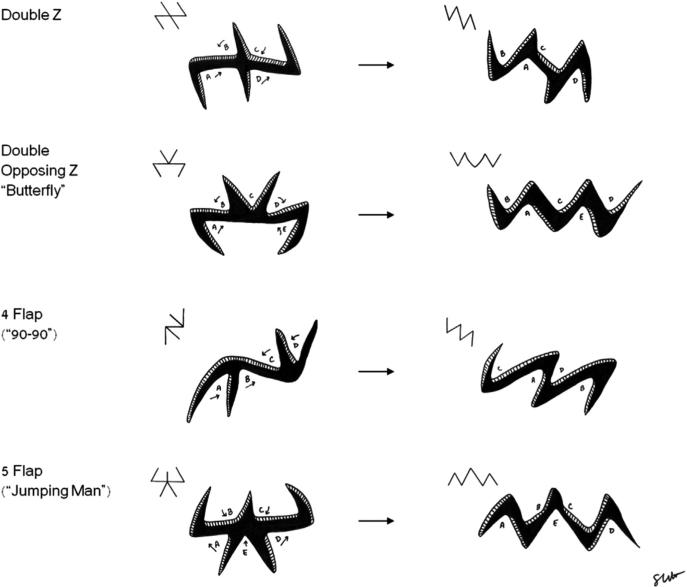BASIC SCIENCE OF SCARS
Introduction
Hypertrophic scarring (HTS) is a common complication of burn injury that can be considered a fibroproliferative disorder (FPD) (Figs. 1 and 2).1 Bombaro and colleagues2 documented an incidence of HTS following burn injury of up to 80% in injured military personnel. Burn patients often require a prolonged period of rehabilitation,3,4 including an average of 12.7 weeks off work for patients with thermal injuries greater than 30% of the total body surface area (TBSA). Much of the rehabilitative phase is related to functional and cosmetic limitations imposed by HTS, including a reduction in range of motion of the extremities and the intense pruritus and heat intolerance often preventing early return to work and school.3,4 Risk factors for HTS include sex, age, racial or genetic factors, and wound location1,5; however, HTS develops most predictably after prolonged inflammation of slowly healing burn wounds.6,7 Unfortunately, HTS responds poorly to current forms of therapy, including pressure garments, topically applied silicone, and intralesional steroids.1,8,9
Fig. 1.
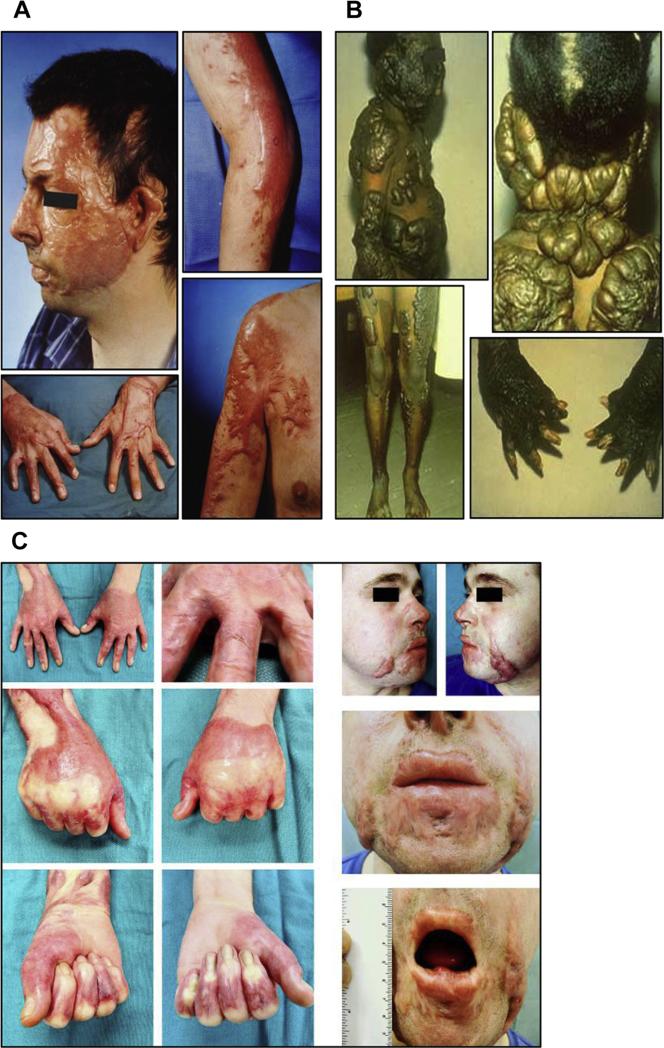
(A) HTS developed on a 34-year-old Caucasian man 8 months after a burn involving 60% of his TBSA. (B) Keloids on a 12-year-old black child following a scald burn including donor sites on lower extremities. (C) A 24-year-old white man, 11 months after a 21% TBSA burn. This patient developed HTS, resulting in cosmetic and functional problems that included restricted opening of mouth and tight web spaces of fingers that limited range of motion on hands.
Fig. 2.
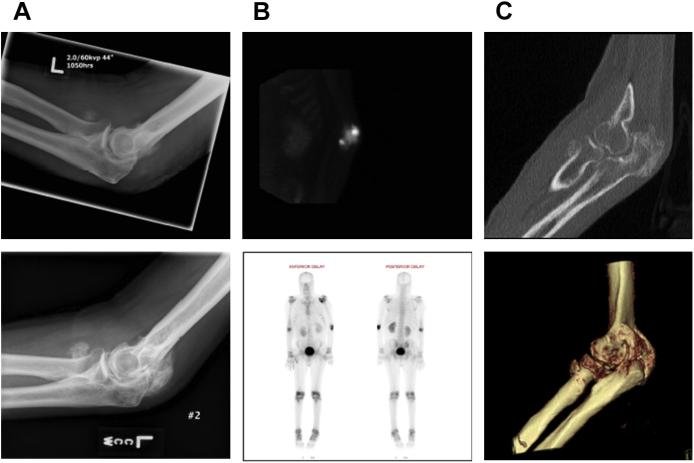
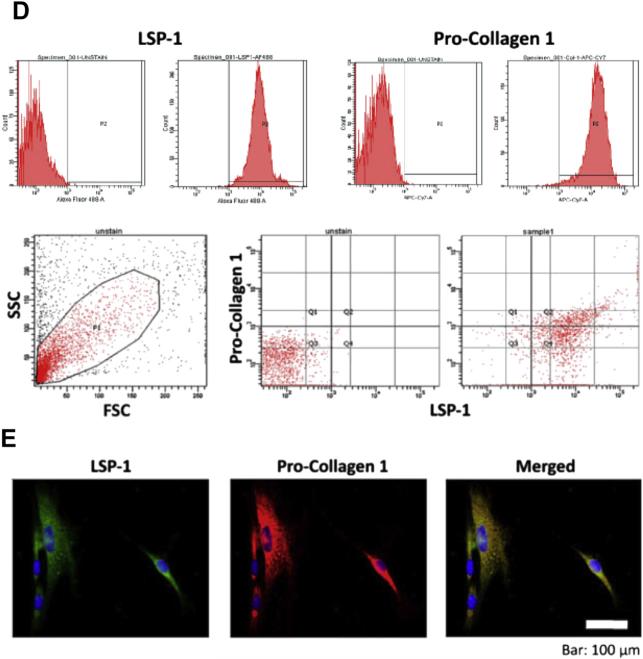
A 26-year-old man with 75% TBSA burns who developed HO in both elbows. (A) Imaging studies of the right elbow at 1.5 months (left) and 5 months (right) after burn injury demonstrate the progression of the HO lesion. (B) Intraoperative views from the same patient showing the surgical approach for HO resection (left) and HO specimen (right). (C) Isolation of bone marrow–derived precursor cells from HO tissue by using cell explantation method. A significant cell subset isolated from HO tissue (~35–65%) exhibits a LSP11/COL11 profile as demonstrated by flow cytometry (D) and immune fluorescence microscopy (E).
The Cellular Basis of HTS
As compared with site-matched normal skin fibroblasts, consistent features of HTS fibroblasts include an increase in procollagen mRNA and protein synthesis, as well as increased transforming growth factor-β (TGF-β), a profibrotic cytokine (Tables 1 and 2).1 HTS fibroblasts have reduced collagenase (matrix metalloproteinase 1 [MMP-1]) activity,10 nitric oxide, and decorin production (a small dermatan sulfate proteoglycan that restores normal collagen fibrillogenesis and binds TGF-β11). Increased numbers of myofibroblasts constitute a persistent component of the hypercellular matrix in HTS and contain microfilament bundles and alpha smooth muscle actin (α-SMA) important for wound contraction, a significant comorbid complication of HTS and other FPD.12 The development of myofibroblasts appears to be induced by TGF-β13 and strongly correlates with the severity of burn injury (TBSA). Myofibroblasts appear to differentiate not only from regional fibroblasts in the wound under the influence of TGF-β, but also from bone marrow-derived blood-borne sources.14
Table 1.
