Abstract
Objective
To examine the prevalence, predictors and effects of nocturia in women, and evaluate overlaps with established urinary tract disorders.
Methods
This was a cross-sectional analysis of 2,016 women, aged 40 years and older, recruited from Kaiser Permanente Northern California from 2008 to 2012. Nocturia and other urinary symptoms were assessed using structured interviewer-administered questionnaires. Nocturia was defined as patient-reported nocturnal voiding of two or more times per night over a typical week.
Results
Thirty-four percent (n=692) reported nocturia, and 40% of women with nocturia reported no other urinary tract symptom. Women with nocturia were older (mean age 58 versus 55) (OR per 5-year increase 1.21 [95% CI, 1.12-1.31]) more likely Black (45%) (OR 1.75, [95% CI, 1.30-2.35]) or Latina (37%) (OR 1.36 [95% CI, 1.02-1.83]) versus non-Latina White (30%), have worse depression (mean Hospital Anxiety and Depression Scale (HADS) score 3.8 versus 2.8)(OR per 1-point increase in HADS score 1.08 [95% CI, 1.04-1.12]) and worse mobility (mean Timed Up-and-Go (TUG) 11.3 versus 10 seconds) (OR per 5-second increase in TUG 1.29 [95% CI, 1.05-1.58]). Nocturia occurred more among women with hysterectomy (53% versus 33%) (OR 1.78 [95% CI, 1.08-2.94]), hot flashes (38% versus 32%) (OR 1.49 [95% CI, 1.19-1.87]), and vaginal estrogen use (42% versus 34%) (OR 1.50 [95% CI, 1.04-2.18]).
Conclusion
Nocturia is common in women and not necessarily attributable to other urinary tract disorders. Factors not linked to bladder function may contribute to nocturia risk, underlining the need for multi-organ prevention and treatment strategies.
Introduction
Nocturia is common among middle-aged and older adults, with up to half reporting waking to urinate at least once per night. (1,2,3) In addition to poor sleep, nocturia has been linked to decreased mental and somatic health, falls and fractures, and increased mortality in some populations.(4,5,6,7)
Among older men, nocturia may occur from impaired bladder emptying due to prostatic hyperplasia,(8) although other factors influencing nocturnal urine production and regulation of bladder function may play a role.(9,10) Among older women, however, the prevalence and severity of nocturia are not as well described, the determinants of nocturia are not as well understood, and many clinicians caring for women are at a loss to explain why some female patients wake up multiple times to urinate but not others.
Nocturia may have received relatively less attention in women's health due to the view of nocturia as resulting from another primary disorder rather than a clinical entity in its own right.(11) Although nocturia can develop in the setting of overactive bladder(12) and polyuria(1), women may also report nocturia without urgency or urinary frequency during the day.
To better understand the prevalence, predictors, and effects of nocturia in women, we examined the age- and race-specific prevalence of nocturnal voiding and the overlap between nocturnal voiding and other common urinary tract symptoms in an ethnically-diverse, community-dwelling population of middle-aged and older women. We also examined duration of nocturia, demographic and clinical factors associated with nocturia, and predictors of greater subjective bother and disruptiveness from nocturia.
Materials and Methods
This research was conducted within a multi-ethnic, observational cohort study of risk factors for urinary tract dysfunction in community-dwelling middle-aged and older women, the Reproductive Risks of Incontinence Study at Kaiser (RRISK). Details about RRISK and participant recruitment have been reported previously.(13) Participants were female members of Kaiser Permanente Northern California (KPNC), an integrated healthcare delivery system serving 25-30% of the northern California population. To be eligible for the study, women had to be at least 40 years of age, to have enrolled in KPNC before age 21, and to have had at least half of any childbirth events at a KPNC facility. Women were randomly sampled from within age and race/ethnicity strata to achieve an overall composition of approximately 20% Black, 20% Latina, 20% Asian and 40% White. No symptoms or history of urinary tract dysfunction were required, but approximately 20% of participants were recruited from the Kaiser Permanente Northern California Diabetes Registry, to enrich the participation in RRISK by women with diabetes. In contrast, the general prevalence of diabetes in California is 13% in those 45-64 years old and 22% in those 65-74 years old.(14)
This study focused on the third wave of RRISK (RRISK3), involving home-based study visits conducted between November 2008 and April 2012.The RRISK cohort was initially interviewed and examined between October 1999 and February 2003 (RRISK1), and a follow-up interview (RRISK2) took place between January 2003 and January 2008. Of the 4,819 women who were contacted to assess eligibility, 3,438 (71%) were found to be preliminarily eligible, 2468 of these (72%) consented to participate, and 2,016 (82%) of these completed a study visit. Fifty-nine percent of eligible women completed the study. Additionally, a subset of 454 (23%) of RRISK3 participants also provided data on nocturia five years earlier as part of RRISK2. All participants provided informed consent before data collection. The institutional review boards of the University of California at San Francisco and the Kaiser Foundation Research Institute approved all study procedures.
