Abstract
Objective
Patients with micrometastasis to para-aortic lymph nodes may benefit from extended field chemoradiation. To determine the rate of para-aortic node micrometastasis in patients with locally advanced cervical cancer undergoing laparoscopic extraperitoneal para-aortic lymphadenectomy
Methods
We prospectively identified consecutive patients diagnosed with stage IB2-IVA biopsy-proven cervical cancer. Eligible patients included those who were candidates for treatment with radiotherapy and concurrent chemotherapy and had no evidence of para-aortic lymphadenopathy (all lymph nodes < 2 cm in diameter) by preoperative computed tomography or magnetic resonance imaging. All patients underwent preoperative positron emission tomography/computed tomography and laparoscopic extraperitoneal para-aortic lymphadenectomy. All lymph nodes were assessed for metastasis by routine hematoxylin-eosin (H&E) staining. Ultrastaging (serial sectioning) and immunohistochemical analysis were performed in H&E-negative specimens.
Results
Thirteen (22%) of 60 consecutive patients had para-aortic lymph node metastases detected on routine H&E staining. Of the remaining 47 patients, one (2.1%) had evidence of micrometastasis, which was detected by ultrastaging. This patient completed whole pelvic radiotherapy and chemotherapy but had a recurrence 27 months after completion of therapy.
Conclusions
The rate of para-aortic node micrometastasis in patients with locally advanced cervical cancer is low. The role of routine ultrastaging and immunohistochemical analysis in such patients remains uncertain. Future studies are needed to determine the clinical impact of para-aortic node micrometastasis in patients with locally advanced cervical cancer.
Introduction
In cervical cancer, metastasis to the para-aortic lymph nodes is associated with worse overall prognosis [1]. The detection of para-aortic lymph node involvement requires extended-field radiation therapy. Current imaging modalities such as CT scan or MRI are limited in their precision to detect metastatic lymphatic disease [2, 3]. Furthermore, clinical staging in locally advanced cervical cancer is often inaccurate [4]; thus, some patients with disease-free lymph nodes according to preoperative imaging evaluation may be undertreated and may benefit from surgical lymphadenectomy to ensure accurate lymph node status. Recently published data supports the fact that there appears to be a survival advantage when comparing treatment based on surgical staging versus treatment based on radiologic imaging prior to the administration of chemotherapy and radiation in patients with locally advanced cervical cancer [5].
The routine approach to assessing whether metastases are present in surgically resected lymph nodes is hematoxylin-eosin (H&E) staining of a single section from each lymph node. Lymph nodes in which no disease is detected on routine H&E staining can also be subjected to ultrastaging or the examination of additional wide intervals by H&E, along with immunohistochemical (IHC) analysis. This additional scrutiny increases the rate of detection of lymph node metastases and can identify so-called, micrometastasis. Ultrastaging along with immunohistochemistry (IHC) for cytokeratin is routinely used for sentinel node evaluation in breast cancer, melanoma and vulvar carcinoma. This is performed because of the potential for a worse prognosis in patients with untreated nodal micrometastasis [6–9].
Currently, there is no standard definition of micrometastases in cervical cancer, but the American Joint Committee on Cancer (AJCC) Cancer Staging Manual includes a standard definition of micrometastasis in breast cancer. Micrometastasis, as defined in the 6th edition AJCC staging system for breast cancer [10], are tumor deposits measuring 0.2 mm to 2 mm. Tumor deposits larger than 2 mm are defined as macrometastasis, and tumor deposits smaller than 0.2 mm are defined as isolated tumor cells or submicrometastasis.
To date, there is limited information on the rate and significance of micrometastasis to para-aortic lymph nodes in women with locally advanced cervical cancer. Ultrastaging could potentially increase the detection rate of malignant cells in para-aortic lymph nodes in locally advanced cervical cancer allowing detection of occult disease otherwise missed on routine H&E staining. Furthermore, detection of micrometastasis in para-aortic lymph nodes would change treatment, as these patients would be candidates for extended field chemoradiation.
In this study, we prospectively evaluated the rate of micrometastases in the para-aortic lymph nodes using ultrastaging and IHC analysis in patients with locally advanced cervical cancer who underwent extraperitoneal lymphadenectomy before the initiation of chemo-radiotherapy. We defined micrometastasis as tumor deposits measuring 0.2 mm to 2 mm.
