Abstract
Background
Behavioral processes and neural systems dysfunctions that put individuals at risk for drug use in general—and stimulant use in particular—are poorly understood. Here, the hypothesis is examined that stimulant-using subjects adjust their decision-making less as a function of errors as evidenced by attenuated behavioral and neural substrate activation patterns.
Methods
Twelve young adults who had used stimulants were compared with 12 education-matched stimulant-naïve comparison subjects. Subjects completed the two-choice prediction task with three fixed error-rate conditions (20%, 50%, or 80% errors) during functional magnetic resonance imaging.
Results
Stimulant users relative to comparison subjects showed less strategy adjustment to different error rates, e.g., they were less likely to stay with winning responses (win-stay) and to shift away from losing responses (lose-shift). These subjects also showed different activation patterns as a function of error rate in left insular and bilateral dorsolateral prefrontal cortex, but not anterior cingulate. The degree to which individuals adjusted switching rate, or win-stay/lose-shift consistent responses, as a function of errors was correlated with the difference in insular cortex activation differences between high and low error rates.
Conclusions
The behavior of stimulant users is less adaptive to the frequency of errors made and fewer brain processing resources are deployed during decision-making to anticipate erroneous performance. These findings could be markers for the predisposition of drug taking; however, their relevance for development of drug dependence needs further study.
Keywords: fMRI, stimulants, decision-making, error processing, insula
Introduction
Stimulant, i.e. amphetamine and cocaine, dependence is an important public health problem with an estimated lifetime prevalence of 1–3% (1–3). The rate of any illicit drug use increases during adolescence and peaks between 18 to 25 years of age, when approximately 20% of college students report some level of illicit drug use (other than marijuana) and approximately 10% report having used stimulants over the past year (4). Results from twin studies are consistent with the notion that genetic factors, which may be disorder-specific (5), predispose to drug use and account for up to 60% of drug using behavior (6).
Studying individuals who have used stimulants but do not have extended experience with them or suffer from significant adverse consequences associated with substance use may provide an opportunity to examine what cognitive and affective characteristics may predispose individuals to transition from recreational use of the drug to abuse or dependence. Previously (7), stimulant-using individuals showed increased activation in the caudate, an area involved in processing salient events (8), in response to uncertainty. Personality measures revealed that users were more impulsive than comparison subjects and that neural response to uncertainty in a number of areas including the thalamus/caudate was positively correlated with impulsivity. These results suggested that these individuals may be subject to more “action pressure”, i.e. have an increased urge to act, when making decisions under uncertainty.
Decision-making consists of a complex set of processes that are presumed to be orchestrated within various brain systems to find an optimal outcome. When people are asked to respond on decision-making tasks in the laboratory, subjects typically choose the response which is associated with the highest anticipated reinforcement, i.e. select an action that is most likely to result in a “correct” outcome or yield the highest reward (9–11). This behavior is consistent with the notion that anticipated “success” or “failure” critically influences decision-making (12). In simple choice decision-making tasks, reinforcement frequency and payoffs primarily determine response selection (13) in such a way that subjects adjust their response strategy consistent with an error-correction set of rules (14). In short, people compute the expected utility, i.e. weighted average between expected success and failure, and choose the alternative that has the most favorable success over failure weight.
The current study was aimed to examine whether stimulant-using individuals show an altered response to different error rates. We hypothesized that behaviorally these individuals would show an attenuated adjustment to different error rates. We expected that this attenuated adjustment would be correlated with altered activation in the anterior insula, anterior cingulate, and/or dorsolateral prefrontal cortex, areas which have been implicated in the anticipatory processing and executive planning of actions following errors (15; 16). Support for these hypotheses would provide further evidence that individuals who have used stimulants show altered decision-making behavior and neural systems activation patterns.
Methods
Participants
This study was approved by the University of California San Diego (UCSD) Institutional Review Board. All subjects were interviewed face to face using a structured diagnostic interview (SCID) (17), modified to enable us to document the use of illicit substances. Twelve young adults (age 20.0, SD 2.6) who had used stimulants at least twice in the last 6 months were compared with 12 education-matched stimulant-naïve comparison subjects (age 18.3, SD 0.9). Stimulant users were recruited from flyers and internet advertisements. They reported having used cocaine, amphetamines, and/or other stimulant drugs when not prescribed, but were neither treatment-seeking nor stimulant-dependent. Subjects were instructed to abstain from illicit substance use for 48 hours prior to the experimental session. All subjects were trained to perform the two-choice prediction task prior to testing during fMRI scanning and received $50 for participation. No restrictions were placed on the consumption of caffeine-containing beverages; none of the subjects were smokers.
Measures
Subjects completed the Zuckerman Sensation Seeking Scale (SSS Form V)(18) and the Barratt Impulsivity Scale (BIS-11)(19). The SSS is a questionnaire to assess engagement in high-risk activities (20). The items are grouped to form the subscales of thrill and adventure seeking, experience seeking, disinhibition, and boredom susceptibility. The BIS measures different components of impulsivity, notably the three subscales of non-planning impulsivity, motor impulsivity, and attention/cognition impulsivity (21).
