Abstract
Genetically engineered mouse models (GEMMs) have been highly instrumental in elucidating gene functions and molecular pathogenesis of human diseases, although their use in studying kidney stone formation or nephrolithiasis remains relatively limited. This review intends to provide an overview of several knockout mouse models that develop interstitial calcinosis in the renal papillae. Included herein are mice deficient for Tamm-Horsfall protein (THP; also named uromodulin), osteopontin (OPN), both THP and OPN, Na+-phosphate cotransporter Type II (Npt2a) and Na+/H+ exchanger regulatory factor (NHERF-1). The baseline information of each protein is summarized, along with key morphological features of the interstitial calcium deposits in mice lacking these proteins. Attempts are made to correlate the papillary interstitial deposits found in GEMMs with Randall’s plaques, the latter considered precursors of idiopathic calcium stones in patients. The pathophysiology that underlies the renal calcinosis in the knockout mice are also discussed wherever information is available. Not all the knockout models are allocated equal space because some are more extensively characterized than others. Despite the inroads already made, the exact physiological underpinning, origin, evolution and fate of the papillary interstitial calcinosis in the GEMMs remain incompletely defined. Greater investigative efforts are warranted in order to pin down the precise role of the papillary interstitial calcinosis in nephrolithiasis using the existing models. Additionally, more sophisticated, second-generation GEMMs that allow gene inactivation in a time-controlled manner and “compound mice” that bear several genetic alterations are urgently needed, in light of mounting evidence that nephrolithiasis is a multifactorial, multi-stage and polygenic disease.
Keywords: urolithiasis, knockout mice, Tamm-Horsfall protein, osteopontin, sodium-phosphate cotransporter 2a, sodium-hydrogen exchanger regulator factor-1
Introduction
The technological advancements that allow engineering of specific genetic alterations into mouse genome has fundamentally changed our way of how gene functions are studied and human diseases are modeled [1–3]. Thus, genes of interest can be transferred into one-cell mouse embryos to be incorporated into mouse chromosomes either randomly or on specific sites, yielding so-called transgenic mouse models. With the help of tissue-specific promoters, expression of transgenes can be confined to a particular tissue, or even to a particular developmental and differentiation stage when aided by inducible strategies [4, 5]. Similarly, through homologous recombination, mutant gene alleles can be introduced into mouse embryonic stem (ES) cells to abrogate the endogenous counterparts, thus giving rise to so-called knockout mice. Together, these genetically engineered mouse models (GEMMs) have provided powerful tools for the research community to systemically interrogate gene functions and molecular pathogenesis of human diseases. The GEMMs offer distinct advantages over test tube-based experiments, cell culture, traditional animal models and analyses of human disease specimens, because they (i) harbor well-defined genetic alterations; (ii) allow systematic analysis of the sequential steps of disease pathogenesis from early onset to advanced stages; (iii) recapitulate critical disease events in an in vivo physiological setting; (iv) permit direct correlation of phenotypes with genotypes in well-controlled genetic backgrounds; and (v) provide a natural anatomic, tissue and cellular context within which a pathologic process arises and evolves. To no great surprise, GEMMs have become an indispensable instigative tool to study almost every known gene and model every disease condition.
Kidney stone disease is very prevalent, painful and costly to manage. Kidney stone formation or nephrolithiasis is believed to be a multifactorial and multistage process involving urinary supersaturation of stone constituents, crystal nucleation, growth, aggregation, crystal/epithelial adhesion (retention) and stone formation [6–17]. Based on the chemical composition, kidney stones can be classified into calcium oxalate, calcium phosphate, struvite, uric acid, cystine, dihydroxyadenine and ammonium acid urate stones. Regardless of the stone type, supersaturation of stone constituents is thought to be the initiation factor that drives urinary salts from a soluble state to an insoluble one. Crystal nucleation ensues; however, small-sized crystals are harmless as they pass freely through the nephron and get eliminated in the urine. On the other hand, under the conducive conditions, certain crystals can grow in size or aggregate to sizes larger than the tubular diameter at which point they get trapped, forming plugging crystals or stones. Alternatively, intratubular crystals of varying sizes can adhere to the luminal surfaces of the renal epithelial cells, thus avoiding elimination [18, 19]. Crystal retention in the kidney is considered a crucially important step for stone formation.
In addition to crystal formation within the renal tubular lumen, mineral crystals are also found frequently in the renal interstitium in stone-bearing kidneys in humans. Of particular interest are the interstitial apatite deposits in the renal papillae of idiopathic calcium oxalate stone formers [20, 21]. Discovered by Alexander Randall and named Randall’s plaques [22], these crystal deposits are more carefully analyzed recently by Evan and collaborators. They found that they are originated in basement membrane zones of the thin limbs of Henle’s loop and later extended to the papillary tip beneath the papillary epithelium [20, 23, 24]. On electron micrograph, the interstitial apatite crystals exhibit distinct tree-trunk appearance with concentric rings interspersed with mineral-dense and -loose materials. Evan et al proposed that the apatite deposits in the papillae can erode the overlaying papillary epithelium and, once exposed, can serve as a nidus over which calcium oxalate crystals accumulate and grow to form calcium oxalate stones [20].
In light of the recent data demonstrating the importance of Randall’s plaques in the pathogenesis of human idiopathic calcium stone formers, it is of particular interest to note that papillary interstitial calcinosis can also occur in knockout mice deficient for specific proteins. This review summarizes the morphological characteristics of the interstitial crystal deposits in mice and compare and contrast them with the Randall’s plaques in humans. The review covers the pathophysiology that potentially underlies the papillary interstitial calcinosis in various knockouts with respect to their shared features and differences. Finally, it discusses the significance and relevance of these crystal deposits to human nephrolithiasis. Highlighted in this review are knockout mice lacking Tamm-Horsfall protein, osteopontin (OPN), THP and OPN, Na+-phosphate cotransporter Type II (Npt2a) and Na+/H+ exchanger regulatory factor (NHERF-1).
Tamm-Horsfall protein knockout mice
Tamm-Horsfall protein (THP) is a primarily kidney-specific protein made by the epithelial cells constituting the thick ascending limb of loop (TAL) of Henle [25–28]. After synthesis in the rough endoplasmic reticulum (rER), THP’s C-terminus (~100 amino acid residues) is removed via proteolytic cleavage and then linked with a glycosylphosphatidylinositol (GPI). It is thought that under the normal circumstances the GPI linkage provides a strong apical targeting signal allowing THP to translocate to the luminal surface of TAL [26]. Once there, THP is released into the urine via proteolytic and/or phospholipase cleavage at the C-terminus in a manner thus far not clearly understood. THP is by far the most abundant of all the urinary proteins, amounting to 100 mg daily. It is worth noting that, while THP is normally apically targeted, it can be detected on the basolateral membrane domain of TAL and adjacent interstitium during experimental ischemia-induced acute kidney injury. Emerging evidence suggests that such “ectopic” THP plays an important role in interacting with and inhibiting inflammatory mediators between TAL and proximal tubules, thus tampering the extent of tubular injury and accelerating recovery [28–31].
THP was “re-discovered” in the late 1980’s in the urine of pregnant women as an immunosuppressant and named uromodulin, but subsequent cloning and sequencing revealed that the primary structures of the two proteins were identical [32–34] (for the sake of brevity, THP will be used henceforth). Mature THP polypeptide contains 48 cysteines many of which are believed to be involved in forming intra-molecule disulfide bridges that are crucial for the proper folding of THP in and exit from the rER. In particular, eight cysteine residues are concentrated in a small, evolutionarily conserved region, forming so-called domain of 8 cysteines (D8C) [35, 36]. Mis-sense mutations that abrogate an existing cysteine or create a de novo cysteine in THP have been causatively linked to certain hereditary hyperuricemic patients, and they cause THP to misfold and be trapped in the rER leading to cytotoxity and failed apical targeting [36–39]. THP also contains eight asparagine-linked glycosylation consensus sites, but only seven are in a good context and were shown to be actually glycosylated [40]. Of these, asparagine 251 is modified by a high-mannose chain that is highly conserved across the species and is responsible for specific interactions between urinary THP and FimH adhesin of type 1-fimbriated E. coli [41–44]. Multiple lines of experimental evidence, including those from the THP KO mice (see below), suggest that THP plays a key role in defending the urinary tract against type 1-fimbriated E. coli from adhering to the uroplakin Ia receptor of the urothelial surface [45–50]. The remaining six asparagine-linked glycosylation sites are known to be occupied by complex-type carbohydrate moieties with sialylated tri- and tetra-antennary structures. Together, the complex-type sugars account for about 30% of total molecular mass of THP [26, 27]. In a normally neutral to acidic urine environment, the sialic acids make THP a highly negatively charged protein, capable of interacting with organic and/or inorganic cations such as calcium and magnesium. Other known domain structures of THP include three epidermal growth factor (EGF) domains, two of which contain putative calcium-binding sites, and zona pellucida repeats at the C-terminus that are responsible for THP polymerization [49, 51].
