Abstract
Background
Thyroid hormones influences glucose homeostasis. The association of insulin resistance in overt hypothyroidism is well proven, but very less information is available about insulin action on subclinical hypothyroidism.
Objective
This study was done to evaluate the association between thyroid hormones and insulin resistance in subclinical hypothyroidism (SCH).
Materials and Methods
Thirty subjects diagnosed as SCH and 30 age matched euthyroids were included. Serum TSH, FT3, FT4, fasting plasma glucose and insulin were estimated. Homeostasis Model Assessment was used to assess insulin resistance (HOMA- IR).
Results and Conclusion
Serum TSH levels were significantly increased in SCH (14.20 ± 5.23 μU/ml) when compared with euthyroids (2.24 ±1.43μU/ml; P< 0.0001). Serum FT3, FT4 levels in SCH (2.96±0.80 pg/ml & 1.15 ± 0.52 ng/dl) were within the normal range.
The mean insulin levels were significantly elevated in SCH (9.07±3.41 μU/ml) when compared with euthyroids (5.28± 2.18 μU/ml; P-value < 0.0001).
The mean HOMA IR was significantly elevated in SCH (2.03 ± 0.95) when compared with euthyroids (1.05±0.45, P-value < 0.0001).
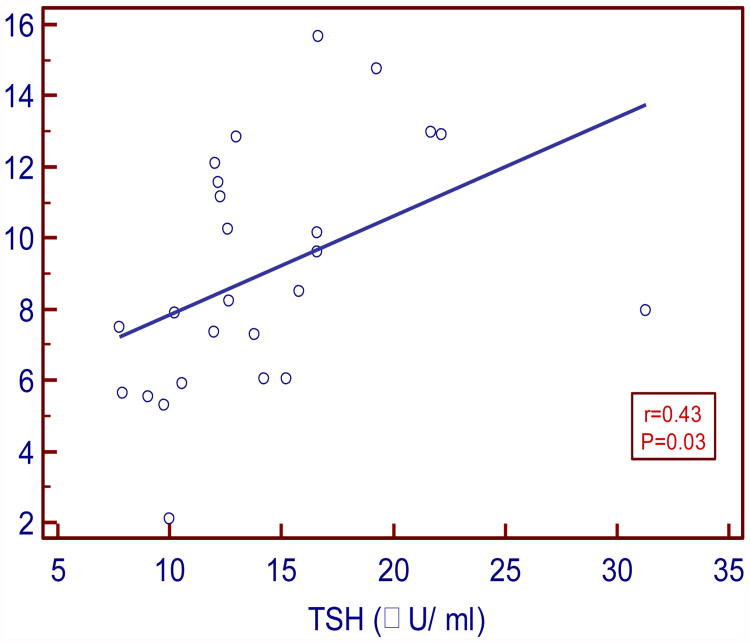
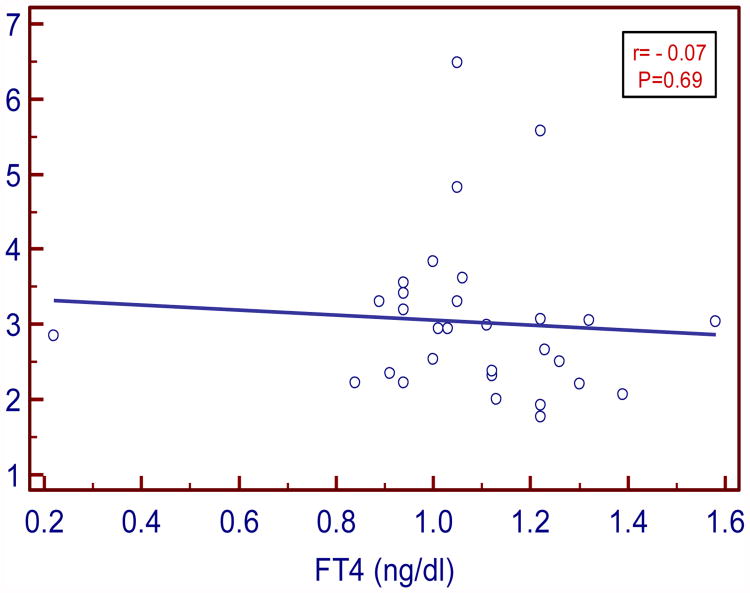
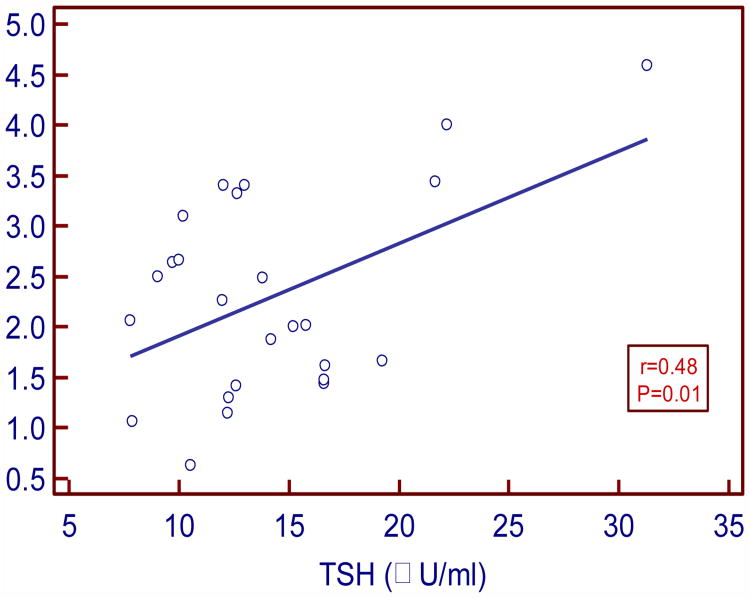
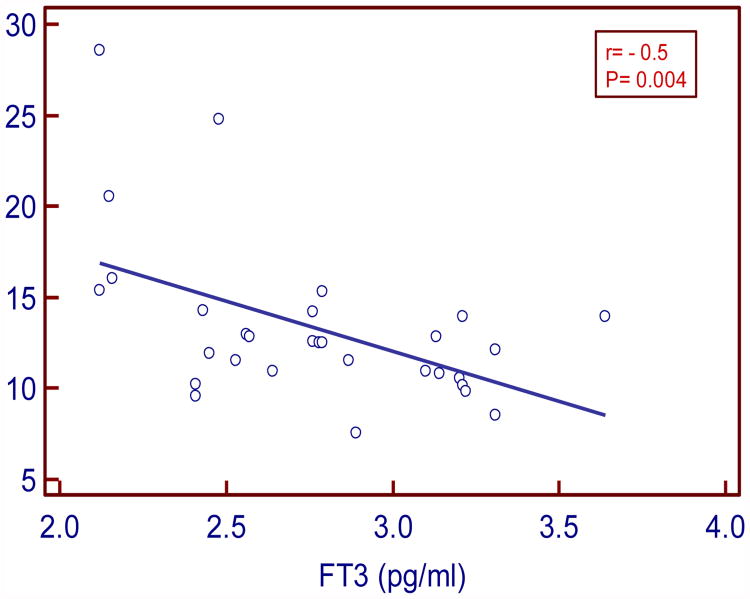
TSH levels positively and moderately correlated with insulin (r= 0.43 P=0.03) and HOMA IR (r =0.48; P= 0.01). FT3 levels negatively and strongly correlated with insulin (r= -0.5, P=0.004) and moderately with HOMA IR (r= -0.38, P= 0.04). FT4 levels negatively and weakly correlated with insulin and IR (r= - 0.11, P=0.54; r= - 0.07, P=0.69 respectively).
To conclude, SCH is associated with insulin resistance. Hence there is an increased risk of insulin resistance associated disorders such as metabolic syndrome, cardiovascular events in SCH.
