Abstract
Background
Chronic rhinosinusitis (CRS) has been defined as inflammation of the paranasal sinuses lasting at least 12-weeks with corresponding two or more “cardinal symptoms” that includes: 1) nasal obstruction, 2) thick nasal discharge, 3) facial pain/pressure, and 4) reduction or loss of sense of smell. Although prior studies have investigated symptoms of CRS after sinus surgery, none have compared the outcomes of these specific symptoms to on-going medical therapy.
Methods
Patients with CRS were prospectively enrolled into a multi-institutional, comparative effectiveness, cohort study. Subjects elected either continued medical management or endoscopic sinus surgery (ESS). Baseline characteristics and objective clinical findings were collected. Cardinal symptoms of CRS were operationalized by four questions on the 22-item SinoNasal Outcome Test (SNOT-22). Symptom improvement was evaluated in subjects with at least 6-month follow-up.
Results
342 subjects were enrolled with 69 (20.2%) electing continued medical management while 273 (79.8%) elected ESS. Subjects electing surgical therapy were more likely to have a higher baseline aggregate SNOT-22 score (44.3(18.9) vs. 53.6(18.8); p<0.001). All subjects improved across all cardinal symptoms; however, subjects undergoing ESS were significantly more likely (p<0.013) to experience improvement in thick nasal discharge (OR=4.36), facial pain/pressure (OR=3.56), and blockage/congestion of nose (OR=2.76). Subjects with nasal polyposis were significantly more likely to report complete resolution of smell/taste following ESS compare to medical management (23.8% vs. 4.0%; p=0.026).
Conclusions
Across a large population, surgical management is more effective at resolving the cardinal symptoms of CRS than ongoing medical management with the exception of sense of smell/taste.
Keywords: Sinusitis, diagnosis, quality of life, endoscopy, therapy
INTRODUCTION
Chronic rhinosinusitis (CRS) is defined in the 2007 Adult Sinusitis Clinical Practice Guidelines1 and the 2012 European Position Paper on Rhinosinusitis2 as inflammation of the nose and paranasal sinuses manifesting with two or more “cardinal” symptoms for 12 weeks with endoscopic and/or computed tomography signs of disease. The cardinal symptoms include nasal obstruction, thick nasal discharge, facial pain/pressure, and reduction or loss of sense of smell. These guidelines are designed to aid clinicians in the diagnosis and management of CRS. These cardinal symptoms were chosen because they are the most common symptoms of CRS1 and are used clinically because they are well understood by both patients and clinicians.
The impact of endoscopic sinus surgery (ESS) on CRS is well documented using a variety of quality-of-life (QOL) measures.3,4 QOL instrument measures are often reported in aggregate (e.g., SNOT-22) 5 or broken down by domain scores (e.g., RSDI, CSS). 3,6 Aggregate and domain scores are effective means to provide a complete view of the impact of ESS, but do not translate well for clinical use and patient-centered decision-making. Aggregate scores may also obfuscate improvements or lack of improvements in specific symptoms7 concealing specific symptomatic changes that may be weighed as more important to each individual patient.
Patients with CRS report interval improvement across all cardinal symptoms following ESS.8 However, specific symptom outcomes have not been compared to a medical cohort, which limits our ability to counsel patients between sinus surgery and continued medical management. The goal of this investigation was to specifically evaluate changes in cardinal symptoms after both continued medical management and sinus surgery.
MATERIALS and METHODS
Patient Population and Inclusion Criteria
Adult patients (> 18 years of age) with a current diagnosis of medically refractory CRS were prospectively enrolled into an on-going, North American, multi-institutional, observational, cohort study between February, 2011 and January, 2014 to compare the effectiveness of treatment outcomes for this chronic disease process. Preliminary findings from this cohort have been previously described.9-12 A current diagnosis of CRS was defined by the 2007 Adult Sinusitis Guideline - endorsed by the American Academy of Otolaryngology-Head and Neck Surgery,1 with subsequent previous treatment with oral, broad spectrum, or culture directed antibiotics (> 2 weeks duration) and either topical nasal corticosteroid sprays (> 3 week duration) or a 5-day trial of systemic steroid therapy during the year prior to enrollment. Enrollment sites consisted of four academic, tertiary care rhinology practices as part of the Oregon Health & Science University (OHSU, Portland, OR, USA), the Medical Univeristy of South Carolina (Charleston, SC, USA), Stanford University (Stanford, CA, USA), and the University of Calgary (Calgary, Alberta, Canada). The Institutional Review Board at each enrollment location provided oversight and annual review the informed consent process and all investigational protocols, while central review and coordination services were conducted at OHSU (eIRB #7198). Study participation did not change the medical therapy regimen or follow-up schedule required for any patient.
Study participants elected either one of two treatment options during the preliminary enrollment meeting as their standard of care. Participants either elected to continue medical management for control of symptoms associated with CRS or ESS procedures based on individual disease processes and intraoperative clinical judgement of the enrolling physician at each site. Surgical procedures consisted of either unilateral or bilateral maxillary antrostomy, partial or total ethmoidectomy, sphenoidotomy, middle or inferior turbinate reduction, frontal sinus procedures (Draf I, IIa/b, or III), or septoplasty. Participants were either primary or revision surgery cases in both treatment groups.