Materials and Methods
This study was conducted as part of a prospective study that evaluated patients with newly diagnosed stage IB2-IVA cervical cancer and negative para-aortic lymph nodes on CT or MRI scans. All patients underwent a PET/CT followed by a laparoscopic extraperitoneal para-aortic lymphadenectomy prior to initiation of radiotherapy and concurrent chemotherapy. The results showing the correlation of preoperative PET/CT scan with histopathologic findings at the time of extraperitoneal laparoscopic para-aortic lymphadenectomy are reported as part of a separate manuscript. The extent of the para-aortic lymph node dissection was to the level of the renal vessels, except in patients with grossly positive nodes. In those patients, once a lymph node was confirmed by frozen-section to have metastatic disease, the procedure was aborted. All histologic subtypes of cervical cancer were eligible. This study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center and informed consent was obtained from all patients. The patients were recruited from 2004 to 2009. All patients were enrolled at MD Anderson or Lyndon Baines Johnson General Hospital.
All lymph nodes were serially sectioned perpendicular to the long axis at 2.0 mm intervals and submitted for routine processing (1 H&E slide per block). If all lymph nodes were negative on routine H&E staining, then an ultrastaging protocol was performed: three consecutive sections (5 μm thick), were obtained at five levels at 40-μm intervals, and these sections were subjected to H&E staining. If all levels were negative by H&E staining, then the case was submitted for IHC analysis. IHC analysis was performed as follows. The polymeric biotin-free horseradish peroxide (HRP) method on (Leica Microsystems, Bannockburn, IL) Bond Max stainer was used for all the immunohistochemical studies. Tissue sections 4 um thick were prepared from formalin-fixed paraffin sections were incubated in the sixty-degree oven for twenty minutes. These sections were placed in the automated Bond Max stainer which pretreated the slides with heat induce epitope retrieval for ten minutes, slides were then incubated for 15 minutes in anti-keratin cocktail (Cytokeratin AE1/AE3, Dako Corp., Carpinteria CA; 1:50, Cytokeratin CAM5.2, BD Biosciences, San Jose, CA; 1:50, Cytokeratin MNF116, Dako Corp., Carpinteria, CA; 1:50 and Cytokeratin 8&18, Invitrogen, South San Francisco, CA ; 1:25). The Refine Polymer Detection kit was used for immunostaining, with 3,3-diaminobenzidine serving as chromagen. Slides were counterstained with Mayer’s hematoxylin. Antibodies were evaluated with known positive and negative tissue controls.
Results
Sixty-five patients were enrolled in the study. Five patients were excluded from the final analysis because blood glucose levels were too high to permit safe PET/CT (n=2); because supraclavicular nodal metastases were detected on PET/CT and surgery was cancelled (n=2); or because the patient agreed to participate in the trial but never returned after giving informed consent (n=1). Patient characteristics for the remaining 60 patients are shown in Table 1. The median age at diagnosis was 48 years (range, 23–84).
Table 1.
Patient characteristics.
| Characteristic | No. of patients (%) |
|---|---|
| Median age (range) | 48 years (23–84) |
| Median body mass index (range) | 26.7 kg/m2 (15.2–41.3) |
| Histology | |
| Squamous cell carcinoma | 48 (80) |
| Adenocarcinoma | 9 (15) |
| Neuroendocrine carcinoma | 2 (3) |
| Transitional cell carcinoma | 1 (2) |
| Stage | |
| IB2 | 16 (27) |
| IIA | 12 (20) |
| IIB | 16 (27) |
| IIIA | 4 (7) |
| IIIB | 12 (20) |
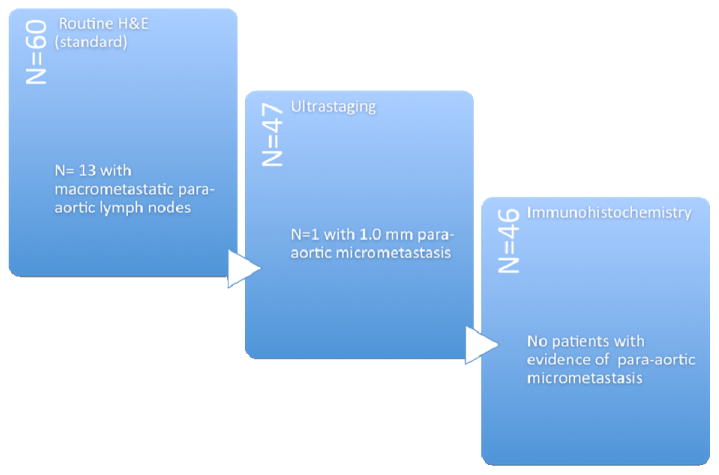
The median number of lymph nodes retrieved was 11 (range, 1–39). Thirteen (22%) of the 60 patients had evidence of metastatic (macrometastasis) disease on routine H&E staining and therefore did not undergo ultrastaging (Figure 1). Of these 13 patients, seven patients had grossly positive lymph nodes. Of the 47 patients with no evidence of metastatic disease on routine H&E staining, one patient (2.1%) had evidence of micrometastasis (measured 1.0 mm) on ultrastaging. The preoperative CT scan and PET/CT of this patient were negative for any evidence of metastatic disease to the para-aortic nodes. Of the remaining 46 patients, none had evidence of micrometastasis on IHC.