Keywords: Subclinical hypothyroidism (SCH), Insulin resistance (HOMA IR)
Introduction
Thyroid hormones T3 and T4 maintain a fine balance of glucose homeostasis by acting as insulin agonistic and antagonistic. Hypothyroidism can break this equilibrium and alter glucose metabolism, which can lead to insulin resistance. [1] Insulin resistance is the central pathophysiological phenomenon underlying the metabolic syndrome, which is a major cardiovascular risk factor. [2] Previous studies have established overt hypothyroidism as a risk factor for insulin resistance. [3,4]
Several studies were done to establish an association between SCH and effects of insulin on glucose homeostasis. [5-8] In some studies no significant difference in insulin and HOMA IR was observed when compared with euthyroids state. [7] However, few studies have reported hyperinsulinemia in SCH. [5,6,8] Controversies still exists.
Hence this study was undertaken to see the relationship between thyroid function and insulin resistance in SCH.
Materials and Methods
This is a comparative cross sectional study done in Mediciti Institute of Medical Sciences, Hyderabad from June 2012-February 2013. Subjects were enrolled prospectively from outpatient department of General Medicine. The study was carried out with permission of Institution's ethics committee and informed consent was obtained from all the participants.
A total of sixty subjects were included in the study. Of the 60 subjects, 30 were grouped based on the thyroid status (an elevated serum TSH levels and FT3 and FT4 levels within normal reference range) as subclinical hypothyroids (SCH). The age and socioeconomic status matched 30 volunteers with normal thyroid status were enrolled as euthyroids.
Patients with diabetes, hypertension, liver and renal disorders, congestive cardiac failure, on thyroid hormone treatment, intake of medicines like of statins, oral contraceptive pills, pregnancy and menopause were excluded from the study
SCH is defined as TSH (Thyroid Stimulating Hormone) levels above the upper defined limits with FT3 and FT4 in reference range. [9] (TSH: 0.35- 5.5 μU/ml, FT3: 2.3-4.2pg/ml, FT4: 0.89-1.76 ng/dl)
Under strict aseptic conditions 5ml of fasting blood sample was collected by venous puncture, into properly labeled plain polystyrene tubes for insulin and thyroid hormone estimation and sodium fluoride tubes for blood glucose estimation. The samples were collected, handled and transported to the lab according to the guidelines given by clinical and laboratory standards institute/NCCLS (National Clinical Chemistry Laboratory Standards). [10,11] Blood samples were centrifuged at 10,000 rpm for 10 min. Plasma glucose was estimated with in 30 min of sample collection. Serum was collected in vials and stored at -80°C till the analysis. Serum for thyroid hormones (TSH, FT3, and FT4) and insulin estimation was done in batches. Frozen samples were thawed to room temperature before analysis.
Serum TSH, FT3 and FT4 were estimated by competitive immunoassay using direct chemiluminiscence technology on fully automated Siemens Advia centaur CP.
The reference range for serum TSH in an apparently healthy individual was 0.35- 5.5 μU/ml. The assay range for serum TSH was 0.01-150 μU/ml. The interassay coefficient of variance (CV) using Randox immunoassay controls level 1,2,3 with target values of 0.078 μU/ml, 2.21 μU/ml, 19.9 μU/ml was 4.2%, 4.8%, 3.6% respectively.
The reference range for serum FT3 in an apparently healthy individual was 2.3-4.2pg/ml. The assay range was 0.2-20 pg/ml. The interassay CV using Randox immunoassay controls level 1,2,3 with target values of 3.47 pg/ml, 0.74 pg/ml, 0.115 pg/ml was 5.2%, 3.9%, 3.8% respectively. The reference range for serum FT4 in an apparently healthy individual was 0.89-1.76 ng/dl. The assay range for was 0.1-12 ng/dl. The interassay CV using Randox immunoassay controls level 1,2,3 with target values of 0.741 ng/dl, 2.19 ng/dl, 3.69 ng/dl was 3.2%, 4.1%, 2.2% respectively.