Features of normal, HTS, and deep dermal fibroblasts
| Normal Fibroblasts | HTS Fibroblasts | Deep Dermal Fibroblasts | |
|---|---|---|---|
| Cell size | + | + | ++ |
| Proliferation rate | ++ | ++ | + |
| Collagen synthesis | + | ++ | ++ |
| Collagenase activity | ++++ | + | + |
| α-SMA expression | + | +++ | +++ |
| Collagen contraction | + | +++ | +++ |
| TGF-β | + | + | + |
| TGF-β T II receptor | + | +++ | +++ |
| CTGF | + | +++ | +++ |
| Osteopontin | + | +++ | +++ |
| Decorin synthesis | ++++ | + | + |
| Fibromodulin | ++++ | + | + |
| Biglycan | + | +++ | +++ |
| Versican | + | +++ | +++ |
| Toll-like receptors | + | +++ | ? |
Table 2.
Z-plasty angles and theoretical gain in length of central limb
| Angle of Z-Plasty Limbs (degrees) | Gain in Length (%) |
|---|---|
| 30 | 25 |
| 45 | 50 |
| 60 | 75 |
| 75 | 100 |
| 90 | 120 |
The role bone marrow cells in wound healing and fibrosis
Bucala and colleagues1,15 have identified an adherent and proliferating population of cells with a fibroblast-like morphology that coexpress collagen I and III, CD13, CD34, and the bone-marrow–derived surface marker CD45, which make up 0.5% of peripheral blood leukocytes, but can constitute 10% of cells infiltrating acute wounds. Migrating fibrocytes are capable of synthesizing extracellular matrix (ECM) proteins, proteases including collagenase, and growth factors, such as TGF-β1, tumor necrosis factor-α, interleukin (IL)-6, and IL-10, but can also present antigens and thereby prime naïve T lymphocytes.1,16,17
Fibrocytes have been identified in burn patients from peripheral blood mononuclear cells (PBMC), where the percentage of type I collagen–positive fibrocytes is significantly higher (up to 10% of PBMC) than for control individuals (normal level <0.5%), which correlated with serum levels of TGF-β.15,18,19 In culture, fibrocytes were derived from CD141 cells, but required TGF-β in the conditioned media from CD14 cells for differentiation.16 Leukocyte-specific protein 1 (LSP-1) is a unique marker for[C0] fibrocytes and is up-regulated in burn patients and remains stable through differentiation.16–18 Double staining with antibodies to LSP-1 and the C-propeptide of type I collagen identified a 300% increase in fibrocytes in HTS tissue located primarily in deeper layers of the papillary dermis. Characteristic morphologic alterations in fibrocytes occur in vitro after exposure to endotoxin, which are corrected by treatment with interferon (IFN)-α2b.20–22 From serial analysis of burn patients with HTS, increased numbers of fibrocytes are present in HTS tissues compared with mature scar and normal skin.20–22 Quantitatively, fibrocytes produce less collagen than hypertrophic scar (HSc) fibro-blasts; however, fibrocytes from burn patients differ from that of normal individuals in their paracrine effects that include stimulating dermal fibroblasts to proliferate, produce, and contract ECM, as well as producing TGF-β and its downstream effector, connective tissue growth factor (CTGF).22 These findings resemble others,23 where the principal source of collagen in other fibroses models appears to be local fibro-blasts, but the presence of bone marrow–derived immune cells resembling fibrocytes persist in the matrix, suggesting an important paracrine role of fibrocytes in HTS and other FPD. It is possible to antagonize many of these fibrogenic effects of fibrocytes in vitro with IFN-α where significantly decreased numbers of fibrocytes were found in the tissues of burn patients in response to systemic IFN treatment in vivo, associated with fibrosis resolution and scar remodeling.21,22 In addition, increased angiogenesis associated with increased vascular endothelial growth factor (VEGF) in HTS is reduced by IFN-α, in part because of suppression of endothelial cell proliferation and tubule formation through reduction in VEGF receptor expression in endothelial cells.22 Coexpression of VEGF mRNA with stromal derived factor 1 (SDF-1) mRNA further implicates fibrocytes in the pathophysiology of interstitial pulmonary fibrosis (IPF) and other fibroses.24
Heterotopic ossification (HO) is a clinical condition whereby mature lamellar bone is formed in damaged tissues, such as muscle, tendon, and fascia.25–28 This condition occurs after burns and traumatic injuries and leads to skin breakdown, soft tissue deformity, joint ankylosis, and chronic pain. In burn patients, the incidence of HO varies between 0.2% and 4%26,27 and is more frequent in patients with extensive burns (>20% TBSA). Although HO may occur in joints unrelated to burn injuries, lesions usually develop under areas of deep burns complicated by HTS, especially in the elbow, and is associated with immobilization, burn wound infection, wound delayed closure, and recurring local trauma (possibly including aggressive passive range of motion).26,27 Local radiation has been recommended, but concerns of long-term side effects and inconsistent results mitigate against its routine use.28,29 There is a need for animal models of HO to develop and test novel diagnostic modalities and therapies before clinical translation.28
Recently, a large population of fibrocytes (LSP-1+/type-1 collagen+) has been identified within HO specimens, as distinctive bone marrow–derived blood-borne cells that traffic to injured areas and interact with resident cells.30,31 Fibrocytes have the potential to differentiate into osteoblasts and chondrocytes and can be reprogrammed into antifibrotic profile cells stimulating the MMP-1 production in dermal fibroblasts, collagen breakdown, and scar remodeling.31 Therefore, HO and FPD such as hyper-trophic scar have common features and appear to be causally related after significant initial local tissue injury, which leads to a systemic inflammatory response, wherein unique PBMCs, including fibrocytes, contribute to the fibrotic and osteogenic matrix in as yet unidentified ways.
The role of the Th1/Th2 paradigm after burn injury
In animal models and humans with acute burn injury, evidence for reduced IL-2 and IFN-γ production and increased Th2 cytokines (IL-4, IL-5, IL-10, IL-13) is emerging.31,32 In burn patients with HTS, a deficiency of circulating lymphocytes exists, which produce IFN-γ very early after injury, and within 3 months, after burn increased numbers of IL-4 containing lymphocytes develop, which persist for up to 1 year after injury, consistent with a polarized Th2 response.33 Significant elevations in IL-10 in the first 2 months after injury persist until 1 year, whereas IL-12 levels were significantly lower and inversely related to IL-10.33 IFN-γ mRNA was not detected in PBMC and in HTS tissues until 6 months after injury, whereas IL-4 was undetected in normal controls, but increased in HTS patients in PBMC within 2 months after injury, as well as in HTS tissues. CD4+/TGF-β+ lymphocytes are present in an increased frequency in the circulating immune cells of burn patients as compared with normal control individuals.34 These cells secrete increased levels of TGF-β, which promotes proliferation of dermal fibroblasts, as well as α-SMA and wound contraction. The development of CD4+TGF-β+cells may contribute to the suppression of Th1 immunity similar to trauma patients, whereby increased T-regulatory CD41CD251 cells, which produce TGF-β, have been found systemically.34 Thus, these findings suggest that after thermal injury, a polarized Th2 environment favors the subsequent development of increased Th3+ cells and fibrocytes, which can induce fibrosis in a paracrine fashion. Pilling and colleagues1,20 have described that Th2 cytokines (IL-4, IL-13) promote, whereas Th1 cytokines (IFN-γ, IL-12) inhibit, fibrocyte differentiation in fibrosis.
Fibroblast heterogeneity and the profibrotic microenvironment
Sorrell and colleagues35 have found that normal adult human skin contains at least 3 separate subpopulations of fibroblasts, which occupy unique niches depending on the depth of the dermis and exhibit distinctive differences when isolated by limited dilution cloning. Fibroblasts associated with hair follicles show distinctive characteristics from cells in the papillary and reticular dermis.36–38 Papillary dermal fibroblasts, which reside in the superficial dermis, are heterogeneous in terms of morphology and proliferation kinetics, whereas reticular fibroblasts in the deep dermis possess myofibroblast-like characteristics by greater collagen lattice contraction and aSMA expression.
