Abstract
Exercise, obesity and type 2 diabetes are associated with elevated plasma concentrations of interleukin-6 (IL-6). Glucagon-like peptide-1 (GLP-1) is a hormone that induces insulin secretion. Here we show that administration of IL-6 or elevated IL-6 concentrations in response to exercise stimulate GLP-1 secretion from intestinal L cells and pancreatic alpha cells, improving insulin secretion and glycemia. IL-6 increased GLP-1 production from alpha cells through increased proglucagon (which is encoded by GCG) and prohormone convertase 1/3 expression. In models of type 2 diabetes, the beneficial effects of IL-6 were maintained, and IL-6 neutralization resulted in further elevation of glycemia and reduced pancreatic GLP-1. Hence, IL-6 mediates crosstalk between insulin-sensitive tissues, intestinal L cells and pancreatic islets to adapt to changes in insulin demand. This previously unidentified endocrine loop implicates IL-6 in the regulation of insulin secretion and suggests that drugs modulating this loop may be useful in type 2 diabetes.
Increased systemic IL-6 concentrations are associated with the pathophysiology of type 2 diabetes, with adipose tissue being the major source of this cytokine (refs. 1-3). Under these conditions, IL-6 is thought to contribute to the induction of insulin resistance and the deterioration of glucose homeostasis4. In contrast to the deleterious actions of systemically elevated IL-6, contracting skeletal muscle during exercise also increases circulating IL-6 concentrations5,6. During exercise, it is proposed that IL-6 promotes nutrient availability and improves whole-body insulin sensitivity7,8. These conflicting observations have led to a debate regarding the role of IL-6 in metabolism9-12.
We recently found that the pancreatic alpha cell is a primary target of IL-6 action13. IL-6 promotes alpha cell proliferation and inhibits apoptosis. In response to a high-fat diet, alpha cell mass expands in an IL-6–dependent manner, corroborating these findings13. Furthermore, whole-body IL-6 knockout mice with no alpha cell expansion show increased glycemia after feeding caused by impaired insulin secretion. Thus, we proposed that alpha cell expansion in response to a high-fat diet may be required for functional beta cell compensation and that systemically increased IL-6 abundance induced by a high-fat diet is an adaptive response necessary to maintain proper insulin secretion and glucose homeostasis13. However, the mechanism linking alpha cell expansion to beta cell adaptation remains enigmatic when considering the accepted role of alpha cells: increased alpha cell mass is normally expected to lead to an increase in glucagon production that would in turn lead to increased hepatic glucose output and a deterioration rather than the observed improvement in metabolic control.
GLP-1 is an incretin hormone secreted from intestinal L cells in response to nutrient intake and acts on beta cells to induce insulin secretion in a glucose-dependent manner14,15. GLP-1 is liberated from its precursor proglucagon in intestinal L cells through processing by the enzyme PC1/3 (refs. 16-19).
In the pancreatic alpha cell, proglucagon is processed by PC2 to yield glucagon20,21. Adult alpha cells are thought to produce little GLP-1. However, induction of diabetes in rodents leads to increased GLP-1 production in alpha cells together with increased alpha cell expression of PC1/3 (refs. 22-26).
In an attempt to explain how elevated IL-6 concentrations during obesity or exercise may improve beta cell insulin secretion, we hypothesized that IL-6 promotes GLP-1 production and secretion from intestinal L cells and pancreatic alpha cells, and here we show that is indeed the case, leading to improved beta cell insulin secretion and glucose tolerance. Thus, IL-6 is a hormone that mediates crosstalk between insulin-sensitive tissues and pancreatic islets through GLP-1.
RESULTS
Exercise induces GLP-1 in an IL-6–dependent manner
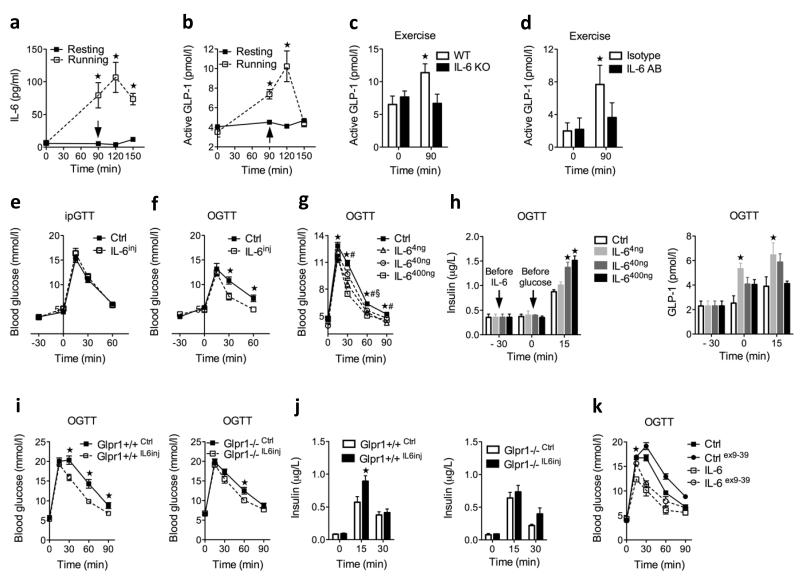
To address whether elevated systemic IL-6 levels have a physiological role in regulating plasma GLP-1 levels, we used exercise (treadmill running) as a model. Systemic IL-6 concentrations increased to 100 ± 20 pg ml−1 (mean ± s.e.m) in response to exercise (Fig. 1a). Together with elevated IL-6 concentrations, we found a 2.5-fold increase in circulating active GLP-1 in response to 90 min of exercise (Fig. 1a). To address whether IL-6 is required for this exercise-induced increase in active GLP-1, we first subjected IL-6 knockout mice to a bout of exercise and found they were unable to increase plasma GLP-1 concentrations in response to this manipulation (Fig. 1b). In another set of experiments, we administered an antibody to IL-6 to block systemic IL-6 actions. We confirmed the specificity of the antibody by showing that it blocked IL-6–stimulated plasma serum amyloid A, whereas the isotype control did not (Supplementary Fig. 1). Indeed, neutralization of plasma IL-6 also inhibited the exercise-induced increase in plasma GLP-1 (Fig. 1b), confirming that systemic increases in IL-6 caused by exercise increase plasma GLP-1 concentrations.
Figure 1.
Effect of acute IL-6 on GLP-1 and insulin secretion in vivo. (a) Plasma IL-6 (left; resting, n = 3 and running, n = 12) and GLP-1 (right; resting, n = 3 and running, n = 4) in resting and running male mice. Arrow at 90 min indicates exhaustion and blood sampling. (b) Plasma GLP-1 in male mice before and after 90 min of running (n = 6). KO, knockout; IL-6AB, antibodies to IL-6. (c) Intraperitoneal glucose tolerance test (ipGTT) (left) and oral glucose tolerance test (OGTT) (right) in female mice after a single injection of NaCl (ctrl) or 400 ng of IL-6 (n = 4). IL-6inj, mice injected with IL-6 .(d) OGTT after a single injection of NaCl or IL-6 in female mice (n = 12). (e) Plasma insulin (left) and GLP-1 (right) concentrations in female mice in response to oral glucose after a single injection of NaCl or IL-6 (n = 4); −30 min indicates the baseline measurement before NaCl or IL-6 injection, and 0 min indicates time point of glucose administration. (f) OGTT in male wild-type (WT) littermates (left) and GLP-1–receptor knockout (Glp1r−/−) (right) mice after a single injection of NaCl or 400 ng of IL-6 (n = 6–10). (g) Oral-glucose–stimulated insulin secretion in male WT littermate (left) and Glp1r−/− (right) mice after a single injection of NaCl or 400 ng of IL-6 (n = 6–10). (h) OGTT in male mice after a single injection of NaCl or 400 ng of IL-6 in the absence and presence of exendin (ex) (9–39) (n = 4). Data represent means ± s.e.m. *P < 0.05, determined by Student’s t test comparing control to IL-6 injection, resting to running or IL-6 to IL-6 plus exendin (9–39). *P < 0.05, $P < 0.05, #P < 0.05, determined by analysis of variance (ANOVA) comparing control to IL-6 injections (d,e).