Figure 1.
Results of evaluations for para-aortic node micrometastasis.
Postoperatively, patients with para-aortic node metastases were recommended treatment with extended-field radiotherapy. Twenty-two patients received radiation therapy to the para-aortic lymph nodes. The total dose to the entire para-aortic chain was 45 Gy in all 22 patients. Three of these 22 patients received radiation therapy using IMRT and the rest were all treat with 4-fields 3-D conformal radiation therapy. All other patients underwent whole pelvic radiation therapy. Radiotherapy dosage data were available for 58 of the 60 patients included in the final analysis. All patients in the study were treated with concurrent chemotherapy in the form of weekly cisplatin (40 mg/m2)
Disease outcome results are shown in Table 2. Of the 46 patients who had negative nodes after ultrastaging and IHC, two were lost to follow up, leaving 44 patients available for analysis of recurrence and survival. Of these 44 patients, eight (18%) had recurrence. The mean time to recurrence was 9.71 months. Five patients (11%) died of disease (Table 2). Of the 13 patients with evidence of metastasis on routine H&E staining, 2 were lost to follow-up, leaving 11 patients available for analysis. Of these 11 patients, six (55%) had recurrence. The mean time to recurrence was 4.16 months. Five (45%) died of disease. The patient with micrometastasis detected was alive at the end of the follow-up period despite her recurrence in the left pelvic area 27 months after completion of therapy. None of the patients had recurrent disease to the para-aortic nodes.
Table 2.
Patient outcomes.
| Outcome | Node negative N= 44 |
Node positive Routine H&E N= 11 |
Micrometastasis Ultrastaging N=1 |
|---|---|---|---|
| Recurrence | 8 (18%) | 6 (55%) | 1 (100%) |
| DOD* | 5 (11%) | 5 (45%) | 0 (0%) |
Dead of disease
Discussion
Our study showed that the rate of detection of para-aortic lymph node micrometastasis in patients with locally advanced cervical cancer is low. One patient (2.1%) had micrometastasis to the para-aortic lymph nodes detected by the ultrastaging protocol, and IHC analysis did not increase the rate of detection of micrometastasis.
In cervical cancer, there are no data from prospective long-term trials on the prognostic value of micrometastasis; the majority of studies examining micrometastasis in cervical cancer have been retrospective analyses in patients with early-stage disease and such studies have only evaluated micrometastases in pelvic lymph nodes [11,12]. Our study is unique in that we focused on determining the rate of micrometastases in the para-aortic nodes of patients with locally advanced disease. Interestingly, most of this retrospective data in cervical cancer show a trend with micrometastasis patients having worse prognosis and higher rates of recurrence than patients without nodal metastases [11–13]. The reported rates of micrometastatic disease in early-stage cervical cancer range from 3.8% to 50% [11,14]. This broad range in incidence is likely a result of the lack of clear definition of micrometastasis in gynecologic cancers and the use of different methods to detect micrometastasis.
Leblanc et al. [15] reported a 45% rate of “microscopic” metastasis in para-aortic nodes using serial sectioning and IHC. The authors defined microscopic metastasis as tumor deposits smaller than 5 mm, which would lead to an overestimation of the rate of micrometastases according to the AJCC definition (tumor deposits measuring 0.2 mm to 2 mm). To our knowledge, our study is the first to evaluate the rate of para-aortic node micrometastasis in locally advanced cervical cancer using the strict definition of the AJCC [10].