Since its discovery in the 1960’s, THP has been proposed to have several physiological functions in the urinary system including salt/water balance of the kidney, immune regulation, defense against urinary tract infection and modulation of kidney stone formation [9, 25–27, 52, 53]. However, in the absence of direct in vivo experimental evidence, none of these presumed functions could be directly substantiated or refuted. In fact, although there were in vitro studies showing that purified THP inhibits aggregation of calcium oxalate and calcium phosphate crystals [54–58], other studies showed the opposite effects of THP in promoting crystal aggregation [59, 60]. The effects of THP on crystallization in the test tubes also vary widely depending on the pH, ion strengths and polymerization state of THP itself. Consequently, despite THP being consistently detected in human renal stones, no consensus existed as to whether THP is an inhibitor or a promoter and plays no role whatsoever in nephrolithiasis.
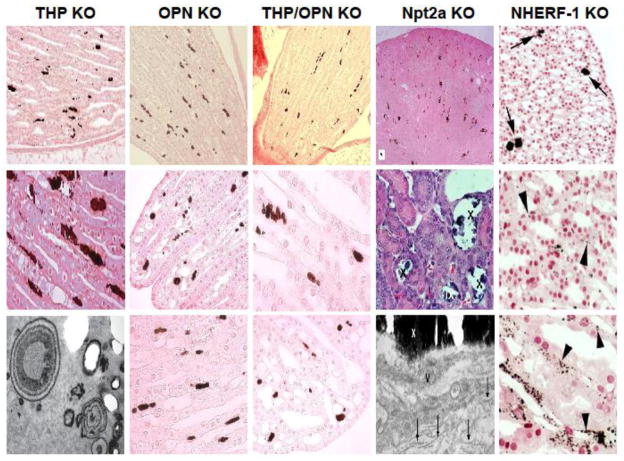
In an effort to improve the understanding of THP’s in vivo role in urinary pathophysiology, Mo and colleagues inactivated the THP gene in mice using a homologous recombination approach that eliminated the 5′-portion of the genomic sequence including a 650-bp proximal promoter region, exons 1 through 4 and partial intron 4 [45]. The resultant THP−/− mice were devoid of THP expression, as evidenced by Northern blotting, RT-PCR, in situ hybridization, Western blotting and polyclonal antibody immunohistochemistry. Initial studies surveying intra-renal calcification were carried out in young (2-month-old) mice using von Kossa (e.g., silver nitrate) histochemical staining [61]. While wild-type (THP+/+) littermates had no crystal in any part of the kidney, whereas THP−/− mice exhibited intratubular crystal aggregates, located mainly in the collecting ducts in deep medulla and renal papillae. Some crystals were seen attached to the luminal surfaces of the tubular epithelial cells. Overall, 4/25 THP−/− mice and 0/25 THP+/+ littermates exhibited intratubular crystals [61]. In a follow-up study, the same group of investigators examined a large cohort comprising 250 THP−/− mice of both genders, young (2-month), middle (5–8-month) and old (15-month) age groups, genetic backgrounds (129/SvEv and C57/BL6) and gene dosages (hetero- and homozygotes) [62]. Several key features were noted. First, renal papillary calcification was age-dependent, i.e., small and infrequent crystals were found in young mice (2 months), whereas larger and frequent crystals were found in aged mice (15 months). Second, in young mice both intratubular and interstitial crystals were present, but in old mice interstitial crystals dominated (Fig. 1). Third, the crystals were largely located in the renal papillary region, with the rest of regions devoid of any crystal. Fourth, by transmission electron microscopy, the interstitial crystals were deposited within the widened basement membrane zone and exhibited tree-truck or onion-ring morphology with electron dense rings (presumably composed of minerals) intersperse with electron-sparse rings (presumably of proteinaceous materials) (Fig. 1; [62]). Fifth, Fourier transform infrared spectroscopy revealed that the interstitial crystals contained primarily calcium phosphate in the form of hydroxyapatite [62]. Sixth, renal calcinosis in the THP KO mice was independent of gender or genetic background, but it was partially gene dosage-dependent, with homozygous THP knockouts (THP−/−) developing more frequent and severe calcinosis than the heterozygous mice (THP+/−). Finally, by light and electron microscopy and by immunohistochemistry using macrophage and neutrophil antibodies, there was no obvious evidence of renal epithelial injury associated with calcium crystal deposition and no inflammatory cell infiltration was present in the vicinity of the interstitial crystals. When urine chemistry was compared between THP−/− and THP+/+, it was apparent that urine of THP−/− was supersaturated with stone precursor brushite and had reduced urinary concentration of stone inhibitor citrate [62]. The urinary supersaturation was much greater in the old KO mice than in the young KO mice. Overall, there was a marked urine volume contraction in the THP−/− mice, particularly in the aged group, due at least partly to a reduced glomerular filtration rate [63]. Calcium concentration was also significantly elevated, along with calcium oxalate concentration, although the latter did not reach statistical significance [62]. Elevated calcium concentration may have to do with reduced urine volume, or decreased transcellular reabsorption of calcium in the distal convoluted tubules as a result of increased endocytosis of TRPV5 and reduced apical TRPV5 in the absence of THP [64].
Figure 1.
Morphological features of renal papillary interstitial calcinosis in knockout (KO) mice lacking Tamm-Horsfall protein (THP), osteopontin (OPN), THP and OPN, Na+-phosphate cotransporter Type II (Npt2a) and Na+/H+ exchanger regulatory factor (NHERF-1). Histochemistry for the detection of calcium crystals was von Kossa method for all the KO mice except for NHERF-1 where Yasue staining was used. The lower panels also contained transmission electron micrographs for THP KO and Npt2a mice. IX and X in the middle and lower panels of Npt2a KO mice denote interstitial crystals. The images were adapted, reoriented and resized from the original publications with permission (THP KO from (ref. 62); OPN KO from (ref. 62); THP/OPN KO from (ref. 62); Npt2a KO from (ref. 105); and NHERF-1 from (ref. 65)).
Clearly the papillary interstitial calcinosis in the THP−/− mice bear strong resemblances to Randall’s plaques of human idiopathic calcium oxalate stone formers, with respect to histological location, chemical composition, ultrastructural features and lack of accompanying inflammation [20, 65]. It differs from Randall’s plaques in that the latter are also found in the basement membranes of the thin loops of Henle. Furthermore, no overgrowth of calcium oxalate stones atop the papillary interstitial crystals has been observed to date despite the long follow-up (up to 15 months) of the THP−/− mice [62].
Although additional studies are clearly needed, the existing data obtained from the THP−/− mice suggest that THP can influence several key steps suggested for human nephrolithiasis. Thus, the absence or reduced levels of THP can lead to urinary calcium phosphate supersaturation [62] which serves as the driving force for crystal nucleation. The lack of THP can also enhance crystal adherence to the renal epithelial cells [66]. The subsequent steps that result in calcium crystals to be deposited in the interstitium, however, remain to be elucidated and will likely require careful dissection of different-aged THP KO mice on an ultrastructural level. More importantly, it will be of considerable interest to see whether the papillary interstitial crystals can be turned into calcium oxalate stones with enhanced supersaturation of various mineral salts. Answers to these and other questions can be greatly facilitated by the development of compound mice that are not only deficient for THP, but have significantly greater mineral supersaturation than that generated by THP knockout alone.
