Abstract
Importance
Anemia is common in patients with advanced chronic kidney disease. While the treatment of anemia in patients with end-stage renal disease (ESRD) has attracted considerable attention, relatively little is known about patterns and trends in the anemia care received by patients before initiating maintenance dialysis or pre-emptive kidney transplantation.
Objective
To determine the trends in anemia treatment received by Medicare beneficiaries approaching ESRD.
Design
Closed cohort study.
Setting
United States using national ESRD registry data (U.S. Renal Data System).
Participants
466,803 patients aged ≥67 years initiated maintenance dialysis or underwent pre-emptive kidney transplantation between 1995 and 2010. All eligible patients had uninterrupted Medicare (A+B) coverage for >2 years before ESRD.
Exposure
Time, defined as calendar year of incident ESRD.
Main Outcomes and Measures
Use of erythropoiesis-stimulating agents (ESA), intravenous iron supplements, and blood transfusions in the two years prior to ESRD; hemoglobin concentration at the time of ESRD. We used multivariable modified Poisson regression to estimate adjusted utilization prevalence ratios (aPR) with 95% confidence intervals (CI).
Results
The proportion of incident ESRD patients receiving any ESA in the two years before increased from 3.2% in 1995 to a peak of 40.8% in 2007; thereafter, ESA use declined modestly to 35.0% in 2010 (compared with 1995: aPR=9.9; CI: 9.0–10.7). Among patients who received an ESA, median time from first recorded ESA use to ESRD increased from 120 days in 1995 to 337 days in 2010. Intravenous iron administration increased from 1.2% (1995) to 12.3% (2010; aPR=8.7; CI: 7.6–10.1). The proportion of patients receiving any blood transfusions increased monotonically from 20.6% (1995) to 40.3% (2010; aPR=1.88; CI: 1.82–1.95). Mean hemoglobin concentrations were 9.5 g/dL in 1995, increased to a peak of 10.3 g/dL in 2006, and then declined moderately to 9.9 g/dL in 2010.
Conclusions and Relevance
Between 1995 and 2010, older adults approaching ESRD were increasingly more likely to be treated with ESAs, to receive intravenous iron, but also more likely to be transfused.
Keywords: chronic kidney disease, end-stage renal disease, dialysis, erythropoietin, iron, blood transfusion
Introduction
Anemia is a hallmark complication of advanced chronic kidney disease (CKD) and a consequence of reduced erythropoietin production by the kidneys, among other factors.1 Using data from the National Health and Nutrition Evaluation Survey III (NHANES; 1988–1994), Hsu et al. found that even among patients who were relatively iron replete (ferritin ≥ 100 ng/mL and transferrin saturation ≥ 20%), 46% (95% confidence interval, CI: 33% to 59%) of men with advanced CKD (creatinine clearance ≤ 30 mL/min) had a hemoglobin concentration <12 g/dL and 21% (95% CI: 15% to 27%) of women had a hemoglobin concentration <11 g/dL.2 While not specifically ascertained in that study, it is unlikely that a sizeable proportion of persons with advanced CKD in the U.S. received any maintenance treatment for anemia as only limited treatment options were available and used in patients with non-dialysis requiring CKD at the time. Iron dextrans of various molecular weights were the only formulations available at that time; relatively few patients with non-dialysis requiring CKD used the iron dextrans for fear of anaphylactoid reactions;3, 4 even fewer used anabolic steroids, which had also been shown to increase hemoglobin concentrations in ESRD.5
Based on its demonstrated ability to raise hemoglobin concentrations and to avoid transfusions,6 epoetin alfa (EPO) was approved for use by the U.S. Food and Drug Administration (FDA) in 1989 for the treatment of anemia associated with chronic renal failure, including patients on dialysis (ESRD) and patients not on dialysis. Comprehensive guidelines on the anemia of kidney disease were published by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) in 1997 in which treatment of anemia using EPO was strongly endorsed, however, mostly based on expert opinion about potential clinical benefit beyond erythropoiesis alone.7 Data from patients with ESRD indicate increased use of ESAs between 1992 and 2005, while transfusion rates declined during that time.8 However, clinical trials that were subsequently published raised serious concerns about more intensive treatment of anemia using ESAs in patients with CKD.9–12 Their results led to more critical assessment of targets of ESA treatment, including changes in ESA labels, and to reduced use of ESAs in patients with CKD requiring dialysis in the most recent years.13 However, little is known about trends in the treatment of anemia in patients with CKD not yet requiring dialysis.
We conducted the present study to examine trends in the use of several types of anemia treatment before the initiation of dialysis or receipt of pre-emptive transplantation in older adults.