Fibroblasts that arise from the deeper layers proliferate at a slower rate,39 but are significantly larger morphologically, and collagenase mRNA is significantly lower in deep dermal fibroblasts. Fibroblasts from the deeper layers produce more TGF-β, CTGF, and heat shock protein 47, a human chaperon protein for type I collagen, compared with those from superficial layers.39 Fibroblasts from the deeper layer produced more α-SMA protein and contracted collagen gels more efficiently.39,40 Fibro-blasts from the deeper layer also produced more collagen, but had less collagenase activity and produced more of the fibrocartilaginous proteoglycan versican, but less decorin. Decorin and other members of the small leucine rich proteoglycans (sLRP) family, fibromodulin and lumican, function to bind type I collagen in the ECM, regulating the kinetics of collagen fibrillogenesis and the diameter and distance between fibrils.40 Decorin and fibromodulin can also bind to and inhibit TGF-β1 activity in vitro and in vivo.41 Fibroblasts isolated from the deep dermis produce less decorin and more large cartilage-like proteoglycans, including versican and aggrecan, that can account for the ultrastructural abnormalities in HTS. Recently, fibrocytes have also been described to produce less sLRPs and more versican, hyaluronan, perlecan, and biglycan in the ECM.42
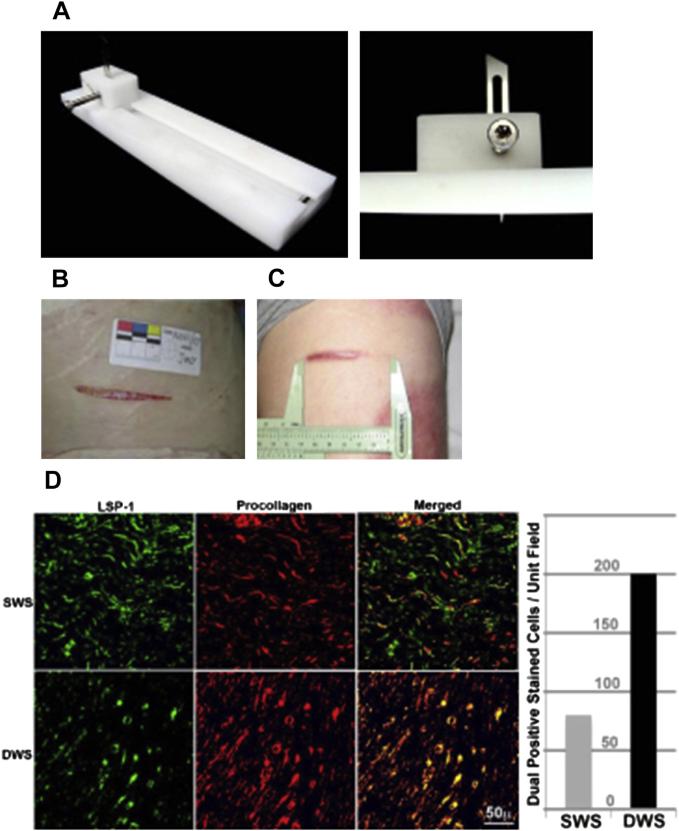
Dunkin and colleagues6 quantified the association between scarring and the depth of dermal injury in human volunteers using a novel jig to create a human dermal scratch model with HTS and normotrophic scar within the same lesion. They found a threshold depth of dermal injury of 0.56 ± 0.03 mm or 33% of the lateral hip thickness, at which scarring develops.6 In patients with thermal injury, Honardoust and colleagues42 found that the superficial one-third of this scratch wound healed normally with minimal scar, whereas the deep dermal end region healed with a thickened wider scar typical of HTS and contained significantly greater numbers of fibrocytes (Figs. 3–5). These data strongly demonstrate that fibroblasts from the deeper layers resemble HTS fibroblasts, suggesting that the activated deeper layer fibroblasts may play a critical role in the formation of HTS.
Fig. 3.
(A) Jig used to make progressive human dermal scratch injury. (B) Progressive wound on day 0. (C) Wound on day 70. (D) Increased number of fibrocytes in the deeper areas of the wound (DWS) as compared with the superficial wound site (SWS).58
Fig. 5.
Diagramic depiction of the design of various z plastids and their variants with the resultant configuration once cut out and flaps transposed.
The role of toll-like receptors signaling in fibrosis
Toll-like receptors (TLR) are a group of highly conserved molecules that allow the immune system to sense pathogen associated molecular patterns (PAMPs) or endogenous molecules, which are released from necrotic tissue, termed damage associated molecular patterns (DAMPs).43 They function as activators of the innate immune system, but, most recently, have increasingly been implicated in the switch from normal wound-healing responses to fibrosis in many different organs and tissues. Ten different members exist that bind specific ligands; however, TLR2 recognizes gram-positive bacteria and TLR4 senses gram-negative bacteria by binding lipopolysaccha-ride (LPS).43 Although the mechanism of fibrosis has not been established in the skin and many other tissues, in liver fibrosis, TLR4-dependent fibrosis is stimulated by LPS directly, targeting fibroblast precursors in the liver, which release chemokines to activate macrophage-like Kupffer cells, resulting in unrestricted TGF-β-mediated activation of hepatic stellate cells, increased deposition of ECM, and promotion of liver fibrosis.44 Recently, HTS fibroblasts have been found to have increased expression of TLR4 mRNA and surface receptors implicating the Toll receptor system in the activation of dermal fibroblasts in HTS (see Fig. 3).45
Newly Evolving Therapies Based on the Pathology of HTS
IFN treatment
With greater understanding of the inflammatory response to thermal injury, newer therapeutics have emerged attempting to shift the systemic Th2 polarized immune response toward a Th1 response.
In a prospective clinical trial evaluating the effect of subcutaneous systemic treatment with IFN-α2β in 9 burn patients with HSc,46 7 of 9 patients demonstrated significant improvement in scar assessment, and 3 of 9 patients demonstrated significant reductions in scar volume compared with the control group. Before IFN treatment, TGF-β levels were significantly higher in burn patients with HSc compared with a control group. With treatment, levels of TGF-β normalized to control levels with no increase following cessation of IFN treatment. Plasma Nτ–methylhistamine levels were significantly elevated in HSc patients compared with controls, and a significant reduction in levels was achieved with treatment. These findings demonstrate an antagonistic relationship between IFN-α2β and TGF-β and reinforce similar findings in vitro.46,47 However, intralesional administration of IFN to HSc has failed to show benefit,48 suggesting that TGF-β and other components of the Th2 response may require systemic administration to shift the inflammatory response toward a Th1 cytokine profile.
Clinically, intralesional injection of IFN-α for the treatment of keloids and hypertrophic scar has been suggested to be effective in preventing recurrence of the scar after excision; however, the authors’ experience and others have found minimal benefit in treating established proliferative scars intralesionally.49 However, systemic IFN-α2β used in dosage regimens similar to the initial treatment of hepatitis C and B, where it is approved as a chemotherapeutic agent, has been found effective in a double-blind placebo-controlled preliminary trial in 21 burn patients with HSc.50 IFN-treated patients demonstrated significant improvements in overall scar assessment and color following treatment. Side effects of IFN treatment in this group of patients include myalgias, low-grade fever, and fatigue; however, depression with IFN therapy is a significant concern requiring careful observation in patients on systemic therapy. Thus, despite early encouraging results, larger phase III trials are required to determine the cost benefit of IFN treatment in patients with HSc and other FPDs before routine off-label use can be advocated.
Chemokines and CXCR4 inhibitors
Chemokines are small 8- to 10-kDa proteins that induce chemotaxis in cells surrounding the sites of injury. They can be divided into 4 types depending on the spacing and location of 2 cysteine residues in the molecules and include CC, CXC, C, and CX3C subfamilies.51–53 Significant increases of the chemokine, macrophage chemotactic peptide1 (MCP-1), expression in fibroblasts from HTS compared with normal fibro-blasts suggest a role for MCP-1 in fibrotic diseases.52
CXCR4 is a CXC chemokine receptor and it exclusively binds to SDF-1, which is unique among receptors because most chemokines have more than one receptor and most receptors have more than one ligand.51,52 Increased expression of SDF-1 in human burn blister fluid has been found with improved wound healing after blocking the SDF-1/CXCR4 pathway.53 Up-regulation of SDF-1/CXCR4 signaling with increased SDF-1 levels in HTS and serum has been described in burn patients, whereby SDF-1/CXCR4 signaling in burn wounds stimulates activated CD14+ CXCR4+ cells to migrate to the injured tissue where they appear to differentiate into fibrocytes and myofibroblasts, contributing to the pathogenesis of HTS.54
Using newly developed antagonists of CXCR4, significant reduction in scar formation in a human skin on nude mice in vivo has be found, in part by reducing the recruitment of macrophages and myofibroblasts, enhancing the remodeling of collagen fibers, and down-regulating gene and protein expression of fibrotic factors in the engrafted human skin.54 Chemotaxis of fibrocyte precursor cells induced by recombinant human SDF-1 and fibroblast-conditioned medium was inhibited by CXCR4 antagonists in vitro, suggesting a potential therapeutic value of this CXCR4 antagonist for the treatment after burn HTS in the future.
Other potential therapeutic agents for HTS in the future
Active research in TGF-β antagonists, including TGF-β antibodies and antisense oligo-nucleotides, suggests potential roles of this approach for HTS after burn injuries in the future. Similarly, strategies using decorin described earlier as a key proteoglycan deficient in HTS fibroblasts and tissues bind TGF-β and have been demonstrated effective in renal fibrosis and after the development of a fusion protein for systemic administration and in skin tissue engineering strategies for burn wounds.55
Current Preventative and Treatment Modalities
Treatment of after burn wounds has classically been thought of as surgical and nonsurgical, with surgical management reserved for wounds thought to be too deep to heal by secondary intention. Traditional methods of nonsurgical management focus on attenuation of the on-going fibrotic response and improvement of scar tissue. Current therapeutic investigations and strategies focus on inhibiting profibrotic responses before abnormal scarring and fibrosis occur.
Although HSc tissue undergoes a degree of spontaneous resolution over time because of gradual ECM remodeling, enhancement of this remodeling process has been viewed as a useful therapeutic strategy.