IL-6 increases insulin secretion through GLP-1
Because systemically elevated IL-6 concentrations during exercise stimulated GLP-1 secretion, we hypothesized that acutely elevated IL-6 may improve oral glucose tolerance through the incretin action of GLP-1. To investigate this hypothesis, we injected a single bolus of 400 ng of IL-6 into mice 30 min before glucose administration (time point −30 min) followed by either intraperitoneal or oral (Fig. 1c) glucose administration (time point 0 min). IL-6 improved oral but not intraperitoneal glucose tolerance, suggesting enhancement of the incretin axis. Dose-response experiments with 4, 40 and 400 ng of IL-6 led to circulating IL-6 concentrations ranging from 10 to 550 pg ml−1 (Supplementary Fig. 2a), similar to the concentrations observed during exercise or after administration of a high-fat diet13 (Fig. 1a). All doses of IL-6 improved glucose tolerance (Fig. 1d), and 40 and 400 ng of IL-6 enhanced insulin secretion in a dose- and glucose-dependent manner (Fig. 1e), along with increasing plasma concentrations of GLP-1 (Fig. 1e) with no impact on insulin sensitivity (Supplementary Fig. 2b). In contrast, in GLP-1–receptor knockout (Glp1r−/−) mice, IL-6 no longer enhanced glucose-stimulated insulin secretion or improved glucose tolerance (Fig. 1f,g), and the GLP-1 receptor antagonist exendin (amino acids 9–39 of GLP-1) prevented IL-6 from improving early glucose excursions during oral glucose tolerance testing (Fig. 1h). These data identify GLP-1 as an essential mediator of IL-6 actions on beta cell function and glucose homeostasis.
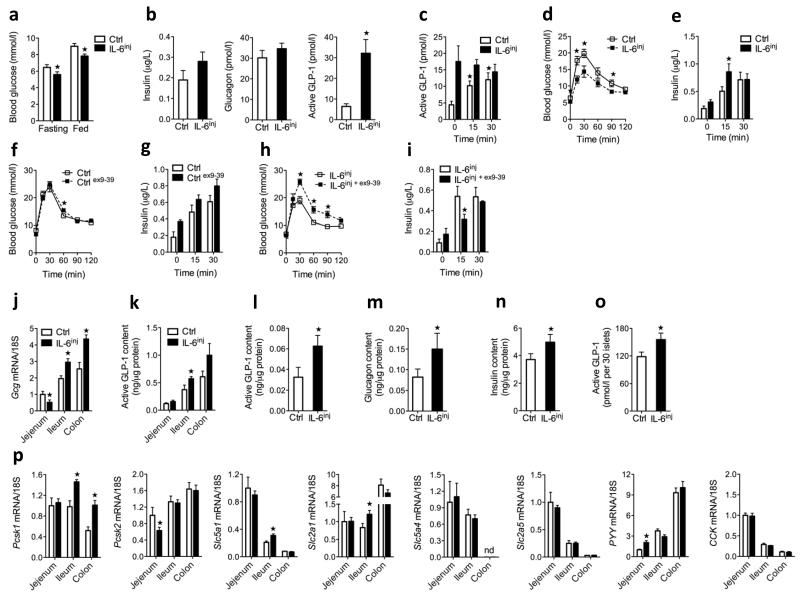
To study the effects of intermittently increased systemic IL-6 concentrations, we injected 400 ng of recombinant mouse IL-6 into mice twice daily for 7 d. We observed peak plasma IL-6 concentrations in response to a single injection after 30 min (baseline, 9.6 ± 2.5 pg ml−1 compared to 30 min after injection, 552 ± 96 pg ml−1 IL-6 concentrations, mean ± s.e.m) (Supplementary Fig. 2c), and the IL-6 concentrations returned to baseline values within 2 h after injection. We obtained all measurements listed below 15–17 h after the last injection of IL-6. After 7 days, both fasting and fed glycemia were lower in IL-6–injected mice compared to saline-injected mice, but we saw little effect on fasting insulin, glucagon (Fig. 2a,b) or GLP-2 (Supplementary Fig. 3). Notably, we detected fivefold higher fasting plasma GLP-1 concentrations in IL-6–injected mice compared to saline-injected mice (Fig. 2b). However, we did not detect further increases in plasma GLP-1 following oral glucose in IL-6–injected mice (Fig. 2c). We reasoned that increased fasting GLP-1 levels might improve insulin secretion during an intraperitoneal glucose tolerance test. Indeed, compared to saline-injected mice, IL-6–injected mice showed improved glucose tolerance during an intraperitoneal glucose tolerance test, as well as enhanced glucose-stimulated insulin secretion (Fig. 2d). IL-6 injections had no effect on insulin sensitivity (Supplementary Fig. 4). To determine whether basally elevated GLP-1 levels in IL-6–injected mice were responsible for the increased glucose-stimulated insulin secretion and the improved glucose tolerance, we performed intraperitoneal glucose tolerance tests in the presence of the GLP-1 receptor antagonist exendin (9–39). Administration of exendin (9–39) prevented the improvement of glucose tolerance and insulin secretion by IL-6 (Fig. 2e,f). Thus, these data show that intermittently increased systemic IL-6 concentrations improve beta cell function and glucose homeostasis by increasing fasting GLP-1 concentrations and thus enhancing intraperitoneal-glucose–stimulated insulin secretion.
Figure 2.
Effect of 1-week IL-6 injections on glucose homeostasis and GLP-1 production. (a) Fasting and fed blood glucose concentrations in male control mice and IL-6inj mice (n = 8). (b) Fasting plasma hormones in male control and IL-6inj mice (n = 6–8). (c) Plasma GLP-1 concentrations in response to oral glucose in male control and IL-6inj mice (n = 8). (d) Intraperitoneal GTT (ipGTT) (left) and plasma insulin in response to intraperitoneal glucose (right) in male control and IL-6inj mice (n = 8). (e) IpGTT (left) and plasma insulin in response to intraperitoneal glucose (right) in male control mice in the absence or presence of exendin (ex) (9–39) (n = 4). (f) IpGTT (left) and plasma insulin in response to intraperitoneal glucose (right) in male IL-6inj mice in the absence or presence of exendin (9–39) (n = 4). (g) Intestinal proglucagon (Gcg) mRNA expression (left) and intestinal GLP-1 content (right) in male control and IL-6inj mice (n = 8). (h) Pancreatic GLP-1 (left), glucagon (middle) and insulin (right) abundance in male control and IL-6inj mice (n = 8). (i) GLP-1 release over 24 h in isolated mouse islets from male control and IL-6inj mice (n = 5 PER GROUP). (j) Intestinal mRNA expression in male control and IL-6inj mice. Data are expressed as a fold of the jejenum control (n = 8). ND, not detectable. Data represent means ± s.e.m. *P < 0.05, determined by Student’s t test comparing control to IL-6inj mice.
IL-6 increases intestinal and pancreatic GLP-1
Next we examined whether IL-6 injections increased tissue Gcg mRNA expression and GLP-1 content. Compared to saline-injected mice, mice injected twice daily with IL-6 for 7 d showed higher Gcg mRNA expression and active GLP-1 content in the distal gut, where most L cells are localized (Fig. 2g). Furthermore, pancreatic GLP-1, glucagon and insulin content were higher after injections of IL-6 compared to saline injections (Fig. 2h). In support of an islet origin for pancreatic GLP-1, isolated islets from IL-6–injected mice showed increased GLP-1 release over 24 h compared to saline-injected mice (Fig. 2i).
Analysis of intestinal tissue gene expression revealed higher PC1/3 (encoded by Pcsk1) mRNA expression in the ileum and colon of IL-6–injected mice compared to controls, with no difference in PC2 (encoded by Pcsk2) mRNA expression (Fig. 2j). IL-6–injected mice also showed higher ileum mRNA expression of sodium glucose transporter 1 (encoded by Slc5a1) and glucose transporter 1 (encoded by Slc2a1) as well as peptide tyrosine tyrosine (also known as peptide YY, encoded by Pyy) in the jejenum compared to saline-injected mice; however, we detected no differences in the levels of mRNA transcripts for sodium glucose transporter 3 (encoded by Slc5a4) and glucose transporter 5 (encoded by Slc2a5) in the same experiments (Fig. 2j). Finally, intestinal dipeptidylpeptidase 4 (encoded by Dpp4) mRNA and plasma Dpp4 activity were not changed by IL-6, supporting IL-6–induced GLP-1 production rather than reduced clearance of GLP-1 (Supplementary Fig. 5a,b).