The serum insulin was estimated by two site sandwich immunoassay using direct chemiluminiscence technology which uses constant amount of two antibodies, on fully automated Siemens Advia centaur CP. The reference range for apparently healthy individual was 3-25 μU/ml. The assay range for serum insulin was 0.5- 300 μU/ml. The interassay CV using Randox immunoassay controls level 1,2,3 with target values of 1.4 μU/ml, 13.2 μU/ml, 17.3 μU/ml was 3.0%, 3.2%, 4.3% respectively.
Blood glucose levels were estimated by hexokinase method on Dade Dimension Xpand plus autoanalyser. The reference range for an apparently healthy individual was 74-106 mg/dl. The assay range was 0-500 mg/dl. The interassay CV using biorad controls level 1,2 with target values of 89.8mg/dl and 273mg/dl was 4.8%, 3.9% respectively.
Insulin resistance (IR) was derived from Homeostatic model assessment (HOMA- IR), method for assessing β-cell function and peripheral insulin resistance from fasting insulin and glucose concentrations. [12]
HOMA - IR is the product of the patient's fasting insulin level times fasting glucose levels in mmol/L divided by 22.5. HOMA = Insulin (μU/ml) × Glucose (mmol/L)/22.5.
Statistical Analysis
The data was processed on MS excel work sheet and analysis was carried out using MedCalc Statistical Software version 12.7.8 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014). The results were analyzed by student‘t’ test and the association between the various parameters in a group were evaluated using Pearson's correlation coefficient. A 2-tailed probability value of 0.05 was considered as statistically significant.
Results
Of the thirty euthyroids involved in the study 24 were females and 6 were males. Of the 30 subclinical hypothyroids (SCH), 21 were females and 9 were males. The mean age and BMI of euthyroids and SCH was not statistically significant. Thyroid hormone levels (FT3, FT4, and TSH), fasting blood sugar and fasting insulin were measured in both the groups. From the values of fasting blood sugar and fasting insulin, the HOMA-IR was calculated.