Materials and Methods
Study Population
Using the United States Renal Data System (USRDS), the national ESRD registry, we identified all patients age 67 years or older who initiated dialysis or who underwent preemptive kidney transplantation in the continental US, Alaska, or Hawaii between 1995 and 2010. We restricted the cohort to all patients who, per payor history file, had uninterrupted Medicare Part A and B coverage for at least two years prior to ESRD, the latter being the study index.
Exposure of Interest
The exposure of interest was calendar year of initiation of treatment for ESRD.
Variables of Interest – Indicators for Anemia Treatment
From two years of Medicare claims prior to initiation of dialysis or receipt of preemptive kidney transplantation, we ascertained receipt of several treatments commonly used to treat anemia in patients with advanced CKD: erythropoiesis stimulating agents (ESA: epoetin alfa, EPO; darbepoetin alfa; DPO), intravenous iron supplements (iron dextran; iron sucrose; sodium ferric gluconate; ferumoxytol), and blood transfusions (for specific codes used refer to the Technical Appendix; Supplemental Table S1). The timing of the earliest recorded receipt of each of these treatments relative to the initiation of ESRD treatment was also noted. For blood transfusions, we defined the distinct number of days on which a transfusion was administered to each patient (the number of transfusions given on any single day cannot be ascertained from claims data). From the Medical Evidence Report (form CMS-2728), we ascertained the hemoglobin concentration or hematocrit reportedly present at initiation of treatment for ESRD. Hematocrit values were converted into approximate hemoglobin concentrations by dividing by 3.
Patient Characteristics
We ascertained the following characteristics from the USRDS patient file: age (on index), sex, race (white, black, Asian, other), and (dual) eligibility for Medicaid. We also ascertained reported kidney function at dialysis initiation (estimated glomerular filtration rate, eGFR), serum albumin, and Quételet’s (body mass) index (BMI) from the Medical Evidence Report.
We additionally identified the following co-morbidities from the Medical Evidence Report and from Medicare claims using previously published algorithms:14 diabetes, hypertension, heart failure, coronary artery disease, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, and cancer. Comorbidities were ascertained in the two-year period prior to the index date and were established by at least one inpatient or two outpatient claims not on the same day.
Statistical Analysis
We described all patient characteristics by incident year (collapsed into 3 eras: 1995–2000; 2001–2005; 2006–2010) using medians and interquartile ranges for continuous variables and percentages for categorical variables. We then tabulated and/or plotted receipt of each category of anemia treatment prior to ESRD over time. We estimated prevalence ratios (PR) and their corresponding 95% confidence intervals (CI) using Poisson regression with a modified variance to get proper inference.15 This was done (1) without any adjustment; (2) with adjustment for demographics; (3) with additional adjustment for presence of diabetes and eGFR and BMI at initiation; and (4) with adjustment for all reported comorbidities. The third model is reported in the results unless otherwise noted. Year was treated as a categorical variable to allow for non-linear changes. The earliest year (1995) served as the referent. A priori, we anticipated an increase of utilization of ESAs over time, but then a decrease following the publication of two trials questioning the benefit and indicating some harm from more aggressive anemia treatment in 2006.10, 11 Therefore, we also conducted analyses that used 2007 as the referent year to quantify any changes following the year in which important safety information on ESA use in CKD became available. For the same reason, we compared the trends for any transfusion use and for number of transfusion days between the 2003–2006 and 2007–2010 eras using interrupted time series analyses.16
Certain variables had incomplete data, especially those on eGFR and BMI at ESRD incidence. Since missingness for the variables considered for multivariable adjustment affected fewer than 10% of patients, we conducted complete case analyses. However, we also conducted sensitivity analyses that employed multiple imputation using chained equation (MICE package in R) with five imputations to test the robustness of our findings; findings were not materially different and are reported in the Technical Appendix (Supplemental Tables S2–S4), but not further discussed.
Analyses were performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC) and R (The R Project for Statistical Computing; Vienna, Austria). The Institutional Review Board of Stanford University approved the study.
Results
We identified 702,192 patients ≥67 years of age who initiated treatment for ESRD between 1995 and 2010. Of those, 471,083 had two years of uninterrupted Medicare fee-for-service (Parts A and B) coverage prior to the index date. Additional 4,275 patients were excluded who resided outside the continental US, yielding the final cohort of 466,803. Trends in patient characteristics over time are illustrated in Table 1. Pertinent to this study of anemia and its treatment, patients initiating treatment for ESRD in more recent years did so with more preserved kidney function (i.e., higher eGFR), higher BMI and a more substantial degree of comorbidity (diabetes, cerebrovascular, and peripheral artery diseases). Patients initiating dialysis in more recent years also had experienced more and earlier care by a nephrologist, as previously reported.17
Table 1.