Nocturnal voiding frequency was assessed using structured, interviewer-administered questionnaire items previously used in large epidemiologic studies.(15) To minimize missing data and encourage accurate responses, trained female interviewers administered the questions about lower urinary tract symptoms and probed participants to clarify responses when appropriate. Participants were asked to indicate the number of times they urinated at night (“from the time you go to bed until the time you get up in the morning”) during a typical week. Consistent with past research suggesting that nocturnal voiding does not become clinically bothersome until it occurs more than once per night,(16,17,18,19) women were considered to have “clinically significant” nocturia if they reported needing to urinate two or more times per night. To further assess the subjective bothersomeness and functional disruptiveness of nocturia, participants were also asked, “How much has this frequency of night-time urination bothered you?” and “How much has this frequency of night-time urination interfered with your day-to-day activities?” with response options including “Not at all,” “Slightly,” “Moderately,” “Quite a bit,” and “Extremely.”
Other urinary tract symptoms were assessed with interviewer-administered structured questionnaire items validated against a detailed bladder diary.(20) To assess urgency incontinence, women were asked about urine leakage associated with an overwhelming urge to urinate or difficulty holding urine. To assess stress incontinence, participants were asked about urine leakage associated with physical activity such as coughing, lifting, sneezing or exercise. Women were considered to have weekly urgency incontinence or stress incontinence if they reported symptoms occurring at least 4 times per month in the past 3 months. Incontinence that occurred at least 4 times per month but was not associated with an urge to urinate or with physical activity was classified as “other or unclassifiable incontinence.” Daytime frequency was defined as urinating 8 or more times during daytime hours (“from the time you get up in the morning to the time that you go to bed at night”) in a typical week.(16)
Demographic and clinical characteristics were assessed using participant-administered questionnaires, physical examination, performance testing, or abstraction of records from KPNC databases. Race/ethnicity was assessed by self-report. Reproductive and gynecologic histories were ascertained by asking participants about all vaginal and cesarean deliveries, history of hysterectomy and oophorectomy, time elapsed since their last menses and whether they experienced hot flashes in the past month. Chronic health conditions were assessed primarily with participant report of prior physician diagnoses, although diagnoses of diabetes were confirmed with review of clinical records indicating use of a glycemic control medication, fasting blood glucose of 126 mg/dL or higher or elevated hemoglobin Alc level greater than 7.0% on one or more occasions. Depression symptoms were assessed using the depression subscale of the Hospital Anxiety and Depression Scale.(21) Participants' height and weight were measured to calculate body mass index. Cognitive function was assessed using the Mini-Mental State Exam.(22) Physical mobility was assessed using the Timed Up-and-Go Test, where participants were timed while asked to rise from a seated position, walk three meters, turn around, walk back to the chair and sit down.(23)
Medication use was abstracted from KPNC electronic pharmacy databases for participants who filled at least 80% of their medications at a KPNC pharmacy. For participants who filled less than 80% of their prescriptions at a KPNC pharmacy, study interviewers reviewed participants' medication lists and bottles in their homes.
Descriptive statistics were used to examine the overall distribution of nocturnal voiding frequency among participants, stratified by age group and race/ethnicity. The prevalence of other urinary tract symptoms among women with and without clinically significant nocturia was compared using chi-square tests. Among participants who reported urinating at least once per night, we examined the distribution of 1) self-reported bother associated with nocturia and 2) impact of nocturia on day-to-day activities, stratified by nocturnal voiding frequency. Among participants reporting clinically significant nocturia in RRISK3, we also examined the proportion who reported nocturia five years year earlier during RRISK2.
Multivariable logistic regression models assessed potential risk factors of nocturia, including demographics (age, race/ethnicity, relationship status, education), gynecologic variables (parity, hysterectomy, oophorectomy, menopausal status, experience of hot flashes in the past month), common chronic medical conditions (diabetes, hypertension, heart disease, stroke, depression), selected medications (diuretics, systemic and vaginal estrogen, tricyclic antidepressants, calcium channel blockers), physical exam and testing (body mass index, Timed Up-and-Go test, Mini-Mental State Exam), and smoking and alcohol use. All potential risk factors were included in multivariable models, regardless of association with nocturia in bivariate analysis.
Finally, additional multivariable logistic regression models examined characteristics associated with being at least “moderately” bothered by nocturia, including a limited set of predictor variables including age, race/ethnicity, relationship status, depression and anxiety, physical mobility, cognitive function, and frequency of nocturnal voiding. All analyses were performed using SAS statistical software version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Demographic and clinical characteristics of the 2,016 participants are summarized in Table 1. Mean age was 56 years (standard deviation of 9 years), and over half of the cohort self-identified as racial or ethnic minorities (22% Black, 23% Latina, 20% Asian). Thirty-four percent (n=692) of women reported clinically significant nocturia, defined as voiding two or more times per night.