Task
The two-choice prediction task (22–24) has been used previously to examine the effect of past history of outcomes and choice behavior on selecting the current choice (for a detailed description see supplemental information). In this version of the task, the number of “correct” or “incorrect” trials in a block of trials, i.e. the block’s error rate was determined a-priori. Three error rate block types were presented in the same order for each subject: high (80% of responses were “incorrect”), chance-level (50% of responses were “incorrect”), and low (20% of responses were “incorrect”). Blocks were separated by a resting condition (presentation of the background stimulus, no response required), which lasted between 6–12 seconds.
Behavioral Measures
For each trial, the participant’s selection (right or left) and response latency, as well as the outcome (right or left), were obtained. Decision-making strategies were assessed using two sets of measures, one for general response biases and one for the influence of prior task events on current responses. General response bias was assessed both by the number of left versus right responses and by the number of stay responses (i.e. same response as previous trial—left following left or right following right) versus switch responses (i.e. response opposite that of previous trial—left following right or right following left). The degree to which a current selection was determined by the previous selection, the previous outcome, or a combination of both was assessed using mutual information functions (25) (for detailed information see supplemental information).
fMRI Protocol and Image Analysis Pathway
Magnetic resonance images were obtained using a 1.5 Tesla whole-body system (Siemens, Erlangen). Anatomical T1-weighted images of the whole brain (MPRAGE, TR = 11.4 ms, TE = 4.4 ms, flip angle 10°, FOV 256 × 256, 1-mm3 voxels) were obtained sagittally. Thirty-two slices of T2*-weighted images were obtained in the axial plane using gradient-recalled echo-planar imaging (TE = 40 ms, flip angle 90°, 64 × 64-pixel FOV = 256 × 256 mm, 4-mm contiguous slice thickness) every 3000 ms for 128 repetitions yielding a voxel size of 4 mm3.
All structural and functional image processing was done with the Analysis of Functional Neuroimages software package (AFNI) package (26). A multivariate regressor approach was used to relate changes in echoplanar image intensity (EPI) to differences in task characteristics. Stimulant subjects did not differ from comparison subjects on motion during scanning (tdroll(22) = −0.737, p =0.469; tdpitch(22) = 1.488, p = 0.151; tdyaw(22) = −0.822, p = 0.420; tdSI(22) = −0.881, p = 0.388; tdLR(22) = −0.316, p = 0.755; and tdAP(22) = −0.940, p = 0.358). The time series of motion parameters were used to obtain an average for these six motion parameters for each subject. For the analysis, only three motion parameters (droll, dpitch, dyaw) were used as regressors to adjust EPI intensity changes due to motion artifacts because for small changes, the change in the roll, pitch and yaw are linearly related to x, z and y changes, respectively. Three regressors of interest (20%, 50%, and 80% error rate blocks) were convolved with a gamma variate function that models a prototypical hemodynamic response and normalized. Percent signal change was calculated from the multiple regressor analysis and used as the main dependent measure. Planned contrasts were for the activation difference between 20% and 80% error-rate were used to determine success versus failurei.e. the change of brain activation as a function of error-rate change. A Gaussian filter with FWHM 6 mm was applied to voxel-wise percentage signal change data or to the linear contrasts to account for individual variations of the anatomical landmarks. A threshold adjustment method based on Monte-Carlo simulations was used to guard against identifying false positive areas of activation (27). A simulation was conducted with a voxelwise p < 0.01, a spatial correlation equal to the FWHM, and a connectivity radius of 4 mm showed that a voxel-wise a-priori probability of 0.01 would result in an a posteriori voxel-wise probability of 4.219 × 10−7 and a corrected cluster-wise probability of false positive detection of 0.01 if a minimum volume of 768 µl (or 12 connected voxels) was considered. The Talairach Demon software (28) was used to determine brain labels for activation clusters.
Statistical Analysis
All behavioral analyses were carried out with SPSS 12.0 (29). A 2×3 mixed model multivariate ANOVA, with error rate (20, 50, or 80%) as the within-subjects factor and group (stimulant user versus control) as the between-subjects factor, was used to analyze the behavioral measures. Averages of neural activation patterns extracted from the functional regions of interest identified by the planned contrasts were subjected to a secondary ANOVA with error rate as a fixed factor and subjects as a random factor. Because there was a slight difference in age across groups, all ANOVA analyses were covaried for age. Parametric correlational analyses were conducted to determine whether differences in brain activation were related to behavioral differences during the task or to personality characteristics of the participants. Significant correlations between brain activation and behavior or personality characteristics were followed up with regression analysis to determine the degree to which lifetime stimulant or THC use affected this relationship. Only those correlations that remained significant after controlling for these factors are reported.
Results
Drug Usage and Behavioral Assessments
Based on SCID and questionnaire life-time assessments, stimulant users had used amphetamine-like stimulants an average of 36 times (range: 0–240) and/or cocaine an average of 14 times (range: 0 – 40); they had used any type of stimulants for an average of 51 times (range: 2– 240). Although individuals were recruited based on their stimulant use, all of these individuals had used other illicit drugs (Table 1). In particular, stimulant users had used THC an average of 316 times (range: 12 – 900). None of the comparison subjects reported having used THC or other illicit drugs. Stimulant users scored higher than comparison subjects on Thrill & Adventure Seeking as well as Experience Seeking, two facets of the Zuckerman Sensation Seeking Scale (Table 2). These individuals also exhibited higher levels of Attentional and Motor impulsiveness, which are subscales of the Barratt Impulsivity Scale.
Table 1.