Prevention of scarring
Several clinicians have demonstrated that HTS following burn injuries develops with high frequency in deep burns that require prolonged time to heal spontaneously.56,57 In progressively deeper scratch wounds, the deep portions of the healed wound, which developed HTS, contained significantly more fibrocytes.58 Activated fibroblasts from the deep regions of the skin very closely resemble HTS fibroblasts. In tissue-engineered models of skin containing deep or superficial fibroblasts, deep fibroblasts are profibrotic and keratinocytes exert paracrine effects that have antifibrotic properties.57,59,60 Therefore, early recognition of deep dermal burn injuries allows early resur-facing and improved quality of wound healing.56,57,59 Using scanning laser Doppler, thermography, and other instruments, many investigators have demonstrated acceptable levels of accuracy in the prediction of deep burn wounds that can be targeted for early skin graft surgery to prevent the development of HTS that would occur if the wounds were allowed to heal spontaneously.61,62 These instruments are common components of surgical decision-making in many burn centers that provide objective information in identifying deep dermal wounds in addition to wound observation and judgment, which have been demonstrated to be subjective and of limited accuracy.
Pressure garment therapy
Compression therapy is thought to enhance ECM remodeling, although the exact mechanism through which it acts is not completely understood.63,64 An in vitro study examining the effect of compression therapy on HSc tissue demonstrated increased MMP-9 activity in samples obtained from HSc tissue cultures following 24 hours of sustained compression.63 Other proposed mechanisms of ECM remodeling stimulated by compression therapy include inhibition of α-SMA expressing cells and generalized induced tissue ischemia leading to cellular damage and reduced collagen synthesis.64 A meta-analysis incorporating 6 clinical trials found that the clinical use of pressure garments after burn injury did not alter global scar scores.65 The study did find a small but statistically significant decrease in scar height with pressure garment therapy, although the clinical relevance of this was undetermined. However, in a 12-year prospective study of moderate to severe HTS in burn patients with forearm injuries using objective outcome measurements, pressure garments led to significant improvements in hardness, color, and thickness of wounds with overall improvements in clinical appearance independent of patient ethnicity.66 Pressure garment therapy is expensive and has recognized complications, including skin breakdown, obstructive sleep apnea, dental alveolar deformation, bony deformity, and patient discomfort, making them difficult to wear for many patients such that adherence is often low.63–66
Silicone gel therapy
Silicone gels are a commonly used treatment modality even though their mechanism of action is poorly understood.67 Silicone sheeting treatment is reported to soften, increase elasticity, and improve the appearance of HSc,68 but conflicting results remain in the literature, which may be attributed to patient compliance.69 The proposed mechanisms of action include increased oxygen delivery to the epidermis and dermis, hydration of the stratum corneum, surface skin temperature, and reduced tissue turgor.67,69,70 In vitro evidence demonstrates decreased TGF-β2 levels and fibroblast-mediated lattice contraction with silicone treatment.65–67,69,70 Meta-analysis of the benefits of silicone gel sheets suggests further research is required for high-level evidence of their benefit for HSc, despite ongoing encouraging trials.68–70
CLINICAL CARE OF SCARS
Introduction
Plastic surgery draws its roots from the reconstruction of patients with challenging burn wounds from military conflicts. Burn wounds are unique in that not only is there a paucity of tissue, but also the tissue remaining has been altered because of thermal denaturation of the cells within these tissues. In response to this thermal injury and the lack of contact inhibition of local cells, burn wounds contract, creating hypertrophic scars. Much of burn reconstruction has focused on removing the damaged tissue and replacing it with tissue from another region of the body. Although in certain instances, the authors agree with the principle of replacing tissue shortage with tissue from another region of the body, in general, they believe that one's original tissues represent the best tissue for reconstruction. Thus, they believe in the principle of “tissue rehabilitation” rather than the old mantra of “tissue replacement.” The approach to burn scars should be multimodal and involved: (1) surgical release of tension, (2) surgical replacement of missing tissues, (3) vascular laser treatment of erythematous scars, and (4) fractional ablative laser treatment of late hypertrophic scars.
The term scar comes from the Greek word “eskara” meaning scab, or eschar caused by a burn injury. Despite its origins, current use of the word scar has been applied to any visible mark after a pathologic wound-healing process. Burn scars are unique because not only is there damage to tissue in the center of the injury as in a wound from a scalpel, but also the surrounding tissues have been altered structurally. Thus, most burn scars will go on to form a hypertrophic appearance. Hypertrophic scars are red, firm, and raised within the confines of the original wound. On a molecular level, hypertrophic scars result from imbalanced and excessive collagen deposition through aberrations in the fundamental wound-healing phases of inflammation, proliferation, and remodeling.71 They tend to form early after the inciting injury, often within the first month, and slowly improve over the course of 6 months, after which point little changes are observed. Hypertrophic scars that occur after surgery are thought to be caused by excessive tension along the incision site.72 Tension is thought to play a central role in burn scars because all patients, to some degree, are short on tissue. If tension exists across a scar that is hypertrophic, the key treatment is surgical release of the tension through surgery. Once tension is released with a procedure such as a Z-plasty, the hypertrophic nature of the scar will often improve. The hypertrophic nature of a scar as well as pruritis that exists after release of scar tension is often best addressed with an ablative laser. Such laser treatment also improves abnormal pigmentation, and the abnormal appearance of a previously meshed skin graft. Despite exciting preliminary data seen with ablative laser treatment of hypertrophic scars, there is a paucity of high-level evidence to validate its use. Scar appearance, scar tension, and pruritis are difficult to quantify objectively and future patient-reported outcomes studies are needed to verify the improvement seen by surgeons and patients.
Immediate Burn Care to Improve Reconstruction (Acute)
Plastic surgeons should be involved in the care of burn patients from the time they arrive in the burn unit. Partial thickness burns should be given time to heal and aggressive excision and grafting should be avoided. In smaller TBSA burns, attention should be paid to aesthetic and functional locations, such as the face and hands. Surgeons should consider that a meshed graft will have an abnormal appearance once it heals. Donor sites should be harvested from inconspicuous locations in case a hypertrophic scar results. Other examples requiring acute reconstructive surgery include eyelid contracture with exposure keratitis and cervical contractures causing airway issues.
Early Scar Rehabilitation (Intermediate)
Once acute grafts and donor sites have healed, patients and physicians should focus on maximizing normal scar healing. Normal wound healing requires a balance in the hydration of the wound and water-based moisturizers should be encouraged. Silicone sheeting or other occlusive dressings can help in early hydration of the wound.73–75 In addition, attempts should be made to minimize tension off of the scar with potential applications of new devices. Compression garments are also commonly used to decrease formation of HTS, although their efficacy is still debated.76,77
Late Scar Reconstruction
Contracture release and Z-plasty
Surgical release of tension, when executed properly, has a profound benefit on the physiology of the burn scar. The central limb is lengthened, decreasing longitudinal tension on the scar, and the width of scarred area is decreased by medial transposition of the lateral flaps. It is important to not make an angle where the lateral flaps intersect the central limb too acute to avoid tip necrosis. The physiology of the Z-plasty is thought to result from improved collagen remodeling after relief of tension.78,79 Z-plasties can be used to flatten a hypertrophic scar or elevate a depressed scar as long as the lateral limbs extend into normal tissue. The classic design of a Z-plasty has a central segment with limbs oriented at 60° (although it can be 30–90°) with all 3 lines of equal length. Widening the angle of the limbs increases the percentage gain in length along the central limb. Multiple Z-plasties can be designed in a series to improve contracture release in large hypertrophic scars.
Contractures are usually limited to the scar or graft and a layer of connective tissue under the skin. Oftentimes underlying structures, such as subcutaneous fat, breast gland, or orbital structures, are displaced. Scar-releasing incisions should be limited to the superficial scarred tissues, allowing the deep tissue to relax and expand back to their original shape. Placing a fishtail dart at the end of the releasing incisions adds additional skin to help prevent a recurrent contracture. A thick split thickness or a thin full-thickness graft should be placed with plenty of redundancy including overlap over the edges of the wound. A bolster in addition to a possible splint or wrap should placed to maximize contact between the skin graft and donor site.
A tissue expander (TE) is an artificial filling device used to grow and expand local tissue to reconstruct an adjacent soft tissue defect. A silicone elastomer reservoir is placed beneath the donor tissue and slowly filled over time with saline, causing the overlying soft tissue envelope to stretch with a net increase in surface area per unit volume. Advantages to TE are that it allows the surgeon to reconstruct “like with like” using donor and recipient tissues that share similarities in color, thickness, texture, and hair-bearing patterns. Larger soft tissue defects that would usually require a local flap for reconstruction can be closed primarily using expanded local tissue, limiting donor site morbidity. A robust angiogenic response is achieved histologically within the expanded local tissue resembling an incisional delay phenomenon. Predictable amounts of donor tissue can be gained through the expansion process. As a reconstructive technique, it is versatile, reliable, and repeatable and can be applied to many regions of the body.
The largest expander possible should be used with a base diameter approximately 2 to 3 times that of the diameter of the soft tissue defect to be reconstructed. If the expander contains a base plate or rigid backing, this side should be placed along the floor of the pocket to guide the direction of expansion outwards. Multiple expanders are sometimes needed to reconstruct a single defect, depending on the availability of donor tissue. Rectangular expanders are useful on the trunk and extremities and result in the greatest amount of actual tissue gain; however, these should be avoided on the scalp (approximately 40% of theoretical tissue gain). Round expanders are most commonly used in breast reconstruction and result in the least amount of actual tissue gain (approximately 25% of theoretical tissue gain). Crescent expanders are useful in scalp reconstruction and gain more tissue centrally than peripherally. Custom expanders are helpful for irregular defects, but may be more expensive.