Table 1. Demographic and clinical characteristics of the study population (N=2016).
| Characteristic | Proportion of study population, No. (%) |
|---|---|
| Demographics | |
| Age, years (Mean ± SD) | 56 (±9) |
| Race/Ethnicity | |
| White | 717 (36%) |
| Black | 434 (22%) |
| Latina | 469 (23%) |
| Asian | 396 (20%) |
| Married | 1306 (65%) |
| Completed college | 767 (38%) |
| Gynecologic history | |
| Parity, deliveries (Mean ± SD) | 2.1 ± 1.5 |
| Postmenopausal | 1653 (82%) |
| Hot flashes in the past month | 803 (40%) |
| Hysterectomy | 165 (8%) |
| Bilateral oophorectomy | 88 (4%) |
| Chronic medical conditions | |
| Hypertension | 1062 (53%) |
| Diabetes | 538 (27%) |
| Heart disease (MI, angina or other heart disease) | 140 (7%) |
| Stroke | 82 (4%) |
| Depression: Hospital Anxiety and Depression Score (HADS)b (Mean±SD) | 3.2 (±3.0) |
| Medications | |
| Diuretics | 759 (38%) |
| Calcium channel blockers | 350 (17%) |
| Systemic estrogen | 178 (9%) |
| Vaginal estrogen | 162 (8%) |
| Tricyclic antidepressant | 174 (9%) |
| Anticholinergics (bladder) | 89 (4%) |
| Examination and testing | |
| Timed Up-and-Go,c seconds (Mean±SD) | 10.5 (±3.4) |
| Mini-Mental State Examd Score (Mean±SD) | 28.6 (±1.7) |
| Body Mass Index (BMI) (Mean±SD) | 30.3 (±7.4) |
| Health-related habits | |
| Current smoker | 111 (6%) |
| Alcohol use (5 or more drinks /week) | 176 (9%) |
Data were missing for 4 women for parity, 1 woman for hysterectomy,7 women for bilateral oophorectomy,1 woman for hypertension, 2 women for heart disease, 3 women for stroke, 10 women for Hospital Anxiety and Depression score,53 women for medications, 164 women for Timed Up-and-Go, 34 women for Mini-Mental State Exam, 9 women for BMI, and 2 women for alcohol use.
Hospital Anxiety and Depression Scale (HADS): Scored from 0-21 with higher score representing more severe depression
Timed Up-and-Go: Time it takes to rise from seated position, walk 3 meters, turn around, walk back to the chair and sit down.
Mini-mental state examination: Scored from 0-30 with lower score representing worse cognitive function
Overall frequency of nocturnal voiding increased with age (p-value for linear trend using ordinal regression by age <0.001) (Table 2). However, more than 20% of women under age 50 still reported clinically significant nocturia. Overall frequency of nocturnal voiding was higher in Black and Latina women compared with White women (p<0.001 for heterogeneity using ordinal regression), with 9% of Black and 7% of Latina women reported needing to urinate 4 or more times per night, compared to 4% of White women.
Table 2. Age and Race-Specific Prevalence of Nocturnal Voiding, n (Row %).
| Characteristic | Self-reported voiding episodes per night | ||||
|---|---|---|---|---|---|
| None n=606 |
One n=718 |
Two n=390 |
Three n=194 |
Four or more n=108 |
|
| Age, in years | |||||
| <50 | 238 (37) | 243 (38) | 108 (17) | 39 (6) | 20 (3) |
| 50-64 | 268 (29) | 338 (36) | 184 (20) | 91 (10) | 49 (5) |
| 65 and older | 100 (22) | 137 (31) | 98 (22) | 64 (15) | 39 (9) |
| Race/Ethnicity | |||||
| White | 221 (31) | 283 (40) | 126 (18) | 60 (8) | 27 (4) |
| Black | 100 (23) | 141 (33) | 100 (23) | 56 (13) | 37 (9) |
| Latina | 149 (32) | 147 (31) | 93 (20) | 48 (10) | 32 (7) |
| Asian | 136 (34) | 147 (37) | 71 (18) | 30 (8) | 12 (3) |
p <0.001 for linear trend of age group on the frequency of nocturnal voiding using ordinal logistic regression. p <0.001 for test of heterogeneity across racial/ethnic groups on the frequency of nocturnal voiding using ordinal logistic regression
The majority of women with clinically significant nocturia reported one or more concomitant lower urinary tract symptoms, including weekly urgency incontinence (32%), stress incontinence (28%), daytime frequency (34%). (Table 3) Nevertheless, over a third of participants had “isolated nocturia” in the absence of other clinically significant urinary symptoms. Furthermore, about half of women without clinically significant nocturia reported one or more of other urinary symptoms.
