Abstract
Caged compounds have widely used by neurophysiologists to study many aspects of cellular signaling in glia and neurons. Biologically inert before irradiation, they can be loaded into cells via patch pipette or topically applied in situ to a defined concentration, photolysis releases the caged compound in a very rapid and spatially defined way. Since caged compounds are exogenous optical probes, they include not only natural products such neurotransmitters, calcium and IP3, but non-natural products such as fluorophores, drugs and antibodies. In this Technical Spotlight we provide a short introduction to the uncaging technique by discussing the nitroaromatic caging chromophores most widely used in such experiments (e.g. CNB1, DMNB, MNI and CDNI). We show that recently developed caging chromophores (RuBi and DEAC450) that are photolyzed with blue light (ca. 430–480 nm range) can be combined with traditional nitroaromatic caged compounds to enable two-color optical probing of neuronal function. For example, one-photon uncaging of either RuBi-GABA or DEAC450-GABA with a 473-nm laser is facile, and can block non-linear currents (dendritic spikes or action potentials) evoked by two-photon uncaging of CDNI-Glu at 720 nm. We also show that two-photon uncaging of DEAC450-Glu and CDNI-GABA at 900 and 720 nm, respectively, can be used to fire and block action potentials. Our experiments illustrate that recently developed chromophores have taken uncaging out of the “monochrome era”, in which it has existed since 1978, so as to enable multichromic interrogation of neuronal function with single synapse precision.
Keywords: uncaging, two-photon, multichromic, dendritic spikes, spines
Caged compounds are organic molecules that are biologically or functionally inert until they are activated by light(Ellis-Davies, 2007). These optical probes are similar to pro-peptides found in cells or pro-drugs made by medicinal chemists in that their activity is latent because of covalent modification of some functionality that is essential for activity. Thermal chemical reactions are used to liberate peptides and drugs from their precursors; the distinctive feature of caged compounds is that light compatible with normal microscopes is used the break the covalent bond (Fig. 1A). The fact that light easily penetrates cell membranes and brain tissue is advantageous as uncaging can evoke rapid responses from single cells in a complex biological environment (Fig. 1B). Since almost all of the biochemistry of cells is mediated by organic molecules, caged compounds have been used to manipulate the concentration of a wide variety of such signaling molecules. Importantly, uncaging can be used for molecules that are not natural products, thus drugs and fluorophores have been caged.
Fig. 1.
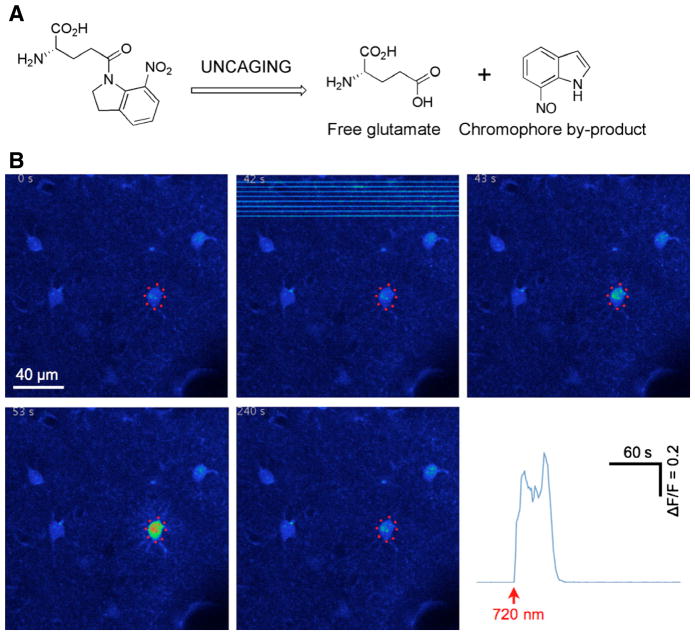
Example of uncaging on living cells. (A) Photorelease of glutamate from MNI-Glu. (B) Example of the rapid biological effects of uncaging. In this experiment MNI-Glu was topically applied to the brain of an anesthetized mouse and uncaging was directed to eight points near an astrocyte. Before applying the caged compound, the dura was removed and a Ca2+-sensitive dye was loaded into the astrocytes (Crowe et al., 2010). Two-photon imaging at 0.8 Hz was at 950 nm and uncaging was at 720 nm (15 mW for 10 ms per point). Warmer colors in the individual image frames represent higher Ca2+ concentration in the targeted cell. The graph shows a plot of the time course of the change in fluorescence in proportion to resting fluorescence.
Most published studies use caged compounds with nitroaromatic chromophores that are activated by light in the 340–410 nm range (Adams & Tsien, 1993; Ellis-Davies, 2008; Klan et al., 2013). Despite the huge success of these probes many other chromophores have been used to cage biological signaling molecules(Klan et al., 2013). An interesting feature of some of these molecules is the extension of the absorption spectrum of uncaging into the blue range (440–500 nm), one that is chromatically complementary to traditional nitroaromatic chromophores. This is proving to be an important addition to the optical arsenal available to neurophysiology, as some recent studies have featured wavelength-selective uncaging of two biological signaling molecules(Lovett-Barron et al., 2012; Chiu et al., 2013; Hayama et al., 2013). In this technical spotlight we discuss the chemical probes that enable such studies. We provide an introduction to uncaging chromophore design and the basics of uncaging for neurophysiological studies. We illustrate these with examples of experiments using whole-cell, single-spine and multi-spine uncaging of neurotransmitters such as glutamate and GABA. Using multi-spine uncaging of glutamate in one optical channel we then show some of the capabilities of two-color uncaging combining either two-photon and one-photon excitation or dual two-photon excitation. Our goals are to provide a clear picture of the advantages and disadvantages of caged compounds for activating neurons and glia, and introduce readers to the new method of two-color uncaging for neuroscience.
Introduction to caged compounds and chromophore design
Chromophores for caged compounds were initially developed by organic chemists for use as photochemical protecting groups in the synthesis of small organic molecules. Thus, the ortho-nitrobenzyl(Barltrop et al., 1966) and the dimethoxy- ortho-nitrobenzyl(Patchornik et al., 1970) photochemical protecting groups were widely used(Binkley & Flechtner; Amit et al., 1974). With the invention of caged compounds (Kaplan et al., 1978) these chromphores became widely used by physiologists for most important signaling molecules such as IP3(Walker et al., 1987), cGMP(Nargeot et al., 1983), calcium(Tsien & Zucker, 1986), neurotransmitters(Milburn et al., 1989), peptides(Walker et al., 1998), lipids(Xia et al., 1997), proteins(Marriott, 1994), RNA(Chaulk & MacMillan, 1998), and DNA(Monroe et al., 1999). Subsequently, novel photochemical protecting groups (or caging chromophores) tended to be developed by biologists(Furuta et al., 1995; Hagen et al., 2001) seeking alternatives to the original nitrobenzyl caging chromophores. The increasing usefulness of caged probes for biology then stimulated chemists to re-enter the area(Zayat et al., 2003; Smirnova et al., 2005; Momotake et al., 2006; Specht et al., 2006; Gug et al., 2008). However, the nitrobenzyl protecting groups are still the most important for organic synthesis(McGall & Christians, 2002) and remain widely used in physiology(Ellis-Davies, 2007; 2008).
The ortho-nitrobenzyl cages(Barltrop et al., 1966; Patchornik et al., 1970) and related nitroaromatic chromophores, MNI (Papageorgiou & Corrie, 2000) and CDNI(Ellis-Davies et al., 2007), absorb light quite well in the 300–380 nm range. The absorption tail of the nitroindolinyl chromophore extends beyond 400 nm, so violet lasers, that are a common part of confocal microscopes, can be used to uncage these probes(Trigo et al., 2009a). Until recently, it has proved difficult to extend the absorption spectrum of such nitroaromatic cages beyond this range(Aujard et al., 2006). Importantly, Specht and co-workers have found that conjugation of an aminophenyl group to the 4-ortho-nitrobenyl chromophore (ANBP) shifts the absorption maximum to ca. 400 nm with the tail extending up to 500 nm(Donato et al., 2012). A similar absorption maximum (385 nm) has been reported for the coumarin-based caging chromophore, DEAC(Hagen et al., 2001). An even larger red shift in the absorption maximum has been reported by Etchenique for the “RuBi” chromophore(Zayat et al., 2003; Zayat et al., 2006). Recently, we have developed a new red-shifted coumarin cage, DEAC450, which also absorbs in a similar region(Olson et al., 2013a). Thus, there is now a variety of caging chromophores and caged compounds with different photochemical properties available for neurophysiological studies. However, only some of these chromophores allow two-color uncaging and we discuss the design considerations for these in the next section.
Chromophore requirements for two-color uncaging
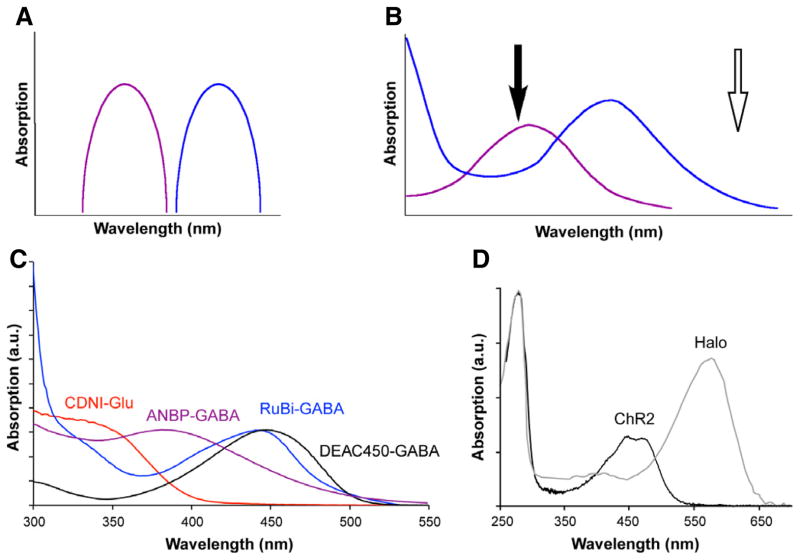
The requirements for wavelength-independent uncaging of two biological signaling molecules are probably obvious to anyone who has used multi-color fluorescence imaging. Not only must each chromophore have a distinct absorption maximum, but there must also be distinct absorption minima for each chromophore so as to prevent optical cross talk when imaging at different wavelengths of light. The same basic constraint applies to caging chromophores. The ideal situation is that each chromophore absorbs light entirely independently of the other (Fig. 2A). It is almost always possible to have a region of unique absorption in the redder part of the absorption spectrum for the longer wavelength caging chromophore (open arrow, Fig. 2B). However in the shorter wavelength range, the ideal is that the longer wavelength chromophore absorption approaches zero as in Fig. 2A. This stipulation is much harder to satisfy completely (we are aware of only one published example (Scott et al., 2009)), as there is normally significant absorption of both chromophores in this region (filled arrow, Fig. 2B). Many recent studies by chemists and biologists have illustrated this overlap problem(Kotzur et al., 2009; Goguen et al., 2011; Menge & Heckel, 2011; Priestman et al., 2011; Schaäfer et al., 2011; Priestman et al., 2012; Rodrigues-Correia et al., 2013). All these reports show that to use two caged molecules in approximately equal concentrations, one must first photolyze the longer wavelength cage completely before photolyzing the short wavelength cage (Box 1). The reason for this is that because there is significant optical cross talk between the two channels, attempts to photolyze the shorter wavelength cage would give co-liberation of both compounds, leading to confounding co-stimulation of two processes. This is analogous to what can be observed in fluorescence imaging with yellow channel emission from blue channel excitation. Interestingly, the absorption spectra of ChR2(Nagel et al., 2003) and Halo(Duschl et al., 1990) have considerable overlap (Fig. 2D), and initial report of bidirectional control of membrane potentials(Han & Boyden, 2007; Zhang et al., 2007) have not been followed by more studies using the probes as partners probably because of the overlap.