Stimulant Users Use Characteristics
| Type | Mean | SD | Range |
|---|---|---|---|
| Amphetamine-like Stimulants (amphetamines, methamphetamine, prescription stimulants, etc) | 38.6 | 66.4 | 0 – 240 |
| Cocaine | 14.1 | 12.7 | 0 – 40 |
| Marijuana | 316.5 | 300.9 | 12 – 900 |
| Hallucinogens (LSD, mescaline, peyote, MDA, DMT, STP, psilocybin, etc) | 3.6 | 1.7 | 0 – 6 |
| PCP | 3.0 | 2.8 | 0 – 5 |
| Barbiturates, Librium-or valium-type drugs or downers (not prescribed) | 2.7 | 2.1 | 0 – 5 |
| Opiates (heroin, prescription pain medicaton etc.) | 4.0 | 4.1 | 0 – 10 |
| Inhalants (glue, aerosols, toluene, gasoline, paint, etc) | 3.7 | 3.8 | 0 – 8 |
Table 2.
Personality Characteristics
| Comparison Subjects |
Stimulant Users |
||||||
|---|---|---|---|---|---|---|---|
| (n=12) | (n=12) | ||||||
| Measures | Mean | SD | Mean | SD | t-test | P value | |
| SS | Thrill & Adventure Seeking | 6.17 | 2.48 | 7.91 | 1.14 | −2.131 | 0.045 |
| Experience Seeking | 4.58 | 2.35 | 7.36 | 1.57 | −3.302 | 0.003 | |
| Disinhibition | 5.08 | 2.97 | 6.84 | 2.59 | −1.506 | 0.147 | |
| Boredom Susceptibility | 1.67 | 1.30 | 1.83 | 1.40 | −0.287 | 0.777 | |
| BIS | Attentional impulsiveness | 17.50 | 6.46 | 23.94 | 5.05 | −2.647 | 0.015 |
| Motor impulsiveness | 23.33 | 4.75 | 27.73 | 3.80 | −2.434 | 0.024 | |
| Non-planning impulsiveness | 21.42 | 3.50 | 24.09 | 3.08 | −1.937 | 0.066 | |
Two-Choice Prediction Task – Behavioral Results
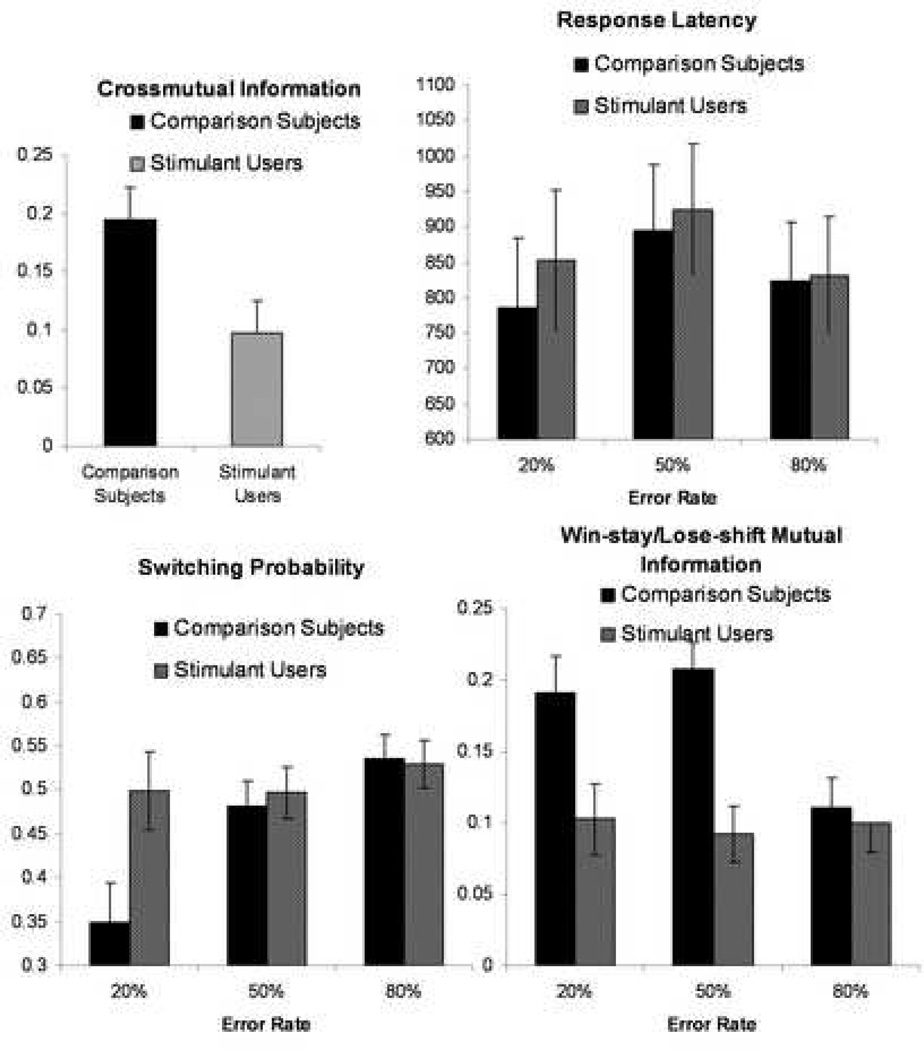
Stimulant users and comparison subjects made an average of 21.3 +/− 1.5 and 21.7 +/− 1.5 responses per error rate block and took an average of 834 +/− 89.5 and 869 +/− 89.5 msecs to make a selection. Neither the number of responses nor the response latency was significantly affected by error rate. Moreover, there was no significant group difference or group by error rate interaction (for F values see Table in Supplemental Material). There was a slight but significant bias for all participants to select right more frequently than left. Although there was no overall effect of error rate on switching rates, there was a significant group by error rate interaction. As shown in Figure 1B, whereas comparison subjects showed the expected increase of switching rate as a function of error rate, stimulant users consistently showed a higher switch rate and differed significantly from comparison subjects for the 20% error rate condition. Moreover, increased switching at 20% error rate was significantly correlated with both thrill and adventure seeking (r=0.519, p = 0.009) and attentional impulsivity (r=0.559, p = 0.006).