IL-6 increases GLP-1 synthesis and secretion in L cells
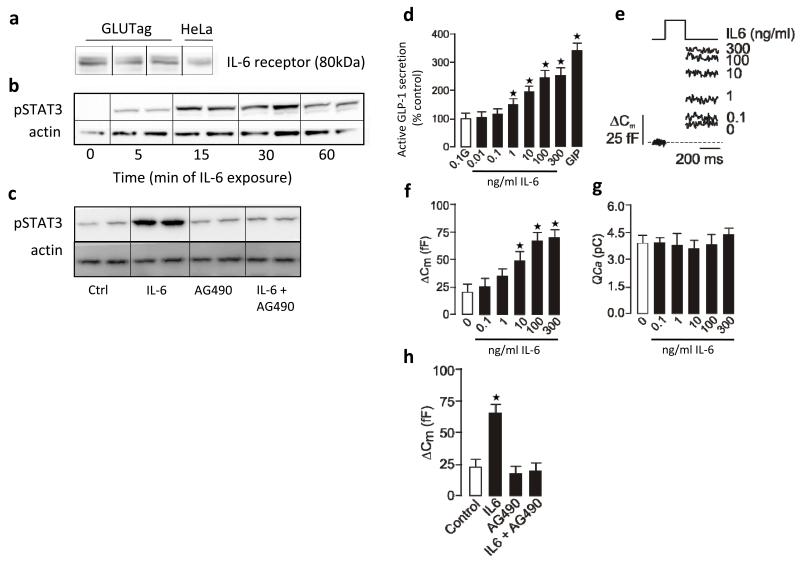
We next investigated the mechanism by which IL-6 promotes GLP-1 secretion and production in the gut by studying the direct effects of IL-6 on the mouse intestinal L cell line GLUTag. Western blot analysis confirmed expression of the IL-6 receptor in GLUTag cells (Fig. 3a); activation of the IL-6 receptor was coupled to increased phosphorylation of signal transducer and activator of transcription 3 (STAT3) (Fig. 3a), and inhibition of janus kinase 2 (JAK2)–phosphorylated STAT3 (pSTAT3) with AG490 blocked IL-6–induced STAT3 phosphorylation (Fig. 3a).
Figure 3.
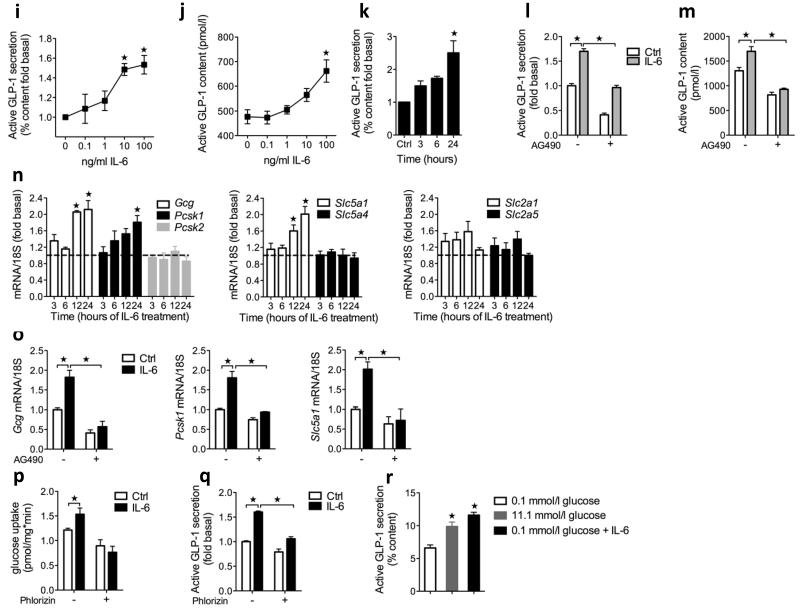
Effects of IL-6 on GLP-1 secretion in GLUTag cells. (a) Western blots of IL-6 receptor (top, with HeLa cell extract as the positive control (ctrl)), pSTAT3 (middle) and actin (bottom). (b) GLP-1 secretion after 15 min of stimulation with IL-6 and 0.1 mM glucose; we used 1 μM glucose-dependent insulinotropic peptide (GIP) as the positive control (n = 5). (c) Capacitance traces following stimulation with IL-6 (top left). Average capacitance following 15 min of stimulation with or without IL-6 (top right, n = 6–11). Ca2+ current following stimulation with or without IL-6 (bottom left, n = 6–11). Average capacitance following 15 min of stimulation with or without IL-6 and AG490 (bottom right, n = 5–9). (d) GLP-1 secretion in response to 2 h of stimulation with 11 mM glucose after 24 h with or without IL-6 (left, n = 3). GLP-1 content after 24 h with or without IL-6 (middle, n = 6). GLP-1 secretion in response to 2 h of stimulation with 11 mM glucose after 0–24 h with or without IL-6 (right, n = 3). (e) GLP-1 secretion in response to 2 h of stimulation with 11 mM glucose after 24 h with or without IL-6 (left, n = 3). GLP-1 content after 24 h with or without IL-6 (right, n = 3). (f) GLUTag mRNA in response to IL-6; data are expressed as a fold of the untreated control (broken line) (n = 3–6). (g) GLUTag mRNA after 24 h with or without IL-6 (n = 3). (h) 2-deoxy-3H-D-glucose (3H-2dG) uptake after 24 h with or without IL-6 (n = 4). (i) GLP-1 secretion in response to 2 h of stimulation with 11 mM glucose after 24 h with or without IL-6 (n = 3). (j) GLP-1 secretion in response to 2 h of stimulation with 0.1 or 11 mM glucose after 24 h with or without IL-6 (n = 6). Data represent means ± s.e.m. *P < 0.05, determined by ANOVA.
To determine whether IL-6 directly stimulates GLP-1 secretion, we incubated GLUTag cells with increasing concentrations of IL-6 in the presence of 0.1 mM glucose (Fig. 3b). IL-6 (1–300 ng ml−1) significantly increased GLP-1 secretion in a dose-dependent manner. We monitored increases in cell capacitance as a measure of exocytosis in individual GLUTag cells. IL-6 increased exocytosis in a dose-dependent manner (Fig. 3c). That capacitance increases after IL-6 stimulation independently of an increase in Ca2+ current (Fig. 3c) suggests that IL-6 enhances GLP-1 secretion beyond Ca2+ entry and thus modulates the exocytotic machinery. Inhibiting STAT3 phosphorylation completely blocked IL-6–induced exocytosis (Fig. 3c).
We next investigated whether IL-6 directly potentiated glucose-stimulated GLP-1 secretion. GLUTag cells treated with 0–100 ng ml−1 of IL-6 for 24 h and then stimulated with 11 mM glucose for 2 h showed significantly increased GLP-1 secretion at 10 and 100 ng ml−1 IL-6 (Fig. 3d; unless otherwise indicated, we used 100 ng ml−1 of IL-6 in all further experiments). Cellular GLP-1 content was also increased in response to IL-6 (Fig. 3d). Time course experiments showed that the effect of IL-6 to potentiate glucose-stimulated GLP-1 secretion was most pronounced at 24 h after treatment (Fig. 3d). The effect of IL-6 on glucose-induced GLP-1 secretion and GLP-1 content was diminished after JAK2-pSTAT3 inhibition (Fig. 3e). Consistent with the importance of JAK2-STAT3 in the L cell27, IL-6 potentiates glucose-stimulated GLP-1 secretion in a JAK2-STAT3–dependent manner.
To further investigate how IL-6 increases GLP-1 secretion and production in L cells, we analyzed the mRNA expression profiles of the candidate genes. Investigation of Gcg, Pcsk1, Psck2, Slc5a1, Slc5a4, Slc2a1 and Slc2a5 from 0 to 24 h after treatment with IL-6 revealed greater amounts of Gcg, Pcsk1 and Slc5a1 mRNA transcripts at 24 h (Fig. 3f). These mRNA effects were all reversed by JAK2-pSTAT3 inhibition (Fig. 3g), whereas the amount of Pcsk2 (which is not regulated by IL-6) mRNA transcripts was not affected by JAK2-STAT3 inhibition (Supplementary Fig. 6).