In humans, down-regulation of THP has been associated with tubular dysfunction including acute tubular necrosis, chronic kidney disease, diabetic and hypertensive nephropathies, kidney transplantation, hyperprostaglandin E syndrome and active lupus nephritis [26, 67–69]. Some of these conditions are known to have an increase risk of kidney stone formation [12, 70]. Recent genome-wide association studies discovered that certain single nucleotide polymorphisms are protective against kidney stones [71]. THP measurements in stone formers versus non-stone formers have been inconclusive among different cohorts, some finding lower THP levels [72–75] with others showing little difference [76]. Some of the inconsistency might be attributable to varied THP partition in soluble versus insoluble fractions. Unless more reproducible sample preparation, storage and quantitation methodologies become a gold standard for different laboratories, such as the one recently published [77], it would be premature and misleading to conclude that quantitative defects of THP do not exist in stone formers. Finally, qualitative deficiencies such as insufficient sialylation of THP have been suggested to cause a functional defect in the inhibition of stone formation [40, 54, 78, 79]. These human-relevant topics are undoubtedly worthy of further exploration.
Osteopontin knockout mice
Unlike THP which is kidney-specific, osteopontin (OPN) is ubiquitous, expressed in a wide range of tissues and cell types and believed to have diverse functions [80–84]. Initially isolated from osteoblasts, OPN exists as an extracellular protein that regulates bone biomineralization and remodeling as well as ectopic calcification [85]. In normal kidneys, OPN is detected by immunohistochemistry in TAL cells and renal papillary epithelium. OPN is markedly acidic and negatively charged at neutral pH, as (i) it contains abundant negatively charged amino acids (e.g., aspartic and glutamic acids that together account for ~30% of the entire amino acid composition); (ii) it is glycosylated with complex-type carbohydrates capped with siliac acids (up to 10 sialic acids); and it is heavily phosphorylated on the serine residues. OPN binds avidly to Ca2+ ions at the crystal surfaces of divergent mineral types including calcium phosphate and calcium oxalate [Rittling, 1999 #15;Giachelli, 2000 #5;[86, 87].
Long suspected to play a role in nephrolithiasis, OPN is found reproducibly in the matrix of human kidney stones [86, 88–91]. When tested in various in vitro settings, it potently inhibits the nucleation of calcium crystals, crystal aggregation and adhesion to renal epithelial cells. Knockout mice of OPN were first reported in 1998 by Liaw and colleagues [92], but their predisposition to renal calcification was not studied until 2003 [93]. Wesson et al found that, when administered 1% ethylene glycol in the drinking water, the OPN mice were significantly more susceptible than the wild-type controls to develop renal calcium oxalate crystallization. The crystals were exclusively intra-tubular and located primarily in the outer medulla. Spontaneous calcinosis, e.g., calcinosis in the absence of chemical treatment, was not noted in this study.
In a more recent study, Mo and colleagues reassessed the spontaneous calcium crystal deposition in an independent cohort of OPN KO mice [66]. von Kossa staining of 2–3 month old male knockouts revealed calcium crystals in the renal papillary region that on higher magnification appeared interstitial (Fig. 1). The size and frequency of these crystals were less severe than those in the THP KO mice, as was the percentage of the knockouts that developed these crystals (10% in OPN KO mice vs. 16% in THP KO mice). Nevertheless, as with the THP KO mice, the papillary interstitial crystals in the OPN mice were of calcium phosphate as evidenced by Fourier transform infrared spectroscopy [66]. The fact that these crystals were not observed in an earlier study of the OPN KO mice could be due to the exclusive location of the crystals in the renal papillae that are of very small sizes in mice thus easily escaping detection.
The Wesson and Mo studies both analyzed the urine chemistry of the OPN mice. Consistently, no increase in urine concentration of calcium, phosphorus and oxalate was observed [66, 93]. Unlike the THP KO mice, brushite was not supersaturated in the OPN KO mice. These data seem to suggest that the papillary interstitial calcinosis in the two KO mice may have different underlying mechanisms.
Single nucleotide polymorphisms (SNPs) of osteopontin have been an active area of investigation. Thus far, OPN SNPs have been associated with a number of disease conditions including kidney stone disease [94–97]. Some SNPs are located in the coding region with others in the regulatory (promoter) region. Of the nine SNPs found in the OPN gene (rs11739060, rs28357094, rs2728127, rs11730582, rs1126772, rs9138, rs2853744, rs4754=p.Asp80Asp, and rs1126616=p.Ala236Ala), rs9138 AA was found to be protective, whereas rs28357094 TT genotype, rs2728127 GG and rs2853744 GG genotypes were predisposing [98]. Since OPN SNPs are also known to be associated with other kidney conditions such as diabetes and hypertension that they themselves increase the risk of kidney stone formation, it remains to be seen as to whether these SNPs directly or indirectly affect human nephrolithiasis.
Tamm-Horsfall protein and osteopontin double knockout mice
The lack of widespread intra-renal calcinosis in mice deficient for either THP or OPN prompted Mo and colleagues to develop double KO mice lacking both proteins [66]. The double KO mice were fully compatible with life with no overt anatomic and physiological deficits. von Kossa histochemistry of 2–3 month old animals detected spontaneous calcium deposits in about 39% of the mice, a significantly higher percentage than THP and OPN single knockouts [66]. Like the single knockouts, the crystals were interstitial and located almost exclusively in the renal papillae (Fig. 1). Not only were the double KO mice more prone to spontaneous calcification, they also developed more frequently, much greater number of and larger renal crystals than the single knockouts upon ethylene glycol overload. It should be noted that the EG-induced crystals were almost exclusively intra-tubular, plugging crystals concentrated in the inner medullary region as well as in severely dilated ducts of Bellini [66]. Interestingly, in the absence of THP, EG triggered an over-expression of OPN in renal epithelial cells that was far greater than in the wild-type mice, suggesting that OPN is an inducible inhibitor of calcium crystallization playing a critical role when THP is deficient. In contrast, THP was not induced upon EG treatment when OPN is absent, suggesting that THP acts as a constitutive inhibitor of calcium crystallization. This, combined with the fact that THP and OPN deficiency has divergent effects on urinary supersaturation and that these proteins exert different effects on nephrolithiasis, raises the interesting possibility that normal THP and OPN are synergistic inhibitors of renal calcinosis.
The urine chemistry of the THP/OPN double KO mice for most part resembled that of the THP KO mice, with significantly elevated urinary concentration of phosphate and increased supersaturation of brushite [66]. The urine chemistry per se therefore does not fully explain why the double KO mice developed interstitial calcinosis more frequently than the single KO mice.
It should be pointed out that the majority of knockouts lacking both THP and OPN did not develop spontaneous renal calcification [66]. On the one hand, it is possible that the mice analyzed were of young age (2–3 months) and that renal calcinosis appeared to be highly age-dependent at least based on available data from the THP KO mice. Age-related studies in genetically engineered mice are extremely labor-intensive and cost-prohibitive, but it will be of major interest, if resources permit, to see if renal calcinosis in THP/OPN double KO mice exacerbates with age so much so that even kidney stones arise. On another hand, one cannot rule out the possibility that macromolecule inhibition of renal stone formation is a highly redundant process and that loss of proteins beyond THP and OPN is necessary to trigger stone formation. Urine is known to contain other macromolecules that show potent inhibitory effects toward the various phases of nephrolithiasis. These include urinary prothrombin fragment 1, inter-alpha-trypsin inhibitor (IAI) family proteins such as bikunin, calgranulin A and B, urinary trefoil factor 1 [8–10, 53, 99], to name a few. Hopefully, knockout mice lacking these proteins will be generated soon, so that their in vivo role in kidney stone formation can also be assessed. Finally, deficiency of macromolecules might need collaboration with enhanced supersaturation of mineral salts such as calcium oxalate and calcium phosphate in order to trigger stone formation. It is now possible to test some of these possibilities by the use of second-generation compound mice that harbor a combination of different genetic alterations (see later).
Na+-phosphate cotransporter Type II knockout mice
Na+-dependent phosphate cotransporter Type II (Npt2a), also classified as solute carrier family 34, member 1 (SLC34A1), is localized on the brusher border membrane of renal proximal convoluted tubule and is responsible for reabsorbing bulk of the phosphate (up to 80%) filtered through the glomeruli [100]. As such, this transporter plays a crucially important role in keeping the phosphate homeostasis. Indeed, mutations of the Npt2a gene in patients have been causatively linked to certain types of hypophosphatemia, hyperphosphaturia and nephrolithiasis [101, 102]. Npt2a is hormonally regulated by parathyroid hormone which promotes the endocytosis of the channel thereby decreasing its luminal presence and reduced phosphate reabsorption [103].