Fig. 2.
Design of chromophore absorption for two-color photochemical actuation. (A) Cartoon of ideal chromatically independent absorption spectra for wavelength-selective two-color actuation. (B) Normally two chromophores with distinct absorption maxima have a non-overlapping region of the electromagnetic spectrum where only the longer wavelength caging chromophore (blue trace) absorbs (open arrow). However, the absorption maximum of the short-wavelength chromophore (purple) typically overlaps significantly with the longer wavelength chromophore (black arrow). (C) Absorption spectra of CDNI-Glu (red), ANBP-GABA (purple), RuBi-GABA (blue) and DEAC450-GABA (black). Note the minimum of DEAC450 is close to the maximum of CDNI. In the remaining figures we ‘color-code’ 1P uncaging with blue, 2P uncaging at 720 nm with red and 2P uncaging at 900 nm with black or white. (D) Absorption spectra of ChR2 and Halo when normalized to their absorption maximum have sizable overlap at 450 nm.
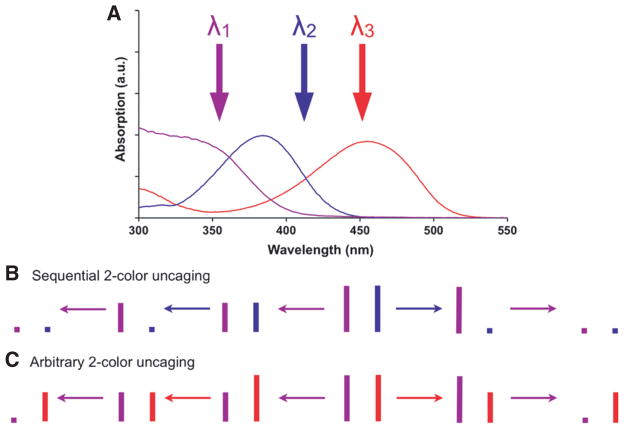
Box 1. Two versions of wavelength-selective two-color uncaging.
(A) Absorption spectra of nitroaromatic (purple), DEAC (blue) and DEAC450 (red) caging chromophores. Note that microscope glass is normally opaque below 330 nm. (B) Irradiation of a mixture of nitroaromatic- and DEAC-caged compounds with λ1 causes uncaging of both cages, whereas irradiation with λ2 enables wavelength-selective photolysis of the DEAC caged compound. Typically, experiments with such chromophore mixtures feature essentially complete photolysis of the DEAC-cage (i.e. the “long wavelength”) with λ2 before applying λ1 to the cells. (C) Irradiation of a mixture of nitroaromatic- and DEAC450-caged compounds with λ1 or λ3 produces wavelength-selective uncaging of either caged compound, no matter which wavelength of light is used first. Thus λ1 and λ3 can be applied in an arbitrary order such that photolysis with λ1 can bracket photolysis with λ3, see (Olson et al., 2013a) and experiments herein.
Recently we have developed a new caging chromophore, called DEAC450, that is the first caging chromophore made to address this problem(Olson et al., 2013a; Olson et al., 2013b; Amatrudo et al., 2014). DEAC450, as the name implies, has a large absorption maximum in the 450–460 nm range, importantly it also has a distinct absorption minimum in the 340–360 nm range (black trace, Fig 2C). Independently of our own work, Jullien’s group recently reported similar findings with another DEAC-based chromophore, 4-thio-DEAC(Fournier et al., 2013). Using DEAC450-caged Glu(Olson et al., 2013b), cAMP(Olson et al., 2013a) and GABA(Amatrudo et al., 2014)we have established that the DEAC450 chromophore may be used for optically independent uncaging at longer wavelengths of light when partnered with the appropriate nitroaromatic caged compound, using one- and two-photon excitation, so as to modulate membrane potential in a bi-directional manner (see below).
One and two-photon uncaging of neurotransmitters on cells and single spines
Most studies of LTP use electrical stimulation of afferent axons onto target neurons to induce the measured change in synaptic efficacy. Uncaging bypasses the presynaptic release machinery used by such methods, providing a well defined stimulation at a known location and as such provides some useful features when compared to traditional stimulation methods(Ellis-Davies, 2007).
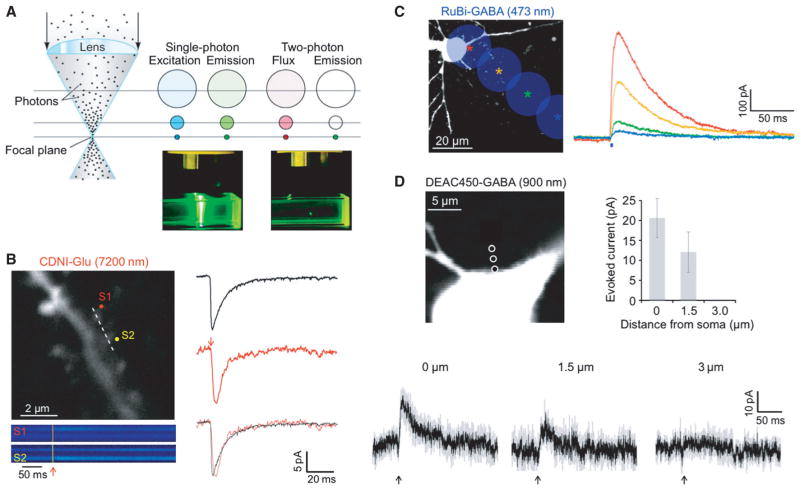
Uncaging of neurotransmitters using one or two-photon excitation (Fig. 3A) has been a core method in many neurophysiological studies (reviews: (Eder et al., 2004; Alvarez & Sabatini, 2007; Ellis-Davies, 2013)). An important feature of both means of photolysis is that they can produce stereotypical responses. Thus, broad scaled functional mapping using one-photon uncaging of neurotransmitter has been used by several groups(Eder et al., 2004). Two-photon uncaging is more useful for high-resolution functional mapping of receptor densities across the surface of individual dendritic segments(Matsuzaki et al., 2001; Asrican et al., 2007; Katona et al., 2011). Within both types of experiments a stereotypical amount of neurotransmitter is uncaged, albeit on different scales, so that the measured responses can be correlated with strength of synaptic connectivity or receptor density. The basic difference between the effects of one-photon and two-photon excitation is the of spatial resolution of the evoked signals, as large quantities of glutamate from one-photon uncaging fire action potentials whereas small quantities from two-photon uncaging mimic quanta (Fig. 3B). For example, when a continuous-wave 473nm-laser was focused onto a microscope objective so as to overfill the back aperture we found that the spatial resolution of GABA uncaging was modest (Fig. 3C) in comparison to the signal evoked by two-photon uncaging (Fig. 3D). Other studies have reported similar resolution for one-photon uncaging of GABA(Chalifoux & Carter, 2011) and two-photon uncaging of GABA(Gross et al., 2013). While other reports feature much more refined resolution for one-photon uncaging of GABA (2 and 7 microns (Trigo et al., 2009b), or 3 and 25 microns(Rial Verde et al., 2008b) in x and z, respectively). Such differences in resolution for these one-photon studies may arise from different laser powers, shutter times and uncaging depths. Careful control of uncaging conditions enable diffraction-limited one-photon uncaging of glutamate in brain slices(DiGregorio et al., 2007). Crucial factors in this were uncaging with low power and that only cells near the surface of the slice (20 microns) were targeted so as to reduce light scatter. However with deeper penetration into tissue such scatter becomes important. Thus, when confocal imaging was compared quantitatively with 2P microscopy, it was found that the former gave comparable signals to latter only close to the tissue surface; at 50–70 microns into tissue only the latter gave bright, clear images(Centonze & White, 1998). Similarly uncaging with near-IR (e.g. 720 nm) or IR light (e.g. 900 nm) enables much more effective depth penetration of the uncaging beam into brain tissue than the much shorter wavelengths of light (e.g. 400–470 nm) used for linear uncaging. For example, Higley and co-workers have reported 2P uncaging at single spines at depths of 200–300 microns(Chiu et al., 2013).
Fig. 3.
Two versions of wavelength-selective two-color uncaging. (A) Absorption spectra of nitroaromatic (purple), DEAC (blue) and DEAC450 (red) caging chromophores. Note that microscope glass is normally opaque below 330 nm. (B) Irradiation of a mixture of nitroaromatic- and DEAC-caged compounds with λ1 causes uncaging of both cages, whereas irradiation with λ2 enables wavelength-selective photolysis of the DEAC caged compound. Typically, experiments with such chromophore mixtures feature essentially complete photolysis of the DEAC cage (i.e. the ‘long wavelength’) with λ2 before applying λ1 to the cells. (C) Irradiation of a mixture of nitroaromatic- and DEAC450-caged compounds with λ1 or λ3 produces wavelength-selective uncaging of either caged compound, no matter which wavelength of light is used first. Thus λ1 and λ3 can be applied in an arbitrary order such that photolysis with λ1 can bracket photolysis with λ3; see Olson et al. (2013a) and experiments herein.