Exclusion Criteria
Study participants diagnosed with a current exacerbation of either recurrent acute sinusitis or ciliary dyskinesia were excluded from the final study cohort due to the heterogeneity of those disease processes. Participants were also excluded from final analyses if they failed to complete all required baseline study evaluations or had not yet entered into the follow-up appointment time window. Subjects originally electing continued medical management and changed treatment course to include ESS during the study period (“crossed over”) were also excluded due to the heterogeneity of the treatment protocols.
Clinical Disease Severity Measures
During the initial clinical / enrollment visit, all study subjects completed a medical history, head and neck clinical examinations, sinonasal endoscopy, and computed tomography (CT) imaging as part of their standard care. Endoscopic examinations were scored using the Lund-Kennedy endoscopy scoring system where higher scores represent worse disease severity (total score range: 0-20).13 This staging system grades bilateral, visual pathologic states within the paranasal sinuses including polyposis, discharge, edema, scarring, and crusting. Computed tomography images were evaluated and staged in accordance with the Lund-Mackay bilateral scoring system where higher scores represent higher severity of disease (total score range: 0-24).14 This scoring system quantifies the degree of image opacification in the maxillary, ethmoidal, sphenoidal, ostiomeatal complex, and frontal sinus regions. All visualizations were subjectively scored by the enrolling physician at each site at the time of enrollment.
Cardinal Symptom Evaluations
To operationalize the cardinal symptoms associated with confirmatory diagnosis of CRS, study participants were asked to complete items included on the 22-item Sinonasal Outcome Test (SNOT-22; Table 1).5 The SNOT-22 is a validated, 22-item treatment outcome measure applicable to chronic sinonasal conditions (©2006, Washington University, St. Louis, MO, USA). Higher scores on the SNOT-22 survey items suggest worse patient functioning or symptom severity (total score range: 0-110). Individual item scores are recorded using patient selected responses on a Likert scale (0 – 5) where higher scores represent worse symptom severity.
Table 1.
Survey items on SNOT-22 instrument used to operationalize cardinal symptoms of CRS
| SNOT-22 Survey Items: | Symptom: |
|---|---|
| Item #6 | “Thick nasal discharge” |
| Item #10 | “Facial pain / pressure” |
| Item #21 | “Sense of smell / taste” |
| Item #22 | “Blockage / congestion of nose” |
SNOT-22, 22-item Sinonasal Outcome Test
Participants were asked to complete the SNOT-22 survey items at both baseline appointments and at least 6-months after continued medical therapy or ESS procedures when possible, with the assistance of a research coordinator at each site. Patients were lost to follow-up if they did not complete any survey evaluations within 18 months after enrollment. The last available follow-up collected for study subjects (at least 6-months) was used to determine interval change in cardinal symptoms. Physicians at each site were blinded to all patient-based survey responses for the study duration.
Study Data Collection
Study participants were required to complete all necessary baseline surveys and informed consent in English. Participants were asked to provide demographic, social and medical history cofactors including, but not limited to: age, gender, race, ethnicity, education (years), insurance status, nasal polyposis, history of prior sinus surgery, asthma, acetylsalicylic acid (ASA) intolerance, chronic obstructive pulmonary disorder (COPD), current tobacco use, alcohol consumption, depression, known allergies (reported by patient history or confirmed skin prick or radioallergosorbent testing), ciliary dyskinesia / cystic fibrosis, and asthma / sinusitis related steroid dependency. All study data was collected at each site using standardized clinical research forms, de-identified, and manually transferred to a centralized, relational database (Access 2007; Microsoft Inc., Redmond, WA, USA).
Data Management and Statistical Analysis
All statistical analysis was performed using a commercially available statistical software program (SPSS v.22.0, IBM Corp., Chicago, IL, USA). Sample size determination were completed assuming a minimum 1.0 mean difference on SNOT-22 item responses between independent treatment modalities, corresponding to a discernable shift in Likert scale responses for each cardinal symptom. Using a conservative 1:4 allocation ratio of patients electing medical therapy to ESS, two-sided t-testing utilized 80% 1-β error probability (power), 0.050 alpha level, and an assumed equal variance of 1.0 for both treatment group values (Table 2). Complete descriptive analysis of clinical disease severity measures, demographics, clinical characteristics, and cardinal symptom survey scores were evaluated for distribution and assumptions of normality where appropriate. Comparisons between study participant characteristics were completed using either two-tailed independent t-tests or chi-square (χ2) analysis for measures of comorbidity and baseline disease severity. The percentage (%) of relative improvement was calculated for each treatment cohort using the formula: [(mean follow-up score – mean baseline score) / mean baseline score] X 100. Differences over time between baseline and follow-up cardinal symptom scores were compared using Wilcoxon Signed-Rank tests. Baseline and follow-up score distributions were evaluated for all symptom item scores to identify potential floor or ceiling effects. Binary logistic regression was used to identify whether treatment modality was a significant predictor of treatment outcome for each cardinal symptom score before and after adjustment for other independent cofactors. Primary model outcomes were considered to be patient-reported indications of complete symptom resolution of cardinal symptom (e.g., SNOT-22 item score of “0” at follow-up evaluation) after removal of subjects reporting “0” at both baseline and follow-up assessments to eliminate potential survey floor effects A total of 23 additional cofactors, including baseline SNOT-22 item scores, were screened for preliminary entry into each of four predictive models at the 0.250 level of significance. Final models were selected using a manual, step-wise procedure with forward inclusion (p<0.100) and backwards elimination (p<0.050) process. Crude and adjusted odds ratios (OR) with 95% confidence intervals are reported. Predictive model goodness-of-fit was evaluated using the Hosmer & Lemeshow χ2 test.15 Statistical associations were set at the 0.050 level of significance.