Anthropometric and biochemical characteristics of euthyroids and SCH are showed in Table 1. In our study the serum TSH, insulin and HOMA IR values were significantly elevated in SCH (14.2±5.23 μU/ml, 9.07 ± 3.14 μU/ml, 2.03 ± 0.95 respectively) when compared with euthyroids (2.24 ± 1.43 μU/ml, 5.28 ± 2.18 μU/ml, 1.05 ± 0.45 respectively).
Table no.1. Anthropometric and biochemical characteristics of euthyroids and Subclinical hypothyroidism.
| Euthyroids (N=30) Mean±SD | SCH (N=30) Mean±SD | P-value | |
|---|---|---|---|
| Age (yrs) | 34.2±10.4 | 36.7±9.1 | 0.33 |
| BMI (kg/m2) | 23.4± 1.8 | 27.8± 2.8 | 0.47 |
| FT3 (pg/ml) | 3.08±0.51 | 2.96 ±0.8 | 0.49 |
| FT4 (ng/dl) | 1.24 ± 0.33 | 1.15±0.52 | 0.43 |
| TSH (μU/ml) | 2.24±1.43 | 14.20±5.23 | <0.0001* |
| FBG (mg/dl) | 81.8 ±9.5 | 101.29 ± 20.8 | <0.0001* |
| Insulin(μU/ml) | 5.28 ± 2.18 | 9.07 ± 3.41 | <0.0001* |
| HOMA-IR | 1.05 ± 0.45 | 2.03 ± 0.95 | <0.0001* |
FBG: Fasting Blood Glucose; HOMA IR- Insulin resistance
Significant P<0.05
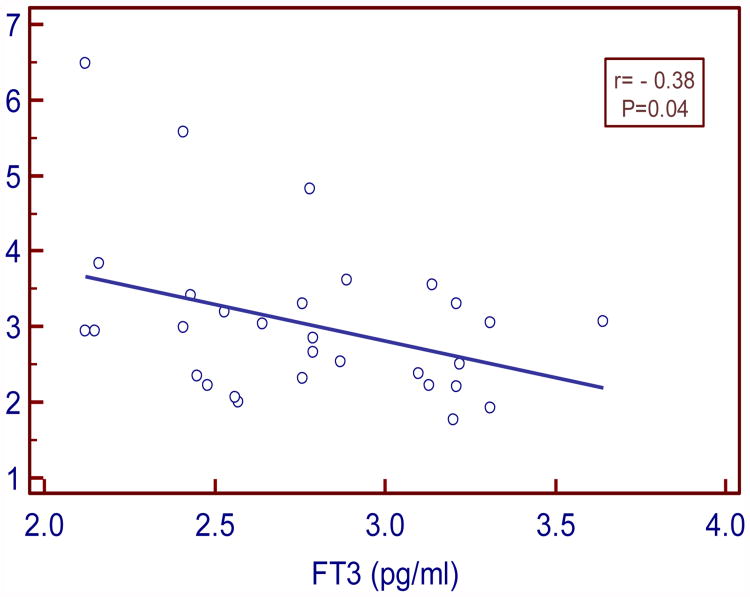
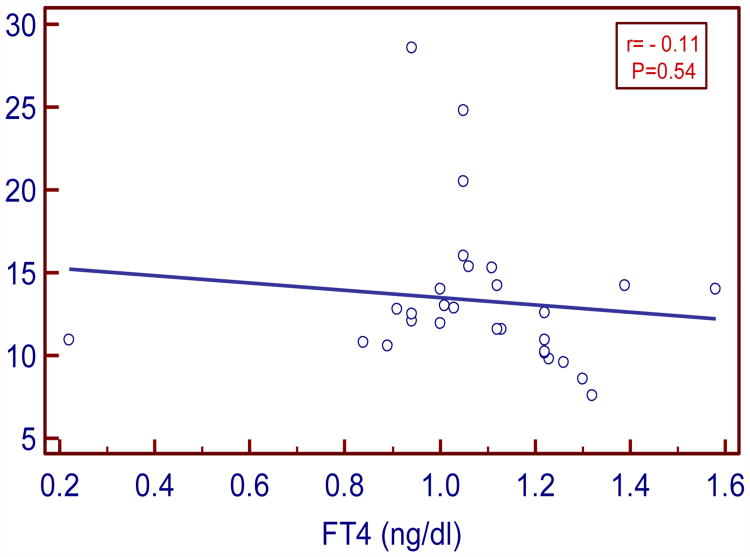
In our study serum TSH levels in SCH moderately and positively correlated with insulin and HOMA IR (r=0.43, P= 0.03 and r= 0.48, P= 0.01 respectively). Serum FT3 levels negatively and strongly correlated with insulin and moderately with HOMA IR (r= - 0.5, P= 0.004; r= - 0.38, P=0.04 respectively). Serum FT4 levels negatively and weakly correlated with insulin and HOMA IR (r= -0.11, P= 0.54 and r= -0.07, P=0.69 respectively).
Pearson's correlation coefficient for the relationships between serum TSH, FT3 and FT4 with insulin and insulin resistance in SCH are shown in Table 2 and graphically represented in figures 1-6.
Table 2. Pearson's correlation between different parameters in Subclinical hypothyroidism.
| Correlation coefficient | P-value | |
|---|---|---|
| TSH vs. Insulin | 0.43 | 0.03* |
| TSH vs. HOMA IR | 0.48 | 0.01* |
| FT3 vs. Insulin | -0.5 | 0.004* |
| FT3 vs. HOMA IR | -0.38 | 0.04* |
| FT4 vs. Insulin | -0.11 | 0.54 |
| FT4 vs. HOMA IR | -0.07 | 0.69 |
HOMA IR- Insulin resistance
Significant P<0.05
Figure:1. Correlation between TSH and insulin in SCH.
Figure:6. Correlation between FT4 and HOMA-IR in SCH.