The first example of diffraction-limited two-photon uncaging was published by Kasai and co-workers(Matsuzaki et al., 2001). Using brief pulses (50 microseconds) of 720nm-light, MNI-Glu was photolyzed on cultured hippocampal neurons using low average power (7 mW) so as to mimic quantal release at single synapses for the first time(Matsuzaki et al., 2001). Interestingly, even though some studies have suggested that uncaging flashes longer than 0.3 ms would reduce the spatial resolution of 2P uncaging due to excited molecule leaving the focal volume before release, or simply by uncaged glutamate diffusing beyond the excited volume(Brown et al., 1999a; Brown et al., 1999b; Kiskin & Ogden, 2002), many studies have reported that diffraction-limited uncaging of glutamate at single spines is possible with longer pulse durations (e.g. 1 ms)(Matsuzaki et al., 2001; Smith et al., 2003; Carter & Sabatini, 2004; Sobczyk et al., 2005; Araya et al., 2006; Beique et al., 2006; Asrican et al., 2007; Katona et al., 2011).
In 2004 Carter and Sabatini used two-photon uncaging of MNI-Glu and two-photon imaging of calcium in medium spiny neurons (MSN) to establish that that Ca2+-permeable AMPA receptors are a major source of spine Ca2+, and that this Ca2+ signal was independent of spine NMDA receptors and voltage-gated Ca2+ channels(Carter & Sabatini, 2004). The amount of Ca2+ conducted through AMPA receptors was found to be “state dependent”. In other words, it was controlled by subthreshold depolarizations of MSN (the up and down states). From a technical point of view, it should be noted this was the first study combining two-photon uncaging and Ca2+ imaging at single spines. Two-photon uncaging of glutamate has been used in conjunction with 2pFRET-FLIM to understand more fully signal cascades involved in LTP and LTD (Harvey & Svoboda, 2007; Harvey et al., 2008; Patterson et al., 2010). For a review of this field see(Patterson & Yasuda, 2011).
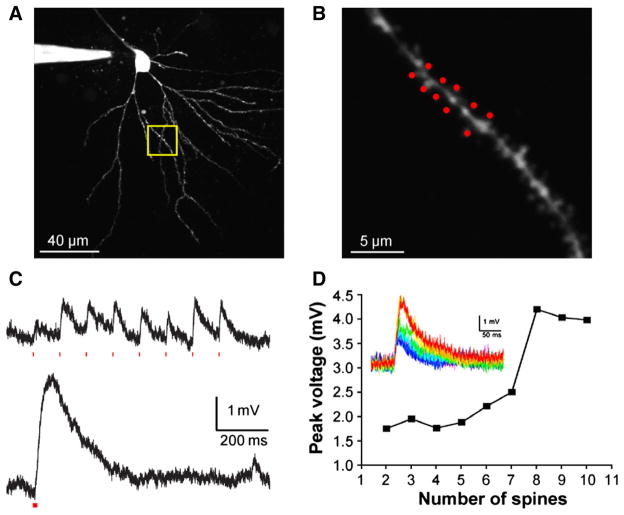
After two-photon uncaging at single spines became an established technique, several studies took advantage of the ability of galvanometer-based beam steering to direct uncaging to several optically designated spine heads in rapid succession (Figs. 4A,B). When the time between uncaging pulses at a clustered group of individual spines is large (e.g. 100 ms) the effect of each pulse is isolated temporally (Fig. 4C, upper panel). However when the inter-pulse interval is reduced to 1 ms, the evoked signal becomes supralinear (Fig. 4C, the integrated signal in the lower panel is 2-fold larger than the upper panel). An alternative means of producing such dendritic non-linearities is gradually increasing the total uncaging energy at a group of clustered spines, an approach that was first reported using 1P uncaging in 2000 by Schiller and co-workers(Schiller et al., 2000), an approach that can be effected using 2P uncaging (see below). However the contribution of single spines, and thus the importance of small voltage changes for dendritic spikes, is seen even more clearly with the experiment shown in (Fig. 4D). Here, the gradually increasing somatic response is plotted when 2–10 spines (Fig. 4B) were irradiated (50 mW), and it showed a marked non-linearity when more than 7 spines were stimulated. This experiment is facile because of the fine three-dimensional resolution that is inherent to two-photon uncaging. As well as one-photon uncaging, local glutamate perfusion or local synaptic stimulation have all been used to produce dendritic spikes. However, such methods have not been used to mimic the temporal summation shown in Figs. 4C or D. It is this feature that several groups have used to study many of the fundamental properties of dendritic integration that had remained closed to synaptic physiology due to the technical inability to stimulate visually defined single spines in an arbitrary manner (Gasparini & Magee, 2006; Losonczy & Magee, 2006; Branco et al., 2010) (Losonczy et al., 2008; Govindarajan et al., 2011; Plotkin et al., 2011; Zhai et al., 2013). For a detailed review see(Major et al., 2013).
Fig. 4.
Introduction to 1P and 2P uncaging. (A) Left, cartoon of a lens focusing light. Middle, 1P excitation occurs throughout the light absorption path and total excitation is equal in each z-section. Right, 2P emission is confined to the focal plane due to the nonlinear nature of the creation of the excited singlet state. (B) Left, image of a dendritic segment of a CA1 pyramidal cell filled with Oregon Green BAPTA-1 (0.2 mM) using 2PE at 820 nm showing spines that were targeted for 2P uncaging of CDNI-Glu (1 mM) with 720 nm light (50 mW). Spine-selective 2P uncaging of CDNI-Glu was revealed by line-scan imaging (dotted line) with a period of 0.7 ms (146 pixels, 4 μs/pixel). Increases in [Ca2+] are shown in pseudocolor traces and reveal that S1 and S2 were stimulated separately. Right, currents (red traces, n = 24 from five cells) from uncaging of CDNI-Glu (1 mM, 720 nm, 1 ms) at single spines closely mimicked miniature EPSCs (black traces, n = 108). (C) A 473-nm laser was targeted on a cell soma and, at 20-μm increments (colored stars), the corresponding current traces from photolysis of RuBi-GABA (10 μM, 2 ms, 20 mW) are shown. (D) Two-photon photolysis of DEAC450-GABA (0.2 mM, 900 nm, 5 ms, 100 mW) evoked outward currents that decreased as the laser was moved away from the cell body. Each trace is an average of three events recorded from three cells, with grey indicating the SEM. For C and D, CA1 pyramidal cells were held at −40 mV. Compared to 1P uncaging, 2P uncaging generates a much smaller spatially restricted input. Brian slices were isolated as described in the Appendix.
Comparison with other methods of actuation
Solution exchange, rapid mixing or “puffing” all offer alternative means to apply agonists to biological tissue so as to evoke a temporally or spatially defined response. It must be noted that these methods do not allow rapid access to the intracellular compartment, so uncaging is almost always the preferred means to evoke responses with intracellular second messengers such as cAMP or Ca2+(Ellis-Davies, 2007), however for application of extracellular messengers “non-photochemical” means of agonist application are extremely useful. Importantly any molecule can be easily applied topically. However, solution exchange and rapid mixing are relatively slow and offer poor spatial control when compared to uncaging. In contrast, puffing can be very fast and highly localized. For example, iontophoresis allows glutamate to be applied to cultured neurons with single synapse spatial resolution so as to evoke quantal-sized EPSCs(Murnick et al., 2002). Importantly it delivers only the natural biomolecule at the correct time and place, without prior bath application of a compound. Using this method one can circumvent the well-documented problem that most caged neurotransmitters have some antagonism towards GABA-A receptors(Rial Verde et al., 2008b; Matsuzaki et al., 2010). (Note, however we have not detected any epileptiform activity in our experiments, even though the caged compounds are bath-applied at concentrations above the IC50.) Another important advantage of puffing in brain slices is that agonists may (easily) be applied to cell processes at different depths or in very different parts of cells(Muller et al., 2012; Oikonomou et al., 2012).
One-photon uncaging of glutamate has also been used to generate dendritic spikes in several studies(Schiller et al., 2000; Jadi et al., 2012) that are very similar to those shown in Fig. 4C,D. Given the very large cost of two-photon lasers, what advantages does the technique have compared to these other methods of local stimulation? First, two-photon uncaging has a slightly superior spatial resolution, signals drop to zero when photostimulation is moved 2–3 microns from the target (Fig. 3D), whereas with other methods this distance is larger, typically 7–10 microns (see (Muller et al., 2012) supplemental figure 2). For studies involving co-activation of small clusters of spines this latter value is quite useful, however the ability to activate a group of single spines in a temporally dispersed or clustered way (Fig. 4C) is inherent to two-photon uncaging such that the study of spatial integration of individual spines in short or long dendritic segments is facile(Gasparini & Magee, 2006; Losonczy & Magee, 2006).
Two-color uncaging
From the very first glutamate uncaging experiment in brain slices(Callaway & Katz, 1993), the potential of two-color uncaging of two signaling molecules was obvious2. From the point of view of neurophysiological studies, wavelength-selective, two-color uncaging of glutamate and GABA would allow sophisticated analysis of the synaptic integration of inputs from these neurotransmitters, in a way that local perfusion does not(Liu, 2004; Hao et al., 2009). Recently a number of studies have shown this is now possible, by combining two-photon uncaging of glutamate with one-photon uncaging of GABA(Lovett-Barron et al., 2012; Chiu et al., 2013; Hayama et al., 2013).