Remote filling ports are connected to the TE via silastic tubing and can either be placed subcutaneously (most common) for percutaneous access or be externalized for direct access. It is crucial not to make the tunnel too wide or the port will fall down back next to the expander, making it difficult to fill. Integrated filling ports are located within the expander, although this design may increase the risk of inadvertent puncture of the outer shell. The expander is usually placed adjacent and parallel to the long axis of the soft tissue defect. If placed in the extremities, the expander should not cross any joints or impinge on joint motion. Donor tissue must be well-vascularized and free of unstable scar. Use cautiously in irradiated tissue or patients with poorly controlled diabetes mellitus, vascular disease, or connective tissue disorders. The expander pocket can be developed in the subcutaneous, submuscular, or subgaleal planes depending on the location of the soft tissue defect. The size of the expander pocket should be individually tailored to allow the expander to lie completely flat with minimal wrinkling.
Excessive dissection should be limited to prevent expander migration postoperatively, and meticulous hemostasis is important to minimize hematoma formation. Incisions are placed radial to the expander pocket to minimize tension on the incision during the expansion process. Undue tension placed on the incision during expansion can cause dehiscence and exposure of the expander. Consider future reconstructive options when planning incision placement such that the incisions can easily be incorporated into planned flaps or the tissue to be resected. Endoscopic-assisted expander placement uses smaller incisions and allows more direct visualization of the expander pocket, but at the expense of a steep learning curve and altered depth perception.
For expansion, a 23-gauge butterfly needle or Huber (noncutting) needle is inserted into the filling port perpendicularly; bigger needles should be avoided because they can cause valve leak because of increased back pressure. At the time of expander placement, an initial volume is infused intraoperatively to gently fill the expander pocket to prevent seroma formation, and in the case of breast reconstruction patients, to maintain the shape of the overlying soft tissue envelope. The expansion process usually begins 2 weeks postoperatively and continues on a weekly basis thereafter. The expander is filled until the patient expresses discomfort or the overlying skin blanches. The expansion process is complete based on surgeon preference when he/she deems there is enough donor tissue available to reconstruct the soft tissue defect. Additional “over”-expansion is often recommended to ensure adequate soft tissue coverage.
Disadvantages to the TE include that it requires multiple operations (at least 2 for placement and removal of the expander) and outpatient visits are required. Definitive reconstruction is delayed secondary to the expansion process. Specific complications related to the presence of foreign material can be as high as 30% (eg, infection, exposure, or extrusion).
Laser Therapy
Background
Until recent years, surgery was the only treatment to help rehabilitate scars. Surgery plays a key role in relieving tension in scar contractures, improving contour abnormalities. Surgery, however, can at times create secondary defects and provide a morbid and extensive option for patients. Following surgical tension relief, the next stage in scar rehabilitation uses laser therapies. Laser, which stands for “light amplification by stimulated emission of radiation,” offers a revolutionary new tool for surgeons to treat hypertrophic and erythematous burn and donor site scars. The lasing cavity comprises 2 parallel mirrors (one totally reflective, the other partially reflective). In between the mirrors, there is a lasing medium that is a compound: gas, solid, or liquid. External power source provides energy to the lasing cavity that starts the light amplification process. Light produced within the cavity exits through the partially reflective mirror. Beam can be guided through a wave-guide, articulated arm, or fiberoptics, and finally, to a hand-piece with a focusing lens. Medium is stimulated to move electrons to a higher excited state. Energy is released in the form of photons when they return to a resting state. Wavelength of the photons is determined by the atoms/molecules in the medium. Wavelengths can be delivered with different strategies. Continuous-wave lasers emit a continuous beam of light with relatively constant power. Pulsed lasers deliver high-energy pulsed light. Energy builds up quickly, tapers off, giving great peak powers with each pulse. Shutters can be used to interrupt light delivery resulting in intermittent exposures. Q-switching produces even shorter light pulses (nanoseconds) using a fast electromagnetic switch. During this process, light amplification continues within the cavity until an extremely high peak power is reached; then, high-energy light is released in extremely short time intervals. Measurements used in laser include energy, power, fluence, and irradiance. Energy is proportional to the number of photons and is measured in Joules (J). Power is the rate of delivery of energy and is measured in Watts (W 5 J/s). Fluence is the energy delivered per unit area (J/cm2), and Irradiance is power per unit area (W/cm2).
Laser tissue interaction
Light that encounters skin may be reflected, transmitted, scattered, or absorbed. Only absorbed light produces tissue effects. The stratum corneum reflects 4% to 7% of light that encounters skin. The dermis predominantly scatters light because of collagen. Choromophores absorb light and include hemoblogin, oxyhemoglobin, and melanin. Absorbed light energy can produce thermal, mechanical, and chemical changes in skin. Thermal effects range from protein denaturation to vaporization and carbon formation. Chemical reactions occur when absorbed light leads to the production of chemically reactive excited state molecules (ie, photodynamic therapy).
Selective photothermolysis is a theory first described in 1983 by Anderson and Parrish and describes 3 variables: wavelength, pulse duration, and fluence. Target tissue damage occurs when light of a specific wavelength is preferentially absorbed by the target tissue during a pulse duration greater thermal relaxation time (Tr) of the target. Tr is the time required for an object to cool to 50% of the initial temperature achieved. Light fluence must be greater than the threshold fluence for tissue destruction. Laser-stimulated tissue remodeling includes acute inflammation, metalloproteinase-mediated turnover of ECM proteins, increased cell proliferation in the epidermis and dermis, recruitment of precursor cell, and sustained production of collagen I, III, and elastin.
Pulsed dye laser
Flash lamp pulsed dye lasers (PDL) are best for erythematous scars.80 This laser has a wavelength of 585 nm or 595 nm and its chromophore is oxyhemoglobin. Millisecond-domain PDLs and similar devices that produce selective photothermolysis of small blood vessels have been used for vascular anomalies.81 PDL technology has improved with the addition of dynamic cryogen cooling for epidermal protection. PDLs emitting 0.4- to 20-ms pulses, are particularly useful for improving inflamed scars with erythema, pruritus, and/or pain. Shorter pulse durations are generally more effective for scar improvement.82 Unlike vascular malformations and hemangiomas, scars also tend to respond better to low or medium PDL fluences, about 4 to 7 J/cm2, than to higher fluences.83 Low, short-pulse duration PDL fluences induce local damage to the vascular endothelium followed by mural platelet thrombi, whereas high PDL fluences at longer pulse durations tend to cause immediate intravascular coagulation with cessation of blood flow.84 Side effects include erythema/purpura for 7 to 14 days, hyperpigmentation, and hypopigmentation. Although these lasers are effective in improving scar erythema, they do not have a substantial effect on the thickness or contour of the scar.
Fractional CO2 ablative laser
Fractional CO2 ablative laser is an ablative laser and is the primary laser used for hypertrophic scars.85–89 Its wavelength is10,600 nm, making its chromophore water. Water absorbs energy, converting light to heat, which vaporizes or ablates tissue. Because all tissues contain water, it will ablate all tissues nonspecifically. Pulsed-wave or continuous-wave modes can be used. In normal skin, microscopic thermal wounds from lasers heal rapidly and without scar.87,88 Ablation threshold of 5 J/cm2 is the necessary amount of energy that achieves tissue vaporization. Only a portion of the epidermis and dermis is treated with columns of energy to create targeted areas of thermal damage (microthermal treatment zones, or MTZs). This microthermal zone disrupts the collagen fibrils that are often disorganized. The untreated areas are a reservoir of collagen and promote tissue regrowth. Fractional lasers, as opposed to nonfractional lasers, allow for greater penetration with decreased risk of scarring. Pattern density is described as the number of MTZs within the treatment area. A greater number of MTZs yields a greater surface of the skin treated at each pass. The ablated microchannels are typically 60 to 250 μm in diameter, surrounded by a thermal coagulation layer 50 to 150 μm thick, and capable of treating the deep thickness of the scar with MTZs of ablation and coagulation to dermal depths of 0.08 to 4.0 mm. Ablative lasers have a greater potential depth of treatment compared with nonablative lasers (4.0 mm compared with 1.8 mm). Ablative lasers appear to be more effective for thicker scars and those associated with restriction.
Tissue ablation literally removes some of the hypertrophic scar mass and apparently induces an immediate mechanical release of tension in some restrictive scars. Timing of treatment traditionally has been described as at least 12 months after injury. Recent experience, however, demonstrates that as early as 6 months postinjury may be beneficial. The settings that can be controlled include depth, density, pulse energy, and pulse shape and size. Depth is controlled by setting the energy delivered per micro-beam and should correspond with scar thickness. Density sets the number of micro-beam exposures per unit of skin. In general, a density of less than 10% is recommended. If higher energies are used, caution must be used with density settings. Pulse energy should be proportional to the scar thickness as estimated by palpation and the desired treatment depth. There are differing opinions as to whether the laser should penetrate beyond the depth of the scar. Repeat delay sets how much time elapses between pulses. The shorter the delay, the more rapidly the laser can be applied. Pulse shape and size can also be adjusted and the square pattern is often used due to its ease of allowing for coverage of a large area completely.