Discussion
Thyroid dysfunction alters glucose and lipid metabolism which is an important risk factor for cardiovascular disorders. [14] Various studies have mentioned an increased insulin levels in hypothyroidism. [5,6,8,13] In our study we tried to observe the serum insulin levels and insulin resistance (HOMA IR) in subclinical hypothyroidism (SCH).
In our study insulin levels and HOMA-IR were significantly higher in SCH when compared with euthyroid. A positive correlation was observed for TSH with insulin and HOMA IR. This is in accordance with the study done by Maratou E et al, [8] ALSayed et al, [13] Lekakis et al, [14] Tuzcu etal. [6] Maratou E etal [8] attributed it to impaired translocation of GLUT-4 transporters on cell surface of adipose tissue and muscle. ALSayed etal [13] in his study found significantly higher insulin levels in SCH when compared with controls, where as HOMA-IR was not statistically significant. Lekakis etal [14] in his study found similar results and stated that flow mediated endothelial vasodilatation is impaired in hypothyroidism which leads to insulin resistance.
Tuzcu etal [6] in his study found insulin resistance and also observed a positive correlation between TSH and insulin in SCH. In this study prolactin was also estimated and was attributed to insulin resistance. It was stated that fasting hyperinsulinemia and insulin resistance in SCH is due to decrement in insulin receptors due to prolactin excess. Few studies have mentioned altered insulin secretary characteristics and hyperinsulinemia with prolactin. [15-17,20] Serum prolactin was not estimated in our study.
In our study thyroid hormones (FT3 & FT4) had negative correlation with insulin and HOMA- IR -which indicates, lower thyroid hormones in plasma lowers the sensitivity of tissues to insulin. Kim etal [18] in his study stated that thyroid hormone-T3 and insulin have a synergistic role in glucose homeostasis at both cellular and molecular levels. It was hypothesized that impaired insulin stimulated glucose disposal results from reduced intracellular content of serumT3. Hence even mild decrease in the levels of thyroid hormones, as in SCH, inversely correlates with the HOMA IR. [19]
Conclusion
In our study we found in SCH is associated with higher insulin levels and insulin resistance which correlates positively with TSH levels and negatively with FT3 and FT4. There is a risk of development of insulin resistance disorders such as metabolic syndrome, cardiovascular disorders in patients with SCH. SCH is diagnosed either on routine TSH screening or when non specific symptoms are evaluated. Hence regular screening by TSH should be done and thyroid treatment should be started at an early stage. Frequent monitoring with glucose and insulin should be done in order to ward off the adverse cardiovascular effects.
The limitation of our study is small sample size; being a case control study the effects of hormone replacement on insulin resistance could not be inferred. As insulin resistance is seen in both subclinical and overt hypothyroidism, a research focusing on insulin signaling cascade mechanism and its effects of thyroid hormone is needed.
Figure:2. Correlation between TSH and HOMA-IR in SCH.
Figure:3. Correlation between FT3 and insulin in SCH.
Figure:4. Correlation between FT3 and HOMA IR in SCH.
Figure:5. Correlation between FT4 and insuilin in SCH.