Table 2.
Sample size estimations for mean changes in SNOT-22 scores between treatment groups
| SNOT-22 Mean Score Difference: | Treatment Group Ratio: | Total Sample Size |
|---|---|---|
| 0.5 | 1:4 | 200 |
| 1.0 | 1:4 | 52 |
| 1.5 | 1:4 | 24 |
| 2.0 | 1:4 | 16 |
| 2.5 | 1:4 | 12 |
| 3.0 | 1:4 | 8 |
SNOT-22, 22-item Sinonasal Outcome Test
RESULTS
Final Study Population
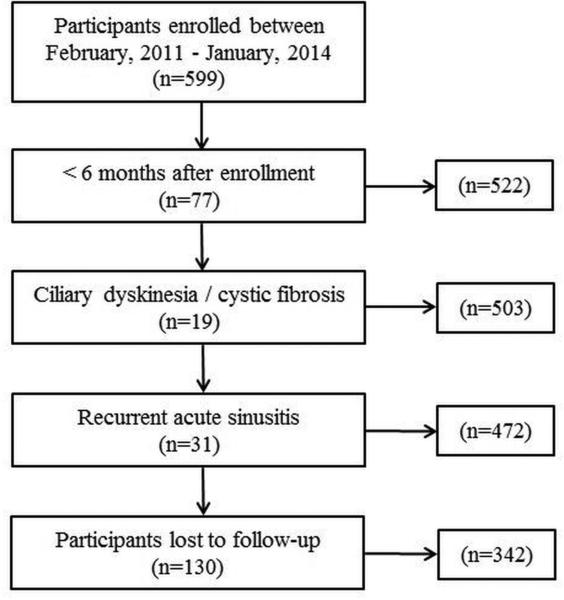
The application of inclusion and exclusion criteria allowed for a total of 342 participants with follow-up in the final analysis enrolled between February, 2011 and January, 2014 (Figure 1). A total of 69 (20.2%) participants elected continued medical management while 273 (79.8%) elected endoscopic sinus surgery. Both medical and surgical cohorts were found to have similar prevalence of follow-up (70.4% vs. 73.0%; p=0.610). Baseline demographics, clinical characteristics, and clinical disease severity measures were compared between treatment modality for participants with follow-up (Table 3). No significant differences between treatment modalities were noted with the exception of average years of education, the prevalence of deviated septum, and SNOT-22 total scores.
Figure 1.
Final cohort selection after inclusion and exclusion criteria.
Table 3.
Baseline characteristics of subjects with follow-up by treatment modality
| Medical management (n=69) | Endoscopic sinus surgery (n=273) | ||||
|---|---|---|---|---|---|
| Demographics: | Mean (SD) | N(%) | Mean (SD) | N (%) | p-value |
| Follow-up duration (months) | 12.8 (5.6) | 13.4 (5.6) | 0.398 | ||
| Age (years) | 52.0 (13.8) | 52.2 (14.6) | 0.937 | ||
| Males | 28 (40.6) | 127 (46.5) | --- | ||
| Females | 41 (59.4) | 146 (53.5) | 0.376 | ||
| White / Caucasian | 58 (84.1) | 231 (84.6) | 0.909 | ||
| Hispanic / Latino | 1 (1.4) | 16 (5.9) | 0.213 | ||
| Education (years) | 15.9 (2.6) | 15.0 (2.8) | 0.014 | ||
| Clinical characteristics: | |||||
| Asthma | 21 (30.4) | 101 (37.0) | 0.309 | ||
| Allergies (skin prick / RAST confirmed) | 27 (39.1) | 102 (37.4) | 0.787 | ||
| ASA sensitivity | 8 (11.6) | 23 (8.4) | 0.413 | ||
| Depression | 13 (18.8) | 48 (17.6) | 0.807 | ||
| Tobacco use/current smoker | 1 (1.4) | 15 (5.5) | 0.211 | ||
| Alcohol consumption | 36 (52.2) | 123 (45.1) | 0.289 | ||
| COPD | 3 (4.3) | 13 (4.8) | >0.999 | ||
| Steroid dependency | 3 (4.3) | 19 (7.0) | 0.587 | ||
| Previous sinus surgery | 40 (58.0) | 142 (52.0) | 0.376 | ||
| Nasal polyposis | 27 (39.1) | 105 (38.5) | 0.919 | ||
| Septal deviation | 15 (21.7) | 119 (43.6) | 0.001 | ||
| Hypertrophy turbinate | 5 (7.2) | 42 (15.4) | 0.115 | ||
| Clinical disease severity measures: | |||||
| SNOT-22 total score | 44.3 (18.9) | 53.6 (18.8) | <0.001 | ||
| Computed tomography score | 13.3 (6.0) | 12.3 (6.0) | 0.265 | ||
| Endoscopy score | 6.6 (4.0) | 6.2 (3.8) | 0.426 | ||
SD, standard deviation; RAST, radioallergosorbent; ASA, acetysalicyclic acid; COPD, chronic Obstructive pulmonary disease; SNOT-22, 22-item SinoNasal Outcome Test
A total of 130 subjects (medical management, n=29; sinus surgery, n=101) were either lost to follow-up or had not entered the first 6 month follow-up evaluation period at study inception. Compared to subjects with at least 6 month follow-up evaluations, patients without follow-up were significantly younger (47.6(14.2) vs. 52.1(14.4) years; p=0.003), reported a higher prevalence of tobacco use (10.8% vs. 4.7%; p=0.015) and were found to have slightly lower baseline CT scores (11.2(3.8) vs. 12.5(6.0); p=0.048). No other statistical differences were found for other demographics, clinical characteristics, or measures of disease severity.