Despite that the fractional laser is primarily used for aesthetic facial rejuvenation in the clinic with minimal sedation, the higher settings used for scar rejuvenation often require more pain control. Smaller areas can be anesthetized with lidocaine in the clinic. However, larger areas often require conscious sedation and general anesthesia; this is particularly true in the pediatric population wherein the laser can be poorly tolerated without general anesthesia.
Timing
In general, it is recommended to allow scars to mature for at least 1 year before intervening surgically. Treating wounds that have recently healed with unstable epidermal coverage can lead to marginal outcomes. Younger, less mature scars are less tolerant of aggressive treatments and are less tolerant to high settings. More recent clinical experiences have now shown that laser intervention as early as 6 months might be warranted in specific patient populations. Once laser treatment is begun, a minimum of 1 to 3 months should elapse between treatments. Patients often require multiple treatments, which should continue until the benefits are seen to plateau. Initial changes in the scar are often noticed as early as 1 to 2 weeks after fractional therapy but changes may continue for several months.
Laser safety
Safety principles to avoid excessive thermal injury include minimizing the number of concurrent therapies, applying fractional treatments at low densities with a relatively narrow beam diameter and pulse width, and minimizing the number of passes. In addition, higher pulse energy settings are typically deeper treatments and require a concomitant decrease in treatment density, and treatments are frequently performed at the lowest density settings.
When using this laser, it is helpful to test the laser density on a tongue depressor before using on the patient. Treatment can include surrounding rim of normal skin (several millimeters). Everyone in the room including the patient should have protective eyewear. Multiple treatments are almost always necessary and changes in the settings should be made based on how the patient tolerated the previous treatment and the outcomes observed by the treating surgeon. Treatments should not be done more frequently than every 3 months. Treatments should not begin if the patient is less than 3 months out from their initial injury.
Laser post-operative care
Postoperative care includes dressings, including a petrolatum-based ointment that is applied immediately after the treatment. This dressing can be removed after laser day 1 or 2, and the patient can continue applying petrolatum-based treatments until the wound is epithelialized (usually day 3 or 4).
The patient can resume normal activity immediately if no other surgery was performed at the time of the laser. The patient should be encouraged to avoid the sun and to use sunblock. Patients should avoid full immersion in water, although showering is permitted once the dressings are removed. Side effects of this laser include erythema, temporary hyperpigmentation, possible yeast, bacterial, viral infections, risk of permanent hypopigmentation (infrequent), risk of scarring (infrequent), and pain. Patients with a history of herpes simplex should receive preoperative antiviral prophylaxis.
Laser Adjuncts
Steroid therapy can be used at the same time as laser therapy. An injection of kenalog intralesionally is the most common adjunct. It is recommended to use 10 to 40 mg/mL injection depending on scar thickness. It is also possible to apply topical steroids immediately after fractional laser treatment, allowing the kenalog to diffuse into the pores created by the laser. Once hypopigmentation or scar atrophy is noted, steroid use should be halted.
KEY POINTS.
Hypertrophic scarring is extremely common and is the source of most morbidity related to burns.
The biology of hypertrophic healing is complex and poorly understood. Multiple host and injury factors contribute, but protracted healing of partial thickness injury is a common theme.
Hypertrophic scarring and heterotopic ossification may share some basic causes involving marrow-derived cells.
Several traditional clinical interventions exist to modify hypertrophic scar. All have limited efficacy.
Laser interventions for scar modification show promise, but as yet do not provide a definitive solution. Their efficacy is only seen when used as part of a multimodality scar management program.
Key Points for Lasers.
Lasers are an effective treatment option for civilian and military adults and children with burn scars
PDLs have a role for early erythematous scars
Fractional lasers have a role in hypertrophic scars and improve surface irregularities, pigmentary abnormalities, hypertrophy, pruritis, and contraction
Scar optimization and rehabilitation is a process that occurs over time with a multimodal approach that includes relieving tension and replacing tissue deficits surgically and targeting erythema and hypertrophy with lasers
Randomized, prospective multi-institutional studies are needed to further define and describe optimal uses of laser for burn reconstruction
SUMMARY.
Despite advancements in burn care, HTS remains a significant clinical problem in burn injury. Understanding of the pathophysiology of the after burn scar and systemic responses to thermal injury have revealed new targets for future therapeutic strategies, such as exogenous Th1 cytokine administration, specific antibody, or antisense mRNA therapy toward fibrogenic factors like TGF-β and CTGF, hold significant promise in preventing the development of HTS. In addition, methods of accurately assessing burn depth are improving, most recently with the advent of laser Doppler imaging.
As such, deep dermal wounds can be recognized and operated on at an early stage, thereby circumventing complications of HTS. Through continued investigation and understanding of the pathogenesis of burn injury and scar formation, advancements in burn surgery and burn laser treatments will continue to improve, leading to improved patient outcomes after burn injury.
Fig. 4.
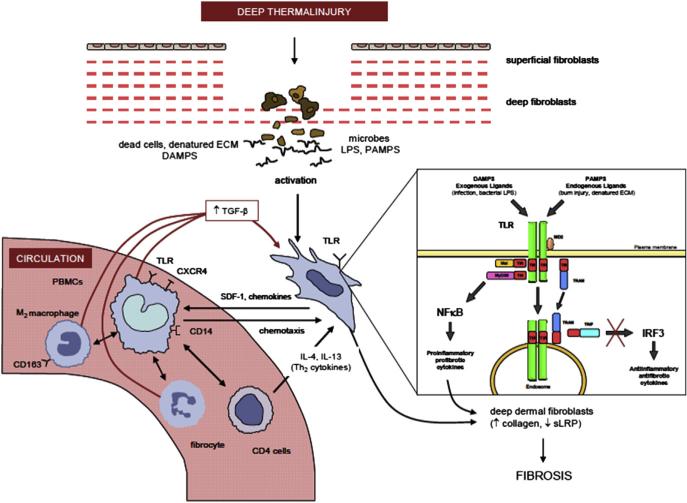
It is hypothesized that burn injury activates fibroblasts in the deep dermis by using PAMPs (ie, LPS) and DAMPs (ie, Biglycan) to stimulate the Toll receptors/NFκB pathway on fibroblasts, which in turn release chemokines and growth factors (ie, TGF-β) recruiting bone marrow–derived monocytes precursors to further activate the production of ECM proteins in deep dermal fibroblasts and subsequently HTS.
Acknowledgments
This work was supported by the Firefighters’ Burn Trust Fund of the University of Alberta Hospital, the Canadian Institutes of Health Research, and the Alberta Heritage Trust Fund for Medical Research. Dr Levi was funded by 1K08GM109105-01 and Plastic Surgery Foundation National Endowment Award.
REFERENCES
- 1.Kwan P, Desmouliere A, Tredget EE. Chapter 45—Molecular and cellular basis of hypertrophic scarring. In: Herndon DN, editor. Total burn care. 3rd edition. Saunders Elsevier; Philadelphia: 2012. pp. 495–505.e5. [Google Scholar]
- 2.Bombaro KM, Engrav LH, Carrougher GJ, et al. What is the prevalence of hyper-trophic scarring following burns? Burns. 2003;29:299–302. doi: 10.1016/s0305-4179(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 3.Engrav LH, Covey MH, Dutcher KD, et al. Impairment, time out of school, and time off from work after burns. Plast Reconstr Surg. 1987;79:927–34. doi: 10.1097/00006534-198706000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Helm P, Herndon DN, Delateur B. Restoration of function. J Burn Care Res. 2007;28(4):611–4. doi: 10.1097/BCR.0B013E318093E4CA. [DOI] [PubMed] [Google Scholar]
- 5.Brown JJ, Bayat A. Genetic susceptibility to raised dermal scarring. Br J Dermatol. 2009;161(1):8–18. doi: 10.1111/j.1365-2133.2009.09258.x. [DOI] [PubMed] [Google Scholar]
- 6.Dunkin CS, Pleat JM, Gillespie PH, et al. Scarring occurs at a critical depth of skin injury: precise measurement in a graduated dermal scratch in human volunteers. Plast Reconstr Surg. 2007;119(6):1722–32. doi: 10.1097/01.prs.0000258829.07399.f0. [DOI] [PubMed] [Google Scholar]
- 7.Jaskille AD, Shupp JW, Jordan MH, et al. Critical review of burn depth assessment techniques: Part I. Historical review. J Burn Care Res. 2009;30(6):937–47. doi: 10.1097/BCR.0b013e3181c07f21. [DOI] [PubMed] [Google Scholar]
- 8.Ripper S, Renneberg B, Landmann C, et al. Adherence to pressure garment therapy in adult burn patients. Burns. 2009;35(5):657–64. doi: 10.1016/j.burns.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Harte D, Gordon J, Shaw M, et al. The use of pressure and silicone in hypertrophic scar management in burns patients: a pilot randomized controlled trial. J Burn Care Res. 2009;30(4):632–42. 29. doi: 10.1097/BCR.0b013e3181ac01a3. [DOI] [PubMed] [Google Scholar]