Combing two-photon uncaging of glutamate with one-photon uncaging of GABA
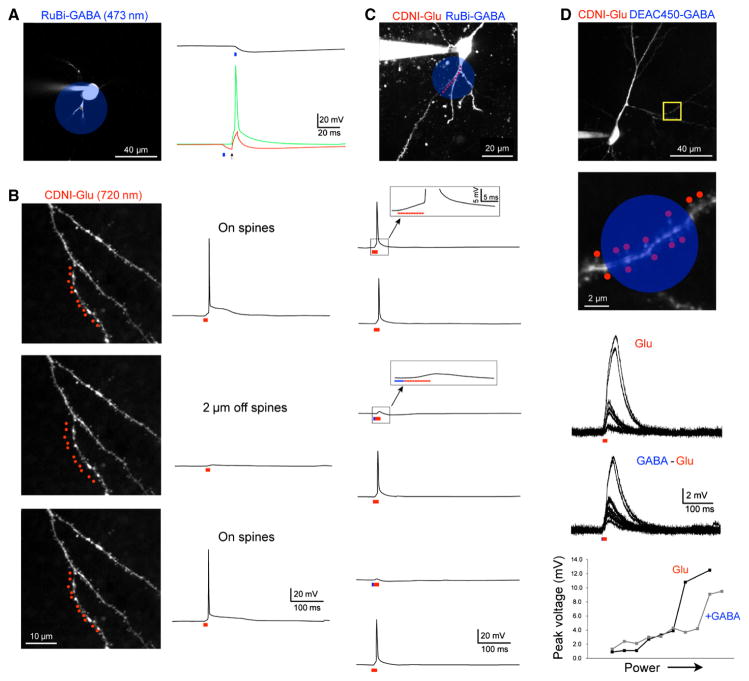
RuBi-GABA is efficiently uncaged with blue light(Rial Verde et al., 2008a). Application of this probe in the micromolar concentration range to brain slices allows one-photon uncaging to produce large currents from activation of GABA-A (Fig. 3C) or GABA-B receptors(Chalifoux & Carter, 2011). These outward currents can be used to block action potentials evoked by current injection from a patch pipette(Rial Verde et al., 2008b) (Fig. 5A). Two-photon irradiation of RuBi-GABA at such low concentrations does not evoke any currents (RuBi compounds have a two-photon absorption maximum at 800 nm, this falls by only 65% at 720 nm, see (Fino et al., 2009a) supplemental information). Since nitroaromatic-caged probes do not absorb blue light (Fig. 2C), two-photon uncaging of MNI- or CDNI-Glu can be easily combined with RuBi-GABA. Thus two-photon uncaging of glutamate at 720 nm at multiple spines on a dendrite can evoke action potentials with excellent spatial resolution (Fig. 5B). In separate experiments we found that the presence of RuBi-GABA has no effect on these spikes unless a brief pulse of blue light preceded the two-photon uncaging (Fig. 5C). Note in the latter experiment the two laser beams were directed at the same dendritic segment, so does not rely on differential distribution of excitatory and inhibitory receptors for the success of such two-color uncaging. In 2012 Losonczy and co-workers combined two-photon uncaging of glutamate with one-photon uncaging of GABA for the first time(Lovett-Barron et al., 2012). This study found that colocalized two-color uncaging on a CA1 radial oblique dendrite (cf. Fig. 5D) was more effective in reducing dendritic non-linearities compared to the effects of GABA uncaging on the cell body. Interestingly, in a study also published in 2012, Schiller and coworkers have arrived at different conclusions by combining one-photon uncaging of glutamate on layer 5 neuron basal dendrites with iontophoresis of GABA on the same two compartments(Jadi et al., 2012). They found that dendritic colocalization reduced the spike threshold, but not height, whereas cell-body inhibition reduced spike height but not threshold. The subtly different conclusions reached in these two reports probably did not arise from the means of glutamate delivery, but may reflect that different neurons were examined.
Fig. 5.
Temporal integration of excitation at multiple spine heads. (A) Two-photon fluorescence image of a CA1 pyramidal cell. (B) Detail of dendrite shown in the yellow box in (A), with a cluster of 10 spines targeted for the experiment in (D). (C) CDNI-Glu (1 mM) was uncaged at 720 nm at eight spines along a dendrite, compressing the inter-pulse interval between uncaging events from 100 ms (upper) to 1 ms (lower), producing a nonlinear response. Individual responses from each spine varied between 0.25 and 1.0 mV, and temporal clustering doubled the integrated voltage response. A similar nonlinear dendritic response (dendritic spike) can be clearly seen in (D), where the inter-pulse interval was 0.12 ms. (D) Plot of the somatic voltage responses from uncaging at two to ten spines, with the associated voltage traces inset. The evoked response was approximately linear for two to seven spines, but showed a marked nonlinearity when uncaging was directed to eight or more spines.
The localization of GABA-A receptors to dendritic spine heads has been known for sometime(Kubota et al., 2007), but the function of such receptors is poorly understood. Higley and co-workers showed recently that selective stimulation of such synapses using two-photon photolysis of CDNI-GABA or ChR2 infected interneurons reduced Ca2+ influx induced by backpropagating action potentials (bAPs) at about 50% of spines on layer 2/3 pyramidal neurons in the PFC. Two-color uncaging of CDNI-Glu and RuBi-GABA in a manner similar to Figs. 5C,D revealed that inhibition was confined to individual spines within a small cluster(Chiu et al., 2013). Kasai and coworkers used two-color uncaging of CDNI-Glu and RuBi-GABA to show that inhibition could help induce LTD within a small cluster of spine heads, after the stimulation of a single spine(Hayama et al., 2013). STDP can be induced at single spines photochemically when two-photon uncaging of glutamate precedes a bAP at 1 Hz for about 1 min ((Harvey & Svoboda, 2007) and follows the “pre before post” timing rule(Feldman, 2012)). However Kasai’s laboratory found that a “post before pre” protocol did not induce LTD unless it was preceded by local uncaging of GABA within 50 ms of the AP. LTD appeared as spine shrinkage, with shrunken spines showing reduced currents. Such effects had single spines as their initiation point, but the LTD effect was seen in spines covering an adjacent 15-micron segment. Phosphorylated cofilin was found to be the molecule that spread from the activated spine to its near neighbors. Interestingly, prior sLTP at single spines within a cluster protected this spine from a subsequent LTD protocol, suggesting that such spines were “write protected” from GABA-mediated spine erasure.
The blue lasers used to uncage RuBi-GABA can also be used to activate ChR2(Chiu et al., 2013). Since ChR2 does not generate experimentally useful currents by non-linear excitation at 720 nm, it has been combined with two-photon uncaging of glutamate at this wavelength in single neurons so as to induce sLTP(Zhang et al., 2008). This is an “all photochemical” version of the pre before post STDP used by the Kasai and Svoboda laboratories. Oertner’s laboratory are the only group to publish this type of two-color experiment, but it is a strong proof of principle that the “old” and “new” methods of neuronal actuation can be combined for neurophysiological studies (e.g.(Yagishita et al., 2014)).
Other compounds for 2P/1P, two-color uncaging
Etchenique and co-workers have synthesized several other RuBi caged compounds, these include dopamine(Araya et al., 2013), tryptamine, serotonin(Zayat et al., 2006), nicotine(Filevich et al., 2010), and glutamate(Fino et al., 2009b). In principle, any amino containing molecule could be caged with this technology, e.g. important amino acids such as glycine and D-aspartate. All RuBi-caged compounds are highly active towards one-photon uncaging with blue lasers, so these caged compounds could all be partnered with two-photon uncaging of MNI- or CDNI-caged neurotransmitters at 720 nm, to enable the optical interrogation of the bimodal interaction of such bio-molecules. Recently we have developed DEAC450-cAMP(Olson et al., 2013a) and used blue laser light to uncage this inside neurons (discussed next section). Since DEAC450 is not effectively photolyzed at 720 nm(Olson et al., 2013b; Amatrudo et al., 2014), this caged second messenger could also be used with two-photon photolysis of caged compounds at 720 nm. DEAC450 could be used to cage other molecules such cGMP, ATP, and IP3. Thus, there is quite an array of compounds (potentially) available for “2P/1P” two-color uncaging experiments.
Two-color, one-photon uncaging
The wide availability of continuous-wave lasers on confocal microscopes makes the development of compounds that can be photolyzed in a wavelength selective manner using only linear excitation a very attractive proposition. As noted above, many such experiments have been attempted, but the “normal” spectral overlap of most caged compounds (filled arrow, Fig. 2B) has prevented such selectivity. Recently we have shown that DEAC450-cAMP can be photolyzed with excellent wavelength selectivity in the presence of CDNI-GABA using two wavelengths of visible light(Olson et al., 2013a). Importantly, irradiation of neurons with both caged compounds present with 355-nm light only manifested effects from CDNI-GABA. Thus violet and blue light could be applied to neurons in an arbitrary order (Box 1) so as to modulate the firing rate of striatal cholinergic interneurons in a bidirectional manner. The two-color, one-photon uncaging method is analogous to partnering one-photon uncaging with iontophoresis(Jadi et al., 2012) and dual iontophoresis(Muller et al., 2012; Oikonomou et al., 2012) in terms of resolution and rate of neuronal response.
Two-color, two-photon uncaging
The development of DEAC450-Glu, which allowed optically selective two-photon uncaging at long wavelengths of light for the first time(Olson et al., 2013b), suggested that this probe could be partnered with nitroaromatic caged compounds like CDNI-GABA(Matsuzaki et al., 2010) so as to enable a clean two-color uncaging experiment using non-linear excitation with two wavelengths of light. In Fig 6 we now show that this is feasible.
Fig. 6.
Two-color uncaging combining 2P and 1P excitation. (A) A 473-nm laser was targeted on a layer 2/3 pyramidal cell soma (blue circle); the hyperpolarization from photolysis of RuBi-GABA (10 μM, 2 ms, 20 mW) is shown (right). This stimulus generated an inhibitory input (top black trace) of sufficient amplitude to block an electrically evoked (1 nA, 1 ms) action potential (bottom green and red traces). Uncaging was 10 ms prior to the electrical stimulus. (B) Two-photon uncaging of CDNI-Glu (1 mM, 720 nm, 1 ms, 70 mW) at 11 spines along the dendrite of a CA1 pyramidal cell generated sufficient depolarization to evoke an action potential (top). When the uncaging flashes were targeted 2 μm away from the dendrite, no action potential was generated (middle); the spike was restored when uncaging was returned to the original position, demonstrating the precise nature of 2P inputs. (C) When CDNI-Glu (1 mM) and RuBi-GABA (10 μM) were co-applied to a slice, rapid bi-directional control of the membrane potential was effected when the 720-nm light was or was not preceded by a flash of 473-nm light (2 ms, 20 mW). Two-photon photolysis was with 720-nm light at eleven points (0.5 ms, 60 mW) along the basal dendrite of a CA1 pyramidal cell (held at −55 mV). Note that both of these inputs are occurring along the same portion of the dendritic tree, demonstrating the optical selectivity these two caged compounds under these conditions. (D) Two-photon fluorescence image of a CA1 pyramidal neuron used for the following physiological experiment, with a more detailed image in the area in the yellow box. Red dots indicate the location of 2P photolysis and the blue circle represents the area for 1P uncaging. CDNI-Glu (1 mM) and DEAC450-GABA (20 μM) were co-applied to the brain slice. Irradiation with 720-nm light of increasing power (55–90 mW) at 14 spines along an oblique dendrite elicited a dendritic spike. The dendritic spike was modulated when the 720-nm light stimulus was preceded by 473-nm light (2 ms, 15 mW). The plots of peak spike amplitude vs. laser power shows how the dendritic spike is altered by inhibition, as the maximal amplitude is reduced and the threshold (i.e. the point at which the signal becomes nonlinear) is increased.