Total Improvement in Cardinal Symptom Scores
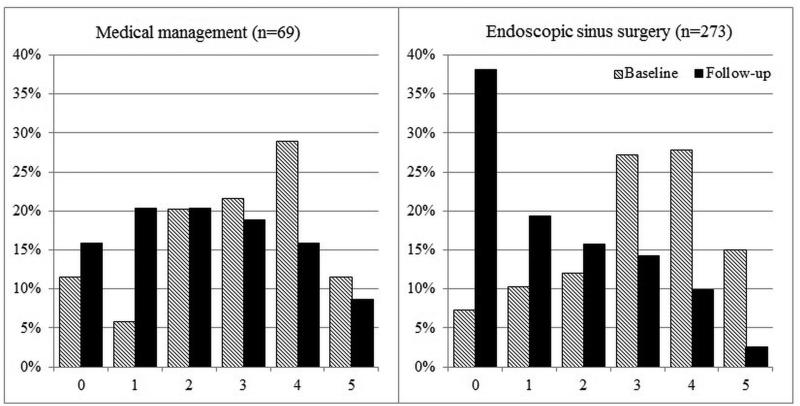
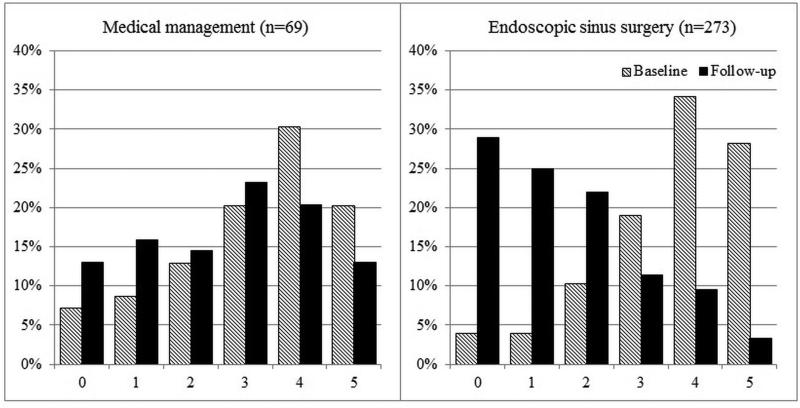
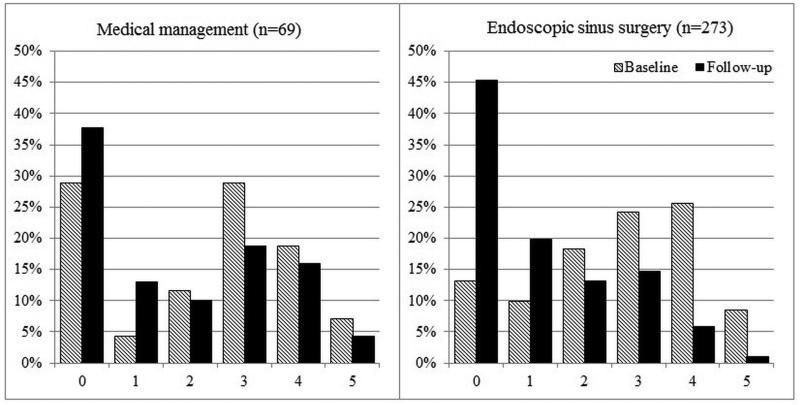
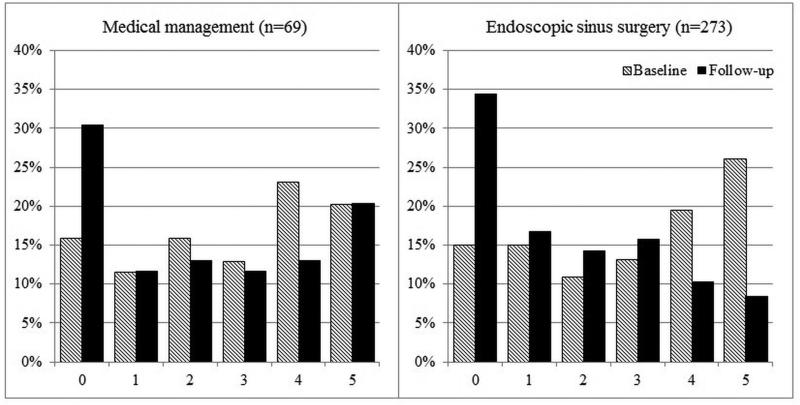
Overall improvement over time between mean baseline and follow-up scores for each cardinal symptom item on the SNOT-22 was evaluated (Table 4). Significant improvement for all cardinal symptoms was reported for both the total cohort and for each treatment modality. On average, participants electing sinus surgery reported significantly greater improvement compared to participants who continued with on-going medical management (Table 5). Additionally, the total cumulative percentages of cardinal symptom item scores between baseline and follow-up evaluations for both treatment cohorts are described in Figures 2-5.