Table 3. Self-reported lower urinary tract symptoms in women with and without clinically significant nocturiab, N (Column %).
| Urinary symptom | Clinically significant nocturiab (N=692) | No clinically significant nocturiab (N=1324) | Pa |
|---|---|---|---|
| Urgency incontinence (weekly)c (N=423) | 222 (32%) | 201 (15%) | <0.001 |
| Stress incontinence (weekly)d (N = 405) | 193 (28%) | 212 (16%) | <0.001 |
| Other or unclassifiable incontinence (weekly)e (N=48) | 17 (2%) | 31 (2%) | 0.87 |
| Day time frequencyf (N=535) | 232 (34%) | 303 (23%) | <0.001 |
| No other significant urinary symptom (N= 971) | 274 (40%) | 785 (59%) | <0.001 |
P-value for Chi-square tests for heterogeneity in the prevalence of different types of urinary symptoms among women with and without clinically significant nocturia
Clinically significant nocturia was defined as urinating 2 or more times from the time of going to bed until getting up in the morning.
Weekly urgency incontinence was defined as leakage associated with overwhelming urge to urinate occurring at least 4 times per month
Weekly stress incontinence was defined as leakage associated with physical activity occurring at least 4 times per month
Other or unclassifiable incontinence was defined as incontinence not associated with an urge to urinate or with physical activity was classified as other or unclassifiable incontinence
Daytime frequency was defined as needing to urinate 8 or more times during the day
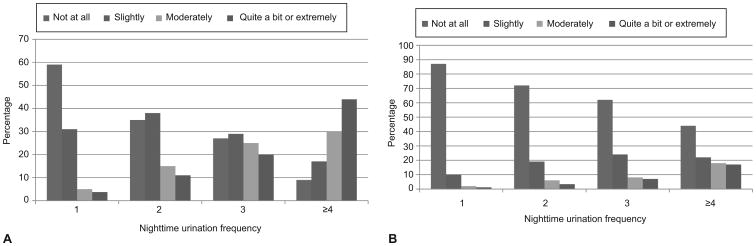
Greater frequency of nocturnal voiding was associated with greater subjective bothersomeness and disruptiveness from nocturia (Figure 1). P-values for the linear trend of nocturnal voiding on being at least moderately bothered and affected day-to-day activities were both <0.001. Of 1407 women who reported at least one voiding episode per night during an average week, 334 (24%) reported being moderately or more bothered by their nocturnal voiding and approximately 122 (9%) of women reported that their nocturnal voiding had at least a moderate effect on their day-to-day activities. Nevertheless, only 12% of those with 4 or more episodes of nocturnal voiding reported being “extremely” bothered, and only 4% of those with this level of nocturia reported that their symptoms interfered “extremely” with their day-to-day activities.
Figure 1. Distribution of bother and disruptiveness from nocturia by voiding frequency.
Response to A) "How much has this frequency of night time urination bothered you?" and B) "How much has this frequency of night time urination affected your day-to-day activities?" P< 0.001 for test of linear trend of nocturnal voiding on the proportion of participants who reported being at least “moderately” bothered by nocturia or who reported that nocturia had at least a “moderate” effect on day-to-day activities using logistic regressions
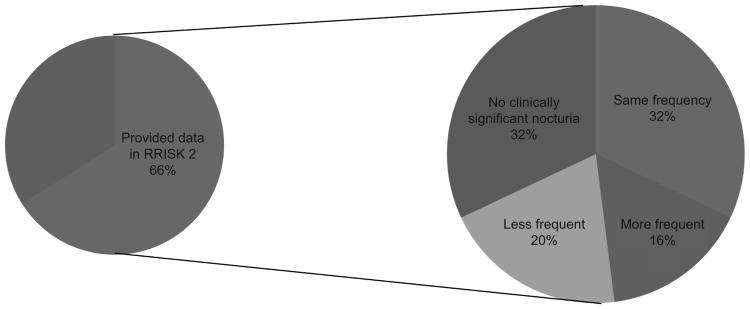
Among the 692 women who reported clinically significant nocturia in RRISK3, a subset of 454 (66%) women also provided data on nocturnal voiding five years earlier as part of RRISK2(13) (Figure 2). Three hundred and eight (68%) of those women also reported clinically significant nocturia during RRISK2. Of these women, 143 (32%) had nocturia that was equally frequent, 74 (16%) had nocturia that was more frequent, and 91 (20%) had nocturia that was less frequent five years ago.
Figure 2. Women with clinically significant nocturia (N=692) who also provided data on nocturnal voiding in RRISK2 (5 years prior).