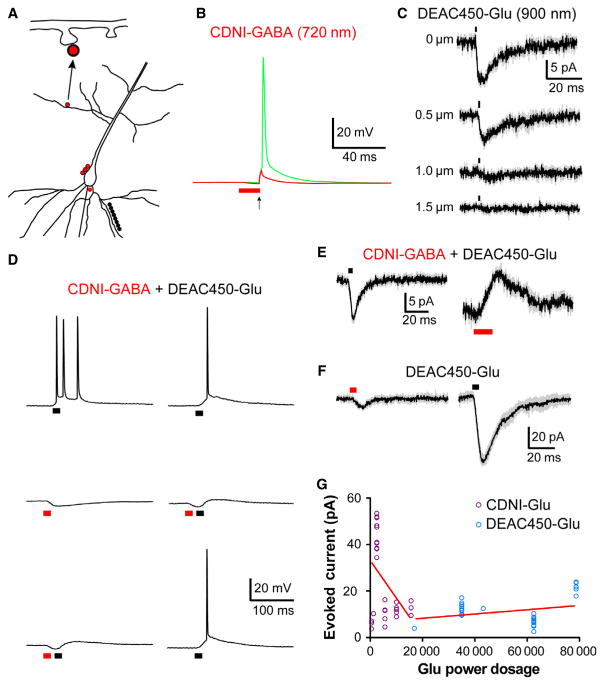
First, we established that DEAC450-Glu could be uncaged at 900 nm with lateral resolution (Fig. 6C) that was comparable to that reported in many studies for MNI-Glu(Matsuzaki et al., 2001; Smith et al., 2003; Carter & Sabatini, 2004; Sobczyk et al., 2005; Araya et al., 2006; Beique et al., 2006; Asrican et al., 2007; Katona et al., 2011). We have recently shown that when DEAC450-GABA was uncaged around the trigger zone of a CA1 neuron with 900 nm irradiation the resulting hyperpolarization can block action potentials(Amatrudo et al., 2014). Similarly, when CDNI-GABA was bath applied to a brain slice, irradiation around the trigger zone at 720 nm could be used to block action potentials generated by current injection from the patch pipette (Fig. 6B). The experiments shown in Figs. 3B,D were performed with only one caged compound present, when DEAC450-Glu (0.6 mM) and CDNI-GABA (1 mM) were co-applied to a brain slice, bidirectional modulation of membrane potential with chromatically selective two-color, two-photon uncaging was possible (Fig. 6D). Irradiation at 15 points along a basal dendrite at 900 nm fired an action potential, this was reversibly blocked by irradiation at 720 nm (Fig. 6D, in separate experiments uncaging CDNI-Glu at 720 nm and DEAC450-GABA at 900 nm produced similar effects, data not shown). Importantly, when the two lasers were directed to the same neuronal segment, only individual, wavelength-specific effects were apparent (Fig. 6E). These experiments show that two-color, two-photon uncaging of Glu and GABA is practical but do not reveal the quantitative color separation that the DEAC450 chromophore enables. Therefore we measured the optical selectivity of such currents by applying the same power at 720 nm or 900 nm at single spine heads, the difference in currents was 30-fold (Fig. 6F), and the power dosage difference for uncaging CDNI-Glu and DEAC450-Glu at 720 nm was found to be highly significant (P < 0.0007, Fig. 6G). In practice, the amount of energy required to see any current from uncaging DEAC450-Glu at 720 nm (50 mW, 5 ms) was much more than was needed for CDNI-Glu uncaging at 720 nm. For the latter, 50–60 mW for 0.5 or 25 mW for 1.0 ms at 720 nm could evoke currents reliably, such powers produced no measurable current from DEAC450-Glu. This series of experiments are similar to those we published in 2010(Kantevari et al., 2010), except with the new DEAC450-caged glutamate almost no photolysis is induced at short wavelengths of light. DCAC-GABA(Kantevari et al., 2010), a DEAC analog, was more photoactive at short wavelengths than long wavelengths of light(Kantevari et al., 2010). With CDNI- and DEAC450-caged compounds we find that it is straightforward to establish conditions in which there is effectively no optical cross talk between uncaging at each wavelength (Box 1). These experiments, we believe, demonstrate the real potential of two-color, two-photon uncaging for the first time. Since it has been established that only two-photon uncaging allows rapid, high-resolution stimulation of single synapses, we suggest that dual-color two-photon uncaging offers unprecedented capabilities in controlling neuronal signaling.
Summary of possible two-color actuation modalities
Wavelength-selective, two-color actuation is based upon, most obviously, the ability of chromophores to absorb in spectrally isolated parts of the electromagnetic spectrum (Fig. 2). The biological responses of the target receptors is also important as “bi-directional weight” of two such receptors acting in concert is probably never equal. Until the development of DEAC450(Olson et al., 2013a), all attempts at multi-color actuation using linear excitation required sequential and complete uncaging of the longer wavelength cage(Kotzur et al., 2009; Goguen et al., 2011; Menge & Heckel, 2011; Priestman et al., 2011; Schaäfer et al., 2011; Priestman et al., 2012; Rodrigues-Correia et al., 2013), or for the caged compounds to be present in very different concentrations(San Miguel et al., 2011; Klan et al., 2013). Initial reports for the genetically encoded probes ChR2 and Halo suggested that retinal-based actuators could circumvent the constraints of the absorption spectra (Fig 2D), and no doubt that for particular circumstances this can be observed. However, a more recent quantitative comparison of the currents generated by spectral variants of several excitatory opsin-based probes revealed that clean actuation at two wavelengths is very difficult to achieve(Prigge et al., 2012). This may go some way to explaining why bi-directional actuation with ChR2 and Halo (or their many equivalents) has been difficult to deliver for in vivo studies of mouse behavior. But note that Gottschalk and co-workers have used ChR2 variants with blue- and red-light sensitivities to fire different classes of neurons in C. elegans so as to control behavior bimodally(Erbguth et al., 2012). Recently Boyden and co-workers have found and mutated new opsin proteins that allow decent power windows for blue and red light activation of spikes in pyramidal cells in brain slices(Klapoetke et al., 2014). However, they do emphasize that all opsins can be activated by blue light.
Another important issue has been raised recently for the partnering of ChR2 and Halo (or equivalents) is that expression of two proteins under different promoters will always produce widely different protein levels in cells, introducing an almost insurmountable problem for consistent bi-directional control. Such problems are not seen with caged compounds, as concentrations are defined. Recently, the discoverers of ChR2(Nagel et al., 2003), Bamberg and co-workers, developed a solution to this problem by linking two opsin probes in one integral membrane protein(Kleinlogel et al., 2011). Many research groups continue to improve the properties of light-gated ion channels and pumps to address these issues(Knopfel et al., 2010).
With all these constraints, it is perhaps no surprise that there have been relatively few two-color neurophysiological actuation experiments(Zhang et al., 2008; Lovett-Barron et al., 2012; Chiu et al., 2013; Hayama et al., 2013). However, these studies show clearly that two-photon uncaging of glutamate at short wavelengths (720–740 nm range) can be partnered with one-photon actuation with blue light (450–473 nm range). This is because nitroaromatic caged glutamate probes are not photolyzed with blue light and RuBi-GABA at low concentrations does not produce any current with non-linear excitation at 720 nm. ChR2 has a much larger two-photon cross-section at 720 nm than MNI-Glu(Andrasfalvy et al., 2010), but the small nature of the excitation volume (Fig. 3A) coupled with the low single channel current of ChR2 mean that point excitation produces no effective current(Rickgauer & Tank, 2009; Prakash et al., 2013). In contrast, RuBi-GABA and ChR2 produce large currents with blue light(Zhang et al., 2008; Chiu et al., 2013).
What other 2P/1P modalities are easily available with commercially available probes? MNI-Glu at 720 nm can be partnered with linear activation of RuBi-dopamine and probably RuBi-Glu, as well C1V1, optoXR, eArch3.0(Yizhar et al., 2011). Unfortunately DEAC450-based probes are not commercially available, but DEAC450-cAMP offers another potential 1P partner for 2P uncaging of MNI-Glu. In terms of mixed 1P/1P modalities, uncaging MNI-Glu at 355 nm could be partnered with Halo and eArch3.0 (450–600 nm light(Yizhar et al., 2011)). Recently, Deisseroth and colleagues have shown that some newer opsin-based probes are usefully two-photon active(Prakash et al., 2013), but only at very long wavelengths (>900 nm), thus these probes could also be partnered for 2P/2P experiments with MNI-Glu at 720 nm or RuBi-Glu at 800 nm. An advantage of two-photon excitation is that its inherent non-linearity can enhance differences in linear absorption. For example, DEAC450-Glu is about 11-fold more active at 450 nm compared to 350 nm(Olson et al., 2013b), and it is about 18-fold more fluorescent at 900 nm compared to 720 nm when two-photon excitation is used. These data suggest that dual two-photon excitation has the capability of always providing better wavelength selectivity for two-color experiments. Certainly dual two-photon uncaging is uniquely powerful for subcellular interrogation of dendritic processes (Fig. 6). Our hope is that this Technical Spotlight will help further the application of two-color actuation experiments, especially using dual two-photon methods of uncaging.
Fig. 7.