Supporting a functional role for the enhanced expression of sodium glucose transporter 1 (encoded by Slc5a1), we observed greater glucose uptake in GLUTag cells after 24 h of IL-6 treatment compared to control cells (Fig. 3h), an effect that was abolished by the sodium glucose transporter 1 inhibitor phlorizin (Fig. 3h). The ability of IL-6 to potentiate glucose-induced GLP-1 secretion was also inhibited by phlorizin (Fig. 3i), suggesting that enhanced glucose uptake is crucial for IL-6 to stimulate GLP-1 secretion. Indeed, 0.1 mM glucose stimulated GLP-1 secretion to a similar degree as 11 mM glucose after IL-6 incubation (Fig. 3j).
In summary, these data show that IL-6 acutely increases GLP-1 secretion from GLUTag cells by directly increasing GLP-1 exocytosis, whereas chronic IL-6 exposure increases glucose-stimulated GLP-1 secretion by increasing GLP-1 biosynthesis and glucose uptake, rendering the L cell more responsive to glucose.
IL-6 increases GLP-1 secretion from human islet alpha cells
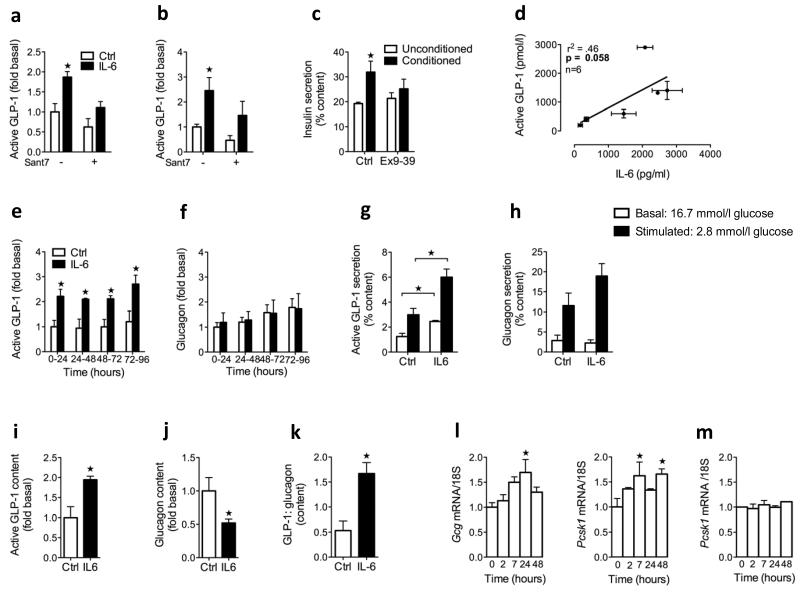
We used intact and FACS-sorted human islet cells to address whether IL-6 directly acts on alpha cells to increase GLP-1 production. Incubating human islets with IL-6 for 4 d with or without the IL-6 receptor antagonist super antagonist 7 (Sant7) indicated that IL-6 enhances both the constitutive release of GLP-1 as well as acute arginine-stimulated GLP-1 secretion (Fig. 4a). These effects were blunted in the presence of Sant7. That Sant7 tended to reduce GLP-1 in the absence of exogenous IL-6 can be explained by the presence of endogenous islet-derived IL-6 (ref. 28).
Figure 4.
Effects of IL-6 on GLP-1 secretion in human islets and human alpha cells. (a) GLP-1 release (left) over 24 h after 4 d with or without IL-6 and with (+) or without (−) the IL-6 receptor antagonist Sant7 (n = 3). GLP-1 secretion (right) in response to 1 h of static incubation in 10 mM arginine after 5 days with or without IL-6 and with (+) or without (−) Sant7 (n = 3). (b) Human islet insulin secretion in response to 1 h of static incubation in unconditioned or conditioned medium from human islets in the absence and presence of exendin (9–39) (n = 3). (c) Basal GLP-1 and IL-6 released from various human islet preparations (n = 6, r2 = 0.46, P = 0.058). (d) GLP-1 (left) and glucagon (right) released over consecutive 24 h intervals with or without IL-6 (n = 4). (e) GLP-1 (left) and glucagon (right) secretion in response to 1-h glucose incubations after 4 d with or without IL-6 (n = 4). (f) GLP-1 content (left), glucagon content (middle) and GLP-1:glucagon content as a molar ratio (right) after 4 days with or without IL-6 (n = 3–4). (g) FACS-enriched human alpha cell mRNA in response to IL-6 (n = 3–6). (h) FACS-sorted human beta cell mRNA in response to IL-6 (n = 4). Data represent means ± s.e.m. *P < 0.05, determined by Student’s t test comparing control to IL-6 or unconditioned to conditioned medium, or determined by ANOVA (g,h) comparing control (at time 0) to IL-6.
To assess whether the GLP-1 released from human islets was biologically active, we performed glucose-stimulated insulin secretion experiments using conditioned medium (cell culture medium from untreated human islets containing 11 mM glucose) in the absence and presence of exendin (9–39). These experiments showed improved insulin secretion stimulated by 11 mM glucose in islets incubated with conditioned medium relative to unconditioned medium, and this improvement was reversed in the presence of the GLP-1 receptor antagonist exendin (9–39) (Fig. 4b). Thus, bioactive GLP-1 released from human islets has the ability to improve insulin secretion in vitro. Furthermore, we observed a positive correlation between basal IL-6 release and basal GLP-1 release in human islets from six different organ donors (Fig. 4c).
FACS-enriched human alpha cells incubated with IL-6 also showed more GLP-1 release compared to control cells (basal GLP-1 release over 0–24 h was 3.1 ± 0.6 nM (mean ± s.e.m), whereas glucagon release was not significantly different in cells incubated with IL-6 and control cells (basal glucagon release over 0–24 h was 2.0 ± 0.7 nM, mean ± s.e.m) (Fig. 4d and Supplementary Table 1). After 4 d of exposure to IL-6, human alpha cells showed increased GLP-1 secretion in response to an acute decrease in glucose from 16.7 to 2.8 mM compared to control cells (Fig. 4e). Stimulated glucagon secretion trended higher at low glucose concentrations (Fig. 4e). In agreement with the increased pancreatic GLP-1 content of IL-6–injected mice (Fig. 2h), the cellular GLP-1 content of enriched human alpha cells was 1.9-fold higher after IL-6 treatment, whereas glucagon content was decreased after treatment compared to controls (Fig. 4f). These data suggest a shift in the processing of proglucagon from glucagon toward GLP-1, as shown by the expression of the cellular content as the molar ratio of GLP-1:glucagon (Fig. 4f). Thus, these data show that IL-6 acts directly on alpha cells to enhance their ability to liberate GLP-1.
Gene expression analysis revealed higher amounts of Gcg and Pcsk1 mRNA in response to IL-6 incubation in FACS-enriched human alpha cells after 24 and 7 h, respectively (Fig. 4g). IL-6 had no effect on Pcsk1 mRNA in purified human beta cells, indicating an alpha cell–specific effect (Fig. 4h). These data support the notion that IL-6 increases alpha cell GLP-1 production by increasing both proglucagon gene transcription and its subsequent processing toward GLP-1 through PC1/3. Overall, IL-6 is able to directly increase GLP-1 secretion from the human islet alpha cell.