- 10.Ghahary A, Shen YJ, Nedelec B, et al. Collagenase production is lower in post-burn hypertrophic scar fibroblasts than in normal fibroblasts and is reduced by insulin-like growth factor-1. J Invest Dermatol. 1996;106(3):476–81. doi: 10.1111/1523-1747.ep12343658. [DOI] [PubMed] [Google Scholar]
- 11.Scott PG, Dodd CM, Ghahary A, et al. Fibroblasts from post-burn hypertrophic scar tissue synthesize less decorin than normal dermal fibroblasts. Clin Sci (Lond) 1998;94:541–7. doi: 10.1042/cs0940541. [DOI] [PubMed] [Google Scholar]
- 12.Nedelec B, Shankowsky H, Scott PG, et al. Myofibroblasts and apoptosis in human hypertrophic scars: the effect of interferon-alpha2b. Surgery. 2001;130:798–808. doi: 10.1067/msy.2001.116453. [DOI] [PubMed] [Google Scholar]
- 13.Moulin V, Castilloux G, Auger FA, et al. Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp Cell Res. 1998;238:283–93. doi: 10.1006/excr.1997.3827. [DOI] [PubMed] [Google Scholar]
- 14.Direkze NC, Hodivala-Dilke K, Jeffery R, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64:8492–5. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Shankowsky HA, Scott PG, et al. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–92. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 16.Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep. 2006;8(2):145–50. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- 17.Abe R, Donnelly SC, Peng T, et al. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Jiao H, Shankowsky HA, et al. Identification of fibrocytes in post-burn hypertrophic scar. Wound Repair Regen. 2005;13(4):398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Jiao H, Stewart TL, et al. Accelerated wound healing in leukocyte-specific, protein 1-deficient mouse is associated with increased infiltration of leukocytes and fibrocytes. J Leukoc Biol. 2007;82:1554–63. doi: 10.1189/0507306. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, Stewart TL, Chen H, et al. Improved scar in post-burn patients following interferon alpha 2b treatment is associated with decreased angiogenesis mediated by vascular endothelial cell growth factor. J Interferon Cytokine Res. 2008;28(7):423–34. doi: 10.1089/jir.2007.0104. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Jiao H, Stewart TL, et al. Improvement in postburn hypertrophic scar after treatment with IFN-alpha2b is associated with decreased fibrocytes. J Interferon Cytokine Res. 2007;27:921–30. doi: 10.1089/jir.2007.0008. [DOI] [PubMed] [Google Scholar]
- 22.Wang JF, Jiao H, Stewart TL, et al. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15(1):113–21. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 23.Higashiyama R, Nakao S, Sibusawa Y, et al. Differential contribution of dermal resident and bone marrow-derived cells to collagen production during wound healing and fibrogenesis. J Invest Dermatol. 2011;131(2):529–36. doi: 10.1038/jid.2010.314. [DOI] [PubMed] [Google Scholar]
- 24.Antoniou KM, Soufla G, Lymbouridou R, et al. Expression analysis of angiogenic growth factors and biological axis CXCL12/CXCR4 axis in idiopathic pulmonary fibrosis. Connect Tissue Res. 2010;51:71–80. doi: 10.3109/03008200903056150. [DOI] [PubMed] [Google Scholar]
- 25.Chen HC, Yang JY, Chuang SS, et al. Heterotopic ossification in burns: our experience and literature reviews. Burns. 2009;35(6):857–62. doi: 10.1016/j.burns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Michelsson JE, Rauschning W. Pathogenesis of experimental heterotopic bone formation following temporary forcible exercising of immobilized limbs. Clin Orthop Relat Res. 1983;(176):265–72. [PubMed] [Google Scholar]
- 27.Medina A, Shankowsky HA, Savaryn B, et al. Characterization of heterotopic ossification in burn patients. J Burn Care Res. 2014;35(3):251–6. doi: 10.1097/BCR.0b013e3182957768. [DOI] [PubMed] [Google Scholar]
- 28.Zuo KJ, Tredget TE. Multiple Marjolin's ulcers arising from irradiated post-burn hypertrophic scars: a case report. Burns. 2013 doi: 10.1016/j.burns.2013.10.008. http://dx.doi.org/10.1016/j.burns.2013.10.008.pii:S0305-4179(13)00337-9. [DOI] [PubMed]
- 29.Nesti LJ, Jackson WM, Shanti RM, et al. Differentiation potential of multipotent progenitor cells derived from war-traumatized muscle tissue. J Bone Joint Surg Am. 2008;90(11):2390–8. doi: 10.2106/JBJS.H.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medina A, Ma Z, Varkey, et al. Fibrocytes participate in the development of heterotopic ossification. J Burn Care Res. doi: 10.1097/BCR.0000000000000102. in press. [DOI] [PubMed] [Google Scholar]
- 31.Medina A, Ghahary A. Transdifferentiated circulating monocytes release exosomes containing 14-3-3 proteins with matrix metalloproteinase-1 stimulating effect for dermal fibroblasts. Wound Repair Regen. 2010;18(2):245–53. doi: 10.1111/j.1524-475X.2010.00580.x. [DOI] [PubMed] [Google Scholar]
- 32.Miller AC, Rashid RM, Elamin EM. The “T” in trauma: the helper T-cell response and the role of immunomodulation in trauma and burn patients. J Trauma. 2007;63(6):1407–17. doi: 10.1097/TA.0b013e31815b839e. [DOI] [PubMed] [Google Scholar]
- 33.Tredget EE, Yang L, Delehanty M, et al. Polarized T helper cells Th2 cytokine production in patients with hypertrophic scar following thermal injury. J Interferon Cytokine Res. 2005;26:179–89. doi: 10.1089/jir.2006.26.179. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Jiao H, Stewart TL, et al. Increased TGF-beta-producing CD41 T lymphocytes in postburn patients and their potential interaction with dermal fibro-blasts in hypertrophic scarring. Wound Repair Regen. 2007;15(4):530–9. doi: 10.1111/j.1524-475X.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 35.Sorrell JM, Baber MA, Caplan AI. Site-matched papillary and reticular human dermal fibroblasts differ in their release of specific growth factors/cytokines and in their interaction with keratinocytes. J Cell Physiol. 2004;200:134–45. doi: 10.1002/jcp.10474. [DOI] [PubMed] [Google Scholar]
- 36.Sorrell JM, Baber MA, Caplan AI. Clonal characterization of fibroblasts in the superficial layer of the adult human dermis. Cell Tissue Res. 2007;327:499–510. doi: 10.1007/s00441-006-0317-y. [DOI] [PubMed] [Google Scholar]
- 37.Ali-Bahar M, Bauer B, Tredget EE, et al. Dermal fibroblasts from different layers of human skin are heterogeneous in expression of collagenase and types I and III procollagen mRNA. Wound Repair Regen. 2004;12:175–82. doi: 10.1111/j.1067-1927.2004.012110.x. [DOI] [PubMed] [Google Scholar]
- 38.Honardoust D, Varkey M, Hori K, et al. Small leucine-rich proteoglycans, decorin and fibromodulin, are reduced in post-burn hypertrophic scar. Wound Repair Regen. 2011;19(3):368–78. doi: 10.1111/j.1524-475X.2011.00677.x. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Dodd C, Shankowsky H, et al. Deep dermal fibroblast may dictate hypertrophic scarring. Lab Invest. 2008;88(12):1278–90. doi: 10.1038/labinvest.2008.101. [DOI] [PubMed] [Google Scholar]
- 40.Scott PG, Dodd CM, Tredget EE, et al. Immunohistochemical localization of the proteoglycans decorin, biglycan and versican and transforming growth factor-beta in human post-burn hypertrophic and mature scars. Histopathology. 1995;26:423–31. doi: 10.1111/j.1365-2559.1995.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 41.Hildebrand A, Romarís M, Rasmussen LM, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–34. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Honardoust D, Varkey M, Hori K, et al. Reduced Decorin, Fibromodullin and TGF-b3 in Deep Dermis lead to Hypertrophic Scar. J Burn Care Res. 2012;33(2):218–27. doi: 10.1097/BCR.0b013e3182335980. [DOI] [PubMed] [Google Scholar]
- 43.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signaling. Mediators Inflamm. 2010;2010:672395. doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Hori K, Ding J, et al. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol. 2011;226(5):1265–73. doi: 10.1002/jcp.22454. [DOI] [PubMed] [Google Scholar]
- 46.Tredget EE, Shankowsky HA, Pannu R, et al. Transforming growth factor-beta in thermally injured patients with hypertrophic scars: effects of interferon alpha-2b. Plast Reconstr Surg. 1998;102(5):1317–28. doi: 10.1097/00006534-199810000-00001. [discussion: 1329–30] [DOI] [PubMed] [Google Scholar]
- 47.Wang R, Ghahary A, Dodd C, et al. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor-beta1 mRNA and protein than normal skin and cells. Wound Repair Regen. 2009;8:128–37. doi: 10.1046/j.1524-475x.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 48.Wong TW, Chiu HC, Yip KM. Intralesional interferon alpha-2b has no effect in the treatment of keloids. Br J Dermatol. 1994;130(5):683–5. doi: 10.1111/j.1365-2133.1994.tb13125.x. [DOI] [PubMed] [Google Scholar]