In multivariable analyses (Table 4), age was an independent predictor of nocturia, with each 5-year increase in age associated with an estimated 21% increase in the odds of reporting nocturia. Additionally, women were more likely to have nocturia if they self-identified as Black or Latina rather than White or had a history of hysterectomy, hot flashes in the past month, worse depressive symptoms, vaginal estrogen use, or decreased physical mobility. No evidence of interaction between postmenopausal status and use of estrogen or bilateral oophorectomy status was detected (p = 0.71 and 0.92 respectively).
Table 4. Adjusted associations between demographic and clinical factors and clinically significant nocturiab.
| Characteristic | Without clinically significant nocturia (Row%) | With clinically significant nocturia (Row%) | Univariate OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|---|---|
| Demographics | ||||
| Age, per 5 year increase | Mean 55 (±9) | Mean 58 (±9) | 1.23 (1.17-1.29) | 1.21 (1.12-1.31) |
| Race/Ethnicity: | ||||
| White | 70 | 30 | Reference | Reference |
| Black | 56 | 45 | 1.89 (1.48-2.43) | 1.75 (1.30-2.35) |
| Latina | 63 | 37 | 1.38 (1.08-1.77) | 1.36 (1.02-1.83) |
| Asian | 72 | 29 | 0.94 (0.72-1.24) | 1.09 (0.79-1.50) |
| Relationship status: | ||||
| Married | 68 | 32 | Reference | Reference |
| Single/Divorced/Widowed | 61 | 39 | 1.34 (1.11-1.62) | 1.09 (0.87-1.36) |
| Education: | ||||
| Completed college | 72 | 28 | Reference | Reference |
| Some college or less | 62 | 38 | 1.57 (1.29-1.90) | 1.17 (0.92-1.48) |
| Gynecologic history | ||||
| Hysterectomy | 47 | 53 | 2.36 (1.71-3.25) | 1.78 (1.08-2.94) |
| Parity, per pregnancy | Mean 2 (±1.4) | Mean 2 (±1.5) | 1.16 (1.09-1.23) | 1.07 (0.99-1.16) |
| Bilateral oophorectomy | 43 | 57 | 2.65 (1.72-4.08) | 1.25 (0.65-2.40) |
| Postmenopausal | 63 | 37 | 2.20 (1.68-2.88) | 1.16 (0.82-1.63) |
| Hot flashes in the past month | 62 | 38 | 1.32 (1.09-1.59) | 1.49 (1.19-1.87) |
| Chronic medical conditions | ||||
| Diabetes | 60 | 40 | 1.44 (1.18-1.77) | 1.07 (0.83-1.39) |
| Hypertension | 60 | 40 | 1.68 (1.40-2.03) | 1.08 (0.81-1.43) |
| Heart disease | 49 | 51 | 2.14 (1.52-3.02) | 1.25 (0.82-1.90) |
| Stroke | 50 | 50 | 1.97 (1.27-3.07) | 1.31 (0.77-2.22) |
| Depression: HADS scorec, per one point increase | Mean 2.8 (±2.9) | Mean 3.8 (±3.1) | 1.10 (1.07-1.14) | 1.08 (1.04-1.12) |
| Medications | ||||
| Diuretics | 59 | 41 | 1.57 (1.30-1.89) | 0.99 (0.75-1.30) |
| Systemic estrogen | 72 | 28 | 0.73 (0.52-1.02) | 0.73 (0.48-1.09) |
| Vaginal estrogen | 58 | 42 | 1.43 (1.03-1.98) | 1.50 (1.04-2.18) |
| Tricyclic antidepressant | 61 | 39 | 1.22 (0.89-1.68) | 1.04 (0.72-1.51) |
| Calcium channel blocker | 53 | 47 | 1.87 (1.48-2.37) | 1.10 (0.82-1.48) |
| Examination and testing | ||||
| Timed Up-and-God, per 5 second increase | Mean 10 (±2) | Mean 11 (±4) | 2.12 (1.77-2.53) | 1.29 (1.05-1.59) |
| MMSEe, per each 1 point decrease | Mean 29 (±2) | Mean 28 (±2) | 1.14 (1.08-1.20) | 1.03 (0.97-1.10) |
| BMI, per 1 unit increase | Mean 30 (±7) | Mean 31 (±8) | 1.02 (1.01-1.04) | 1.01 (0.99-1.02) |
| Health habits | ||||
| Current smoker | 68 | 32 | 0.91 (0.61-1.38) | 0.84 (0.53-1.35) |
| Alcohol use (5 or more drinks /week) | 70 | 30 | 0.81 (0.58-1.13) | 0.97 (0.66-1.43) |
Total sample size N=1813
Clinically significant nocturia: urinating two or more times from the time one goes to sleep until one gets up in the morning.
HADS (Hospital Anxiety and Depression Scale): Scored from 0-21 with higher score representing more severe depression.