Consistent with its proposed function in tubular phosphate reaborption, Npt2a knockout mice developed hyperphosphaturia and hypophosphatemia [104]. A compensatory increase of calcium reabsorption from the intestine also led to hypercalcemia and hypercalciuria. Khan and Canales analyzed the renal calcification of Npt2a KO mice aged 2 days to one year using light, scanning and transmission electron microscopy together with electron diffraction and energy dispersive x-ray microanalyses (Fig. 1) [105, 106]. They detected intratubular calcium phosphate deposits throughout the nephron with some crystals in the collecting ducts. Although also of concentric appearance, the crystals observed in the Npt2a knockout mice seemed much less organized and compact than those in the THP KO mice [106]. Interstitial crystals were also noted, albeit somewhat infrequently and in small areas where collecting tubules were completely occluded. The authors concluded that the interstitial calcinosis in the Npt2a knockout differ from those of the Randall’s plaques in humans.
As with the THP, OPN and THP/OPN knockouts, no bona-fide kidney stone was ever noted in these mice. In addition, urinary supersaturation of phosphate seemed to improve over time in the Npt2a knockout mice [104], a trend opposite to what occurs in THP knockout where supersaturation progressively worsens with the advanced age.
Na+/H+ exchanger regulatory factor (NHERF-1) knockout mice
Na+/H+ exchanger regulatory factor (NHERF-1), encoded by solute carrier family 9 isoform A3 regulatory factor 2 (SLC9A3R2) gene, is a member of the sodium-hydrogen exchanger regulatory factor family found on the brush border membranes of renal proximal tubule and intestinal epithelia [107, 108]. These proteins contain PSD-95/Dlg/ZO-1 (PDZ) domains and act as scaffolds that link membrane channels to cytoplasmic ezrin-moesin-radixin family proteins and the microfilament [109]. A very important function of these scaffold proteins is therefore to regulate the apical surface targeting and stability of the membrane channels. NHERF-1 is highly expressed at the brush border membranes of renal proximal tubule. By inactivating the NHERF-1 gene in mice, Weinman’s group found a significant increase of cytoplasmic Npt2a and a marked reduction of Npt2a at the apical surface of proximal tubule [110]. This corresponded with a three-fold increase of urinary excretion of phosphate over the wild-type mice. Similar to what occurred in the Npt2a knockout, the urinary phosphate loss led to a compensatory increase of serum 1,25 (OH)2 vitamin D that in turn caused an increased intestinal reabsorption of calcium and hypercalciuria.
Like the Npt2a knockout mice, NHERF-1 knockout mice developed renal papillary calcinosis as shown by von Kossa staining [111]. The crystals appeared to be primarily interstitial in both male and female mice, but the number and size of the crystal deposits were relatively small. In another cohort, Evan and collaborators performed a more in-depth analysis of the renal calcinosis in NHERF-1 knockout mice focusing on old-age animals (17–19 months) (Fig. 1) [65]. The authors detected using Yasue staining a greater number of calcium crystals than originally reported. The crystals were interstitial in the basement zone located near thin loops of Henle as well as inner medullary collecting tubules. No tubular injury or inflammation cells were seen accompanying the crystals. Transmission electron microscopy and chemical composition analyses of the crystals were not carried out.
Similarities and implications of papillary calcinosis in different knockout strains
A shared feature of the papillary calcinosis in different knockout mice covered in this review is the interstitial location of the calcium crystal deposition (Table 1; Fig. 1). Because of their lack of accessibility to the tubular lumen, these crystals cannot possibly pass through the renal tubules to be eliminated in the urine. In the absence of accompanying inflammation, the crystals are also unlikely to be ingested and cleared by phagocytosis. As a result, the papillary interstitial crystals in these knockout mice will likely persist, thereby providing opportunities for further growth and aggregation. Another commonality is that with the exception of OPN KO mice all other KO mice had increased urinary concentrations of phosphate and/or calcium [65, 66, 93, 104, 111]. For instance, phosphate and brushite were significantly supersaturated in the THP KO mice [66]. The concentrations of both phosphate and calcium were elevated in Npt2 and NHERF-1 [104, 111]. The data from these knockouts are therefore very much in line with the notion conceived in human nephrolithiasis that mineral salt supersaturation drives crystallization and nucleation [8, 10]. Nevertheless, the fact that OPN KO mice did not have apparent urinary supersaturation points to another possible scenario where other (non-supersaturation-related) abnormalities lead to interstitial calcification. With respect to the chemical composition, apatite seems to be the predominant type among the crystals whose chemical composition has been determined including those in the THP, OPN and Npt2a mice [66, 105]. This stands in stark contrast to THP or OPN knockout mice treated with ethylene glycol that subsequently developed exclusively calcium oxalate crystals [61, 66, 93]. Of note, all the calcium oxalate crystals in THP, OPN and, THP/OPN mice under hyperoxaluric conditions were intra-tubular. It appears therefore that the development of interstitial apatite calcinosis (without signs of tubular injury) relies on functional renal epithelial cells, whereas extensive tubular damage caused by ethylene glycol leads to mainly intra-tubular crystallization.
Table 1.
Spontaneous Renal Calcinosis in Knockout Mice
| Main features | THP KO | OPN KO | THP/OPN KO | Npt2a KO | NHERF-1 KO |
|---|---|---|---|---|---|
| Normal protein location | Apical membrane of TAL of kidney | Ubiquitous/cytoplasmic | NA | Brush border of Renal PCT | Brush border of PCT & small intestine |
| Proposed role in nephrolithiasis | Macromolecule inhibitor | Macromolecule inhibitor | Macromolecule inhibitors | Ion channel | Scaffold protein |
| Crystal location in KO mice | Papilla | Papilla | Papilla | Nephron & papilla | Papilla |
| Intratubular/interstitial | Intratubular in young mice; interstitial in old mice | Interstitial | Interstitial | Mainly intratubular/occasionally interstitial | Interstitial |
| Chemical composition | Hydroxyapatite | Hydroxyapatite | ND | Apatite | ND |
| TEM | Compact concentric rings | ND | ND | Loose concentric rings | ND |
| Renal injury | Not visible | Not visible | Not visible | Not visible | Not visible |
| Inflammation adjacent to crystals | Not seen | Not seen | Not seen | Not seen | Not seen |
| Age-dependence | Highly dependent | ND | ND | Not systematically compared | Not systematically compared |
| Urinary supersaturation | Phosphorus, brushite | None | Phosphorus, brushite | Phosphorus, calcium | Phosphorus, calcium uric acid |
| Urinary stone formation | Bladder struvite stones in old mice | None | None | None | None |
| Mutations and stone risk in humans | ND | ND | NA | Yes | Yes |
| SNPs and stone risk in humans | Protective | Protective & predisposing | NA | Predisposing | Predisposing |
NA: not applicable
TAL: thick ascending limb of loop of Henle
PCT: proximal convoluted tubule
ND: not determined
TEM: transmission electron microscopy
Key remaining questions
Although the development of papillary interstitial calcinosis in several KO mouse models is of considerable interest as they bear strong resemblance to Randall’s plaques of idiopathic kidney calclium stone patients, key questions remain. The most obvious ones are how these apatite crystals form in the first place and what they will eventually become. With respect to the first question, it does not appear that the elevated levels of urinary phosphate and/or calcium in the THP, THP/OPN, Npt2a and NHERF-1 KO mice are the direct cause of interstitial calcification because, if this were the case, crystals should have formed primarily in the tubular lumen. That interstitial calcinosis occurs in the OPN KO mice without apparent hyperphosphoturia or hypercalciuria also suggests that a yet-to-be discovered mechanism exists that underlie interstitial calcinosis. With respect to the second question, none of the knockout mouse strains has developed full-fledge stones equivalent to the human counterparts. It is possible that the time required for the KO mice to develop bona-fide kidney stones far-exceeds what has been attempted. In the case of THP KO mice, there is indeed a progressive increase of the severity of interstitial calcification with age. However, mice have a finite life and maintaining a large number of old KO mice is prohibitively expensive. It is impractical to find out whether stones eventually arise in very old KO animals. Besides age-related issues, one certainly cannot rule out the possibility that stone formation requires pathogenic events besides what was initially created in the KO mice. Clearly, further investigation is needed, and answers to these questions should shed light on whether the papillary interstitial calcinosis is truly a precursor of calcium oxalate stones.
