011, Wu & Bunney 1990). In summary, disproportionate REM sleep observed in major depression may trigger excess next-day blunting of the adrenergic system, and further provides a mechanistic explanation for the anti-depressant effects (including the restoration of emotional salience discrimination) of both pharmacologically enhancing noradrenaline availability and decreasing REM sleep characteristics.
Conclusion
A causal and bi-directional relationship appears to exist between sleep and emotional brain function. Without sleep, the ability to adequately regulate and express emotions is compromised at both a brain and behavioral level, common to both the positive and negative domains of the emotional spectrum. In contrast, sleep, and specifically REM sleep, provides a restoration of appropriate next-day emotion reactivity and salience discrimination. Beyond processes basic emotion stimulus responsivity, sleep, and most consistently REM sleep, additionally promotes the offline processing and consolidation of emotional memories.
Uniting these empirical findings with the basic neurobiological features of REM sleep, an emotional homeostasis framework emerges in which REM sleep provides at least two discreet functional benefits (a) the therapeutic depotentiation of emotion from prior affective experiences, and (b) a next-day recalibration of noradrenergic salience signaling by the brain, restoring optimal emotion sensitivity and specificity. Moreover, these model offer clarifying neurobiological insights underlying the neural etiology and expressed symptomatology of PTSD and major depression, as well as novel mechanistic routes through which pharmacological interventions currently used in PTSD and major depression may operate.
While considerable progress has been made in understanding the relationship between sleep and affective brain function, the field is still only in its infancy, with much yet to understand. We currently know little about the interplay between peripheral and central nervous system mechanisms leading to abnormalities of emotion processing caused by sleep deprivation. Similarly, the precise combination of sleep factors that reset the balance for optimal next-day emotional brain function is unclear. Is it the stages of sleep, their quantity or quality, their brain oscillations, their neurochemistry? Or the cycling nature of sleep, and/or the timing of sleep? Conversely, why do some emotional events we experience while awake impact our sleep at night, while others do not? Is it their intensity, novelty, salience, valence, temporal proximity to sleep, relationship with past experiences or degree of unresolved understanding? We need to look no further than mood disorders to appreciate this wake-sleep reciprocity that modulates affective states. What does seems clear, however, is that the clinical, professional and public health ramifications of this emerging association between sleep and affective brain function are substantive.
References
- Anderson C, Platten CR. Sleep deprivation lowers inhibition and enhances impulsivity to negative stimuli. Behav Brain Res. 2011;217:463–6. doi: 10.1016/j.bbr.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Appelhans B, Luecken L. Heart rate variability as an index of regulated emotional responding. Review of General Psychology. 2006;10:229–40. [Google Scholar]
- Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr Scand Suppl. 2007:104–15. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. J Addict Dis. 2007;26:41–54. doi: 10.1300/J069v26n04_06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colombo C. Sleep deprivation in mood disorders. Neuropsychobiology. 2011;64:141–51. doi: 10.1159/000328947. [DOI] [PubMed] [Google Scholar]
- Benedict C, Brooks SJ, O'Daly OG, Almen MS, Morell A, et al. Acute sleep deprivation enhances the brain's response to hedonic food stimuli: an fMRI study. J Clin Endocrinol Metab. 2012;97:E443–7. doi: 10.1210/jc.2011-2759. [DOI] [PubMed] [Google Scholar]
- Berger RH, Miller AL, Seifer R, Cares SR, LeBourgeois MK. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J Sleep Res. 2012;21:235–46. doi: 10.1111/j.1365-2869.2011.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernert RA, Joiner TE. Sleep disturbances and suicide risk: A review of the literature. Neuropsychiatr Dis Treat. 2007;3:735–43. doi: 10.2147/ndt.s1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 1991;11:3135–45. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, Briley M. The noradrenergic symptom cluster: clinical expression and neuropharmacology. Neuropsychiatr Dis Treat. 2011;7:15–20. doi: 10.2147/NDT.S19613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Roth T, Burduvali E, Kapke A, Schultz L, Roehrs T. Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psychiatry. 2004;61:508–16. doi: 10.1001/archpsyc.61.5.508. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Perron BE. Sleep disturbance as a universal risk factor for relapse in addictions to psychoactive substances. Med Hypotheses. 2010;74:928–33. doi: 10.1016/j.mehy.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ. Insomnia, depression and aging. Assessing sleep and mood interactions in older adults. Geriatrics. 2004;59:47–51. quiz 52. [PubMed] [Google Scholar]