Figure 1. Behavioral Differences during Two-choice Prediction Task.
Stimulant using individuals relative to comparison subjects showed a reduced influence of the prior stimulus (car) on their decision-making irrespective of error-rate condition (upper left) and adjust both switching behavior (lower left) and win-stay/lose-shift consistent responses less as a function of error rate (lower right), but did not differ on response latency (upper right).
A significant group effect for cross-mutual information, i.e. the mutual information between participant selections and prior outcomes, was due to the fact that choices by stimulant users were significantly less influenced by the previous trial outcome than those of comparison subjects. In comparison, there was no group difference in the degree to which the previous response influenced the selection on the current trial. Stimulant users made significantly fewer selections according to a win-stay/lose-shift strategy than comparison subjects, i.e. selected less win-stay and lose-shift consistent responses (see Supplemental Table). Stimulant users did not modulate the degree to which they selected a win-stay/lose-shift consistent response as a function of error rate (Figure 1C). In contrast, comparison subjects selected a win-stay/lose-shift consistent response significantly less often during high (80%) error rate blocks compared to the 20% and 50% blocks, reflected by a significant group by error interaction (see Supplemental Table). Thus, stimulant users changed response options less as a function of varying error rates and were less influenced by a win-stay/lose-shift strategy.
Functional neuroimaging results
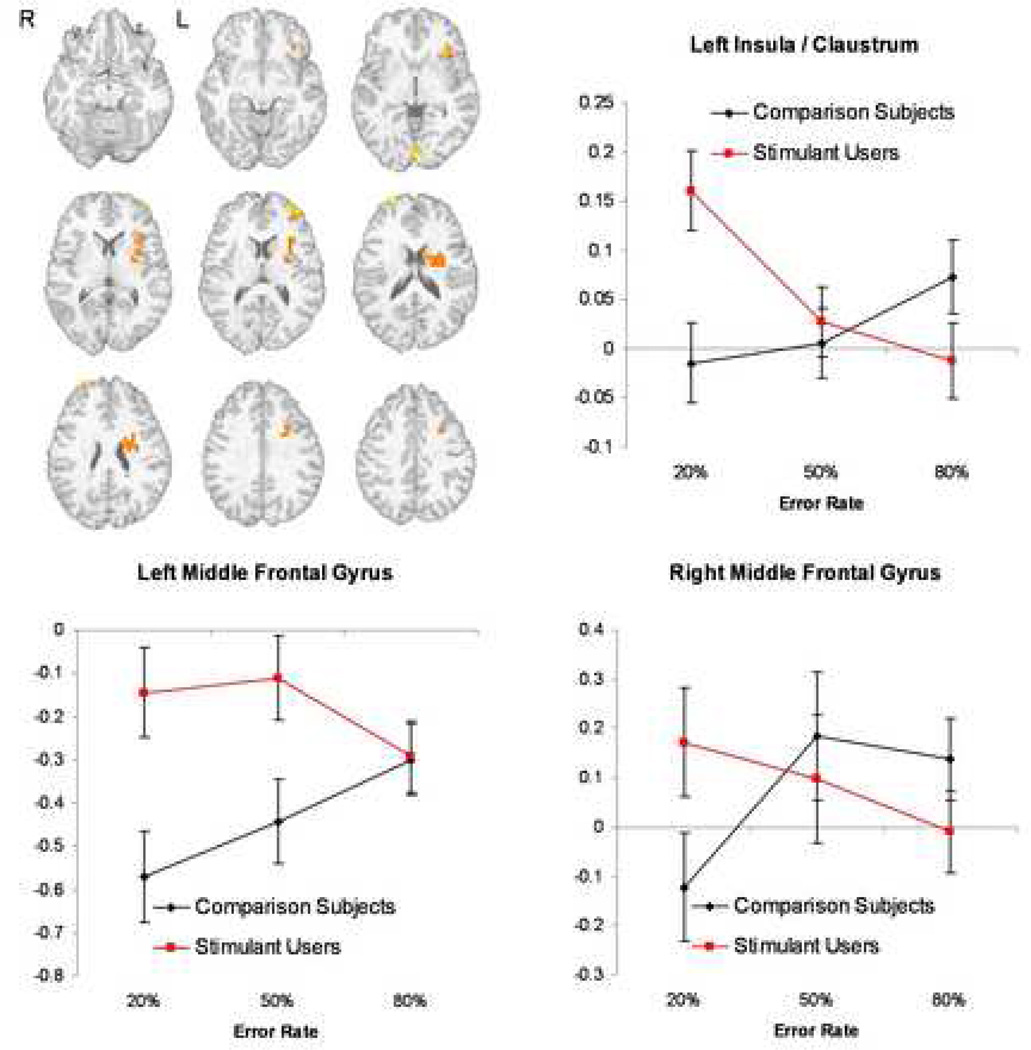
Both groups showed activation differences as a function of error rate in bilateral caudate and bilateral temporal gyrus (Table 3). Similar to previous studies (30; 31), activation in these structures was significantly less for the 80% relative to 20% error rate. There was a group effect in error-rate related activation in left insula and bilateral middle frontal gyrus. Whereas comparison subjects showed an increase of left insula activation as a function of error rate (greater activation at high error rates), stimulant users showed the reverse pattern, a greater activation at the low as compared to the high error rates (Figure 2). Moreover, comparison subjects but not stimulant users showed an error-rate dependent increase in bilateral middle frontal gyrus. There was no significant activation difference in the anterior cingulate cortex.