Timed Up-and-Go: Time it takes to rise from seated position, walk 3 meters, turn around, walk back to the chair and sit down.
Mini-mental state examination: Scored from 0-30 with lower score representing worse cognitive function.
In multivariate analyses examining factors associated with more subjective bother from nocturia, women were more likely to report being at least moderately bothered if they had more frequent nocturnal voiding (OR 2.63, 95% CI 2.08-3.31 per each additional episode of nocturnal voiding) or higher depression scores (OR 1.09, 95% CI 1.02-1.15 per each 1-point increase in HADS depression subscale score), controlling for age, race or ethnicity, relationship status, physical mobility, and cognitive function. Women who were at least moderately bothered had a mean of 3 (±1) episodes of voiding per night compared with 2 (±1) among women who were not at all or slightly bothered. Mean Hospital Anxiety and Depression Scale score was 4.3 (±3.3) among women who were at least moderately bothered versus 3.4 (±3.0) among women who were not at all or slightly bothered. Among women with “isolated nocturia,” multivariate analyses controlling for the same covariates revealed the same two independent risk factors for at least moderate bothersomeness from “isolated nocturia”: number of episodes of nocturia (OR 2.69; 95% CI, 1.72-4.21 per each additional nocturnal voiding episode) and depression (OR per each 1-point increase in HADS depression subscale scores 1.19; CI, 1.04-1.34). Women with “isolated nocturia” who were at least moderately bothered had a mean of 3 (±1) episodes of voiding per night compared with 2 (±3) among women who were not at all or slightly bothered. Mean Hospital Anxiety and Depression Scale score was 4.7 (± 3.2) among women who were at least moderately bothered versus 3.1 (± 2.7) among women who were not at all or slightly bothered.
Discussion
In our study, over a third of women reported clinically significant nocturia, indicating that nocturia may be as prevalent as other lower urinary tract symptoms that currently receive more attention in women's health. Although nocturnal voiding frequency was higher in older women and those from certain racial or ethnic minority groups, over 20% of women under age 50 and at least 30% of women from all racial or ethnic groups reported nocturnal voiding of at least two episodes in a typical night. Furthermore, approximately a third of women with clinically significant nocturia reported no other urinary tract symptoms, suggesting that nocturia is not exclusively explained by other established urinary tract disorders. Among those reporting clinically significant nocturia, over 60% also reported nocturia 5 years earlier, suggesting that their nocturia was a longstanding or recurrent problem. These results highlight the need for increased awareness, screening, or both for nocturia in women, even in the absence of other urinary symptoms.
Consistent with prior studies suggesting that the effects of nocturia become significant only when individuals void two or more times per night,(16,17,18,19) our data indicate that the bothersomeness and disruptiveness of nocturia increase substantially at this threshold. However, we observed a wide distribution of bother at each level of nocturnal voiding frequency, with some women having very frequent nocturia yet finding their symptoms only mildly bothersome or disruptive. Furthermore, depression was strongly associated with finding nocturia to be bothersome, independent of nocturnal voiding frequency, suggesting that factors unrelated to bladder function may shape women's experience of nocturia.
Our study agrees with prior studies that show increased odds of nocturia with age, racial/ethnic minority status, and history of hysterectomy.(5,24,25,26,27) We also found other factors, such as depression, use of vaginal estrogen, and physical mobility that may contribute to risk of nocturia in community-dwelling women. Rather than focusing predominantly on women's urogynecologic history, clinicians may need to conduct a more comprehensive assessment.
Prior studies have found gynecologic factors such as parity (24,27,28) and postmenopausal status(28) to be associated with nocturia, which did not emerge as independent predictors of nocturia in our analyses. However, those studies were conducted in younger females, (27) in less diverse populations, (24) or had a smaller number of participants who reported nocturia (28). Other medical conditions and health factors such as obesity, (25,29,30) diabetes, (18,19,25) and diuretic use(18,25) have also been reported to be associated with nocturia in prior research. Those studies were generally restricted to geriatric populations,(18,26) included lower numbers of women with nocturia, (19) or used a lower threshold of one or more episodes of nighttime voiding. (25).
There are several limitations of this research. First, assessment of nocturia and other urinary tract symptoms was based on structured questionnaires rather than voiding diaries or frequency volume charts. However, the questionnaire measures used in this research have been used in other large epidemiologic studies and have been shown to have good overall correlation with voiding diaries. (20) Second, our outcome measure could not determine whether women were woken up by the need to urinate or were already awake due to other factors (such as pain, anxiety, hot flash, bed partner activity, or pre-existing insomnia) at the time they decided to void. Third, several risk factors that we identified for nocturia (eg, depression) are also known risk factors for insomnia (31), and their effects on women's risk of nocturia may possibly be mediated by direct effects on sleep quality. Fourth, medical conditions other than diabetes mellitus were assessed primarily by patient report rather than chart review. However, there is no intrinsic reason to believe that recall or report of other comorbid conditions would differ between women who have nocturia versus women who do not have nocturia. Lastly, our study population consisted of long-time female enrollees in an integrated health care system in northern California, and was enriched with women from racial or ethnic minorities and women with diabetes, which may affect the generalizability of our findings to women in other settings.