Two-color 2P uncaging. (A) A schematic representation of the various photolysis positions on CA1 pyramidal neurons of the two mode-locked Ti: sapphire lasers used in (B)–(F). The two uncaging wavelengths are color-coded in each part with red representing the 720-nm laser and black the 900-nm laser. Neurons were imaged with a third laser at 1070 nm. For (C) and (F) the same points are targeted with both uncaging lasers. (B) CDNI-GABA (0.2 mM) was uncaged with 720 nm light (2 ms, 60 mW) at 12 points around the axon hillock, which generated an inhibitory input capable of blocking (red trace) electrically evoked action potentials. The green trace represents a control action potential in which only the electrical stimulus (2 ms, 500 pA) was applied. The pyramidal cell was held at −55 mV. (C) DEAC450-Glu (1 mM) was uncaged with 900-nm light (1 ms, 35 mW) at a single spine head. Each trace represents an average of 5 or 10 responses from one cell (grey represents SEM), which is representative of four cells on which this experiment was performed. (D) CDNI-GABA (1 mM) and DEAC450-Glu (0.6 mM) were co-applied to a slice and a CA1 pyramidal cell (held at −60 mV) was recorded from. Irradiation at 900 nm at 10 spines along a basal dendrite (1 ms, 120 mW) produced sufficient depolarization to evoke a burst of action potentials (top left trace). Irradiation at 720 nm at three points adjacent to the soma (5 ms, 125 mW) generated an inhibitory input of several millivolts (middle left). When these two inputs were paired in rapid succession, the action potentials were blocked (bottom left trace). Action potentials were blocked reversibly by interleafing dual-color irradiation at 720 and 900 nm (right middle trace) with 900 nm photolysis (right top and bottom traces). (F) CDNI-GABA (1 mM) and DEAC450-Glu (0.6 mM) were co-applied to a slice, and selectively activated at three points along a CA1 pyramidal cell soma (held at −50 mV). Stimuli were 720 nm, 5 ms for 90 mW and 900 nm, 1 ms for 50 mW. Traces are an average of five responses from a cell with a holding potential of −50 mV, and are representative of responses observed in four cells (grey indicates SEM). (F) DEAC450-Glu (0.5 mM) was uncaged at single spine heads; the same stimulus (5 ms, 50 mW) was used at both wavelengths. Uncaging at 900 nm evoked 30-fold larger response than that at 720 nm. Each trace is an average of five events from one cell, representative of six cells on which this experiment was performed (grey indicates SEM). Note that when a stimulus duration of 1 ms was used, DEAC450-Glu did not generate a measurable response at 720 nm (data not shown). Furthermore, the typical power dosage at 720 nm used to uncage CDNI-Glu (0.5–1 ms, 25–60 mW) did not produce any detectable 2P EPSCs from DEAC450-Glu. (G) Summary of all uncaging experiments for CDNI-Glu and DEAC450-Glu when uncaged at 720 nm at single spine heads. The evoked currents from many cells for CDNI-Glu and DEAC450-Glu uncaging at 720 nm (hence red lines for both data groups) are plotted vs. the ‘Glu power dosage’ (the caged compounds are color-coded according to their absorption spectra). This latter quantity is the product of the (power)2 × (time) × [cage]. As DEAC450 is ~ 10× more photoactive for 2P excitation (Olson et al., 2013b) this adjustment factor is also used. The graph shows an n of 35 for each cage compound. The linear regression line for each compound was computed and an analysis of covariance showed the two slopes were significantly different (F = 12.76, P = 0.00075).
Acknowledgments
This work was supported by grants from the NIH (GM053395, DA035612 and NS069720), and the HFSP (RG0089/2009C) to GCRE-D. We thank Ernst Bamberg for the ChR2 absorption spectrum, Janos Lanyi for permission to use the Halo absorption spectrum and Jeff Haines for the data analysis in Fig. 6G. GCRE-D thanks Craig Deitelhoff (Prairie Technologies) for help in the original configuration of the three-laser, two-photon microscope.
Appendix
Materials and methods
Caged compounds and fluorescent dyes
MNI-Glu (Papageorgiou & Corrie, 2000) (Matsuzaki et al., 2001), CDNI-Glu(Ellis-Davies et al., 2007), CDNI-GABA(Matsuzaki et al., 2010; Ellis-Davies, 2011), PEG-DEAC450-GABA(Amatrudo et al., 2014)] and DEAC450-Glu(Olson et al., 2013b) were prepared as previously described. RuBi-GABA was purchased from Tocris. Alexa-594 and Oregon Green BAPTA-1 were purchased from Molecular Probes.
Animals and brain slice preparation
All animal handling was performed in accordance with NIH guidelines approved by institutional IACUC review. Under isoflurane anesthesia, mice were decapitated and transverse brain slices (300–350 microns thick) were cut in ice-cold external solution, bubbled with 95% O2 and 5% CO2, containing (in mM): 124 NaCl, 25 NaHCO3, 1 KCl, 2 KH2PO4, 10 glucose, 2.5 CaCl2, 1.3 MgCl2. Slices were then transferred to artificial cerebrospinal fluid (ACSF) containing (in mM): 127 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 1 MgCl2, 2 CaCl2 and 20 glucose, bubbled with 95% O2 and 5% CO2. The slices were maintained at 20–22°C for at least 60 minutes until use.
Two-photon microscopy and physiology
Imaging and uncaging experiments were performed as described previously(Crowe et al., 2010; Kantevari et al., 2011; Amatrudo et al., 2014). An Olympus BX61 microscope (Penn Valley, PA, USA) fitted with a Prairie Technologies (Middleton, WI, USA) Ultima dual-galvo scan head (Haydon et al., 2008) controlled by Prairie View 5 and three Chameleon Ti:sapphire lasers (Coherent, Palo Alto, CA, USA) modulated by 350–80 Pockels cells (Conoptics, Danby, CT, USA), a continuous-wave 473-nm laser (Laserglow, Toronto, ON, Canada), an EPC9 or EPC10 amplifier (Heka Instruments, Bellmore, NY, USA) and an Olympus 60x lens (LUMFLN60XW, 1.1 NA) was used. Whole-cell patch-clamp recordings were obtained from cortical pyramidal cells identified with IR differential interference contrast microscopy. Patch-clamp recordings were made with glass electrodes (3–5 MΩ) filled with (in mM): potassium gluconate (135), HEPES (10), MgCl2 (4), Na2ATP (4), NaGTP (0.4), and sodium creatine phosphate (10), adjusted to pH 7.3 with KOH. Unless other wise noted in the figure legend, the internal solution contained Alexa-594 (0.1 mM) for visualization at 1070 nm. Caged compounds were dissolved in a small volume of ACSF and bath-applied to brain slices by recirculation. Off-line data analysis was performed using IgorPro, imageJ, Excel, or Prism.
Footnotes
Abbreviations used: CNB, α-carboxy-ortho-nitrobenyl; DMNB, dimethoxynitrobenzyl; MNI, 4-methoxy-7-nitroindolinyl, CDNI, 4-carboxymethoxy-7-nitroindolinyl; RuBi, rutheniumbipyridial; DEAC, 7-diethylaminocoumarin; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; NMDA, N-methyl-D-aspartate; ANBP, aminonitrobiphenyl; ChR2, channelrhodpsin-2; Halo, halorhodopsin; MSN, medium spiny neurons; NA, numerical aperture; LTP, long-term potentiation; uEPSP, unitary excitatory postsynaptc potential; EPSC, excitatory postsynaptic currents; FRET, fluorescence resonance energy transfer; FLIM, fluorescence life-time imaging microscopy; 2P or 2p; two-photon; sLTP, structural long-term potentiation; LTD, long-term depression; STDP, spike-timing dependent plasticity; DCAC, diethylaminocoumarin.
GCRE-D discussed the idea with the late Larry Katz at Cold Spring Harbor in 2003.
Author contributions. MNI-Glu was made by GCRE-D. DEAC450-Glu, PEG-DEAC450-GABA and CDNI-GABA were made by JPO. CDNI-Glu was made by HKA. All electrophysiology experiments were carried out by JMA. GCRE-D wrote the paper. All authors approved the manuscript.
Conflict of interest. GCRE-D has a US patent for dinitroindolinyl-caged compounds.
References
- Adams SR, Tsien RY. Controlling cell chemistry with caged compounds. Annu Rev Physiol. 1993;55:755–784. doi: 10.1146/annurev.ph.55.030193.003543. [DOI] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Amatrudo JM, Olson JP, Lur G, Chiu CQ, Higley MJ, Ellis-Davies GCR. Wavelength-selective one- and two-photon uncaging of GABA. ACS Chem Neurosci. 2014;5:64–70. doi: 10.1021/cn400185r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit B, Zehavi U, Patchornik A. Photosensitive protecting groups – a review. Isreal J Chem. 1974;12:103–113. [Google Scholar]
- Andrasfalvy BK, Zemelman BV, Tang J, Vaziri A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. P Natl Acad Sci Usa. 2010;107:11981–11986. doi: 10.1073/pnas.1006620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Andino-Pavlovsky V, Yuste R, Etchenique R. Two-photon optical interrogation of individual dendritic spines with caged dopamine. ACS Chem Neurosci. 2013;4:1163–1167. doi: 10.1021/cn4000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Eisenthal KB, Yuste R. Dendritic spines linearize the summation of excitatory potentials. Proc Natl Acad Sci U S A. 2006;103:18799–18804. doi: 10.1073/pnas.0609225103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrican B, Lisman J, Otmakhov N. Synaptic strength of individual spines correlates with bound Ca2+-calmodulin-dependent kinase II. J Neurosci. 2007;27:14007–14011. doi: 10.1523/JNEUROSCI.3587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujard I, Benbrahim C, Gouget M, Ruel O, Baudin JB, Neveu P, Jullien L. o-nitrobenzyl photolabile protecting groups with red-shifted absorption: syntheses and uncaging cross-sections for one- and two-photon excitation. Chemistry, Eur J. 2006;12:6865–6879. doi: 10.1002/chem.200501393. [DOI] [PubMed] [Google Scholar]
- Barltrop JA, Plant PJ, Schofield P. Photosensitive protective groups. Chem Comm. 1966:822–823. [Google Scholar]
- Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci U S A. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binkley RW, Flechtner TW. Photoremovable protecting groups. Plenum; London: [Google Scholar]