Table 3.
Center of mass for volume-thresholded mixed ANOVA clusters of task-related activation for task effect and the error-rate effect. Labels and Brodman Area (BA) designations are based on Talairach Demon software (Lancaster et al., 2000).
| Error-rate Effect | Volume | x | y | z | L/R | Description | BA |
|---|---|---|---|---|---|---|---|
| 2048 | −32 | −8 | −1 | L | Caudate/Putamen | ||
| 1856 | 38 | −51 | 5 | R | Middle Temporal Gyrus | 39 | |
| 1280 | 38 | −33 | 37 | R | Inferior Parietal Lobule | 40 | |
| 1024 | 40 | −68 | 17 | R | Middle Temporal Gyrus | 39 | |
| 896 | 44 | −48 | 18 | R | Superior Temporal Gyrus | 13 | |
| 896 | 3 | 34 | 48 | R | Superior Frontal Gyrus | 8 | |
| 832 | 18 | 8 | 13 | R | Caudate | ||
| 768 | −51 | −18 | 9 | L | Transverse Temporal Gyrus | 41 | |
| Group × Error-rate | |||||||
| 6016 | −27 | 6 | 16 | L | Insula/Claustrum | 13 | |
| 896 | −4 | −83 | 3 | L | Lingual Gyrus | 18 | |
| 896 | −34 | 47 | 14 | L | Middle Frontal Gyrus | 10 | |
| 768 | 24 | 60 | 23 | R | Middle Frontal Gyrus | 10 |
Figure 2. Different Activation to Error rate.
Stimulant using individuals show an inverse relationship between error rate and brain activation in left insula (upper right) and bilateral middle frontal gyrus (lower left and right).
Brain-Behavior Relationships
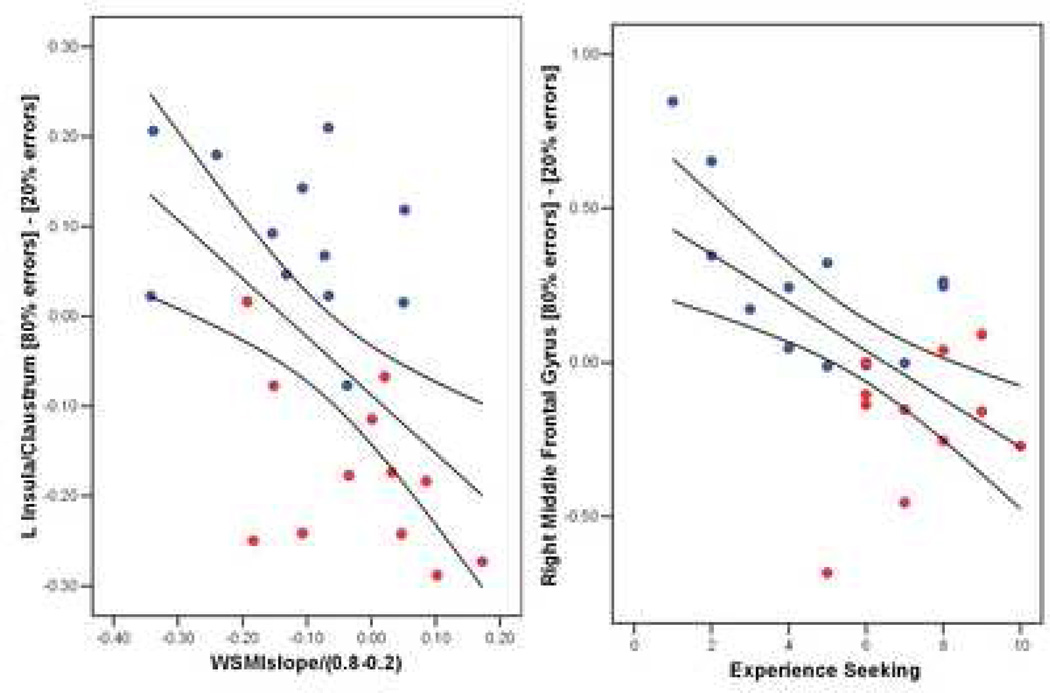
Correlational analyses (followed by regression analyses to covary for the amount of stimulants and THC used) were performed between, on the one hand, areas of significant brain activation difference across groups and, on the other hand, personality measures and behavioral response patterns in the two choice prediction task. The degree to which individuals reduced win-stay/lose-shift consistent responses as a function of error rate was inversely correlated with adjustments in left insular cortex activation according to error rate (r= −0.53, p = 0.007, Figure 3A). Specifically, the more individuals abandoned a win-stay/lose shift consistent response at higher error rates the greater insular cortex activation attenuated as a function of error rate. This relationship remained significant when controlling for lifetime use of marijuana (beta = −0.142, p = 0.45) or lifetime use of stimulants (beta = −0.365, p = 0.05), which had a marginally significant effect. Lower Experience Seeking was associated with more modulation of bilateral prefrontal cortex activation (right: r=−0.51, p = 0.01) as a function of error rate (Figure 3B), which remained significant after controlling for lifetime use of marijuana (beta = −0.091, p = 0.66) or lifetime use of stimulants (beta = −0.224, p = 0.26).