Perspectives
Relative to the prevalence and importance of kidney stone disease, genetically engineered mouse models available to study its pathogenesis remain far and in between. Some investigators continue to hold the opinion that, since mice do not develop usual kidney stones, they are not relevant to studying human nephrolithiasis. This view is off the mark. The rationale behind the creation of GEMMs to study kidney stone formation is precisely because normal mice have a very low incidence of spontaneous kidney stones. Should normal mice frequently develop kidney stones, they would not have been suitable for the GEMMs. This is akin to the tumorigenesis studies where a low incidence of spontaneous tumors in normal mice is a prerequisite for developing transgenic and knockout tumor models. Granted, the existing models do not recapitulate all the aspects of human nephrolithiasis, but this is exactly why additional work is necessary in order to create human-relevant models. Human kidneys bearing full-fledged stones may no longer contain some of the earliest pathogenic steps. This is where the GEMMs can help fill the void. There is ample evidence from many other organ systems that mouse and human studies can be highly complementary and mutually reinforcing, and together they are much more informative than each approach pursued alone [1, 3, 4, 28, 112, 113].
When it comes to the expanded use of GEMMs to study kidney stone formation, there are reasons to believe that a great deal of information will be forthcoming in the next few years. More sophisticated gene knockout strategies can now be employed, especially the temporally controlled gene inactivation strategy. For instance, all the 5 GEMMs reviewed here were generated in a constitutive manner, i.e., gene ablation begins during an embryonic stage. A major limitation of this approach is that cells are very plastic during embryogenesis, to the extent that the loss of one gene could lead to the compensatory induction of another gene with similar functions [113, 114]. This can mask the phenotype(s) that would otherwise have manifested. This is a relevant issue with regards to macromolecule stone inhibitors which may be highly redundant – a situation occurring in the THP KO mice where OPN is strongly induced under hyperoxaluric conditions [61, 66]. A similar compensatory event could explain the age-related levering-off of elevated urinary phosphate levels in NHERF-1 KO mice [111]. It will be interesting to know if more severe phenotypes will develop when some of these genes are abrogated abruptly in adult mice with the inducible knockout systems (e.g., triggered by tetracycline or estrogen inducers).
Deeper insights into idiopathic kidney stone formation should also come from the development of compound mice that bear multiple genetic alterations. As with many complex diseases, it seems unlikely that idiopathic kidney stones are caused by a single genetic defect. Family history and data from twins support the polygenic nature of human idiopathic kidney stones [7, 115–117]. In this regard, intercrossing different knockout strains to yield compound mice that exhibit multiple defects in functionally complementary pathways should be extremely informative about the collaborative relationship among different genetic defects in nephrolithiais. These complex mouse models should also offer new opportunities to trace whether the papillary interstitial calcinosis can gradually transit to become renal pelvic stones.
Since the completion of mouse genome sequencing project, a concerted effort is underway under the auspices of International Knockout Mouse Consortium (http://www.knockoutmouse.org/) to generate knockout mice for every known mouse gene. There is no reason why the kidney stone research field should be left out of this fast-moving effort. Instead of continuing to be skeptical of the usefulness and prematurely dismissing the need to develop more kidney-stone-related GEMMs, a more productive approach would be to keep an open mind and embrace the exciting and inevitable developments that lie ahead.
Acknowledgments
The author would like to acknowledge the grant support from the National Institutes of Health (DK056903) and the Veterans Affairs Research and Development Service (Merit Review). He also would like to thank his research associates and collaborators who contributed to the generation and/or characterization of THP, OPN and THP/OPN knockout mice discussed in this review. The author apologizes to those authors whose work cannot be cited due to space limitation.
Footnotes
Conflict of Interest
The author declared no conflict of interest.
References
- 1.Doyle A, McGarry MP, Lee NA, Lee JJ. The construction of transgenic and gene knockout/knockin mouse models of human disease. Transgenic Res. 2012;21:327–349. doi: 10.1007/s11248-011-9537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh M, Murriel CL, Johnson L. Genetically engineered mouse models: closing the gap between preclinical data and trial outcomes. Cancer Res. 2012;72:2695–2700. doi: 10.1158/0008-5472.CAN-11-2786. [DOI] [PubMed] [Google Scholar]
- 3.Kohan DE. Progress in gene targeting: using mutant mice to study renal function and disease. Kidney Int. 2008;74:427–437. doi: 10.1038/ki.2008.146. [DOI] [PubMed] [Google Scholar]
- 4.Bockamp E, Maringer M, Spangenberg C, Fees S, Fraser S, Eshkind L, Oesch F, Zabel B. Of mice and models: improved animal models for biomedical research. Physiol Genomics. 2002;11:115–132. doi: 10.1152/physiolgenomics.00067.2002. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H, Liu Y, He F, Mo L, Sun TT, Wu XR. Temporally and spatially controllable gene expression and knockout in mouse urothelium. Am J Physiol Renal Physiol. 2010;299:F387–395. doi: 10.1152/ajprenal.00185.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaeger P. Genetic versus environmental factors in renal stone disease. Curr Opin Nephrol Hypertens. 1996;5:342–346. doi: 10.1097/00041552-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Robertson WG. Pathophysiology of stone formation. Urol Int. 1986;41:329–333. doi: 10.1159/000281232. [DOI] [PubMed] [Google Scholar]
- 8.Coe FL, Evan A, Worcester E. Kidney stone disease. J Clin Invest. 2005;115:2598–2608. doi: 10.1172/JCI26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci. 2004;9:1450–1482. doi: 10.2741/1347. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Lieske JC. Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hypertens. 2006;15:374–380. doi: 10.1097/01.mnh.0000232877.12599.f4. [DOI] [PubMed] [Google Scholar]
- 11.Mandel N. Crystal-membrane interaction in kidney stone disease. J Am Soc Nephrol. 1994;5:S37–45. doi: 10.1681/ASN.V55s37. [DOI] [PubMed] [Google Scholar]
- 12.Sakhaee K, Maalouf NM, Sinnott B. Clinical review. Kidney stones 2012: pathogenesis, diagnosis, and management. J Clin Endocrinol Metab. 2012;97:1847–1860. doi: 10.1210/jc.2011-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 14.Holmes RP, Ambrosius WT, Assimos DG. Dietary oxalate loads and renal oxalate handling. J Urol. 2005;174:943–947. doi: 10.1097/01.ju.0000169476.85935.e2. discussion 947. [DOI] [PubMed] [Google Scholar]
- 15.Bushinsky DA, Frick KK, Nehrke K. Genetic hypercalciuric stone-forming rats. Curr Opin Nephrol Hypertens. 2006;15:403–418. doi: 10.1097/01.mnh.0000232881.35469.a9. [DOI] [PubMed] [Google Scholar]