- Branco T, Clark BA, Häusser M. Dendritic discrimination of temporal input sequences in cortical neurons. Science. 2010;329:1671–1675. doi: 10.1126/science.1189664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EB, Shear JB, Adams SR, Tsien RY, Webb WW. Photolysis of caged calcium in femtoliter volumes using two-photon excitation. Biophys J. 1999a;76:489–499. doi: 10.1016/S0006-3495(99)77217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EB, Wu ES, Zipfel W, Webb WW. Measurement of molecular diffusion in solution by multiphoton fluorescence photobleaching recovery. Biophys J. 1999b;77:2837–2849. doi: 10.1016/S0006-3495(99)77115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Katz LC. Photostimulation using caged glutamate reveals functional circuitry in living brain slices. Proc Natl Acad Sci U S A. 1993;90:7661–7665. doi: 10.1073/pnas.90.16.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Centonze VE, White JG. Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys J. 1998;75:2015–2024. doi: 10.1016/S0006-3495(98)77643-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABAB receptor modulation of voltage-sensitive calcium channels in spines and dendrites. J Neurosci. 2011;31:4221–4232. doi: 10.1523/JNEUROSCI.4561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaulk SG, MacMillan AM. Caged RNA: photo-control of a ribozyme reaction. Nucleic Acids Res. 1998;26:3173–3178. doi: 10.1093/nar/26.13.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Lur G, Morse TM, Carnevale NT, Ellis-Davies GCR, Higley MJ. Compartmentalization of GABAergic inhibition by dendritic spines. Science. 2013;340:759–762. doi: 10.1126/science.1234274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe SE, Kantevari S, Ellis-Davies GCR. Photochemically Initiated Intracellular Astrocytic Calcium Waves in Living Mice Using Two-Photon Uncaging of IP3. ACS Chemical Neuroscience. 2010;1:575–585. doi: 10.1021/cn100052v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGregorio DA, Rothman JS, Nielsen TA, Silver RA. Desensitization properties of AMPA receptors at the cerebellar mossy fiber granule cell synapse. J Neurosci. 2007;27:8344–8357. doi: 10.1523/JNEUROSCI.2399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato L, Mourot A, Davenport CM, Herbivo C, Warther D, Leonard J, Bolze F, Nicoud JF, Kramer RH, Goeldner M, Specht A. Water-soluble, donor-acceptor biphenyl derivatives in the 2-(o-nitrophenyl)propyl series: highly efficient two-photon uncaging of the neurotransmitter gamma-aminobutyric acid at lambda = 800 nm. Angew Chem Int Ed Engl. 2012;51:1840–1843. doi: 10.1002/anie.201106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duschl A, Lanyi JK, Zimányi L. Properties and photochemistry of a halorhodopsin from the haloalkalophile, Natronobacterium pharaonis. J Biol Chem. 1990;265:1261–1267. [PubMed] [Google Scholar]
- Eder M, Zieglgansberger W, Dodt HU. Shining light on neurons--elucidation of neuronal functions by photostimulation. Rev Neurosci. 2004;15:167–183. doi: 10.1515/revneuro.2004.15.3.167. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies GC, Matsuzaki M, Paukert M, Kasai H, Bergles DE. 4-Carboxymethoxy-5,7-dinitroindolinyl-Glu: an improved caged glutamate for expeditious ultraviolet and two-photon photolysis in brain slices. J Neurosci. 2007;27:6601–6604. doi: 10.1523/JNEUROSCI.1519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies GCR. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nature methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies GCR. Neurobiology with caged calcium. Chem Rev. 2008;108:1603–1613. doi: 10.1021/cr078210i. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies GCR. A practical guide to the synthesis of dinitroindolinyl-caged neurotransmitters. Nature protocols. 2011;6:314–326. doi: 10.1038/nprot.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies GCR. A chemist and biologist talk to each other about caged neurotransmitters. Beilstein J Org Chem. 2013;9:64–73. doi: 10.3762/bjoc.9.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbguth K, Prigge M, Schneider F, Hegemann P, Gottschalk A. Bimodal activation of different neuron classes with the spectrally red-shifted channelrhodopsin chimera C1V1 in Caenorhabditis elegans. PLoS One. 2012;7:e46827. doi: 10.1371/journal.pone.0046827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filevich O, Salierno M, Etchenique R. A caged nicotine with nanosecond range kinetics and visible light sensitivity. J Inorg Biochem. 2010;104:1248–1251. doi: 10.1016/j.jinorgbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Fino E, Araya R, Peterka DS, Salierno M, Etchenique R, Yuste R. RuBi-Glutamate: Two-Photon and Visible-Light Photoactivation of Neurons and Dendritic spines. Front Neural Circuits. 2009a;3:2. doi: 10.3389/neuro.04.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Araya R, Peterka DS, Salierno M, Etchenique R, Yuste R. RuBi-Glutamate: Two-Photon and Visible-Light Photoactivation of Neurons and Dendritic spines. Frontiers in neural circuits. 2009b;3:2. doi: 10.3389/neuro.04.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier L, Gauron C, Xu L, Aujard I, Le Saux T, Gagey-Eilstein N, Maurin S, Dubruille S, Baudin JB, Bensimon D, Volovitch M, Vriz S, Jullien L. A Blue-Absorbing Photolabile Protecting Group for in Vivo Chromatically Orthogonal Photoactivation. ACS Chem Biol. 2013;7:1528–1536. doi: 10.1021/cb400178m. [DOI] [PubMed] [Google Scholar]
- Furuta T, Torigai H, Sugimoto M, Iwamura M. Photochemical Pproperties of New Photolabile cAMP Derivatives in a Physiological Saline Solution. J Org Chem. 1995;60:3953–3956. [Google Scholar]
- Gasparini S, Magee JC. State-dependent dendritic computation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26:2088–2100. doi: 10.1523/JNEUROSCI.4428-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguen BN, Aemissegger A, Imperiali B. Sequential activation and deactivation of protein function using spectrally differentiated caged phosphoamino acids. J Am Chem Soc. 2011;133:11038–11041. doi: 10.1021/ja2028074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Israely I, Huang SY, Tonegawa S. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69:132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GG, Junge JA, Mora RJ, Kwon HB, Olson CA, Takahashi TT, Liman ER, Ellis-Davies GCR, McGee AW, Sabatini BL, Roberts RW, Arnold DB. Recombinant probes for visualizing endogenous synaptic proteins in living neurons. Neuron. 2013;78:971–985. doi: 10.1016/j.neuron.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gug S, Bolze F, Specht A, Bourgogne C, Goeldner M, Nicoud JF. Molecular engineering of photoremovable protecting groups for two-photon uncaging. Angew Chem Int Ed Engl. 2008;47:9525–9529. doi: 10.1002/anie.200803964. [DOI] [PubMed] [Google Scholar]
- Hagen V, Bendig J, Frings S, Eckardt T, Helm S, Reuter D, Kaupp UB. Highly Efficient and Ultrafast Phototriggers for cAMP and cGMP by Using Long-Wavelength UV/Vis-Activation. Angew Chem Int Ed Engl. 2001;40:1045–1048. doi: 10.1002/1521-3773(20010316)40:6<1045::aid-anie10450>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Wang XD, Dan Y, Poo MM, Zhang XH. An arithmetic rule for spatial summation of excitatory and inhibitory inputs in pyramidal neurons. Proc Natl Acad Sci U S A. 2009;106:21906–21911. doi: 10.1073/pnas.0912022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey C, Yasuda R, Zhong H, Svoboda K. The Spread of Ras Activity Triggered by Activation of a Single Dendritic Spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450:1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama T, Noguchi J, Watanabe S, Takahashi N, Hayashi-Takagi A, Ellis-Davies GCR, Matsuzaki M, Kasai H. GABA promotes the competitive selection of dendritic spines by controlling local Ca signaling. Nat Neurosci. 2013 doi: 10.1038/nn.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG, MS, RE-DGC . Photolytic Activation of Receptors, Signaling Pathways, and Cellular Networks. In: HK, editor. Optical Control of Neural Excitability Society for Neuroscience. Washington, DC: 2008. pp. 101–109. [Google Scholar]
- Jadi M, Polsky A, Schiller J, Mel BW. Location-dependent effects of inhibition on local spiking in pyramidal neuron dendrites. PLoS Comput Biol. 2012;8:e1002550. doi: 10.1371/journal.pcbi.1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantevari S, Buskila Y, Ellis-Davies GCR. Synthesis and characterization of cell-permeant 6-nitrodibenzofuranyl-caged IP(3) Photochem Photobiol Sci. 2011 doi: 10.1039/c1pp05155e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantevari S, Matsuzaki M, Kanemoto Y, Kasai H, Ellis-Davies GCR. Two-color, two-photon uncaging of glutamate and GABA. Nature methods. 2010;7:123–125. doi: 10.1038/nmeth.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JH, Forbush B, 3rd, Hoffman JF. Rapid photolytic release of adenosine 5′-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978;17:1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- Katona G, Kaszas A, Turi GF, Hajos N, Tamas G, Vizi S, Rozsa B. Roller Coaster Scanning reveals spontaneous triggering of dendritic spikes in CA1 interneurons. P Natl Acad Sci Usa. 2011;108:2148–2153. doi: 10.1073/pnas.1009270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskin NI, Ogden D. Two-photon excitation and photolysis by pulsed laser illumination modelled by spatially non-uniform reactions with simultaneous diffusion. Eur Biophys J. 2002;30:571–587. doi: 10.1007/s00249-001-0186-y. [DOI] [PubMed] [Google Scholar]
- Klan P, Solomek T, Bochet CG, Blanc A, Givens R, Rubina M, Popik V, Kostikov A, Wirz J. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem Rev. 2013;113:119–191. doi: 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine-Paton M, Wong GK, Boyden ES. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinlogel S, Terpitz U, Legrum B, Gökbuget D, Boyden ES, Bamann C, Wood PG, Bamberg E. A gene-fusion strategy for stoichiometric and co-localized expression of light-gated membrane proteins. Nature methods. 2011;8:1083–1088. doi: 10.1038/nmeth.1766. [DOI] [PubMed] [Google Scholar]
- Knopfel T, Lin MZ, Levskaya A, Tian L, Lin JY, Boyden ES. Toward the second generation of optogenetic tools. J Neurosci. 2010;30:14998–15004. doi: 10.1523/JNEUROSCI.4190-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzur N, Briand B, Beyermann M, Hagen V. Wavelength-selective photoactivatable protecting groups for thiols. J Am Chem Soc. 2009;131:16927–16931. doi: 10.1021/ja907287n. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Hatada S, Kondo S, Karube F, Kawaguchi Y. Neocortical inhibitory terminals innervate dendritic spines targeted by thalamocortical afferents. J Neurosci. 2007;27:1139–1150. doi: 10.1523/JNEUROSCI.3846-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci. 2004;7:373–379. doi: 10.1038/nn1206. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Magee JC. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun XH, Nicoud JF, Zemelman BV, Sternson SM, Losonczy A. Regulation of neuronal input transformations by tunable dendritic inhibition. Nat Neurosci. 2012;15:423–430. S421–423. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- Major G, Larkum ME, Schiller J. Active properties of neocortical pyramidal neuron dendrites. Annu Rev Neurosci. 2013;36:1–24. doi: 10.1146/annurev-neuro-062111-150343. [DOI] [PubMed] [Google Scholar]