Effect of acutely elevated IL-6 in animal models of diabetes
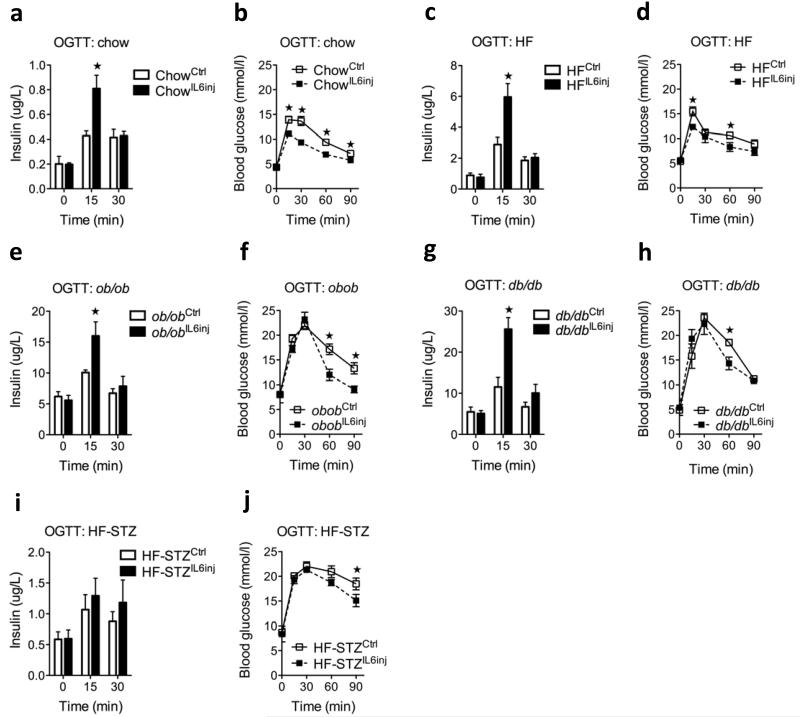
Because plasma concentrations of IL-6 are chronically increased in mouse models of obesity and diabetes13,29-31 we questioned whether these mice still responded to an acute increase in IL-6 by improving beta cell function. Indeed, compared to a saline injection, a single bolus of IL-6 significantly increased glucose-stimulated insulin secretion in mice fed chow (Fig. 5a), mice fed a high-fat diet (Fig. 5b), ob/ob mice (Fig. 5c) and db/db mice (Fig. 5d). In contrast, a high-fat diet model with direct beta cell destruction by streptozotocin (STZ) and IL-6 failed to enhance insulin secretion (Fig. 5e). Overall, the effect of IL-6 on glucose tolerance (Fig. 5 a–e) varied to a greater extent than the effect on insulin secretion, probably because of varying degrees of insulin resistance in the models used.
Figure 5.
Effect of acute IL-6 on insulin secretion in animal models of prediabetes and diabetes. (a) Plasma insulin (left) and oral glucose tolerance test (OGTT) (right) in response to oral glucose in male chow-fed mice after a single injection of NaCl (ctrl) or 400 ng of IL-6 (n = 8). (b) Plasma insulin (left) and OGTT (right) in response to oral glucose in male mice fed a high-fat diet (HF) for 18 weeks after a single injection of NaCl or 400 ng of IL-6 (n = 8). (c) Plasma insulin (left) and OGTT (right) in response to oral glucose in male ob/ob mice after a single injection of NaCl or 400 ng of IL-6 (n = 8). (d) Plasma insulin (left) and OGTT (right) in response to oral glucose in male db/db mice after a single injection of NaCl or 400 ng of IL-6 (n = 5). (e) Plasma insulin (left) and OGTT (right) in response to oral glucose in mice fed a high-fat diet and treated with STZafter a single injection of NaCl or 400 ng of IL-6 (n = 5). Data represent means ± s.e.m. *P < 0.05, determined by Student’s t test comparing control to IL-6 injection.
IL-6 reprograms alpha cells in response to obesity
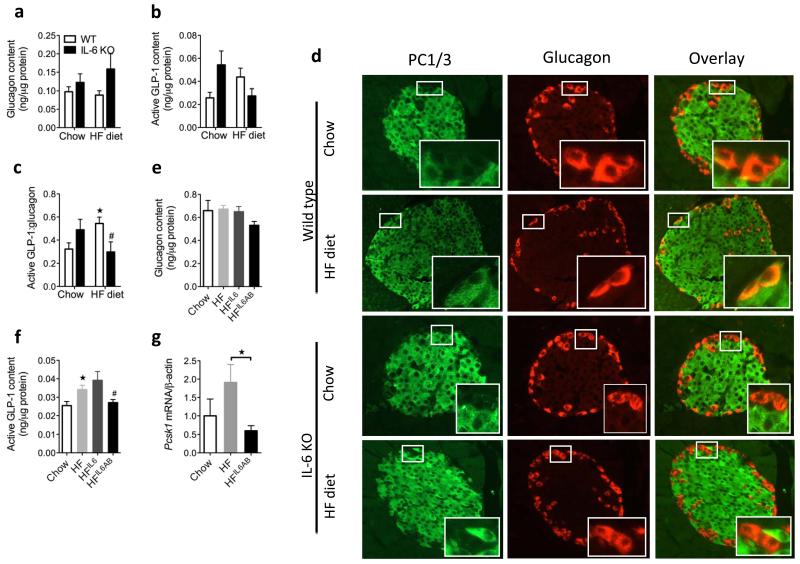
We previously reported that a high-fat diet leads to an IL-6–dependent expansion of alpha cell mass13. However, the expansion of alpha cell mass in wild-type mice was not associated with changes in pancreatic glucagon content (Fig. 6a), whereas the trend for pancreatic GLP-1 content was to increase (P = 0.08) (Fig. 6a), leading to a significant increase in the GLP-1:glucagon content in wild-type but not in IL-6 knockout mice (Fig. 6a). This high-fat diet–induced increase in the pancreatic GLP-1:glucagon ratio suggests a shift in proglucagon processing in alpha cells and was associated with increased PC1/3 co-localization to alpha cells in wild-type mice, whereas PC1/3 remained undetectable in alpha cells of IL-6 knockout mice on either a chow or a high-fat diet (Fig. 6b).
Figure 6.
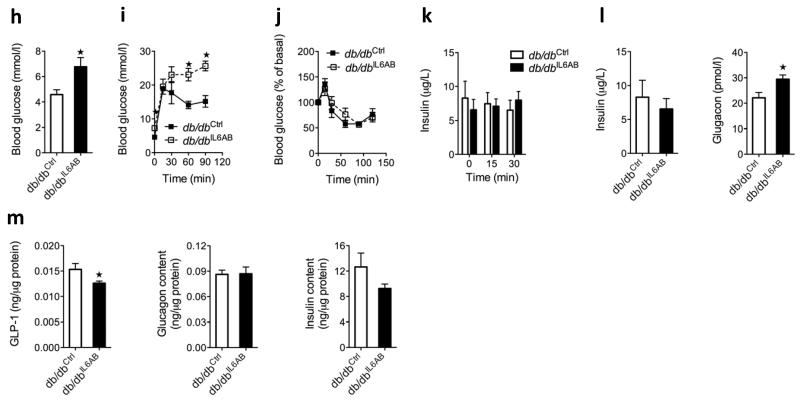
Effect of IL-6 antagonism in mice fed a high-fat diet and in db/db mice. (a) Pancreatic glucagon (left), GLP-1 (middle) and GLP-1:glucagon content as a molar ratio (right) in male WT and IL-6 knockout (KO) mice after 18 weeks of chow or high-fat diet (HF) (n = 8). (b) Immunohistochemistry of pancreatic tissue sections using antibodies against PC1, PC3 and glucagon (representative image of n = 5) (c) Pancreatic glucagon (left) and GLP-1 (right) in male mice fed chow or a high-fat diet for 15 weeks (n = 7–8). Mice on a high-fat diet and injected with IL-6 (HF IL-6inj) received IL-6 twice daily for the last 7 days of the study, and mice fed on a high-fat diet and injected with an antibody to IL-6 (HF IL-6AB) received an antibody that neutralized IL-6 for the last 4 weeks of the study. (d) Pcsk1 mRNA in FACS-sorted alpha cells from male mice expressing a yellow fluorescent protein under the control of the glucagon promoter after 20 weeks on chow or a high-fat diet (n = 5–8). Blood glucose (e), ipGTT (left), ITT (middle) and plasma insulin (right) (f) in response to intraperitoneal glucose in male db/db mice after 4 weeks without (db/db ctrl) or with (db/db IL-6AB) treatment with antibodies to IL-6 (n = 4–5). (g) Fasting plasma hormones in male db/db mice with or without treatment with antibodies to IL-6 (n = 4–5). (h) Pancreatic hormone content in male db/db mice with or without treatment with antibodies to IL-6 (n = 4–5). Data represent means ± s.e.m. *,#P < 0.05, determined by ANOVA, where the asterisk compares chow to a high-fat diet, and # compares genotypes on a high-fat diet only (a) or high-fat diet to a high-fat diet plus antibodies to IL-6 (c). In e–h, *P < 0.05, determined by Student’s t test comparing control mice to mice injected with antibodies to IL-6.