- 16.Park S, Pearle MS. Pathophysiology and management of calcium stones. Urol Clin North Am. 2007;34:323–334. doi: 10.1016/j.ucl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Holmes RP, Assimos DG, Goodman HO. Molecular basis of inherited renal lithiasis. Curr Opin Urol. 1998;8:315–319. doi: 10.1097/00042307-199807000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Lieske JC, Toback FG. Renal cell-urinary crystal interactions. Curr Opin Nephrol Hypertens. 2000;9:349–355. doi: 10.1097/00041552-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Koul HK, Koul S, Fu S, Santosham V, Seikhon A, Menon M. Oxalate: from crystal formation to crystal retention. J Am Soc Nephrol. 1999;10(Suppl 14):S417–421. [PubMed] [Google Scholar]
- 20.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest. 2003;111:607–616. doi: 10.1172/JCI17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagga HS, Chi T, Miller J, Stoller ML. New insights into the pathogenesis of renal calculi. Urol Clin North Am. 2013;40:1–12. doi: 10.1016/j.ucl.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randall A. THE ORIGIN AND GROWTH OF RENAL CALCULI. Ann Surg. 1937;105:1009–1027. doi: 10.1097/00000658-193706000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evan AP, Lingeman JE, Coe FL, Shao Y, Parks JH, Bledsoe SB, Phillips CL, Bonsib S, Worcester EM, Sommer AJ, Kim SC, Tinmouth WW, Grynpas M. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int. 2005;67:576–591. doi: 10.1111/j.1523-1755.2005.67114.x. [DOI] [PubMed] [Google Scholar]
- 24.Matlaga BR, Coe FL, Evan AP, Lingeman JE. The role of Randall’s plaques in the pathogenesis of calcium stones. J Urol. 2007;177:31–38. doi: 10.1016/j.juro.2006.08.088. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Muchmore A. Tamm-Horsfall protein--uromodulin (1950–1990) Kidney Int. 1990;37:1395–1401. doi: 10.1038/ki.1990.128. [DOI] [PubMed] [Google Scholar]
- 26.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis. 2003;42:658–676. doi: 10.1016/s0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 27.Rampoldi L, Scolari F, Amoroso A, Ghiggeri G, Devuyst O. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int. 2011 doi: 10.1038/ki.2011.134. [DOI] [PubMed] [Google Scholar]
- 28.El-Achkar TM, Wu XR. Uromodulin in kidney injury: an instigator, bystander, or protector? Am J Kidney Dis. 2012;59:452–461. doi: 10.1053/j.ajkd.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol. 2008;295:F534–544. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, Wu XR. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. Am J Physiol Renal Physiol. 2011;300:F999–1007. doi: 10.1152/ajprenal.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, Wu XR. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. Am J Physiol Renal Physiol. 2013;304:F1066–1075. doi: 10.1152/ajprenal.00543.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hession C, Decker JM, Sherblom AP, Kumar S, Yue CC, Mattaliano RJ, Tizard R, Kawashima E, Schmeissner U, Heletky S, et al. Uromodulin (Tamm-Horsfall glycoprotein): a renal ligand for lymphokines. Science. 1987;237:1479–1484. doi: 10.1126/science.3498215. [DOI] [PubMed] [Google Scholar]
- 33.Muchmore AV, Decker JM. Uromodulin: a unique 85-kilodalton immunosuppressive glycoprotein isolated from urine of pregnant women. Science. 1985;229:479–481. doi: 10.1126/science.2409603. [DOI] [PubMed] [Google Scholar]
- 34.Pennica D, Kohr WJ, Kuang WJ, Glaister D, Aggarwal BB, Chen EY, Goeddel DV. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science. 1987;236:83–88. doi: 10.1126/science.3453112. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Wu C, Zhao S, Guo J. Identification and characterization of D8C, a novel domain present in liver-specific LZP, uromodulin and glycoprotein 2, mutated in familial juvenile hyperuricaemic nephropathy. FEBS Lett. 2004;578:236–238. doi: 10.1016/j.febslet.2004.10.092. [DOI] [PubMed] [Google Scholar]
- 36.Ma L, Liu Y, El-Achkar TM, Wu XR. Molecular and cellular effects of Tamm-Horsfall protein mutations and their rescue by chemical chaperones. J Biol Chem. 2012;287:1290–1305. doi: 10.1074/jbc.M111.283036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nasr SH, Lucia JP, Galgano SJ, Markowitz GS, VDDA Uromodulin storage disease. Kidney Int. 2008;73:971–976. doi: 10.1038/sj.ki.5002679. [DOI] [PubMed] [Google Scholar]
- 38.Scolari F, Caridi G, Rampoldi L, Tardanico R, Izzi C, Pirulli D, Amoroso A, Casari G, Ghiggeri GM. Uromodulin storage diseases: clinical aspects and mechanisms. Am J Kidney Dis. 2004;44:987–999. doi: 10.1053/j.ajkd.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Bleyer AJ, Hart PS, Kmoch S. Hereditary interstitial kidney disease. Semin Nephrol. 2010;30:366–373. doi: 10.1016/j.semnephrol.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serafini-Cessi F, Monti A, Cavallone D. N-Glycans carried by Tamm-Horsfall glycoprotein have a crucial role in the defense against urinary tract diseases. Glycoconj J. 2005;22:383–394. doi: 10.1007/s10719-005-2142-z. [DOI] [PubMed] [Google Scholar]
- 41.Serafini-Cessi F, Dall’Olio F, Malagolini N. High-mannose oligosaccharides from human Tamm-Horsfall glycoprotein. Biosci Rep. 1984;4:269–274. doi: 10.1007/BF01119663. [DOI] [PubMed] [Google Scholar]
- 42.van Rooijen JJ, Voskamp AF, Kamerling JP, Vliegenthart JF. Glycosylation sites and site-specific glycosylation in human Tamm-Horsfall glycoprotein. Glycobiology. 1999;9:21–30. doi: 10.1093/glycob/9.1.21. [DOI] [PubMed] [Google Scholar]
- 43.Pak J, Pu Y, Zhang ZT, Hasty DL, Wu XR. Tamm-Horsfall protein binds to type 1 fimbriated Escherichia coli and prevents E. coli from binding to uroplakin Ia and Ib receptors. J Biol Chem. 2001;276:9924–9930. doi: 10.1074/jbc.M008610200. [DOI] [PubMed] [Google Scholar]
- 44.Cavallone D, Malagolini N, Monti A, Wu XR, Serafini-Cessi F. Variation of high mannose chains of Tamm-Horsfall glycoprotein confers differential binding to type 1-fimbriated Escherichia coli. J Biol Chem. 2004;279:216–222. doi: 10.1074/jbc.M308821200. [DOI] [PubMed] [Google Scholar]
- 45.Mo L, Zhu XH, Huang HY, Shapiro E, Hasty DL, Wu XR. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. Am J Physiol Renal Physiol. 2004;286:F795–802. doi: 10.1152/ajprenal.00357.2003. [DOI] [PubMed] [Google Scholar]
- 46.Bates JM, Raffi HM, Prasadan K, Mascarenhas R, Laszik Z, Maeda N, Hultgren SJ, Kumar S. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 2004;65:791–797. doi: 10.1111/j.1523-1755.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 47.Dou W, Thompson-Jaeger S, Laulederkind SJ, Becker JW, Montgomery J, Ruiz-Bustos E, Hasty DL, Ballou LR, Eastman PS, Srichai B, Breyer MD, Raghow R. Defective expression of Tamm-Horsfall protein/uromodulin in COX-2-deficient mice increases their susceptibility to urinary tract infections. Am J Physiol Renal Physiol. 2005;289:F49–60. doi: 10.1152/ajprenal.00134.2004. [DOI] [PubMed] [Google Scholar]
- 48.Zhou G, Mo WJ, Sebbel P, Min G, Neubert TA, Glockshuber R, Wu XR, Sun TT, Kong XP. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J Cell Sci. 2001;114:4095–4103. doi: 10.1242/jcs.114.22.4095. [DOI] [PubMed] [Google Scholar]
- 49.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. Uroplakins in urothelial biology, function, and disease. Kidney Int. 2009;75:1153–1165. doi: 10.1038/ki.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu XR, Sun TT, Medina JJ. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc Natl Acad Sci U S A. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jovine L, Qi H, Williams Z, Litscher E, Wassarman PM. The ZP domain is a conserved module for polymerization of extracellular proteins. Nat Cell Biol. 2002;4:457–461. doi: 10.1038/ncb802. [DOI] [PubMed] [Google Scholar]
- 52.Weichhart T, Haidinger M, Horl WH, Saemann MD. Current concepts of molecular defence mechanisms operative during urinary tract infection. Eur J Clin Invest. 2008;38(Suppl 2):29–38. doi: 10.1111/j.1365-2362.2008.02006.x. [DOI] [PubMed] [Google Scholar]