Figure 3. Brain Behavior Relationships.
Greater reduction of win-stay lose-shift mutual information (WSMI) as a function of error rate (fraction of errors: 0.8 – 0.2) was associated with greater attenuation of insular cortex activation as a function of error rate (left). Individuals with higher experience seeking scores adjusted right middle frontal gyrus less as a function of error rate (right). Both results were independent of stimulant or cannabinoid use.
Relationship between Behavioral Characteristics or Brain Activation and Total Amount of Drug Use
Correlational analyses conducted with stimulant using individuals between either total amount of stimulant use (combined amphetamine-like stimulants or cocaine) or total amount of THC use and behavioral characteristics during the two-choice prediction task or brain activation pattern differences did not yield correlations with p < 0.01.
Discussion
This investigation yielded four main results. First, stimulant users relative to comparison subjects adjusted their choices less as a function of different error rates. Second, these subjects also showed an altered activation pattern in the left insular and bilateral dorsolateral prefrontal cortex, but not the anterior cingulate, as a function of error rate. Third, brain pattern differences were associated with an attenuated change in win-stay/lose-shift consistent responses. Fourth, relatively more experience seeking, as part of increased tendency to engage in sensation seeking, was associated with less prefrontal cortical activation adjustment as a function of error rates. Therefore, when stimulant users relative to comparison subjects chose responses that led to an erroneous prediction they showed less behavioral adjustment and fewer changes in brain processing resources. Together with increased activation to uncertainty (7), this attenuated error processing may constitute a second altered cognitive process during decision-making.
The behavioral results of an attenuated switching response as a function of error rate and fewer win-stay/lose-shift consistent responses in stimulant-using individuals relative to comparison subjects are in contrast with behaviors previously observed in methamphetamine dependent individuals (25). There are several possible explanations. First, the group of stimulant-using individuals was not dependent and had relatively few exposures to stimulant. Therefore, the findings with methamphetamine dependent individuals could point toward a long-term use effect. Second, only about one out of seven individuals who use stimulants goes on to develop stimulant dependence (32). Thus, the current group of users may comprise individuals who have different behavioral characteristics than those individuals who have experienced many years of use and dependence. Users in this study exhibited an increased switching rate regardless of previous outcomes and error rates, which could be related to impulsivity and sensation-seeking. Moreover, because only a subset of individuals develops dependence, one may speculate that the opposite behavioral pattern found here may actually point towards resiliency factors. Specifically, the behavioral characteristics of the current group may represent mostly individuals who are not going to develop dependence and may therefore not show the same behavioral patterns as those observed in dependent users. To summarize, attenuated error processing and decreased use of win-stay/lose-shift consistent responses may reflect characteristic of drug initiation behaviors but not maintenance or misuse behaviors. However, these hypotheses can only be tested longitudinally by comparing those individuals who develop dependence with those who do not.
The degree to which failure or success influence behavioral change, particularly response selection during decision-making, may be an important process that needs to be further investigated in stimulant-using subjects. The inverse relationship between the degree of behavioral change and insular functioning, even after controlling for the amount of stimulant and THC use, may point toward an attenuated or altered signal in this structure. There is accumulating evidence that the insular cortex is important for processing both aversive (33) and reward-related stimuli (34). The insular cortex is important for subjective feeling states and interoceptive awareness (35; 36). Within the insular cortex, a multidimensional representation and integration of the current and possibly the predicted (37) body state provides the individual with a temporal representation of a “global moment in time” (38). Importantly, this interoceptive network processes information in a homeostatic manner, i.e. the valence of the information fundamentally depends on the nature of the individual’s current state. Thus, altered insular cortex activity as a function of error rate may point toward altered interoceptive processing in response to errors. A recent study with brain lesion individuals showed that those who had insular damage were more likely to experience a disruption of cigarette addiction, including abolition of the urge to smoke (39). The insular cortex is also part of inhibitory processing together with the middle and inferior frontal gyri, frontal limbic areas, and the inferior parietal lobe (40). In the context of an inhibitory task, individuals with high error rates showed increased insula response (41). Error-related processing during a gonogo task was associated with activation in the insula among other neural structures such as the anterior cingulate cortex, pre-supplementary motor area, thalamus and right inferior parietal lobule (42). Others have suggested that left and right anterior insular cortex are active only during error processing but not during response competition, inhibition, selection, or execution (43). Taken together, these imaging studies support the notion that the insular cortex may have a monitoring function for correct performance and appropriate response strategies to adjust to the subject’s homeostatic state.