Similar to studies in IL-6 knockout mice, high-fat diet feeding alone or in combination with IL-6 injections or IL-6 neutralization did not change the pancreatic glucagon content (Fig. 6c). In contrast, mice fed a high-fat diet had a higher pancreatic GLP-1 content compared to mice fed on chow (Fig. 6c), and, although IL-6 injections had no statistically_significant additive effect on pancreatic GLP-1 content, treatment with an antibody to IL-6 blocked this high-fat diet–induced pancreatic GLP-1 (Fig. 6c). Supporting previous data27, high-fat diet feeding was associated with higher GLP-1 content in the colon, an effect that was IL-6 dependent (chow, 1.2 ± 0.1 active GLP-1 ng μg−1 protein; high-fat diet, 1.7 ± 0.1 active GLP-1 ng μg−1 protein; and high-fat diet plus antibody to IL-6, 0.8 ± 0.2 active GLP-1 ng μg−1 protein, mean ± s.e.m). Moreover, IL-6 antagonism in mice fed on a high-fat diet worsened fasting glycemia (Supplementary Fig. 7a). Fasting plasma concentrations of GLP-1 were unchanged in response to a high-fat diet and interventions with IL-6 (chow, 7.4 ± 1.9 pM active GLP-1; high-fat diet, 3.7 ± 0.3 pM active GLP-1; high-fat diet plus injected IL-6 3.9 ± 0.4 pM active GLP-1; and high-fat diet plus antibodies to IL-6, 3.7 ± 0.5 pM active GLP-1, mean ± s.e.m). Glucose-stimulated insulin secretion in response to intraperitoneal glucose revealed an enhanced insulin response in mice fed a high-fat diet compared to mice fed chow, and IL-6 injections further increased this response (Supplementary Fig. 7b). As was the case in chow-fed mice, in mice fed high-fat diet IL-6 interventions did not affect insulin tolerance (Supplementary Fig. 7c). Taken together, these data show that short-term antagonism of IL-6 signaling during high-fat diet feeding impairs the ability of alpha cells to increase GLP-1 production and also impairs glycemia.
Increasing high-fat diet–induced IL-6 concentrations by exogenous IL-6 injections revealed lower islet Tnf (also known as Tnf-α) mRNA expression and higher Ins1, Ins2 and Pdx1 (also known as Ipf1) mRNA expression (Supplementary Fig. 8).Finally, FACS sorting of alpha cells from transgenic mice fed on a high-fat diet expressing a yellow fluorescent protein under the control of the glucagon promoter32 showed that alpha cell PC1/3 mRNA expression was significantly lower in mice treated with antibodies to IL-6 compared to control mice fed a high-fat diet (Fig. 6d). Thus, high-fat diet–induced IL-6 promotes Pcsk1 mRNA expression, PC1/3 protein expression and GLP-1 production in alpha cells. These data suggest that elevated systemic IL-6 concentrations during obesity increase alpha cell PC1/3 expression, causing a shift from glucagon toward GLP-1 production.
IL-6 antagonism deteriorates glycemia in db/db mice
Next we examined whether blocking increased levels of endogenous IL-6 in db/db mice29,33 precipitates diabetes. We treated db/db mice with an antibody to IL-6 for 4 weeks and found impaired fasting glycemia relative to control mice (Fig. 6e) and a severe deterioration of glucose tolerance without any difference in insulin tolerance (Fig. 6f). Beta cell responses to intraperitoneal glucose were absent in these 9- to 10-week-old mice, and antagonizing IL-6 had no effect on insulin secretion (Fig. 6f). Fasting plasma insulin was unchanged, whereas glucagon was increased (Fig. 6g), and GLP-1 concentrations were undetectable in mice treated with antibodies to IL-6 compared to control mice. The insulin and glucagon concentrations in the pancreatic tissue were not different, whereas the GLP-1 content was lower in mice receiving antibody to IL-6 compared to control mice (Fig. 6h).
DISCUSSION
We identified IL-6 as a key regulator of glucose homeostasis through effects on L cell and alpha cell GLP-1 production and secretion and subsequent improvements in insulin secretion. Acute effects are caused by an IL-6–mediated increase in GLP-1 exocytosis in L cells, whereas chronic effects are caused by an IL-6–mediated increase in glucose responsiveness and GLP-1 production in L and alpha cells.
Exercise is accompanied by an increase in plasma GLP-1 concentrations34-37. Here we show that this increase is mediated by skeletal-muscle–derived IL-6. Elevated GLP-1 in response to exercise may be physiologically relevant through its effects on gut motility and satiety, whereas GLP-1 may improve beta cell function after physical training38. Of note, the potentiating actions of GLP-1 on insulin secretion are glucose dependent and require blood glucose concentrations above ~4–5 mM (ref. 15). Therefore, during exercise, IL-6–induced GLP-1 will not acutely affect insulin secretion in subjects without diabetes. In line with this concept, GLP-1 was found to determine the future insulin secretory response; that is, basal GLP-1 levels help prepare the beta cell for the subsequent meal, resulting in a potentiating effect on glucose-stimulated insulin secretion39-41. Therefore, IL-6-induced GLP-1 released during exercise will promote insulin secretion during a post exercise meal.
Compared to weight-matched wild type mice on high-fat diet IL-6 knockout mice on a high-fat diet show glucose intolerance caused by impaired insulin secretion13,42. In this study, we show that these mice have reduced pancreatic GLP-1 and that neutralization of IL-6 in wild-type mice fed on a high-fat diet and in db/db mice impairs this obesity-induced alpha cell adaptation associated with increased glycemia. This suggests that IL-6 released by adipose tissues has a compensatory role under conditions of obesity by increasing islet GLP-1 production to augment insulin secretion in order to adapt to insulin resistance and prevent diabetes.
GLP-1 produced by L cells is thought to act on beta cells through the circulation. We show that IL-6 increases GLP-1 production in the alpha cell during obesity, probably through increased proglucagon transcription and PC1/3 expression. Previous work has shown that alpha cells can be a source of GLP-1 and increased PC1/3 expression under conditions of beta cell stress22-26. A study describing the architecture in human islets reported direct intercellular contacts between alpha and beta cells, supporting the notion that alpha cell products can act in a paracrine manner to regulate the beta cell43. In support hereof, a recent study shows that acetylcholine secreted by alpha cells acts in a paracrine manner to prime the beta cell to respond optimally to subsequent increases in glucose44. Along these lines, alpha cell-derived GLP-1 may act locally on the beta cell to potentiate glucose-induced insulin secretion and to promote survival, while L cell-derived GLP-1 will act locally to decrease gut motility and enhance satiety via neuronal stimulation. Considering the short half-life of bioactive plasma GLP-1 (< 2 min)45 a paracrine rather than an endocrine action of GLP-1 seems more sensible. Prolonging the half-life of GLP-1 using DPP4 inhibitors is a widely used anti-diabetic treatment. It remains to be shown whether the main action of DPP4 inhibition occurs at plasma or tissue level.
GLP-1 is quite short-lived and it seems unlikely that GLP-1 produced in the gut acts on beta cells in the pancreas. So it’s more likely the incretin action of GLP-1 is mediated by GLP-1 produced locally in the pancreas, whereas the gut GLP-1 more likely is devoted to gut motility regulation (which is also likely to be regulated during exercise). Also you could spend a sentence to cover why a pro-inflammatory cytokine is beneficial for metabolism – perhaps that the energy demands of fighting off infection also increases the demand for insulin or that IL-6 goes up as an adaptive response to inflammation-induced hyperglycemia. And finally there is the notion that chronically elevated IL-6 is probably bad while modest levels of oscillatory IL-6 is probably good. Since the paper won’t have a News & Views please take the extra words to cover these issues.]
Several studies have uncovered IL-6 as a cytokine that regulates glucose tolerance, obesity and insulin action46-50. However, there exists little consensus on whether IL-6 is beneficial or detrimental. We enhanced systemic IL-6 concentrations through exercise, a high-fat diet and acute and intermittent IL-6 injections, and we observed consistent beneficial effects. Thus, IL-6 may adapt the metabolism to exercise and obesity, and during proinflammatory states IL-6 may contribute to adjust the metabolism to the requirement of the immune system.
In this study, we describe an adipose-tissue and skeletal-muscle enteroendocrine-islet axis. This crosstalk between insulin-sensitive tissues and insulin-producing cells is mediated through IL-6 acting on L cells and alpha cells to promote GLP-1 secretion and production, thereby allowing for adaptation to increased insulin demand during obesity and improved beta cell function in response to physical training. Failure to adapt to enhanced insulin demand leads to type 2 diabetes. Thus, understanding this endocrine loop may open the door to new therapeutic approaches or lead to a more judicious use of existing drugs modulating IL-6 and GLP-1.