Acknowledgments
This project is ICMR Short Term Student ship (STS) programme 2012. Research reported in this publication was conducted by scholars in the Fogarty International Center of the National Institutes of Health training program under Award Number D43 TW 009078. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: nil
References
- 1.Gabriela Brenta. Why Can Insulin Resistance Be a Natural Consequence of Thyroid Dysfunction? J of Thyroid Research. 2011;152850:1–9. doi: 10.4061/2011/152850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.Dimitriadis G, Mitrou P, Lambadiari V, et al. Insulin action in adipose tissue and muscle in hypothyroidism. Journal of Clinical Endocrinology and Metabolism. 2006;91:4930–4937. doi: 10.1210/jc.2006-0478. [DOI] [PubMed] [Google Scholar]
- 4.Cettour-Rose P, Theander-Carrillo C, Asensio C, et al. Hypothyroidism in rats decreases peripheral glucose utilization, a defect partially corrected by central leptin infusion. Diabetologia. 2005;48:624–633. doi: 10.1007/s00125-005-1696-4. [DOI] [PubMed] [Google Scholar]
- 5.Singh BM, Goswami B, Mallika V. Association between Insulin Resistance and Hypothyroidism in Females attending a Tertiary Care Hospital. Indian Journal of Clinical Biochemistry. 2010;25(2):141–145. doi: 10.1007/s12291-010-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuzcu A, Bahceci M, Gokalp D, et al. Subclinical hypothyroidism may be associated with elevated high sensitive C-reactive protein (low grade inflammation) and fasting hyperinsulinemia. Endocr J. 2005;52:89–94. doi: 10.1507/endocrj.52.89. [DOI] [PubMed] [Google Scholar]
- 7.Owecki M, Nikisch E, Sowina ski J. Hypothyroidism has no impact on insulin sensitivity assessed with HOMA IR in totally thyroidectimised patients. Act clinica belgica. 2006;61(2):69–73. doi: 10.1179/acb.2006.013. [DOI] [PubMed] [Google Scholar]
- 8.Maratou E, Hadjidakis D, Kollias A, et al. Studies of insulin resistance in patients with clinical and sub clinical hypothyroidism. Eur J Endocrinol. 2009;160:785–790. doi: 10.1530/EJE-08-0797. [DOI] [PubMed] [Google Scholar]
- 9.SCH: Scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–38. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 10.Procedures for the collection of diagnostic blood specimens by venipuncture: Approved standard. 4th. Wayne PA: National committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 11.Procedures for handling and transport of domestic diagnostic specimens and etiologic agents: Approved standard. 3rd. Wayne PA: National committee for Clinical Laboratory Standards; 1994. [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor, et al. Homeostasis model assessment: insulin resistance and β cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Sayed Al, Ali Nadia, Abbas Y, et al. Subclinical hypothyroidism is associated with insulin resistance in Kuwaiti women. Endocrine journal. 2006;53(5):653–57. doi: 10.1507/endocrj.k06-018. [DOI] [PubMed] [Google Scholar]
- 14.Lekakis J, Papamichael C, Alevizaki M, et al. Flow-mediated, endothelium-dependent vasodilatation is impaired in subjects with hypothyroidism, borderline hypothyroidism, and high normal serum thyrotrophic (TSH) values. Thyroid. 1997;7:411–414. doi: 10.1089/thy.1997.7.411. [DOI] [PubMed] [Google Scholar]
- 15.Tuzcu A, Bahceci M, Dorsum M, et al. Insulin senstivity and hyperprolactinemia. J Endocrinol invest. 2003;26:341–346. doi: 10.1007/BF03345182. [DOI] [PubMed] [Google Scholar]
- 16.Bahceci M, Tuzcu A, Bahceci S, et al. Is hyperprolactinemia is associated with insulin resistance in non obese patient with polycystic ovarian syndrome. J Endocrinol invest. 2003;26:655–659. doi: 10.1007/BF03347025. [DOI] [PubMed] [Google Scholar]
- 17.Schernthaner G, Prager R, Punzengruber C, et al. Severe hyperprolactinemia is associated with decreased insulin binding in vitro and insulin resistance in vivo. Diabetologia. 1985;28:138–142. doi: 10.1007/BF00273860. [DOI] [PubMed] [Google Scholar]
- 18.Kim SR, Tull ES, Talbott EO, et al. A hypothesis of synergism: the interrelationship of T3 and insulin to disturbances in metabolic homeostasis. Medical Hypotheses. 2002;59:660–666. doi: 10.1016/s0306-9877(02)00211-6. [DOI] [PubMed] [Google Scholar]
- 19.Roos A, Bakker SJ, Links TP, et al. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. Journal of Clinical Endocrinology and Metabolism. 2007;92:491–496. doi: 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- 20.Seki K, Nagata I. Levels of glucose and insulin during twenty four hours in hyperprolactinemic women with pituitary microadenoma. Gynecol Obstet Invest. 1991;31:222–225. doi: 10.1159/000293163. [DOI] [PubMed] [Google Scholar]