Stimulant use in our group was found to occur in the context of other drug use, which is consistent with other studies in both drug users (44; 45) and drug dependent individuals (46). Therefore, one has to be cautious in directly relating the findings of this study to the use of amphetamine-like stimulants or cocaine. Moreover, other studies have shown that stimulant users who also used other illicit drugs had increased odds of progressing into dependence (46). Although the stimulant user sample for this study was selected based on a history of any lifetime use, the use data indicate that, on average, individuals had used stimulants (combined amphetamine-like stimulants or cocaine) only 50 times, on average, in their lives. Thus, it is unlikely that the personality and symptom differences between the groups are a consequence of drug use.
This investigation has several limitations. First, the group sizes were relatively small, which may affect our ability to detect smaller effects and to delineate relationships between behavior brain activation patterns and stimulant use. Second, the two-choice prediction task has been criticized for not providing explicit rewards (e.g. money or primary reinforces for correct versus incorrect choices) and the fact that individual’s response patterns do not affect the degree to which subjects can make successful predictions. Nevertheless, we have repeatedly shown that despite the lack of an optimal response strategy, individuals do not engage in random choices but tend to follow simple but predictable response patterns (24; 47; 48). Third, the current investigation implemented a block-design version of this task, which does not enable us to delineate different processes involved in decision-making. We have argued previously (49) that fast or slow event-related designs will be necessary to better determine whether stimulant users show altered assessment, selection, or outcome processing abnormalities. Fourth, although individuals were instructed to not use any substances within 48 hours of scanning and reported that they had not, we cannot rule out the possibility that acute substance use contributed to the findings because we did not obtain a urine toxicology screen at time of testing.
In conclusion, the current study shows that stimulant using individuals who are not dependent adjust behavior and neural substrates less than comparison subjects in response to errors on a simple decision-making task. The attenuated representation of an adverse outcome has two consequences. First, it fails to reduce the likelihood to select the same choice in the future, i.e. alter switching behavior as a function of error rates. Second, it facilitates the selection of responses with potential adverse outcomes, i.e. deviate from a win-stay/lose shift consistent response strategy towards risk-taking behavior. Future investigations using larger groups will need to determine the degree to which these findings are specific for individuals using stimulants or whether these behavioral and neural substrate patterns are a factor contributing to drug use in general.
Supplementary Material
Acknowledgments
We would like to acknowledge the invaluable help of Heather Donovan, Esti Arce, and Alan Simmons. This research was supported by grants from NIDA (R01DA016663, R01DA018307) and by a VA Merit Grant and an NIH training grant (5T32MH18399: DL).
Dr. Paulus has received grant support from GlaxoSmithKline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: Drs. Leland and Wittmann as well as Mrs. Lovero have no financial conflicts to report.
Reference List
- 1.Warner LA, Kessler RC, Hughes M, Anthony JC, Nelson CB. Prevalence and correlates of drug use and dependence in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:219–229. doi: 10.1001/archpsyc.1995.03950150051010. [DOI] [PubMed] [Google Scholar]
- 2.Schuckit MA. Drug and alcohol abuse : a clinical guide to diagnosis and treatment. New York: Kluwer Academic/Plenum Publishers; 2000. [Google Scholar]
- 3.Grant BF. Prevalence and correlates of drug use and DSM-IV drug dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1996;8:195–210. doi: 10.1016/s0899-3289(96)90249-7. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration. (Results from the 2003 National Survey on Drug Use and Health: National Findings. Rockville, MD: Office of AppliedStudies,NSDUH Series H-25,DHHS Publication No.SMA 04-3964; [Google Scholar]
- 5.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 6.Karkowski LM, Prescott CA, Kendler KS. Multivariate assessment of factors influencing illicit substance use in twins from female-female pairs. Am J Med Genet. 2000;96:665–670. [PubMed] [Google Scholar]
- 7.Leland DS, Arce E, Feinstein JS, Paulus MP. Young adult stimulant users' increased striatal activation during uncertainty is related to impulsivity. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
- 9.Calfee RC. Choice behavior during long-term probabilistic reinforcement schedules. J Comp Physiol Psychol. 1968;65:232–237. doi: 10.1037/h0025565. [DOI] [PubMed] [Google Scholar]
- 10.Goulet LR, Barclay A. Guessing behavior of normal and retarded children under two random reinforcement conditions. Child Dev. 1967;38:545–552. [PubMed] [Google Scholar]
- 11.Ludvigson HW. Response units in the prediction of simple event patterns. J Exp Psychol. 1966;72:335–360. [PubMed] [Google Scholar]
- 12.Egelman DM, Person C, Montague PR. A computational role for dopamine delivery in human decision-making. J Cogn Neurosci. 1998;10:623–630. doi: 10.1162/089892998563022. [DOI] [PubMed] [Google Scholar]
- 13.Swensson RG, Edwards W. Response Strategies in A Two-Choice Reaction Task with A Continuous Cost for Time. Journal of Experimental Psychology. 1971;88:67–81. [Google Scholar]