Currently, various methods are available for detecting micrometastasis. We used the ultrastaging protocol as previously described in a study from our own institution [16]. In that study by Euscher et al. [16], the investigators evaluated 48 patients with Stage IA to IB2 cervical cancer who underwent sentinel node biopsy. The results showed that ultrastaging improved the detection rate of metastatic disease by 25%. Another method for detecting micrometastasis is to use reverse transcriptase-polymerase chain reaction (RT-PCR) assay to detect cytokeratin 19 mRNA. Use of this highly sensitive method resulted in detection of cytokeratin 19 transcription in 50% of patients with stage 1A2 to 1B2 cervical cancer after radical hysterectomy and pelvic lymphadenectomy [14]. The authors suggested that these findings could suggest occult micrometastases. However, this method measures not the size of the tumor deposit but rather the number of cytokeratin 19 mRNA copies in lymph node tissue. The authors also report that the method of RT-PCR may be flawed by numerable inconsistencies. Furthermore, the clinical significance of PCR-detected micrometastasis requires further prospective validation. Currently, the majority of studies of the detection of micrometastasis in patients with cervical cancer have used serial sectioning and/or IHC [11, 13, 15, 16].
Fregnani et al. [11] performed a retrospective analysis of 289 patients with stage IB to IIA cervical cancer and found the rate of micrometastasis to be 3.8%. Compared with node-negative patients, patients with micrometastasis had a higher rate of recurrence (3.2 times as high as that of node-negative patients) and reduced disease free survival. Furthermore, compared with patients with macrometastasis, patients with micrometastasis had a lower 5-year disease-free survival rate (50.4% vs. 80.4%, p<0.001) because they were less likely to receive postoperative radiotherapy (36.4% vs. 97.2%, P<0.001).
Juretzka et al. [17] performed a retrospective study of 49 patients with early-stage cervical cancer (stages 1A2 to 1B2) who underwent radical hysterectomy and pelvic lymphadenectomy to determine the rate of micrometastasis using IHC analysis for cytokeratin AE1 and CAM 5.2. The authors detected micrometastasis in 8.1% of the patients (4 of 49). The recurrence rate was 50% (2 of 4 patients) for those with micrometastasis and 6.7% (3 of 45 patients) for those without micrometastasis. In that study, the authors did not report the tumor metastasis size.
Horn et al. [12] performed “three step sections” on lymph nodes without the use of IHC in patients with early-stage (IB to IIB) cervical cancer and found a 22.2% rate of micrometastasis (59/266 patients). Patients with micrometastasis had statistically significant decreased 5-year overall survival and recurrence-free survival compared to node-negative patients. Marchiole et al. [13] performed a case-control study of 292 patients diagnosed with early-stage cervical cancer and treated by laparoscopic vaginal radical hysterectomy during the same time period. Two paired series were selected. The cases were 26 patients who had recurrence (at a median follow-up time of 36.8 months), and the controls were 26 patients matched for age, histologic subtype, surgico-pathologic stage, and maximal tumor diameter who did not have recurrence (median follow-up time for controls, 122 months). All the lymph node blocks that were initially considered as uninvolved were submitted for serial sectioning (3 sections at 200 μm intervals). One section underwent H&E, and the other two sections underwent IHC using AE1/AE3. Lymph-vascular space invasion (LVSI) was twice more frequent and micrometastases ten-fold more frequent in the group of patients who recurred: 20/26 (77%) versus 9/26 (35%) and 11/26 (42%) versus 1/26 (4%); respectively. The relative risk of recurrence was 2.64 (1.67–5.49, P < 0.01) in the presence of LVSI and 2.44 (1.58–3.78, P < 0.01) in the presence of micrometastases.
The clinical impact of the detection and treatment of micrometastases to the para-aortic nodes remains a subject of debate. The role of extended-field radiation combined with chemotherapy needs to be explored in the setting of isolated micrometastases to the para-aortic nodes in patients with locally advanced disease. At MD Anderson Cancer Center, we are currently developing a prospective randomized trial to evaluate the impact of surgical staging in patients with locally advanced cervical cancer. In that study, patients with locally advanced cervical cancer will undergo a PET/CT, those patients with positive pelvic nodes and negative para-aortic nodes will then be randomized to treatment based on PET/CT findings alone versus treatment based on surgical staging and histopathologic findings. The primary endpoints of the trial will be an analysis of disease-free survival and overall survival.
Future studies are needed to evaluate the role of micrometastasis as a predictor of overall outcome in patients with locally advanced cervical cancer. Therefore, the role of routine ultrastaging and IHC remains unclear. Furthermore, we propose there needs to be a standard definition of micrometastasis as there are various definitions used in the cervical cancer literature and a consistent term is needed to compare results and validate future results.
Acknowledgments
The authors would like to thank Ms. Stephanie Deming for her editorial contribution to this manuscript.