- Cahill L. Neurobiological mechanisms of emotionally influenced, long-term memory. Prog Brain Res. 2000;126:29–37. doi: 10.1016/S0079-6123(00)26004-4. [DOI] [PubMed] [Google Scholar]
- Calohan J, Peterson K, Peskind ER, Raskind MA. Prazosin treatment of trauma nightmares and sleep disturbance in soldiers deployed in Iraq. J Trauma Stress. 2010;23:645–8. doi: 10.1002/jts.20570. [DOI] [PubMed] [Google Scholar]
- Cape EG, Jones BE. Differential modulation of high-frequency gamma-electroencephalogram activity and sleep-wake state by noradrenaline and serotonin microinjections into the region of cholinergic basalis neurons. J Neurosci. 1998;18:2653–66. doi: 10.1523/JNEUROSCI.18-07-02653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–77. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–66. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol. 2009;73:88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. The impact of inadequate sleep on children's daytime cognitive function. Semin Pediatr Neurol. 1996;3:44–50. doi: 10.1016/s1071-9091(96)80028-3. [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Schabus M, Desseilles M, Sterpenich V, Bonjean M, Maquet P. Functional neuroimaging insights into the physiology of human sleep. Sleep. 2010;33:1589–603. doi: 10.1093/sleep/33.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3:33–6. [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102:2626–31. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mansari M, Guiard BP, Chernoloz O, Ghanbari R, Katz N, Blier P. Relevance of norepinephrine-dopamine interactions in the treatment of major depressive disorder. CNS Neurosci Ther. 2010;16:e1–17. doi: 10.1111/j.1755-5949.2010.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Curr Top Behav Neurosci. 2010;2:251–77. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10:473–81. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170:372–82. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Buysse DJ, Nofzinger E. Sleep-specific mechanisms underlying posttraumatic stress disorder: Integrative review and neurobiological hypotheses. Sleep Medicine Reviews. 2008;12:185–95. doi: 10.1016/j.smrv.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, James J, Insana S, Herringa RJ, Mammen O, et al. A window into the invisible wound of war: functional neuroimaging of REM sleep in returning combat veterans with PTSD. Psychiatry Res. 2013;211:176–9. doi: 10.1016/j.pscychresns.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AN, Greer SM, Saletin JM, Harvey AG, Nitschke JB, Walker MP. Tired and apprehensive: anxiety amplifies the impact of sleep loss on aversive brain anticipation. J Neurosci. 2013;33:10607–15. doi: 10.1523/JNEUROSCI.5578-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesmann C, Gottesman I. The neurobiological characteristics of rapid eye movement (REM) sleep are candidate endophenotypes of depression, schizophrenia, mental retardation and dementia. Prog Neurobiol. 2007;81:237–50. doi: 10.1016/j.pneurobio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Commun. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol. 1999;108:134–42. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Gujar N, McDonald SA, Nishida M, Walker MP. A role for REM sleep in recalibrating the sensitivity of the human brain to specific emotions. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011;31:4466–74. doi: 10.1523/JNEUROSCI.3220-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habukawa M, Uchimura N, Maeda M, Kotorii N, Maeda H. Sleep findings in young adult patients with posttraumatic stress disorder. Biol Psychiatry. 2007;62:1179–82. doi: 10.1016/j.biopsych.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Hamon M, Blier P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:54–63. doi: 10.1016/j.pnpbp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Gianaros PJ, Critchley HD. The embodiment of emotional feelings in the brain. J Neurosci. 2010;30:12878–84. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–46. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian functioning: critical mechanisms in the mood disorders? Annu Rev Clin Psychol. 2011;7:297–319. doi: 10.1146/annurev-clinpsy-032210-104550. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Jones C, Schmidt DA. Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev. 2003;23:377–407. doi: 10.1016/s0272-7358(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Murray G, Chandler RA, Soehner A. Sleep disturbance as transdiagnostic: consideration of neurobiological mechanisms. Clin Psychol Rev. 2011;31:225–35. doi: 10.1016/j.cpr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–3. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- Holshoe JM. Antidepressants and sleep: a review. Perspect Psychiatr Care. 2009;45:191–7. doi: 10.1111/j.1744-6163.2009.00221.x. [DOI] [PubMed] [Google Scholar]