- 14.Myung IJ, Busemeyer JR. Criterion learning in a deferred decisionmaking task. American Journal of Psychology. 1989;102:1–16. [Google Scholar]
- 15.Paulus MP, Feinstein JS, Leland D, Simmons AN. Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. Neuroimage. 2005;25:607–615. doi: 10.1016/j.neuroimage.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 16.Corlett PR, Aitken MR, Dickinson A, Shanks DR, Honey GD, Honey RA, Robbins TW, Bullmore ET, Fletcher PC. Prediction error during retrospective revaluation of causal associations in humans: fMRI evidence in favor of an associative model of learning. Neuron. 2004;44:877–888. doi: 10.1016/j.neuron.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 17.First Michael B, Spitzer RL, Gibbon Miriam, Williams Janet B. (Structured clinical interview for DSM IV Axis I disorders - Patient edition (SCID-I/P, vers 2.0) New York State Psychiatric Institute: New York, Biometrics Research Department; [Google Scholar]
- 18.Zuckerman M. The psychophysiology of sensation seeking. J Pers. 1990;58:313–345. doi: 10.1111/j.1467-6494.1990.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 19.Fossati A, Di Ceglie A, Acquarini E, Barratt ES. Psychometric properties of an Italian version of the Barratt Impulsiveness Scale-11 (BIS-11) in nonclinical subjects. J Clin Psychol. 2001;57:815–828. doi: 10.1002/jclp.1051. [DOI] [PubMed] [Google Scholar]
- 20.Zuckerman M. Sensation seeking and the endogenous deficit theory of drug abuse. NIDA Res Monogr. 1986;74:59–70. [PubMed] [Google Scholar]
- 21.Barratt ES, Stanford MS, Dowdy L, Liebman MJ, Kent TA. Impulsive and premeditated aggression: a factor analysis of self-reported acts. Psychiatry Res. 1999;86:163–173. doi: 10.1016/s0165-1781(99)00024-4. [DOI] [PubMed] [Google Scholar]
- 22.Paulus MP, Geyer MA, Braff DL. The assessment of sequential response organization in schizophrenic and control subjects. Prog Neuropsychopharmacol Biol Psychiatry. 1994;18:1169–1185. doi: 10.1016/0278-5846(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 23.Paulus MP, Geyer MA, Braff DL. Use of methods from chaos theory to quantify a fundamental dysfunction in the behavioral organization of schizophrenic patients. Am J Psychiatry. 1996;153:714–717. doi: 10.1176/ajp.153.5.714. [DOI] [PubMed] [Google Scholar]
- 24.Paulus MP, Hozack N, Zauscher B, McDowell JE, Frank L, Brown GG, Braff DL. Prefrontal, Parietal, and Temporal Cortex Networks Underlie Decision-Making in the Presence of Uncertainty. Neuroimage. 2001;13:91–100. doi: 10.1006/nimg.2000.0667. [DOI] [PubMed] [Google Scholar]
- 25.Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with errorrate-independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- 26.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 27.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 28.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norusis MJ. SPSS base system user's guide. Chicago: SPSS Inc.; 1990. [Google Scholar]
- 30.Verney SP, Brown GG, Frank L, Paulus MP. Error-rate-related caudate and parietal cortex activation during decision making. Neuroreport. 2003;14:923–928. doi: 10.1097/01.wnr.0000072842.93264.b6. [DOI] [PubMed] [Google Scholar]
- 31.Paulus MP, Hozack N, Frank L, Brown GG. Error Rate and Outcome Predictability Affect Neural Activation in Prefrontal Cortex and Anterior Cingulate during Decision-Making. Neuroimage. 2002;15:836–846. doi: 10.1006/nimg.2001.1031. [DOI] [PubMed] [Google Scholar]
- 32.Wagner FA, Anthony JC. From First Drug Use to Drug Dependence. Developmental Periods of Risk for Dependence upon Marijuana, Cocaine, and Alcohol. Neuropsychopharmacology. 2002;26:479–488. doi: 10.1016/S0893-133X(01)00367-0. [DOI] [PubMed] [Google Scholar]
- 33.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Asahi T, Uwano T, Eifuku S, Tamura R, Endo S, Ono T, Nishijo H. Neuronal responses to a delayed-response delayed-reward go/nogo task in the monkey posterior insular cortex. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 36.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 37.Paulus MP, Stein MB. An Insular View of Anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 38.A.D. Craig, personal communication.
- 39.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crawford TJ, Puri BK, Nijran KS, Jones B, Kennard C, Lewis SW. Abnormal saccadic distractibility in patients with schizophrenia: a 99mTc-HMPAO SPET study. Psychological Medicine. 1996;26:265–277. doi: 10.1017/s0033291700034668. [DOI] [PubMed] [Google Scholar]
- 42.Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- 43.Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gledhill-Hoyt J, Lee H, Strote J, Wechsler H. Increased use of marijuana and other illicit drugs at US colleges in the 1990s: results of three national surveys. Addiction. 2000;95:1655–1667. doi: 10.1046/j.1360-0443.2000.951116556.x. [DOI] [PubMed] [Google Scholar]
- 45.McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- 46.Wu LT, Schlenger WE. Psychostimulant dependence in a community sample. Subst Use Misuse. 2003;38:221–248. doi: 10.1081/JA-120017246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paulus MP. Long-range interactions in sequences of human behavior. Physical Review E. 1997;55:3249–3256. [Google Scholar]
- 48.Paulus MP, Geyer MA, Braff DL. Long-range correlations in choice sequences of schizophrenic patients. Schizophr Res. 1999;35:69–75. doi: 10.1016/s0920-9964(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 49.Ernst M, Paulus MP. Neurobiology of Decision Making: A Selective Review from a Neurocognitive and Clinical Perspective. Biol Psychiatry. 2005;58:596–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.