Table 4.
Improvement in mean cardinal symptom scores over time
| Cardinal Symptoms: | Baseline | Follow-up | Improvement | |
|---|---|---|---|---|
| Total cohort (n=342) | Mean (SD) | Mean (SD) | Mean (SD) | p-value |
| “Thick nasal discharge” | 3.0 (1.5) | 1.6 (1.5) | −1.4 (1.7) | <0.001 |
| “Facial pain/pressure” | 2.6 (1.5) | 1.3 (1.4) | −1.3 (1.5) | <0.001 |
| “Sense of smell/taste” | 2.8 (1.8) | 1.9 (1.7) | −1.0 (1.8) | <0.001 |
| “Blockage/congestion of nose” | 3.5 (1.4) | 1.8 (1.5) | −1.7 (1.9) | <0.001 |
| Medical management (n=69) | ||||
| “Thick nasal discharge” | 2.9 (1.5) | 2.2 (1.6) | −0.6 (1.4) | 0.001 |
| “Facial pain/pressure” | 2.3 (1.7) | 1.8 (1.7) | −0.5 (1.2) | 0.002 |
| “Sense of smell/taste” | 2.8 (1.8) | 2.3 (1.9) | −0.5 (1.9) | 0.044 |
| “Blockage/congestion of nose” | 3.2 (1.5) | 2.6 (1.6) | −0.6 (1.8) | 0.009 |
| Endoscopic sinus surgery (n=273) | ||||
| “Thick nasal discharge” | 3.0 (1.4) | 1.5 (1.5) | −1.6 (1.7) | <0.001 |
| “Facial pain/pressure” | 2.7 (1.5) | 1.2 (1.4) | −1.5 (1.5) | <0.001 |
| “Sense of smell/taste” | 2.9 (1.8) | 1.8 (1.7) | −1.1 (1.8) | <0.001 |
| “Blockage/congestion of nose” | 3.6 (1.3) | 1.6 (1.4) | −2.0 (1.7) | <0.001 |
SD, standard deviation
Table 5.
Comparison of average and relative score improvement between treatment modalities
| Medical management (n=69) | Endoscopic sinus surgery (n=273) | ||||
|---|---|---|---|---|---|
| Cardinal Symptoms: | Mean (SD) Improvement | Relative improvement (%) | Mean (SD) Improvement | Relative improvement (%) | p-value |
| “Thick nasal discharge” | −0.6 (1.4) | 24.1 | −1.6 (1.7) | 50.0 | <0.001 |
| “Facial pain/pressure” | −0.5 (1.2) | 21.7 | −1.5 (1.5) | 55.6 | <0.001 |
| “Sense of smell/taste” | −0.5 (1.9) | 17.9 | −1.1 (1.8) | 37.9 | 0.007 |
| “Blockage/congestion of nose” | −0.6 (1.8) | 18.8 | −2.0 (1.7) | 55.6 | <0.001 |
SD, standard deviation
Figure 2.
Frequency of symptom scores for SNOT-22 item “Thick nasal discharge”
Figure 5.
Frequency of symptom scores for SNOT-22 item “Blockage/congestion of nose”
Prevalence of Reported Symptom Resolution
Participants reporting no indications (eg. score of “0”) of each separate cardinal symptom item at both baseline and follow-up assessments were removed due to the potential for healthy user bias. As the primary outcome of interest, the frequency of remaining participants describing complete symptom resolution for each cardinal symptom were compared between medical and surgical modalities, as well as between subjects with and without nasal polyposis (Table 6). Overall, participants electing ESS reported a significantly higher frequency of complete symptom resolution in three of four cardinal symptoms, with the exception of improved sense of smell and/or taste.
Table 6.