ONLINE METHODS
Mice
Male and female C57BL/6J mice (Harlan), male C57BL/6J wild-type and IL-6 knockout B6;129S2-Il6tm1Kopf/J mice, male db/db and ob/ob mice (Jackson Laboratories), male wild-type (Glp1r+/+) and GLP-1–receptor knockout (Glp1r−/−) littermates and male transgenic mice expressing a yellow fluorescent protein under the control of the glucagon promoter were used. We followed the guidelines for laboratory animals from the University of Zurich and Mt. Sinai Hospital, Toronto and the Zurich, Mt. Sinai Hospital Cantonal Animal Experimentation Committee granted ethical approval. The UK Home Office and Local Ethical Committee approved the work done in Cambridge. We fed mice standard chow or a high-fat diet for 15 to 22 weeks13. After 3 weeks, we injected some of the mice with 130 mg per kg of body weight of STZ and continued the high-fat diet.
Injections of IL-6 and antibodies to IL-6
We injected mice either twice daily for 7 d with 400 ng of carrier-free recombinant IL-6 (R&D Systems or saline (NaCl) or with a single bolus of 4, 40 or 400 ng of IL-6 30 min before administration of glucose or insulin. We injected mice fed on a high-fat diet and db/db mice with 500 μg of an antibody that neutralizes IL-6 (R&D Systems) or PBS twice weekly for the final 2–4 weeks of the experiment. One hour before treadmill running, we injected mice with 500 μg of antibody that neutralizes IL-6 or an isotype control. We used a Luminex kit (Millipore) to measure plasma IL-6 concentrations.
Glucose and insulin tolerance tests
We performed glucose tolerance tests by administrating 2 g of glucose per kg of body weight orally (gavage) or by injecting 2 g of glucose per kg of body weight intraperitoneally. In some experiments, we injected 25 nM per kg body weight exendin (9–39) (a GLP-1 receptor antagonist; Bachem) either 1 min (for ipGTTs) or 15 min (for OGTTs) before glucose administration. To analyze the plasma, we used insulin ELISA (Millipore and ALPCO), GLP-1 Luminex and GLP-1 ELISA (Millipore), the in-house Novo Nordisk assay26 and GLP-2 ELISA (Labodia). We performed insulin tolerance tests injecting 0.75–4 U of insulin per kg of body weight (Novo Nordisk).
Treadmill running
Mice ran 10 m per min at a 0° inclination for 30 min followed by a gradual increase by 3 m per min and a 2.5° inclination every 10 min for 90 min until exhaustion. Exhaustion has been defined previously49.
Tissue content
We determined the hormone content in homogenized pancreatic and intestinal tissues after overnight HCl-EtOH (0.18 M HCl in 70% ethanol) extraction at 4 °C. We diluted the supernatant in H2O and measured active GLP-1 and glucagon using the in-house Novo Nordisk kit26 and radioimmunoassay (Millipore). Insulin was measured using ELISA (Mercodia), and protein concentration was measured using a bicinchoninic acid assay (Pierce).
Immunohistochemistry
We used pancreatic paraffin-embedded tissue sections for double immunofluorescence50,51 with primary antibodies to PC1, PC3 (Millipore) and glucagon (Linco).
Human islets
We isolated human islets from pancreata of organ donors at the University of Geneva Medical Center, Geneva, Switzerland, and cultured them as previously described28. We used 1 mM diprotin A (Dpp4 inhibitor; Sigma), 200 ng ml−1 recombinant human IL-6 (R&D Systems), 200 ng ml−1 Sant7 (an IL-6 receptor antagonist provided by Sigma-Tau52) and 100 nM exendin (9–39). We used an IL-6 ELISA (R&D Systems) to measure IL-6 concentrations.
Fluorescence-activated cell sorting
We sorted human beta cells by FACS after labeling with Newport Green53 giving rise to a cell population consisting of on average 96% beta cells) We also used the FACS-sorted non–beta-cell fraction enriched in alpha cells (on average, 50% alpha cells). We cultured cells as human islets and assessed active GLP-1 and glucagon secretion by 1 h static incubations in 16.7 and 2.8 mM glucose. We extracted the cellular content with HCl-EtOH. We separated fluorescent alpha cells from transgenic mice by FACS as previously described32.
Mouse islets
We isolated and cultured mouse pancreatic islets as described28 in the presence of 1 mM diprotin A.
GLUTag cells
We cultured GLUTag cells as described54 and treated them with 100 ng ml−1 mouse IL-6 (unless otherwise indicated) and 1 mM diprotin A. We assessed active GLP-1 secretion by 2 h static incubations in 0.1 or 11.1 mM glucose and used 50 μM of the JAK2-pSTAT3 inhibitor AG490 and 100 μM phlorizin (Fluka). GLP-1 and glucagon radioimmunoassays (Millipore) were used for all in vitro experiments. We used the perforated-patch whole-cell configuration of the patch-clamp technique and an EPC9 patch-clamp amplifier (Heka Elektronik) to monitor changes in cell capacitance as a measure of exocytosis.
Western blotting
We separated proteins (20–50 μg) on 4–12% NuPAGE gels (Invitrogen), blotted them onto nitrocellulose membranes (Bio-Rad) and incubated them with antibodies against pSTAT3 (Tyr705) (Cell Signaling), actin and IL-6 receptor (Santa Cruz).
RNA extraction and real-time PCR
We extracted tissue and cellular RNA according to the manufacturer’s instructions (QIAGEN and MACHEREY-NAGEL respectively, or Ambion for FACS fluorescent alpha cells) and performed real-time PCR using primers and the ABI 7500 and 7900 systems (Applied Biosystems). We calculated changes in mRNA expression using differences of CT values compared to housekeeping genes (18S or β actin).
2-deoxy−3H-D-glucose uptake
We incubated GLUTag cells with or without 100 ng ml−1 of IL-6 and determined uptake of 2-deoxy-3H-D-glucose (3H-2dG) (10 μM) as reported55 with or without 100 μM phlorizin (Fluka).
Statistics
Data are expressed as means ± s.e.m. We considered a value of P < 0.05 to be statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge O. Madsen and D. Steiner for their suggestion to investigate the role of IL-6 on GLP-1 and PC1/3. We thank R. Prazak and M. Borsigova for technical assistance and M. Niessen for discussions. This work was supported by grants from the Swiss National Science Foundation and by the Merck Investigator Studies Program. J.A.E. was supported by grants from the Hartman Muller organization, the Child and Family Research Institute and the University of British Columbia and has salary support from the Canadian Diabetes Association. We obtained human islets thanks to grant 31-2008-416 from the Juvenile Diabetes Research Foundation. D.J.D. is supported by the Canada Research Chair in Regulatory Peptides, the Banting and Best Diabetes Centre–Novo Nordisk Chair in Incretin Biology and Canadian Institutes of Health Research operating grant 93749. F.M.G., F.R. and A.M.H. are supported by grants from the Wellcome Trust (WT088357 to F.M.G. and WT084210 to F.R.) and EU FP7 grant 266408.