- 53.Worcester EM. Inhibitors of stone formation. Semin Nephrol. 1996;16:474–486. [PubMed] [Google Scholar]
- 54.Chen WC, Lin HS, Chen HY, Shih CH, Li CW. Effects of Tamm-Horsfall protein and albumin on calcium oxalate crystallization and importance of sialic acids. Mol Urol. 2001;5:1–5. doi: 10.1089/109153601750124186. [DOI] [PubMed] [Google Scholar]
- 55.Erwin DT, Kok DJ, Alam J, Vaughn J, Coker O, Carriere BT, Lindberg J, Husserl FE, Fuselier H, Jr, Cole FE. Calcium oxalate stone agglomeration reflects stone-forming activity: citrate inhibition depends on macromolecules larger than 30 kilodalton. Am J Kidney Dis. 1994;24:893–900. doi: 10.1016/s0272-6386(12)81057-2. [DOI] [PubMed] [Google Scholar]
- 56.Fellstrom B, Danielson BG, Ljunghall S, Wikstrom B. Crystal inhibition: the effects of polyanions on calcium oxalate crystal growth. Clin Chim Acta. 1986;158:229–235. doi: 10.1016/0009-8981(86)90286-x. [DOI] [PubMed] [Google Scholar]
- 57.Grover PK, Moritz RL, Simpson RJ, Ryall RL. Inhibition of growth and aggregation of calcium oxalate crystals in vitro--a comparison of four human proteins. Eur J Biochem. 1998;253:637–644. doi: 10.1046/j.1432-1327.1998.2530637.x. [DOI] [PubMed] [Google Scholar]
- 58.Hess B, Nakagawa Y, Coe FL. Inhibition of calcium oxalate monohydrate crystal aggregation by urine proteins. Am J Physiol. 1989;257:F99–106. doi: 10.1152/ajprenal.1989.257.1.F99. [DOI] [PubMed] [Google Scholar]
- 59.Hess B. Tamm-Horsfall glycoprotein--inhibitor or promoter of calcium oxalate monohydrate crystallization processes? Urol Res. 1992;20:83–86. doi: 10.1007/BF00294343. [DOI] [PubMed] [Google Scholar]
- 60.Hess B. Tamm-Horsfall glycoprotein and calcium nephrolithiasis. Miner Electrolyte Metab. 1994;20:393–398. [PubMed] [Google Scholar]
- 61.Mo L, Huang HY, Zhu XH, Shapiro E, Hasty DL, Wu XR. Tamm-Horsfall protein is a critical renal defense factor protecting against calcium oxalate crystal formation. Kidney Int. 2004;66:1159–1166. doi: 10.1111/j.1523-1755.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Mo L, Goldfarb DS, Evan AP, Liang F, Khan SR, Lieske JC, Wu XR. Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm-Horsfall protein. Am J Physiol Renal Physiol. 2010;299:F469–478. doi: 10.1152/ajprenal.00243.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, El-Achkar TM, Wu XR. Tamm-Horsfall protein regulates circulating and renal cytokines by affecting glomerular filtration rate and acting as a urinary cytokine trap. J Biol Chem. 2012;287:16365–16378. doi: 10.1074/jbc.M112.348243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolf MT, Wu XR, Huang CL. Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int. 2013;84:130–137. doi: 10.1038/ki.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evan AP, Weinman EJ, Wu XR, Lingeman JE, Worcester EM, Coe FL. Comparison of the pathology of interstitial plaque in human ICSF stone patients to NHERF-1 and THP-null mice. Urol Res. 2010;38:439–452. doi: 10.1007/s00240-010-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mo L, Liaw L, Evan AP, Sommer AJ, Lieske JC, Wu XR. Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Horsfall protein, or both. Am J Physiol Renal Physiol. 2007;293:F1935–1943. doi: 10.1152/ajprenal.00383.2007. [DOI] [PubMed] [Google Scholar]
- 67.Mollsten A, Torffvit O. Tamm-Horsfall protein gene is associated with distal tubular dysfunction in patients with type 1 diabetes. Scand J Urol Nephrol. 2010 doi: 10.3109/00365599.2010.504190. [DOI] [PubMed] [Google Scholar]
- 68.Sejdiu I, Torffvit O. Decreased urinary concentration of Tamm-Horsfall protein is associated with development of renal failure and cardiovascular death within 20 years in type 1 but not in type 2 diabetic patients. Scand J Urol Nephrol. 2008;42:168–174. doi: 10.1080/00365590701644691. [DOI] [PubMed] [Google Scholar]
- 69.Chakraborty J, Below AA, Solaiman D. Tamm-Horsfall protein in patients with kidney damage and diabetes. Urol Res. 2004;32:79–83. doi: 10.1007/s00240-003-0374-6. [DOI] [PubMed] [Google Scholar]
- 70.Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res. 2012;40:95–112. doi: 10.1007/s00240-011-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gudbjartsson DF, Holm H, Indridason OS, Thorleifsson G, Edvardsson V, Sulem P, de Vegt F, d’Ancona FC, den Heijer M, Wetzels JF, Franzson L, Rafnar T, Kristjansson K, Bjornsdottir US, Eyjolfsson GI, Kiemeney LA, Kong A, Palsson R, Thorsteinsdottir U, Stefansson K. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS Genet. 2010;6:e1001039. doi: 10.1371/journal.pgen.1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar V, Pena de la Vega L, Farell G, Lieske JC. Urinary macromolecular inhibition of crystal adhesion to renal epithelial cells is impaired in male stone formers. Kidney Int. 2005;68:1784–1792. doi: 10.1111/j.1523-1755.2005.00595.x. [DOI] [PubMed] [Google Scholar]
- 73.Glauser A, Hochreiter W, Jaeger P, Hess B. Determinants of urinary excretion of Tamm-Horsfall protein in non-selected kidney stone formers and healthy subjects. Nephrol Dial Transplant. 2000;15:1580–1587. doi: 10.1093/ndt/15.10.1580. [DOI] [PubMed] [Google Scholar]
- 74.Romero MC, Nocera S, Nesse AB. Decreased Tamm-Horsfall protein in lithiasic patients. Clin Biochem. 1997;30:63–67. doi: 10.1016/s0009-9120(96)00136-1. [DOI] [PubMed] [Google Scholar]
- 75.Jaggi M, Nakagawa Y, Zipperle L, Hess B. Tamm-Horsfall protein in recurrent calcium kidney stone formers with positive family history: abnormalities in urinary excretion, molecular structure and function. Urol Res. 2007;35:55–62. doi: 10.1007/s00240-007-0083-7. [DOI] [PubMed] [Google Scholar]
- 76.Pourmand G, Nasseh H, Sarrafnejad A, Mehrsai A, Hamidi Alamdari D, Nourijelyani K, Shekarpour L. Urinary Tamm-Horsfall protein and citrate: a case-control study of inhibitors and promoters of calcium stone formation. Urol J. 2005;2:79–85. [PubMed] [Google Scholar]
- 77.Youhanna S, Weber J, Beaujean V, Glaudemans B, Sobek J, Devuyst O. Determination of uromodulin in human urine: influence of storage and processing. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft345. [DOI] [PubMed] [Google Scholar]
- 78.Viswanathan P, Rimer JD, Kolbach AM, Ward MD, Kleinman JG, Wesson JA. Calcium oxalate monohydrate aggregation induced by aggregation of desialylated Tamm-Horsfall protein. Urol Res. 2011;39:269–282. doi: 10.1007/s00240-010-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knorle R, Schnierle P, Koch A, Buchholz NP, Hering F, Seiler H, Ackermann T, Rutishauser G. Tamm-Horsfall glycoprotein: role in inhibition and promotion of renal calcium oxalate stone formation studied with Fourier-transform infrared spectroscopy. Clin Chem. 1994;40:1739–1743. [PubMed] [Google Scholar]
- 80.Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 2004;26:179–184. [PubMed] [Google Scholar]
- 81.Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J. Osteopontin--a molecule for all seasons. Qjm. 2002;95:3–13. doi: 10.1093/qjmed/95.1.3. [DOI] [PubMed] [Google Scholar]
- 82.Rittling SR, Denhardt DT. Osteopontin function in pathology: lessons from osteopontin-deficient mice. Exp Nephrol. 1999;7:103–113. doi: 10.1159/000020591. [DOI] [PubMed] [Google Scholar]
- 83.Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–622. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 84.Xie Y, Sakatsume M, Nishi S, Narita I, Arakawa M, Gejyo F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int. 2001;60:1645–1657. doi: 10.1046/j.1523-1755.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 85.Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–696. doi: 10.1152/ajpendo.00552.2003. [DOI] [PubMed] [Google Scholar]