- Marriott G. Caged protein conjugates and light-directed generation of protein activity: preparation, photoactivation, and spectroscopic characterization of caged G-actin conjugates. Biochemistry (Mosc) 1994;33:9092–9097. doi: 10.1021/bi00197a010. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Hayama T, Kasai H, Ellis-Davies GCR. Two-photon uncaging of gamma-aminobutyric acid in intact brain tissue. Nature chemical biology. 2010;6:255–257. doi: 10.1038/nchembio.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGall GH, Christians FC. High-density genechip oligonucleotide probe arrays. Adv Biochem Eng Biotechnol. 2002;77:21–42. doi: 10.1007/3-540-45713-5_2. [DOI] [PubMed] [Google Scholar]
- Menge C, Heckel A. Coumarin-caged dG for improved wavelength-selective uncaging of DNA. Org Lett. 2011;13:4620–4623. doi: 10.1021/ol201842x. [DOI] [PubMed] [Google Scholar]
- Milburn T, Matsubara N, Billington AP, Udgaonkar JB, Walker JW, Carpenter BK, Webb WW, Marque J, Denk W, Mccray JA, Hess GP. Synthesis, Photochemistry, and Biological-Activity of a Caged Photolabile Acetylcholine-Receptor Ligand. Biochemistry. 1989;28:49–55. doi: 10.1021/bi00427a008. [DOI] [PubMed] [Google Scholar]
- Momotake A, Lindegger N, Niggli E, Barsotti RJ, Ellis-Davies GC. The nitrodibenzofuran chromophore: a new caging group for ultra-efficient photolysis in living cells. Nat Methods. 2006;3:35–40. doi: 10.1038/nmeth821. [DOI] [PubMed] [Google Scholar]
- Monroe WT, McQuain MM, Chang MS, Alexander JS, Haselton FR. Targeting expression with light using caged DNA. J Biol Chem. 1999;274:20895–20900. doi: 10.1074/jbc.274.30.20895. [DOI] [PubMed] [Google Scholar]
- Muller C, Beck H, Coulter D, Remy S. Inhibitory control of linear and supralinear dendritic excitation in CA1 pyramidal neurons. Neuron. 2012;75:851–864. doi: 10.1016/j.neuron.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Murnick JG, Dube G, Krupa B, Liu G. High-resolution iontophoresis for single-synapse stimulation. J Neurosci Methods. 2002;116:65–75. doi: 10.1016/s0165-0270(02)00028-6. [DOI] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargeot J, Nerbonne JM, Engels J, Lester HA. Time course of the increase in the myocardial slow inward current after a photochemically generated concentration jump of intracellular cAMP. Proc Natl Acad Sci U S A. 1983;80:2395–2399. doi: 10.1073/pnas.80.8.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou KD, Short SM, Rich MT, Antic SD. Extrasynaptic glutamate receptor activation as cellular bases for dynamic range compression in pyramidal neurons. Front Physiol. 2012;3:334. doi: 10.3389/fphys.2012.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JP, Banghart MR, Sabatini BL, Ellis-Davies GCR. Spectral evolution of a photochemical protecting group for orthogonal two-color uncaging with visible light. J Am Chem Soc. 2013a;135:15948–15954. doi: 10.1021/ja408225k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JP, Kwon HB, Takasaki KT, Chiu CQ, Higley MJ, Sabatini BL, Ellis-Davies GCR. Optically selective two-photon uncaging of glutamate at 900 nm. J Am Chem Soc. 2013b;135:5954–5957. doi: 10.1021/ja4019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou G, Corrie JET. Effects of aromatic substituents on the photocleavage of 1-acyl-7-nitroindolines. Tetrahedron. 2000;56:8197–8205. [Google Scholar]
- Patchornik A, Amit B, Woodward RB. Photosensitive protecting groups. J Am Chem Soc. 1970;92:6333–6335. [Google Scholar]
- Patterson M, Yasuda R. Signalling pathways underlying structural plasticity of dendritic spines. Br J Pharmacol. 2011;163:1626–1638. doi: 10.1111/j.1476-5381.2011.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MA, Szatmari EM, Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. P Natl Acad Sci Usa. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin JL, Day M, Surmeier DJ. Synaptically driven state transitions in distal dendrites of striatal spiny neurons. Nat Neurosci. 2011;14:881–888. doi: 10.1038/nn.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Yizhar O, Grewe B, Ramakrishnan C, Wang N, Goshen I, Packer AM, Peterka DS, Yuste R, Schnitzer MJ, Deisseroth K. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nature methods. 2013;9:1171–1179. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestman MA, Shell TA, Sun L, Lee HM, Lawrence DS. Merging of confocal and caging technologies: selective three-color communication with profluorescent reporters. Angew Chem Int Ed Engl. 2012;51:7684–7687. doi: 10.1002/anie.201202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestman MA, Sun L, Lawrence DS. Dual wavelength photoactivation of cAMP- and cGMP-dependent protein kinase signaling pathways. ACS chemical biology. 2011;6:377–384. doi: 10.1021/cb100398e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge M, Schneider F, Tsunoda SP, Shilyansky C, Wietek J, Deisseroth K, Hegemann P. Color-tuned channelrhodopsins for multiwavelength optogenetics. J Biol Chem. 2012;287:31804–31812. doi: 10.1074/jbc.M112.391185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial Verde EM, Zayat L, Etchenique R, Yuste R. Photorelease of GABA with Visible Light Using an Inorganic Caging Group. Front Neural Circuits. 2008a;2:2. doi: 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial Verde EM, Zayat L, Etchenique R, Yuste R. Photorelease of GABA with Visible Light Using an Inorganic Caging Group. Frontiers in neural circuits. 2008b;2:2. doi: 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickgauer JP, Tank DW. Two-photon excitation of channelrhodopsin-2 at saturation. P Natl Acad Sci Usa. 2009;106:15025–15030. doi: 10.1073/pnas.0907084106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Correia A, Weyel XM, Heckel A. Four levels of wavelength-selective uncaging for oligonucleotides. Org Lett. 2013;15:5500–5503. doi: 10.1021/ol402657j. [DOI] [PubMed] [Google Scholar]
- San Miguel Vn, Bochet CG, Del Campo An. Wavelength-Selective Caged Surfaces: How Many Functional Levels Are Possible? J Am Chem Soc. 2011;133:5380–5388. doi: 10.1021/ja110572j. [DOI] [PubMed] [Google Scholar]
- Schaäfer F, Joshi KB, Fichte MAH, Mack T, Wachtveitl J, Heckel A. Wavelength-Selective Uncaging of dA and dC Residues. Org Lett. 2011;13:1450–1453. doi: 10.1021/ol200141v. [DOI] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000;404:285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- Scott TF, Kowalski BA, Sullivan AC, Bowman CN, McLeod RR. Two-color single-photon photoinitiation and photoinhibition for subdiffraction photolithography. Science. 2009;324:913–917. doi: 10.1126/science.1167610. [DOI] [PubMed] [Google Scholar]
- Smirnova J, Woll D, Pfleiderer W, Steiner U. Synthesis of caged nucleosides with photoremovable protecting groups linked to intramolecular antennae. Helv Chim Acta. 2005;88:891–904. [Google Scholar]
- Smith MA, Ellis-Davies GC, Magee JC. Mechanism of the distance-dependent scaling of Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol. 2003;548:245–258. doi: 10.1113/jphysiol.2002.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht A, Thomann JS, Alarcon K, Wittayanan W, Ogden D, Furuta T, Kurakawa Y, Goeldner M. New photoremovable protecting groups for carboxylic acids with high photolytic efficiencies at near-UV irradiation. Application to the photocontrolled release of L-glutamate. Chembiochem. 2006;7:1690–1695. doi: 10.1002/cbic.200600111. [DOI] [PubMed] [Google Scholar]
- Trigo FF, Corrie JE, Ogden D. Laser photolysis of caged compounds at 405 nm: photochemical advantages, localisation, phototoxicity and methods for calibration. J Neurosci Methods. 2009a;180:9–21. doi: 10.1016/j.jneumeth.2009.01.032. [DOI] [PubMed] [Google Scholar]
- Trigo FF, Papageorgiou G, Corrie JE, Ogden D. Laser photolysis of DPNI-GABA, a tool for investigating the properties and distribution of GABA receptors and for silencing neurons in situ. J Neurosci Methods. 2009b;181:159–169. doi: 10.1016/j.jneumeth.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Tsien RY, Zucker RS. Control of cytoplasmic calcium with photolabile tetracarboxylate 2-nitrobenzhydrol chelators. Biophys J. 1986;50:843–853. doi: 10.1016/S0006-3495(86)83525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JW, Gilbert SH, Drummond RM, Yamada M, Sreekumar R, Carraway RE, Ikebe M, Fay FS. Signaling pathways underlying eosinophil cell motility revealed by using caged peptides. Proc Natl Acad Sci U S A. 1998;95:1568–1573. doi: 10.1073/pnas.95.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JW, Somlyo AV, Goldman YE, Somlyo AP, Trentham DR. Kinetics of smooth and skeletal muscle activation by laser pulse photolysis of caged inositol 1,4,5-trisphosphate. Nature. 1987;327:249–252. doi: 10.1038/327249a0. [DOI] [PubMed] [Google Scholar]
- Xia J, Huang XP, Sreekumar R, Walker JW. Photolabile ‘caged’ fatty acids containing a 1-(2′-nitrophenyl)-1,2-ethanediol moiety. Bioorg Med Chem Lett. 1997;7:1243–1248. [Google Scholar]
- Yagishita S, Hayashi-Takagi A, Ellis-Davies GCR, Urakubo H, Ishii S, Kasai H. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science. 2014;345:1616–1620. doi: 10.1126/science.1255514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Zayat L, Calero C, Alborés P, Baraldo L, Etchenique R. A new strategy for neurochemical photodelivery: metal-ligand heterolytic cleavage. J Am Chem Soc. 2003;125:882–883. doi: 10.1021/ja0278943. [DOI] [PubMed] [Google Scholar]
- Zayat L, Salierno M, Etchenique R. Ruthenium(II) bipyridyl complexes as photolabile caging groups for amines. Inorg Chem. 2006;45:1728–1731. doi: 10.1021/ic0512983. [DOI] [PubMed] [Google Scholar]
- Zhai S, Ark ED, Parra-Bueno P, Yasuda R. Long-distance integration of nuclear ERK signaling triggered by activation of a few dendritic spines. Science. 2013;342:1107–1111. doi: 10.1126/science.1245622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Holbro N, Oertner TG. Optical induction of plasticity at single synapses reveals input-specific accumulation of alphaCaMKII. Proc Natl Acad Sci U S A. 2008;105:12039–12044. doi: 10.1073/pnas.0802940105. [DOI] [PMC free article] [PubMed] [Google Scholar]