Footnotes
Note: Supplementary information is available on the http://www.nature.com/naturemedicine/ website.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Spranger J, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 2.Herder C, et al. Association of systemic chemokine concentrations with impaired glucose tolerance and type 2 diabetes: results from the Cooperative Health Research in the Region of Augsburg Survey S4 (KORA S4) Diabetes. 2005;54(Suppl 2):S11–S17. doi: 10.2337/diabetes.54.suppl_2.s11. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed-Ali V, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J. Clin. Endocrinol. Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 4.Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- 5.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J. Physiol. (Lond.) 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 7.Febbraio MA, et al. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J. Physiol. (Lond.) 2003;549:607–612. doi: 10.1113/jphysiol.2003.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carey AL, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 9.Mooney RA. Counterpoint: interleukin-6 does not have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 2007;102:816–818. doi: 10.1152/japplphysiol.01208a.2006. discussion 818-819. [DOI] [PubMed] [Google Scholar]
- 10.Jansson JO, Wallenius V. Point-counterpoint: interleukin-6 does/does not have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 2007;102:821. doi: 10.1152/japplphysiol.01353.2006. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen BK, Febbraio MA. Point: interleukin-6 does have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 2007;102:814–816. doi: 10.1152/japplphysiol.01208.2006. [DOI] [PubMed] [Google Scholar]
- 12.Weigert C, Lehmann R, Schleicher ED. Point-counterpoint: interleukin-6 does/does not have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 2007;102:820–821. doi: 10.1152/japplphysiol.01353.2006. author reply 825. [DOI] [PubMed] [Google Scholar]
- 13.Ellingsgaard H, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc. Natl. Acad. Sci. USA. 2008;105:13163–13168. doi: 10.1073/pnas.0801059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creutzfeldt W. The incretin concept today. Diabetologia. 1979;16:75–85. doi: 10.1007/BF01225454. [DOI] [PubMed] [Google Scholar]
- 15.Kieffer TJ, Habener JF. The glucagon-like peptides. Endocr. Rev. 1999;20:876–913. doi: 10.1210/edrv.20.6.0385. [DOI] [PubMed] [Google Scholar]
- 16.Lund PK, Goodman RH, Dee PC, Habener JF. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc. Natl. Acad. Sci. USA. 1982;79:345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983;302:716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- 18.Rouillé Y, Kantengwa S, Irminger JC, Halban PA. Role of the prohormone convertase PC3 in the processing of proglucagon to glucagon-like peptide 1. J. Biol. Chem. 1997;272:32810–32816. doi: 10.1074/jbc.272.52.32810. [DOI] [PubMed] [Google Scholar]
- 19.Zhu X, et al. Disruption of PC1/3 expression in mice causes dwarfism and multiple neuroendocrine peptide processing defects. Proc. Natl. Acad. Sci. USA. 2002;99:10293–10298. doi: 10.1073/pnas.162352599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouillé Y, Martin S, Steiner DF. Differential processing of proglucagon by the subtilisin-like prohormone convertases PC2 and PC3 to generate either glucagon or glucagon-like peptide. J. Biol. Chem. 1995;270:26488–26496. doi: 10.1074/jbc.270.44.26488. [DOI] [PubMed] [Google Scholar]
- 21.Furuta M, et al. Severe defect in proglucagon processing in islet A-cells of prohormone convertase 2 null mice. J. Biol. Chem. 2001;276:27197–27202. doi: 10.1074/jbc.M103362200. [DOI] [PubMed] [Google Scholar]
- 22.Thyssen S, Arany E, Hill DJ. Ontogeny of regeneration of beta-cells in the neonatal rat after treatment with streptozotocin. Endocrinology. 2006;147:2346–2356. doi: 10.1210/en.2005-0396. [DOI] [PubMed] [Google Scholar]
- 23.De León DD, et al. Role of endogenous glucagon-like peptide-1 in islet regeneration after partial pancreatectomy. Diabetes. 2003;52:365–371. doi: 10.2337/diabetes.52.2.365. [DOI] [PubMed] [Google Scholar]
- 24.Nie Y, et al. Regulation of pancreatic PC1 and PC2 associated with increased glucagon-like peptide 1 in diabetic rats. J. Clin. Invest. 2000;105:955–965. doi: 10.1172/JCI7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic alpha-cells in mouse models of ss-cell regeneration. Islets. 2010;2:149–155. doi: 10.4161/isl.2.3.11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen AM, et al. Upregulation of alpha cell glucagon-like peptide 1 (GLP-1) in Psammomys obesus-an adaptive response to hyperglycaemia? Diabetologia. 2011;54:1379–1387. doi: 10.1007/s00125-011-2080-1. [DOI] [PubMed] [Google Scholar]
- 27.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 28.Ehses JA, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 29.Li M, et al. Successful modulation of type 2 diabetes in db/db mice with intra-bone marrow-bone marrow transplantation plus concurrent thymic transplantation. J. Autoimmun. 2010;35:414–423. doi: 10.1016/j.jaut.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabio G, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wueest S, et al. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J. Clin. Invest. 2010;120:191–202. doi: 10.1172/JCI38388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reimann F, et al. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–539. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi K, et al. Blockade of IL-6 signaling exacerbates liver injury and suppresses antiapoptotic gene expression in methionine choline-deficient diet-Fed db/db mice. Lab. Invest. 2011;91:609–618. doi: 10.1038/labinvest.2011.2. [DOI] [PubMed] [Google Scholar]
- 34.Ueda SY, et al. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J. Endocrinol. 2009;201:151–159. doi: 10.1677/JOE-08-0500. [DOI] [PubMed] [Google Scholar]
- 35.Adam TC, Westerterp-Plantenga MS. Activity-induced GLP-1 release in lean and obese subjects. Physiol. Behav. 2004;83:459–466. doi: 10.1016/j.physbeh.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 36.Martins C, Morgan LM, Bloom SR, Robertson MD. Effects of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 2007;193:251–258. doi: 10.1677/JOE-06-0030. [DOI] [PubMed] [Google Scholar]
- 37.Chanoine JP, et al. GLP-1 and appetite responses to a meal in lean and overweight adolescents following exercise. Obesity (Silver Spring) 2008;16:202–204. doi: 10.1038/oby.2007.39. [DOI] [PubMed] [Google Scholar]
- 38.Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2004;287:E1024–E1031. doi: 10.1152/ajpendo.00056.2004. [DOI] [PubMed] [Google Scholar]
- 39.Delmeire D, et al. Prior in vitro exposure to GLP-1 with or without GIP can influence the subsequent beta cell responsiveness. Biochem. Pharmacol. 2004;68:33–39. doi: 10.1016/j.bcp.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 40.Hinke SA, Hellemans K, Schuit FC. Plasticity of the beta cell insulin secretory competence: preparing the pancreatic beta cell for the next meal. J. Physiol. (Lond.) 2004;558:369–380. doi: 10.1113/jphysiol.2004.064881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holz G.G.t., Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Gregorio GB, Hensley L, Lu T, Ranganathan G, Kern PA. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am. J. Physiol. Endocrinol. Metab. 2004;287:E182–E187. doi: 10.1152/ajpendo.00189.2003. [DOI] [PubMed] [Google Scholar]
- 43.Bosco D, et al. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez-Diaz R, et al. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 2011;17(7):888–92. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kieffer TJ, et al. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136(8):3585–96. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 46.Holmes AG, et al. Prolonged interleukin-6 administration enhances glucose tolerance and increases skeletal muscle PPARα and UCP2 expression in rats. J. Endocrinol. 2008;198:367–374. doi: 10.1677/JOE-08-0113. [DOI] [PubMed] [Google Scholar]
- 47.Franckhauser S, et al. Overexpression of Il6 leads to hyperinsulinaemia, liver inflammation and reduced body weight in mice. Diabetologia. 2008;51:1306–1316. doi: 10.1007/s00125-008-0998-8. [DOI] [PubMed] [Google Scholar]
- 48.Sadagurski M, et al. Human IL6 enhances leptin action in mice. Diabetologia. 2010;53:525–535. doi: 10.1007/s00125-009-1580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wallenius V, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 50.Weigert C, et al. Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J. Biol. Chem. 2006;281:7060–7067. doi: 10.1074/jbc.M509782200. [DOI] [PubMed] [Google Scholar]
ONLINE METHODS REFERENCES
- 49.Schuler B, et al. Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc. Natl. Acad. Sci. USA. 2010;107:419–423. doi: 10.1073/pnas.0912924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jevdjovic T, et al. Effects of insulin-like growth factor-I treatment on the endocrine pancreas of hypophysectomized rats: comparison with growth hormone replacement. Eur. J. Endocrinol. 2004;151:223–231. doi: 10.1530/eje.0.1510223. [DOI] [PubMed] [Google Scholar]
- 51.Jevdjovic T, et al. The effect of hypophysectomy on pancreatic islet hormone and insulin-like growth factor I content and mRNA expression in rat. Histochem. Cell Biol. 2005;123:179–188. doi: 10.1007/s00418-005-0760-y. [DOI] [PubMed] [Google Scholar]
- 52.Sporeno E, et al. Human interleukin-6 receptor super-antagonists with high potency and wide spectrum on multiple myeloma cells. Blood. 1996;87:4510–4519. [PubMed] [Google Scholar]
- 53.Parnaud G, et al. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51:91–100. doi: 10.1007/s00125-007-0855-1. [DOI] [PubMed] [Google Scholar]
- 54.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–1154. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- 55.Rudich A, et al. Indinavir uncovers different contributions of GLUT4 and GLUT1 towards glucose uptake in muscle and fat cells and tissues. Diabetologia. 2003;46:649–658. doi: 10.1007/s00125-003-1080-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.