- Horne JA. Sleep function, with particular reference to sleep deprivation. Ann Clin Res. 1985;17:199–208. [PubMed] [Google Scholar]
- Hu P, Stylos-Allen M, Walker MP. Sleep facilitates consolidation of emotionally arousing declarative memory. Psychol Sci. 2006;17:891–98. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol. 2010;22:355–61. doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167:151–60. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani H, Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life Sciences. 1990;47:421–6. doi: 10.1016/0024-3205(90)90300-g. [DOI] [PubMed] [Google Scholar]
- Kamphuis J, Meerlo P, Koolhaas JM, Lancel M. Poor sleep as a potential causal factor in aggression and violence. Sleep Med. 2012;13:327–34. doi: 10.1016/j.sleep.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Keane PE, Candy JM, Bradley PB. The role of endogenous catecholamines in the regulation of electrocortical activity in the encephale isole cat. Electroencephalogr Clin Neurophysiol. 1976;41:561–70. doi: 10.1016/0013-4694(76)90001-8. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Schwab ZJ, Weber M, Kipman M, Deldonno SR, et al. Daytime sleepiness affects prefrontal regulation of food intake. Neuroimage. 2013;71:216–23. doi: 10.1016/j.neuroimage.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Kleim B, Wilhelm FH, Temp L, Margraf J, Wiederhold BK, Rasch B. Sleep enhances exposure therapy. Psychol Med. 2013:1–9. doi: 10.1017/S0033291713001748. [DOI] [PubMed] [Google Scholar]
- Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–7. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345:1825–32. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- Lavie P, Hefez A, Halperin G, Enoch D. Long-term effects of traumatic war-related events on sleep. Am J Psychiatry. 1979;136:175–8. doi: 10.1176/ajp.136.2.175. [DOI] [PubMed] [Google Scholar]
- Libedinsky C, Smith DV, Teng CS, Namburi P, Chen VW, et al. Sleep deprivation alters valuation signals in the ventromedial prefrontal cortex. Front Behav Neurosci. 2011;5:70. doi: 10.3389/fnbeh.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick BN, Singh A. REM sleep loss increases brain excitability: role of noradrenaline and its mechanism of action. Sleep Med Rev. 2011;15:165–78. doi: 10.1016/j.smrv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Portas C, Mascia MS, Casu MA, Fa M, et al. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Research. 1995;671:329–32. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–75. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- McGlinchey EL, Talbot LS, Chang KH, Kaplan KA, Dahl RE, Harvey AG. The effect of sleep deprivation on vocal expression of emotion in adolescents and adults. Sleep. 2011;34:1233–41. doi: 10.5665/SLEEP.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neurosci Biobehav Rev. 2012;36:1750–62. doi: 10.1016/j.neubiorev.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna BS, Dickinson DL, Orff HJ, Drummond SP. The effects of one night of sleep deprivation on known-risk and ambiguous-risk decisions. J Sleep Res. 2007;16:245–52. doi: 10.1111/j.1365-2869.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Hipolito MM. Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectr. 2006;11:611–5. doi: 10.1017/s1092852900013663. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 1995;38:174–9. doi: 10.1016/0006-3223(94)00238-X. [DOI] [PubMed] [Google Scholar]
- Mellman TA, Nolan B, Hebding J, Kulick-Bell R, Dominguez R. A polysomnographic comparison of veterans with combat-related PTSD, depressed men, and non-ill controls. Sleep. 1997;20:46–51. doi: 10.1093/sleep/20.1.46. [DOI] [PubMed] [Google Scholar]
- Mendl M, Burman OH, Paul ES. An integrative and functional framework for the study of animal emotion and mood. Proc Biol Sci. 2010;277:2895–904. doi: 10.1098/rspb.2010.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menz MM, Rihm JS, Salari N, Born J, Kalisch R, et al. The role of sleep and sleep deprivation in consolidating fear memories. Neuroimage. 2013;75:87–96. doi: 10.1016/j.neuroimage.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Minkel J, Htaik O, Banks S, Dinges D. Emotional expressiveness in sleep-deprived healthy adults. Behav Sleep Med. 2011;9:5–14. doi: 10.1080/15402002.2011.533987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkel JD, Banks S, Htaik O, Moreta MC, Jones CW, et al. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12:1015–20. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi S, Misaki M, Kan S, Fukunaga T, Koike T. Human brain activity time-locked to rapid eye movements during REM sleep. Exp Brain Res. 2009;192:657–67. doi: 10.1007/s00221-008-1579-2. [DOI] [PubMed] [Google Scholar]