Comparison of complete symptom resolution frequency between treatment modalities
| Total cohort: | Medical management | Endoscopic sinus surgery | p-value |
|---|---|---|---|
| “Thick nasal discharge” (n=324) | 8 (12.1%) | 89 (34.5%) | <0.001 |
| “Facial pain/pressure” (n=292) | 8 (15.7%) | 92 (38.2%) | 0.002 |
| “Sense of smell/taste” (n=305) | 12 (20.0%) | 66 (26.9%) | 0.270 |
| “Blockage/congestion of nose” (n=333) | 8 (11.8%) | 71 (26.8%) | 0.009 |
| CRSwNP: | |||
| “Thick nasal discharge” | 3 (11.5%) | 35 (34.3%) | 0.029 |
| “Facial pain/pressure” | 2 (6.9%) | 41 (47.1%) | 0.004 |
| “Sense of smell/taste” | 1 (4.0%) | 24 (23.8%) | 0.026 |
| “Blockage/congestion of nose” | 3 (11.1%) | 33 (32.0%) | 0.032 |
| CRSsNP: | |||
| “Thick nasal discharge” | 5 (12.5%) | 54 (34.6%) | 0.006 |
| “Facial pain/pressure” | 6 (18.8%) | 51 (33.1%) | 0.109 |
| “Sense of smell/taste” | 11 (31.4%) | 42 (29.2%) | 0.793 |
| “Blockage/congestion of nose” | 5 (12.2%) | 38 (23.5%) | 0.137 |
CRSwNP, chronic rhinosinusitis with nasal polyposis; CRSsNP, chronic rhinosinusitis without nasal polyposis
Binary Logistic Regression
Four multivariate logistic regression models were then performed to assess the predictive ability of treatment modality type (main independent exposure variable) on reported complete symptom resolution (primary outcome measure). Crude and adjusted OR values are listed for each model, with corresponding 95% confidence intervals and p-values (Table 7). After removal of participants reporting “No problem” at baseline for each separate model the odds of subjects reporting complete symptom resolution for “Thick nasal discharge” are 4.36X better for subjects undergoing ESS than for participants electing continued medical management after adjustment for significant cofactors. Similarly, the odds of participants reporting symptom resolution for “Facial pain and/or pressure” are 3.56X better for subjects undergoing ESS compared to participants electing continued medical management. Treatment modality was not found to be a significant predictor of symptom resolution associated with “Sense of smell/taste” after adjustment for several independent predictive factors (OR: 1.50; p=0.306). Lastly, the odds of subject reporting complete resolution for “Blockage / congestion of the nose” are 2.76X higher for subjects electing ESS compared to continued medical management.
Table 7.
Logistic regression findings for endoscopic sinus surgery to result in resolution of cardinal symptoms compared to continued medical management.
| Cardinal Symptom Resolution: | Unadjusted OR | Adjusted OR | 95% CI | p-value | H-L χ2 |
|---|---|---|---|---|---|
| “Thick nasal discharge” | 3.82 | 4.361 | [1.90, 10.04] | 0.001 | 3.03* |
| “Facial pain/pressure” | 3.32 | 3.562 | [1.48, 8.55] | 0.005 | 8.00* |
| “Sense of smell/taste” | 1.48 | 1.503 | [0.69, 3.24] | 0.306 | 10.70* |
| “Blockage/congestion of nose” | 2.75 | 2.764 | [1.24, 6.13] | 0.013 | 12.24* |
OR, odds ratio. CI, confidence interval. H-L, Hosmer-Lemeshow test statistic.
Hosmer-Lemeshow chi-square tests indicate adequate goodness-of-fit for all models (p>0.050).
Adjusted for significant independent predictors (p<0.050) including: age, enrollment site, previous sinus surgery, and baseline SNOT-22 item score
Adjusted for significant independent predictors (p<0.050) including: enrollment site, previous sinus surgery, nasal polyposis, COPD, and baseline SNOT-22 item score.
Adjusted for significant independent predictors (p<0.050) including: enrollment site, previous sinus surgery, and baseline CT score
Adjusted for significant independent predictors (p<0.050) including: age, previous sinus surgery, and nasal polyposis
DISCUSSION
The present study describes the impact of both medical and surgical management on the cardinal symptoms of CRS. Subjects in both the medical and surgical cohorts improved across all the cardinal symptoms; however, subjects electing surgical therapy experience greater mean gains in all cardinal symptoms except for olfaction. A subgroup analysis of the total cohort, though, highlights a treatment differential in the subgroup of subjects with CRS with nasal polyposis (CRSwNP) with more improvement in smell and taste after surgery in contrast to subjects without nasal polyposis (CRSsNP). The frequency that subjects experience complete resolution of each cardinal symptom is greater in the surgical cohort with the exception of olfaction. Subjects undergoing surgical intervention are 3-4 times more likely to experience complete resolution of thick nasal discharge, facial pain/pressure and blockage/congestion of the nose when compared to subjects undergoing continued medical management.