- 49.Berman B, Viera MH, Amini S, et al. Prevention and management of hypertrophic scars and keloids after burns in children. J Craniofac Surg. 2008;19(4):989–1006. doi: 10.1097/SCS.0b013e318175f3a7. [DOI] [PubMed] [Google Scholar]
- 50.Tredget EE, Adewale AJ, Matthey S, et al. A double-blind placebo controlled trial using subcutaneous Intron A for the treatment of hypertophic scarring. Can J Plast Surg. 2008:116. [abstract: 66] [Google Scholar]
- 51.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Mora A, Shim H, et al. Role of the SDF-1/CXCR4 axis in the pathogenesis of lung injury and fibrosis. Am J Respir Cell Mol Biol. 2007;37(3):291–9. doi: 10.1165/rcmb.2006-0187OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avniel S, Arik Z, Maly A, et al. Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. J Invest Dermatol. 2006;126(2):468–76. doi: 10.1038/sj.jid.5700069. [DOI] [PubMed] [Google Scholar]
- 54.Ding J, Hori K, Zhang R, et al. Stromal cell-derived factor 1 (SDF-1) and its receptor CXCR4 in the formation of postburn hypertrophic scar (HTS). Wound Repair Regen. 2011;19(5):568–78. doi: 10.1111/j.1524-475X.2011.00724.x. [DOI] [PubMed] [Google Scholar]
- 55.Kwan P, Hori K, Ding J, et al. Scar and contracture: biological principles. Hand Clin. 2009;25(4):511–28. doi: 10.1016/j.hcl.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 56.Heimbach D, Engrav L, Grube B, et al. Burn depth: a review. World J Surg. 1992;16(1):10–5. doi: 10.1007/BF02067108. [DOI] [PubMed] [Google Scholar]
- 57.Stewart TL, Ball B, Schembri PJ, et al. The use of laser Doppler imaging as a predictor of burn depth and hypertrophic scar post-burn injury. J Burn Care Res. 2012;33(6):764–71. doi: 10.1097/BCR.0b013e318257db36. [DOI] [PubMed] [Google Scholar]
- 58.Honardoust D, Varkey M, Marcoux Y, et al. Reduced decorin, fibromodulin, and transforming growth factor-b3 in deep dermis leads to hypertrophic scarring. J Burn Care Res. 2012;33(2):218–27. doi: 10.1097/BCR.0b013e3182335980. [DOI] [PubMed] [Google Scholar]
- 59.Varkey M, Ding J, Tredget EE. Superficial dermal fibroblasts enhance basement membrane and epidermal barrier formation in tissue-engineered skin: implications for treatment of skin basement membrane disorders. Tissue Eng Part A. 2014;20(3–4):540–52. doi: 10.1089/ten.tea.2013.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varkey M, Ding J, Tredget EE. Fibrotic remodeling of tissue-engineered skin with deep dermal fibroblasts is reduced by keratinocytes. Tissue Eng Part A. 2014;20(3–4):716–27. doi: 10.1089/ten.TEA.2013.0434. [DOI] [PubMed] [Google Scholar]
- 61.Bray R, Forrester K, Leonard C, et al. Laser Doppler imaging of burn scars: a comparison of wavelength and scanning methods. Burns. 2003;29(3):199–206. doi: 10.1016/s0305-4179(02)00307-8. [DOI] [PubMed] [Google Scholar]
- 62.Hoeksema H, Van de Sijpe K, Tondu T, et al. Accuracy of early burn depth assessment by laser Doppler imaging on different days post burn. Burns. 2009;35(1):36–45. doi: 10.1016/j.burns.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Reno F, Grazianetti P, Stella M, et al. Release and activation of matrix metalloproteinase-9 during in vitro mechanical compression in hypertrophic scars. Arch Dermatol. 2002;138(4):475–8. doi: 10.1001/archderm.138.4.475. [DOI] [PubMed] [Google Scholar]
- 64.Costa AM, Peyrol S, Porto LC, et al. Mechanical forces induce scar remodeling. Study in non-pressure-treated versus pressure-treated hypertrophic scars. Am J Pathol. 1999;155(5):1671–9. doi: 10.1016/S0002-9440(10)65482-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anzarut A, Olson J, Singh P, et al. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. J Plast Reconstr Aesthet Surg. 2009;62(1):77–84. doi: 10.1016/j.bjps.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 66.Engrav LH, Heimbach DH, Rivara FP, et al. 12-year within-wound study of the effectiveness of pressure garment therapy. Burns. 2010;36:975–83. doi: 10.1016/j.burns.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 67.Borgognoni L. Biological effects of silicone gel sheeting. Wound Repair Regen. 2002;10(2):118–21. doi: 10.1046/j.1524-475x.2002.00205.x. [DOI] [PubMed] [Google Scholar]
- 68.Lim AF, Weintraub J, Kaplan EN, et al. The embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast Reconstr Surg. 2014;133:398. doi: 10.1097/01.prs.0000436526.64046.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nedelec B, Carter A, Forbes L, et al. Practice guidelines for the application of non-silicone or silicone gels and gel sheets after burn injury. J Burn Care Res. 2014;35:207–11. doi: 10.1097/BCR.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 70.O'Brien L, Pandit A. Silicon gel sheeting for preventing and treating hypertrophic and keloid scars. Cochrane Database Syst Rev. 2006;(1):CD003826. doi: 10.1002/14651858.CD003826.pub2. Review. Update in: Cochrane Database Syst Rev 2013;(9):CD003826. [DOI] [PubMed] [Google Scholar]
- 71.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raju DR, Shaw TE. Results of simple scar excision and layered repair with elevation in facial scars. Surg Gynecol Obstet. 1979;148:699–702. [PubMed] [Google Scholar]
- 73.Tandara AA, Mustoe TA. The role of the epidermis in the control of scarring: evidence for mechanism of action for silicone gel. J Plast Reconstr Aesthet Surg. 2008;61:1219. doi: 10.1016/j.bjps.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 74.Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg. 2008;32:82. doi: 10.1007/s00266-007-9030-9. [DOI] [PubMed] [Google Scholar]
- 75.Saulis AS, Chao JD, Telser A, et al. Silicone occlusive treatment of hypertrophic scar in the rabbit model. Aesthet Surg J. 2002;22:147. doi: 10.1067/maj.2002.123023. [DOI] [PubMed] [Google Scholar]
- 76.Steinstraesser L, Flak E, Witte B, et al. Pressure garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: randomized controlled trial with intraindividual comparison. Plast Reconstr Surg. 2011;128:306e. doi: 10.1097/PRS.0b013e3182268c69. [DOI] [PubMed] [Google Scholar]
- 77.Van den Kerckhove E, Stappaerts K, Fieuws S, et al. The assessment of erythema and thickness on burn related scars during pressure garment therapy as a preventive measure for hypertrophic scarring. Burns. 2005;31:696–702. doi: 10.1016/j.burns.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 78.Longacre JJ, Berry HK, Basom CR, et al. The effects of Z plasty on hypertrophic scars. Scand J Plast Reconstr Surg. 1976;10:113. doi: 10.3109/02844317609105199. [DOI] [PubMed] [Google Scholar]
- 79.Davis JS. The relaxation of scar contractures by means of the Z-, or reversed Z-type incision: stressing the use of scar infiltrated tissues. Ann Surg. 1931;94:871. doi: 10.1097/00000658-193111000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donelan MB, Parrett BM, Sheridan RL. Pulsed dye laser therapy and z-plasty for facial burn scars: the alternative to excision. Ann Plast Surg. 2008;60:480. doi: 10.1097/SAP.0b013e31816fcad5. [DOI] [PubMed] [Google Scholar]
- 81.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 82.Manuskiatti W, Wanitphakdeedecha R, Fitzpatrick RE. Effect of pulse width of a 595-nm flashlamp-pumped pulsed dye laser on the treatment response of keloidal and hypertrophic sternotomy scars. Dermatol Surg. 2007;33:152. doi: 10.1111/j.1524-4725.2006.33033.x. [DOI] [PubMed] [Google Scholar]
- 83.Manuskiatti W, Fitzpatrick RE, Goldman MP. Energy density and numbers of treatment affect response of keloidal and hypertrophic sternotomy scars to the 585-nm flashlamp-pumped pulsed-dye laser. J Am Acad Dermatol. 2001;45:557. doi: 10.1067/mjd.2001.116580. [DOI] [PubMed] [Google Scholar]
- 84.Garden JM, Tan OT, Kerschmann R, et al. Effect of dye laser pulse duration on selective cutaneous vascular injury. J Invest Dermatol. 1986;87:653. doi: 10.1111/1523-1747.ep12456368. [DOI] [PubMed] [Google Scholar]
- 85.Waibel J, Beer K. Fractional laser resurfacing for thermal burns. J Drugs Dermatol. 2008;7:59. [PubMed] [Google Scholar]
- 86.Waibel J, Beer K. Ablative fractional laser resurfacing for the treatment of a third-degree burn. J Drugs Dermatol. 2009;8:294. [PubMed] [Google Scholar]
- 87.Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med. 2004;34:426. doi: 10.1002/lsm.20048. [DOI] [PubMed] [Google Scholar]
- 88.Tierney EP, Hanke CW. Fractionated carbon dioxide laser treatment of photoaging: prospective study in 45 patients and review of the literature. Dermatol Surg. 2011;37:1279. doi: 10.1111/j.1524-4725.2011.02082.x. [DOI] [PubMed] [Google Scholar]
- 89.Stebbins WG, Hanke CW. Ablative fractional CO2 resurfacing for photoaging of the hands: pilot study of 10 patients. Dermatol Ther. 2011;24:62. doi: 10.1111/j.1529-8019.2010.01379.x. [DOI] [PubMed] [Google Scholar]