Our study highlights that nocturia is common and persistent among middle-aged and older women and a substantial proportion of women develop this problem in the absence of other established urinary tract disorders, suggesting that nocturia should not simply be viewed as a manifestation of another primary urinary tract disorder, but as a potential clinical entity in its own right. Strategies for preventing and treating nocturia should address the wide array of factors that may exert indirect effects on urinary function.
Acknowledgments
Supported by the resources and facilities of the San Francisco VA Medical Center and Kaiser Permanente Northern California. Funding was provided by the Office of Research on Women's Health Specialized Center of Research (Grant # P50 DK064538) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (Grant # DK53335). Alison J. Huang, MD is additionally supported by the Paul B. Beeson Career Development Award in Aging Research from the National Institute on Aging (1K23 AG038335) and the American Federation for Aging Research. Louise C. Walter is supported by National Institute on Aging Mid career Mentoring Award for Patient-Oriented Research in Aging (1K24 AG041180). Sei Lee, MD is supported by the Paul B. Beeson Career Development Award in Aging Research from National Institute on Aging (K23 AG040779) and the American Federation for Aging Research.
Alison J. Huang has received research funding from Pfizer, Inc., through grants awarded through the University of California to conduct research unrelated to this article. Jeanette S. Brown is a Scientific Consultant for Allergan and Scientific Advisor for Astellas.
Footnotes
Source of the work: RRISK (Reproductive Risks of Incontinence Study at Kaiser) 3
Presented as an oral presentation at the Society of General Internal Medicine Annual Meeting from April 23-26, 2014 and as a poster at The American Geriatrics Society Annual Meeting from May 14-17, 2014.
Financial Disclosure: The other authors did not report any potential conflicts of interest.
References
- 1.Bosch JL, Weiss JP. The prevalence and causes of nocturia. J Urol. 2013;189:S86–92. doi: 10.1016/j.juro.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Wehrberger C, Madersbacher S, Jungwirth S, Fischer P, Tragl KH. Lower urinary tract symptoms and urinary incontinence in a geriatric cohort - a population-based analysis. BJU Int. 2012;110:1516–21. doi: 10.1111/j.1464-410X.2012.11022.x. [DOI] [PubMed] [Google Scholar]
- 3.van Kerrebroeck P, Abrams P, Chaikin D, Donovan J, Fonda D, Jackson S, et al. The standardisation of terminology in nocturia: Report from the standardisation sub-committee of the international continence society. Neurourol Urodyn. 2002;21:179–83. doi: 10.1002/nau.10053. [DOI] [PubMed] [Google Scholar]
- 4.Asplund R, Marnetoft SU, Selander J, Akerstrom B. Nocturia in relation to somatic health, mental health and pain in adult men and women. BJU Int. 2005;95:816–9. doi: 10.1111/j.1464-410X.2005.05407.x. [DOI] [PubMed] [Google Scholar]
- 5.Coyne KS, Zhou Z, Bhattacharyya SK, Thompson CL, Dhawan R, Versi E. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 2003;92:948–54. doi: 10.1111/j.1464-410x.2003.04527.x. [DOI] [PubMed] [Google Scholar]
- 6.Stewart RB, Moore MT, May FE, Marks RG, Hale WE. Nocturia: A risk factor for falls in the elderly. J Am Geriatr Soc. 1992;40:1217–20. doi: 10.1111/j.1532-5415.1992.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 7.Kupelian V, Wei JT, O'Leary MP, Norgaard JP, Rosen RC, McKinlay JB. Nocturia and quality of life: Results from the boston area community health survey. Eur Urol. 2012;61:78–84. doi: 10.1016/j.eururo.2011.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanker MH, Bohnen AM, Groeneveld FP, Bernsen RM, Prins A, Ruud Bosch JL. Normal voiding patterns and determinants of increased diurnal and nocturnal voiding frequency in elderly men. J Urol. 2000;164:1201–5. [PubMed] [Google Scholar]
- 9.van Doorn B, Kok ET, Blanker MH, Westers P, Bosch JL. Determinants of nocturia: The krimpen study. J Urol. 2014;191:1034–9. doi: 10.1016/j.juro.2013.10.105. [DOI] [PubMed] [Google Scholar]
- 10.Oelke M, Bachmann A, Descazeaud A, Emberton M, Gravas S, Michel MC, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64:118–40. doi: 10.1016/j.eururo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Van Kerrebroeck PE, Dmochowski R, FitzGerald MP, Hashim H, Norgaard JP, Robinson D, et al. Nocturia research: Current status and future perspectives. Neurourol Urodyn. 2010;29:623–8. doi: 10.1002/nau.20913. [DOI] [PubMed] [Google Scholar]
- 12.Robinson D, Cardozo L. Overactive bladder: Diagnosis and management. Maturitas. 2012;71:188–93. doi: 10.1016/j.maturitas.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Thom DH, van den Eeden SK, Ragins AI, Wassel-Fyr C, Vittinghof E, Subak LL, et al. Differences in prevalence of urinary incontinence by race/ethnicity. J Urol. 2006;175:259–64. doi: 10.1016/S0022-5347(05)00039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.California - Percentage of Adults with Diagnosed Diabetes, By Age, 1994-2010. http://apps.nccd.cdc.gov/DDTSTRS/Index.aspx?stateId=6&state=California&cat=prevalence&Data=data&view=A&trend=prevalence&id=1.