Defining clinically significant improvement in symptoms is a critical step in translating QOL research to clinical care. One-half of a standard deviation from baseline symptoms has been deemed a universally detectable change in symptoms across disease processes and has been applied to CRS QOL investigations.4,16 This definition allows for building logistic models and defining research outcomes, but is challenging to articulate to patients. Other studies have found 0.8 in a single symptom on the SNOT-2017 or 10 points on the total SNOT-22 score5 to represent a minimally clinically detectable change based on comparisons to patient-reported transition scales. We elected to define ‘success’ as complete resolution of symptoms to avoid any concern over establishing what is meant by ‘clinically’ meaningful. Our standard of complete resolution of symptoms, although an extremely high-standard, carries no ambiguity, and allows for determination of odds ratio between treatment modalities (Table 7). These odds ratios are easily articulated to patients and help translate CRS outcomes research to clinical care when counseling patients with CRS. An important caveat to these odds ratios is that they were derived from a sampling of patients spanning several academic referral centers and care should be taken when applying these findings to individual patients. Furthermore, our regression models were built to isolate the impact of treatment modality on outcomes, not to identify other clinical factors which could potentially skew the probability of success for an individual.
The present study represents the largest prospective cohort study to investigate the impact of different therapies on the cardinal symptoms of CRS. The current available literature investigating individual symptom scores is dominated by smaller cohort studies at single institutions. A meta-analysis of these prior studies demonstrated that ESS successfully improves all cardinal symptoms.8 Interestingly, patient-reported olfactory dysfunction improved less than the other cardinal symptoms. The shortcomings of available interventions for olfaction may result from an irreversible olfactory neuron end-organ damage that has been described in the presence of long-standing inflammation.18 Recovering durable olfactory function may require more than just control of inflammation in patients with impaired olfaction using available treatment modalities. The focus of this study was to investigate patient-based clinical responses to different treatment modalities due to the fact that quantifiable, objective measures of olfaction are cost prohibitive and rarely employed in standard clinical practice for this patient population. Additionally, subjectively measured olfaction correlates only weakly with objective measures of olfaction and is neither highly sensitive nor specific in detecting olfactory loss when compared to subjective olfaction.19 Prior evaluation of objective olfactory outcomes, in a subset of the present cohort, found that only about 40% of subjects regain olfaction.12 Similarly, subjects with CRSwNP were more likely to regain sense of smell and taste, which may reflect the impairment of odorant conduction in this subgroup. Prior study has also demonstrated that nasal polyposis is associated with greater olfactory gains.19 Patients with CRS should have cautious expectations about recovering olfactory function after either medical or surgical management.
Prior study has identified that baseline QOL scores can be a significant predictor of patient-elected treatment modality.9 In fact, baseline QOL scores predicts treatment selection better than perceived social support, patient personality profile and physician-patient relationship. Patients are driven by symptoms to elect surgical management yet we do not understand the differential effects of medical and surgical therapy on symptom-specific scores and if these differentials in treatment efficacy parallel the symptoms driving patients to elect surgery. The concern would be that a patient electing surgical therapy over ongoing medical therapy in the hopes of improving a particular symptom might assume that all CRS patients have the same likelihood of improving. Indeed, subjective improvement of smell/taste is no more likely to improve with endoscopic sinus surgery than continued medical management. Although there is great convenience and value to comparing aggregate scores at a population level, a greater degree of transparency adds important clinical value. We elected to examine the cardinal symptoms of CRS since current guidelines1 have highlighted these as diagnostic criteria; however, future studies illuminating other symptoms associated with treatment selection and differential improvement after treatment would similarly add value to clinical decision-making.
The present study has some important limitations which warrant discussion. The ability of patients to self-select on-going medical therapy or surgical intervention, with physician guidance, introduces a possible source of treatment selection bias. This is inherent to the study design and ideally would be avoided through a strict randomization process. There are some important factors that preclude randomizing study participants that have been discussed elsewhere.20 In short, after failing typical medical management many patients may be reluctant to enroll in a study where chance alone would determine if a surgical procedure is performed. The differential enrollment rate (20.2% vs 79.8%) between treatment cohorts reflects this potential bias with study subjects having already failed ‘maximal’ medical management. Despite the lack of randomization, we were able to identify and account for significant confounders between the two cohorts through regression modeling procedures. Additionally, a subset of the originally enrolled medical cohort elected to crossover from medical therapy to surgical treatment. These subjects likely crossed over to surgical management because of a failure to achieve QOL gains; therefore, by excluding crossover subjects from analysis in the medical cohort there is an introduction of bias that favors medical therapy. Regardless, the data favor surgical management thus we did not pursue further analysis of the crossover cohort. Finally, the observational nature of the study precludes tight control over medical therapies and surgical philosophies between sites and patients. By allowing for this heterogeneity this data reflects a more ‘real world’ milieu providing greater external validity of these findings to other tertiary referral centers.