- Motomura Y, Kitamura S, Oba K, Terasawa Y, Enomoto M, et al. Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS One. 2013;8:e56578. doi: 10.1371/journal.pone.0056578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida, Pearsall, Buckner, Walker REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb Cortex. 2009;19:1158–66. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–10. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA. Functional neuroimaging of sleep. Seminars in Neurology. 2005;25:9–18. doi: 10.1055/s-2005-867070. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, et al. Bottom-up and top-down processes in emotion generation: common and distinct neural mechanisms. Psychol Sci. 2009;20:1322–31. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway GA, Schenk J, Stockmeier CA, May W, Klimek V. Elevated agonist binding to alpha2-adrenoceptors in the locus coeruleus in major depression. Biol Psychiatry. 2003;53:315–23. doi: 10.1016/s0006-3223(02)01728-6. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Hellman K, Abel T, Thomas SA. Adrenergic signaling plays a critical role in the maintenance of waking and in the regulation of REM sleep. J Neurophysiol. 2004;92:2071–82. doi: 10.1152/jn.00226.2004. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Milad MR, Orr SP, Rauch SL, Stickgold R, Pitman RK. Sleep promotes generalization of extinction of conditioned fear. Sleep. 2009;32:19–26. [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Stickgold R, Muzur A, Wigren PE, Ward AS, et al. Sleep quality deteriorates over a binge--abstinence cycle in chronic smoked cocaine users. Psychopharmacology (Berl) 2005;179:873–83. doi: 10.1007/s00213-004-2088-z. [DOI] [PubMed] [Google Scholar]
- Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: State of the art. Sleep Med Rev. 2013;17:377–90. doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. Int Rev Psychiatry. 2005;17:229–36. doi: 10.1080/09540260500104524. [DOI] [PubMed] [Google Scholar]
- Pare D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends Cogn Sci. 2002;6:306–14. doi: 10.1016/s1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Park SP. In vivo microdialysis measures of extracellular norepinephrine in the rat amygdala during sleep-wakefulness. J Korean Med Sci. 2002;17:395–9. doi: 10.3346/jkms.2002.17.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–7. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Payne JD, Chambers AM, Kensinger EA. Sleep promotes lasting changes in selective memory for emotional scenes. Front Integr Neurosci. 2012;6:108. doi: 10.3389/fnint.2012.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JD, Kensinger EA. Sleep's Role in the Consolidation of Emotional Episodic Memories. Current Directions in Psychological Science. 2010;19:290–95. [Google Scholar]
- Peterson MJ, Benca RM. Sleep in mood disorders. Psychiatr Clin North Am. 2006;29:1009–32. doi: 10.1016/j.psc.2006.09.003. abstract ix. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Plutchik R. Outward and inward directed aggressiveness: the interaction between violence and suicidality. Pharmacopsychiatry. 1995;28(Suppl 2):47–57. doi: 10.1055/s-2007-979620. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133:725–46. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Prather AA, Bogdan R, Hariri AR. Impact of sleep quality on amygdala reactivity, negative affect, and perceived stress. Psychosom Med. 2013;75:350–8. doi: 10.1097/PSY.0b013e31828ef15b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–36. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–34. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–3. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R. Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res. 2011;45:577–87. doi: 10.1016/j.jpsychires.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29:14299–308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108:68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- Schwarz JF, Popp R, Haas J, Zulley J, Geisler P, et al. Shortened night sleep impairs facial responsiveness to emotional stimuli. Biol Psychol. 2013;93:41–4. doi: 10.1016/j.biopsycho.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shouse MN, Staba RJ, Saquib SF, Farber PR. Monoamines and sleep: microdialysis findings in pons and amygdala. Brain Res. 2000;860:181–9. doi: 10.1016/s0006-8993(00)02013-8. [DOI] [PubMed] [Google Scholar]