Footnotes
Conflict of Interest:
The authors declare that they have no conflict of interest pertaining to the material reported in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stehman FB, Bundy BN, DiSaia PJ, et al. Carcinoma of the cervix treated with radiation therapy: a multivariate analysis of prognostic variables in the Gynecologic Oncology Group. Cancer. 1991;67:2776–85. doi: 10.1002/1097-0142(19910601)67:11<2776::aid-cncr2820671111>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Vidauretta J, Bermudez A, Di Paola G, et al. Laparoscopic staging in locally advanced cervical carcinoma: a new possible philosophy? Gynecol Oncol. 1999;75:366–71. doi: 10.1006/gyno.1999.5597. [DOI] [PubMed] [Google Scholar]
- 3.Hertel H, Kohler C, Elhawary T, et al. Laparoscopic staging compared with imaging techniques in staging of advanced cervical cancer. Gynecol Oncol. 2002;87:46–51. doi: 10.1006/gyno.2002.6722. [DOI] [PubMed] [Google Scholar]
- 4.Lagasse LD, Creasman WT, Shingleton HM, et al. Results and complications of operative staging in cervical cancer: Experience of the Gynecologic Oncology Group. Gynecol Oncol. 1980;9:90–8. doi: 10.1016/0090-8258(80)90013-x. [DOI] [PubMed] [Google Scholar]
- 5.Gold MA, Tian C, Whitney CW, et al. Surgical versus radiographic determination of para-aortic lymph node metastases before chemoradiation for locally advanced cervical carcinoma: a GOG study. Cancer. 2008;112:1954–63. doi: 10.1002/cncr.23400. [DOI] [PubMed] [Google Scholar]
- 6.Sakorafas GH, Geraghty J, Pavlakis G. The clinical significance of axillary lymph node micrometastases in breast cancer. Eur J Surg Oncol. 2004;30:807–16. doi: 10.1016/j.ejso.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Grabau G. Breast cancer patients with micrometastases only: is a basis provided for tailored treatment? Surg Oncol. 2008;17:211–7. doi: 10.1016/j.suronc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Scolyer RA, Murali R, Satzger I, et al. The detection and significance of melanoma micrometastases in sentinel nodes. Surg Oncol. 2008;17:165–74. doi: 10.1016/j.suronc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Narayansingh GV, Miller ID, Sharma M, et al. The prognostic significance of micrometastases in node negative squamous cell carcinoma of the vulva. Br J Cancer. 2005;92:222–24. doi: 10.1038/sj.bjc.6602343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Joint Committee on Cancer. AJCC cancer staging manual. 6. New York (NY): Springer-Verlag; 2002. [Google Scholar]
- 11.Fregnani J, Latorre M, Novik PR, et al. Assessment of pelvic lymph node micrometastatic disease in stages IB and IIA carcinoma of the uterine cervix. Int J Gynecol Cancer. 2006;16:1188–94. doi: 10.1111/j.1525-1438.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 12.Horn LC, Hentschel B, Fischer U, et al. Detection of micrometastases in pelvic lymph nodes in patients with carcinoma of the cervix uteri using step sectioning: frequency, topographic distribution and prognostic impact. Gynecol Oncol. 2008;111:276–81. doi: 10.1016/j.ygyno.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Marchiole P, Buenerd A, Benchaib N, et al. Clinical significance of lympho vascular space involvement and lymph node micrometastases in early stage cervical cancer: a retrospective case-control surgico-pathological study. Gynecol Oncol. 2005;97:727–32. doi: 10.1016/j.ygyno.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Van Trappen PO, Gyselman VG, Lowe DG, et al. Molecular quantification and mapping of lymph node micrometastases in cervical cancer. Lancet. 2001;357:15–20. doi: 10.1016/S0140-6736(00)03566-2. [DOI] [PubMed] [Google Scholar]
- 15.Leblanc E, Narducci F, Frumovitz M, et al. Therapeutic value of pretherapeutic extraperitoneal laparoscopic staging of locally advanced cervical carcinoma. Gynecol Oncol. 2007;105:304–11. doi: 10.1016/j.ygyno.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Euscher ED, Malpica A, Atkinson EN, et al. Ultrastaging improves detection of metastases in sentinel lymph nodes of uterine cervix of squamous cell carcinoma. Am J Surg Pathol. 2008;32:1336–43. doi: 10.1097/PAS.0b013e31816ecfe4. [DOI] [PubMed] [Google Scholar]
- 17.Juretzka MM, Jensen KC, Longacre TA, et al. Detection of pelvic lymph node micrometastasis in stage IA2-IB2 cervical cancer by immunohistochemical analysis. Gynecol Oncol. 2004;93:107–11. doi: 10.1016/j.ygyno.2003.11.033. [DOI] [PubMed] [Google Scholar]