CONCLUSION
Surgical intervention was found to be more effective at resolving thick nasal discharge, nasal obstruction and facial pain/pressure than continued medical therapy in patients with CRS. Patient-reported sense of smell/taste showed no differential improvement between medical and surgical cohorts with the exception of the CRSwNP subjects on subgroup analysis. Subjects electing surgical intervention were more likely to have worse aggregate baseline QOL scores than subjects electing continued medical management. Further investigation into which symptoms motivate patients to elect surgical therapy would help elucidate which symptoms patients are trying to resolve by electing surgical interventions. Coupled with further study of the other symptoms classically associated with CRS a profile of what symptoms are best treated surgically could help guide both physicians and patients in selecting the ideal treatment modality.
Figure 3.
Frequency of symptom scores for SNOT-22 item “Facial pain/pressure”
Figure 4.
Frequency of symptom scores for SNOT-22 item “Sense of smell/taste”
Acknowledgments
Financial Disclosures: Timothy L. Smith, Jess C. Mace, and Zachary M. Soler are supported by a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD (R01 DC005805; PI/PD: TL Smith). Public clinical trial registration (www.clinicaltrials.gov) ID# NCT01332136. Timothy L. Smith is also a consultant for IntersectENT, Inc (Menlo Park, CA.) which is not affiliated with this investigation. Richard R. Orlandi is a consultant for Medtronic ENT (Jacksonville, FL.) which is not affiliated with this research.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Rosenfeld RM, Andes D, Neil B, et al. Clinical practice guideline: Adult sinusitis. Otolaryngol Head Neck Surg. 2007;137(3 suppl):S1–S31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 2.Fokkens WJ, Lund VJ, Mullol J, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50(1):1–12. doi: 10.4193/Rhino12.000. [DOI] [PubMed] [Google Scholar]
- 3.Gliklich RE, Metson R. Effect of sinus surgery on quality of life. Otolaryngol Head Neck Surg. 1997;117(1):12–17. doi: 10.1016/S0194-59989770199-2. [DOI] [PubMed] [Google Scholar]
- 4.Smith TL, Litvack JR, Hwang PH, et al. Determinants of Outcomes of Sinus Surgery: A Multi-Institutional Prospective Cohort Study. Otolaryngol Head Neck Surg. 2010;142(1):55–63. doi: 10.1016/j.otohns.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34(5):447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 6.Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg. 1997;123(11):1175–1179. doi: 10.1001/archotol.1997.01900110025004. [DOI] [PubMed] [Google Scholar]
- 7.Browne JP, Hopkins C, Slack R, Cano SJ. The Sino-Nasal Outcome Test (SNOT): Can we Make it More Clinically Meaningful? Otolaryngol Head Neck Surg. 2007;136(5):736–741. doi: 10.1016/j.otohns.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Chester AC, Antisdel JL, Sindwani R. Symptom-specific outcomes of endoscopic sinus surgery: A systematic review. Otolaryngol Head Neck Surg. 2009;140(5):633–639. doi: 10.1016/j.otohns.2008.12.048. [DOI] [PubMed] [Google Scholar]
- 9.Soler ZM, Rudmik L, Hwang PH, Mace JC, Schlosser RJ, Smith TL. Patient-centered decision making in the treatment of chronic rhinosinusitis. Laryngoscope. 2013;123(10):2341–2346. doi: 10.1002/lary.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alt JA, Mace JC, Buniel MCF, Soler ZM, Smith TL. Predictors of olfactory dysfunction in rhinosinusitis using the brief smell identification test. Laryngoscope. 2014 doi: 10.1002/lary.24587. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alt JA, Smith TL, Mace JC, Soler ZM. Sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope. 2013;123(10):2364–2370. doi: 10.1002/lary.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeConde AS, Mace JC, Alt JA, Schlosser R, Smith TL, Soler Z. Impact of treatment modality on olfaction in chronic rhinosinusitis: a prospective, multi-institutional study. Int Forum Allergy Rhinol. 2014;4(9):725–733. doi: 10.1002/alr.21350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3 Pt 2):S35–40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 14.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31(4):183–184. [PubMed] [Google Scholar]
- 15.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. John Wiley & Sons; Hoboken, NJ: 2000. [Google Scholar]
- 16.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 200341(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 17.Piccirillo JF, Merritt MG, Jr, Richards ML. Psychometric and clinimetric validity of the 20-Item Sino-Nasal Outcome Test (SNOT-20). Otolaryngol Head Neck Surg. 2002;126(1):41–471. doi: 10.1067/mhn.2002.121022. [DOI] [PubMed] [Google Scholar]
- 18.Kern RC. Candidate's Thesis: Chronic Sinusitis and Anosmia: Pathologic Changes in the Olfactory Mucosa. Laryngoscope. 2000;110(7):1071–1077. doi: 10.1097/00005537-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Litvack JR, Mace J, Smith TL. Does olfactory function improve after endoscopic sinus surgery? Otolaryngol Head Neck Surg. 2009;140(3):312–319. doi: 10.1016/j.otohns.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith TL, Kern RC, Palmer JN, et al. Medical therapy vs surgery for chronic rhinosinusitis: a prospective, multi-institutional study. Int Forum Allergy Rhinol. 2011;1(4):235–241. doi: 10.1002/alr.20063. [DOI] [PubMed] [Google Scholar]