- 15.Huang AJ, Moore EE, Boyko EJ, Scholes D, Lin F, Vittinghoff E, et al. Vaginal symptoms in postmenopausal women: Self-reported severity, natural history, and risk factors. Menopause. 2010;17:121–6. doi: 10.1097/gme.0b013e3181acb9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukacz ES, Whitcomb EL, Lawrence JM, Nager CW, Luber KM. Urinary frequency in community-dwelling women: What is normal? Am J Obstet Gynecol. 2009;200:552.e1–552.e7. doi: 10.1016/j.ajog.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tikkinen KA, Johnson TM, 2nd, Tammela TL, Sintonen H, Haukka J, Huhtala H, et al. Nocturia frequency, bother, and quality of life: How often is too often? A population-based study in finland. Eur Urol. 2010;57:488–96. doi: 10.1016/j.eururo.2009.03.080. [DOI] [PubMed] [Google Scholar]
- 18.Burgio KL, Johnson TM, 2nd, Goode PS, Markland AD, Richter HE, Roth DL, et al. Prevalence and correlates of nocturia in community-dwelling older adults. J Am Geriatr Soc. 2010;58:861–6. doi: 10.1111/j.1532-5415.2010.02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Zhang J, Chen J, Zhang C, Li Q, Xu T, et al. Prevalence and risk factors of nocturia and nocturia-related quality of life in the chinese population. Urol Int. 2011;86:173–8. doi: 10.1159/000321895. [DOI] [PubMed] [Google Scholar]
- 20.Bradley CS, Brown JS, Van Den Eeden SK, Schembri M, Ragins A, Thom DH. Urinary incontinence self-report questions: Reproducibility and agreement with bladder diary. Int Urogynecol J. 2011;22:1565–71. doi: 10.1007/s00192-011-1503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. an updated literature review. J Psychosom Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Podsiadlo D, Richardson S. The timed “up & go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 24.Brieger GM, Yip SK, Hin LY, Chung TK. The prevalence of urinary dysfunction in hong kong chinese women. Obstet Gynecol. 1996;88:1041–4. doi: 10.1016/S0029-7844(96)00335-3. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald MP, Litman HJ, Link CL, McKinlay JB BACH Survey Investigators. The association of nocturia with cardiac disease, diabetes, body mass index, age and diuretic use: Results from the BACH survey. J Urol. 2007;177:1385–9. doi: 10.1016/j.juro.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 26.Asplund R. Nocturia in relation to sleep, somatic diseases and medical treatment in the elderly. BJU Int. 2002;90:533–6. doi: 10.1046/j.1464-410x.2002.02975.x. [DOI] [PubMed] [Google Scholar]
- 27.Tikkinen KA, Auvinen A, Tiitinen A, Valpas A, Johnson TM, 2nd, Tammela TL. Reproductive factors associated with nocturia and urinary urgency in women: A population-based study in finland. Am J Obstet Gynecol. 2008;199:153.e1–153.12. doi: 10.1016/j.ajog.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 28.Alling Moller L, Lose G, Jorgensen T. Risk factors for lower urinary tract symptoms in women 40 to 60 years of age. Obstet Gynecol. 2000;96:446–51. [PubMed] [Google Scholar]
- 29.Tikkinen KA, Auvinen A, Johnson TM, 2nd, Weiss JP, Keranen T, Tiitinen A, et al. A systematic evaluation of factors associated with nocturia--the population-based FINNO study. Am J Epidemiol. 2009;170:361–8. doi: 10.1093/aje/kwp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schatzl G, Temml C, Schmidbauer J, Dolezal B, Haidinger G, Madersbacher S. Cross-sectional study of nocturia in both sexes: Analysis of a voluntary health screening project. Urology. 2000;56:71–5. doi: 10.1016/s0090-4295(00)00603-8. [DOI] [PubMed] [Google Scholar]
- 31.Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30:873–80. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]