- Siegel JM, Rogawski MA. A function for REM sleep: regulation of noradrenergic receptor sensitivity. Brain Res. 1988;472:213–33. doi: 10.1016/0165-0173(88)90007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: Secondary symptom or core feature? Sleep Medicine Reviews. 2008;12:169–84. doi: 10.1016/j.smrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Schroter MS, Andrade KC, Dresler M, Kiem SA, et al. Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Hum Brain Mapp. 2012;33:2362–76. doi: 10.1002/hbm.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker VI, Sturm A, Andrade KC, Schroter MS, Goya-Maldonado R, et al. The neural correlates and temporal sequence of the relationship between shock exposure, disturbed sleep and impaired consolidation of fear extinction. J Psychiatr Res. 2010;44:1121–8. doi: 10.1016/j.jpsychires.2010.04.017. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–24. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern WC, Morgane PJ. Theoretical view of REM sleep function: maintenance of catecholamine systems in the central nervous system. Behav Biol. 1974;11:1–32. doi: 10.1016/s0091-6773(74)90145-x. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Boly M, Vandewalle G, Darsaud A, et al. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biology. 2007;5:e282. doi: 10.1371/journal.pbio.0050282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn JR, Geracioti TD., Jr Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–71. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol Psychiatry. 1999;46:1205–18. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63:629–32. doi: 10.1016/j.biopsych.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribl GG, Wetter TC, Schredl M. Dreaming under antidepressants: a systematic review on evidence in depressive patients and healthy volunteers. Sleep Med Rev. 2013;17:133–42. doi: 10.1016/j.smrv.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Daly EJ. Treatment strategies to improve and sustain remission in major depressive disorder. Dialogues Clin Neurosci. 2008;10:377–84. doi: 10.31887/DCNS.2008.10.4/mhtrivedi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–69. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm E, Gujar N, Walker MP. Sleep deprivation impairs the accurate recognition of human emotions. Sleep. 2010;33:335–42. doi: 10.1093/sleep/33.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helm E, Yao J, Dutt S, Rao V, Saletin JM, Walker MP. REM sleep depotentiates amygdala activity to previous emotional experiences. Curr Biol. 2011;21:2029–32. doi: 10.1016/j.cub.2011.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Helm EW, MP Sleep and Affective Brain Regulation. Soc Personal Psychol Compass. 2012;6:773–91. [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biol Psychiatry. 2009;66:649–55. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Overeem S, Fernandez G. The effect of exogenous cortisol during sleep on the behavioral and neural correlates of emotional memory consolidation in humans. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30:603–9. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, et al. Hyperstimulation of striatal D2 receptors with sleep deprivation: Implications for cognitive impairment. Neuroimage. 2009;45:1232–40. doi: 10.1016/j.neuroimage.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Degirmenci M, Drosopoulos S, Perras B, Born J. Effects of cortisol suppression on sleep-associated consolidation of neutral and emotional memory. Biol Psychiatry. 2005;58:885–93. doi: 10.1016/j.biopsych.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Wagner U, Gais S, Born J. Emotional memory formation Is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learning and Memory. 2001;8:112–9. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner U, Hallschmid M, Rasch B, Born J. Brief sleep after learning keeps emotional memories alive for years. Biological Psychiatry. 2006;60:788–90. doi: 10.1016/j.biopsych.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–97. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135:731–48. doi: 10.1037/a0016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, Moore D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J Clin Psychol. 2011;67:1240–58. doi: 10.1002/jclp.20845. [DOI] [PubMed] [Google Scholar]
- Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147:14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep - a prefrontal amygdala disconnect. Current Biology. 2007;17:R877–8. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zhong X, Hilton HJ, Gates GJ, Jelic S, Stern Y, et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 2005;98:2024–32. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epstein R, Lavie P. The effects of sleep loss on medical residents' emotional reactions to work events: a cognitive-energy model. Sleep. 2005;28:47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]