- 86.Kleinman JG, Wesson JA, Hughes J. Osteopontin and calcium stone formation. Nephron Physiol. 2004;98:43–47. doi: 10.1159/000080263. [DOI] [PubMed] [Google Scholar]
- 87.De Yoreo JJ, Qiu SR, Hoyer JR. Molecular modulation of calcium oxalate crystallization. Am J Physiol Renal Physiol. 2006;291:F1123–1131. doi: 10.1152/ajprenal.00136.2006. [DOI] [PubMed] [Google Scholar]
- 88.Kohri K, Yasui T, Okada A, Hirose M, Hamamoto S, Fujii Y, Niimi K, Taguchi K. Biomolecular mechanism of urinary stone formation involving osteopontin. Urol Res. 2012;40:623–637. doi: 10.1007/s00240-012-0514-y. [DOI] [PubMed] [Google Scholar]
- 89.Atmani F, Glenton PA, Khan SR. Identification of proteins extracted from calcium oxalate and calcium phosphate crystals induced in the urine of healthy and stone forming subjects. Urol Res. 1998;26:201–207. doi: 10.1007/s002400050047. [DOI] [PubMed] [Google Scholar]
- 90.Atmani F, Khan SR. Quantification of proteins extracted from calcium oxalate and calcium phosphate crystals induced in vitro in the urine of healthy controls and stone-forming patients. Urol Int. 2002;68:54–59. doi: 10.1159/000048418. [DOI] [PubMed] [Google Scholar]
- 91.McKee MD, Nanci A, Khan SR. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J Bone Miner Res. 1995;10:1913–1929. doi: 10.1002/jbmr.5650101211. [DOI] [PubMed] [Google Scholar]
- 92.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wesson JA, Johnson RJ, Mazzali M, Beshensky AM, Stietz S, Giachelli C, Liaw L, Alpers CE, Couser WG, Kleinman JG, Hughes J. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol. 2003;14:139–147. doi: 10.1097/01.asn.0000040593.93815.9d. [DOI] [PubMed] [Google Scholar]
- 94.Chiocchetti A, Orilieri E, Cappellano G, Barizzone N, SDA, GDA, Lorini R, Ravazzolo R, Cadario F, Martinetti M, Calcaterra V, Cerutti F, Bruno G, Larizza D, Dianzani U. The osteopontin gene +1239A/C single nucleotide polymorphism is associated with type 1 diabetes mellitus in the Italian population. Int J Immunopathol Pharmacol. 2010;23:263–269. doi: 10.1177/039463201002300124. [DOI] [PubMed] [Google Scholar]
- 95.Zhao F, Chen X, Meng T, Hao B, Zhang Z, Zhang G. Genetic polymorphisms in the osteopontin promoter increases the risk of distance metastasis and death in Chinese patients with gastric cancer. BMC Cancer. 2012;12:477. doi: 10.1186/1471-2407-12-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marciano R, D’Annunzio G, Minuto N, Pasquali L, Santamaria A, Di Duca M, Ravazzolo R, Lorini R. Association of alleles at polymorphic sites in the Osteopontin encoding gene in young type 1 diabetic patients. Clin Immunol. 2009;131:84–91. doi: 10.1016/j.clim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 97.Naito M, Matsui A, Inao M, Nagoshi S, Nagano M, Ito N, Egashira T, Hashimoto M, Mishiro S, Mochida S, Fujiwara K. SNPs in the promoter region of the osteopontin gene as a marker predicting the efficacy of interferon-based therapies in patients with chronic hepatitis C. J Gastroenterol. 2005;40:381–388. doi: 10.1007/s00535-005-1558-3. [DOI] [PubMed] [Google Scholar]
- 98.Safarinejad MR, Shafiei N, Safarinejad S. Association between polymorphisms in osteopontin gene (SPP1) and first episode calcium oxalate urolithiasis. Urolithiasis. 2013;41:303–313. doi: 10.1007/s00240-013-0582-7. [DOI] [PubMed] [Google Scholar]
- 99.Chutipongtanate S, Nakagawa Y, Sritippayawan S, Pittayamateekul J, Parichatikanond P, Westley BR, May FE, Malasit P, Thongboonkerd V. Identification of human urinary trefoil factor 1 as a novel calcium oxalate crystal growth inhibitor. J Clin Invest. 2005;115:3613–3622. doi: 10.1172/JCI25342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tenenhouse HS. Regulation of phosphorus homeostasis by the type iia na/phosphate cotransporter. Annu Rev Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- 101.Iwaki T, Sandoval-Cooper MJ, Tenenhouse HS, Castellino FJ. A missense mutation in the sodium phosphate co-transporter Slc34a1 impairs phosphate homeostasis. J Am Soc Nephrol. 2008;19:1753–1762. doi: 10.1681/ASN.2007121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prie D, Huart V, Bakouh N, Planelles G, Dellis O, Gerard B, Hulin P, Benque-Blanchet F, Silve C, Grandchamp B, Friedlander G. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002;347:983–991. doi: 10.1056/NEJMoa020028. [DOI] [PubMed] [Google Scholar]
- 103.Courbebaisse M, Leroy C, Bakouh N, Salaun C, Beck L, Grandchamp B, Planelles G, Hall RA, Friedlander G, Prie D. A new human NHERF1 mutation decreases renal phosphate transporter NPT2a expression by a PTH-independent mechanism. PLoS One. 2012;7:e34764. doi: 10.1371/journal.pone.0034764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci U S A. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Khan SR, Canales BK. Ultrastructural investigation of crystal deposits in Npt2a knockout mice: are they similar to human Randall’s plaques? J Urol. 2011;186:1107–1113. doi: 10.1016/j.juro.2011.04.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Khan SR, Glenton PA. Calcium oxalate crystal deposition in kidneys of hypercalciuric mice with disrupted type IIa sodium-phosphate cotransporter. Am J Physiol Renal Physiol. 2008;294:F1109–1115. doi: 10.1152/ajprenal.00620.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khundmiri SJ, Ahmad A, Bennett RE, Weinman EJ, Steplock D, Cole J, Baumann PD, Lewis J, Singh S, Clark BJ, Lederer ED. Novel regulatory function for NHERF-1 in Npt2a transcription. Am J Physiol Renal Physiol. 2008;294:F840–849. doi: 10.1152/ajprenal.00180.2007. [DOI] [PubMed] [Google Scholar]
- 108.Lederer ED, Khundmiri SJ, Weinman EJ. Role of NHERF-1 in regulation of the activity of Na-K ATPase and sodium-phosphate co-transport in epithelial cells. J Am Soc Nephrol. 2003;14:1711–1719. doi: 10.1097/01.asn.0000072744.67971.21. [DOI] [PubMed] [Google Scholar]
- 109.Gisler SM, Pribanic S, Bacic D, Forrer P, Gantenbein A, Sabourin LA, Tsuji A, Zhao ZS, Manser E, Biber J, Murer H. PDZK1: I. a major scaffolder in brush borders of proximal tubular cells. Kidney Int. 2003;64:1733–1745. doi: 10.1046/j.1523-1755.2003.00266.x. [DOI] [PubMed] [Google Scholar]
- 110.Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci U S A. 2002;99:11470–11475. doi: 10.1073/pnas.162232699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weinman EJ, Mohanlal V, Stoycheff N, Wang F, Steplock D, Shenolikar S, Cunningham R. Longitudinal study of urinary excretion of phosphate, calcium, and uric acid in mutant NHERF-1 null mice. Am J Physiol Renal Physiol. 2006;290:F838–843. doi: 10.1152/ajprenal.00374.2005. [DOI] [PubMed] [Google Scholar]
- 112.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nat Rev Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 113.Wu XR. Biology of urothelial tumorigenesis: insights from genetically engineered mice. Cancer Metastasis Rev. 2009;28:281–290. doi: 10.1007/s10555-009-9189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.He F, Mo L, Zheng XY, Hu C, Lepor H, Lee EY, Sun TT, Wu XR. Deficiency of pRb family proteins and p53 in invasive urothelial tumorigenesis. Cancer Res. 2009;69:9413–9421. doi: 10.1158/0008-5472.CAN-09-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watts RW. Idiopathic urinary stone disease: possible polygenic aetiological factors. Qjm. 2005;98:241–246. doi: 10.1093/qjmed/hci041. [DOI] [PubMed] [Google Scholar]
- 116.Zerwekh JE, Reed-Gitomer BY, Pak CY. Pathogenesis of hypercalciuric nephrolithiasis. Endocrinol Metab Clin North Am. 2002;31:869–884. doi: 10.1016/s0889-8529(02)00028-2. [DOI] [PubMed] [Google Scholar]
- 117.Goodman HO, Brommage R, Assimos DG, Holmes RP. Genes in idiopathic calcium oxalate stone disease. World J Urol. 1997;15:186–194. doi: 10.1007/BF02201856. [DOI] [PubMed] [Google Scholar]