Characteristics of Older Patients Initiating Treatment for End-stage Renal Disease, by Era
| 1995–2000 N=157,492 |
2001–2005 N=159,726 |
2006–2010 N=149,585 |
|
|---|---|---|---|
| Patient Demographics | |||
| Patient age (years) | 75 (71–80) | 76 (72–81) | 77 (72–82) |
| Female | 50.1 | 48.7 | 45.9 |
| Race | |||
| White | 75.8 | 76.6 | 78.0 |
| Black | 21.1 | 20.0 | 18.1 |
| Asian | 2.0 | 2.3 | 3.0 |
| Other | 1.1 | 1.1 | 0.8 |
| Missing | 0.1 | 0.1 | 0.0 |
| Medicaid (“dual”) beneficiary | 18.2 | 19.7 | 18.8 |
| Cause of ESRD | |||
| Diabetes | 38.3 | 39.8 | 38.5 |
| Hypertension | 35.4 | 36.2 | 36.8 |
| Glomerulonephritis | 8.0 | 5.9 | 5.3 |
| Other | 15.8 | 16.5 | 17.7 |
| Missing | 2.5 | 1.6 | 1.6 |
| Pre-emptive kidney transplantation | 0.1 | 0.3 | 0.6 |
| Pre-ESRD Nephrology Care | |||
| Earliest outpatient visit prior to ESRD | |||
| None | 47.4 | 39.5 | 31.7 |
| >18 months | 16.6 | 24.5 | 34.8 |
| 18–12 months | 7.3 | 8.5 | 8.6 |
| 12–6 months | 9.1 | 9.3 | 8.8 |
| <6 months | 19.6 | 18.3 | 16.2 |
| Number of outpatient visits in 2 years prior to ESRD | 7 (3–17) | 8 (3–19) | 9 (4–23) |
| Comorbidities | |||
| Diabetes | 56.1 | 61.2 | 64.2 |
| Hypertension | 94.2 | 96.6 | 98.1 |
| Heart failure | 72.0 | 71.5 | 70.1 |
| Coronary artery disease | 46.4 | 45.5 | 44.0 |
| Cerebrovascular disease | 33.6 | 34.9 | 36.9 |
| Peripheral artery disease | 41.9 | 42.9 | 43.6 |
| Chronic obstructive pulmonary disease | 10.1 | 11.8 | 13.3 |
| Cancer | 20.9 | 22.3 | 24.6 |
| Estimated GFR at ESRD (mL/min per 1.73 m2) | 8.2 (6.2–11.0) | 9.8 (7.3–13.3) | 11.1 (9.3–14.6) |
| Missing | 7.3 | 3.5 | 3.7 |
| Body mass index at ESRD (kg/m2) | 24.1 (21.1–27.8) | 25.4 (22.2–29.5) | 26.4 (23.0–30.9) |
| Missing | 15.0 | 2.5 | 2.9 |
| Serum albumin at ESRD (g/dL) | 3.2 (2.8–3.6) | 3.2 (2.7–3.6) | 3.2 (2.7–3.6) |
| Missing | 28.9 | 27.0 | 24.8 |
All values are presented as percent or median (interquartile range).
ESRD – end-stage renal disease; GFR – glomerular filtration rate.
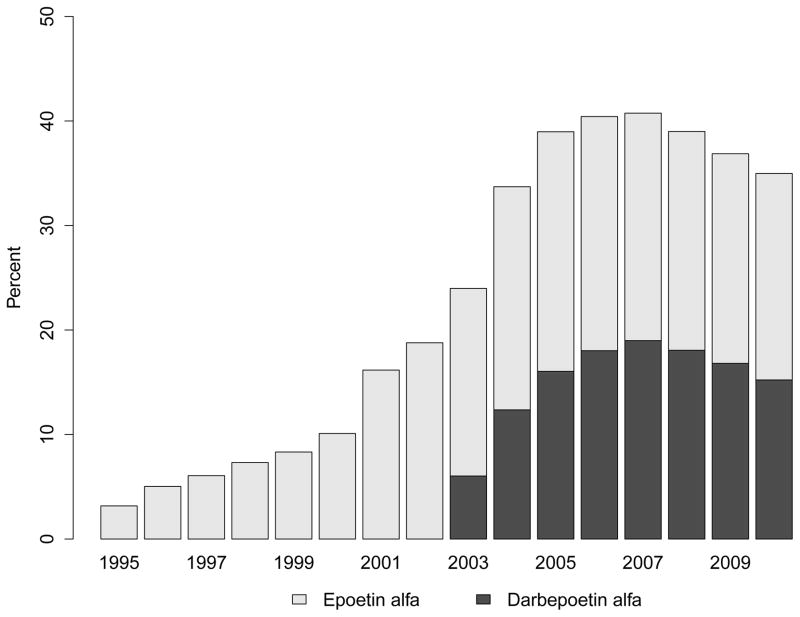
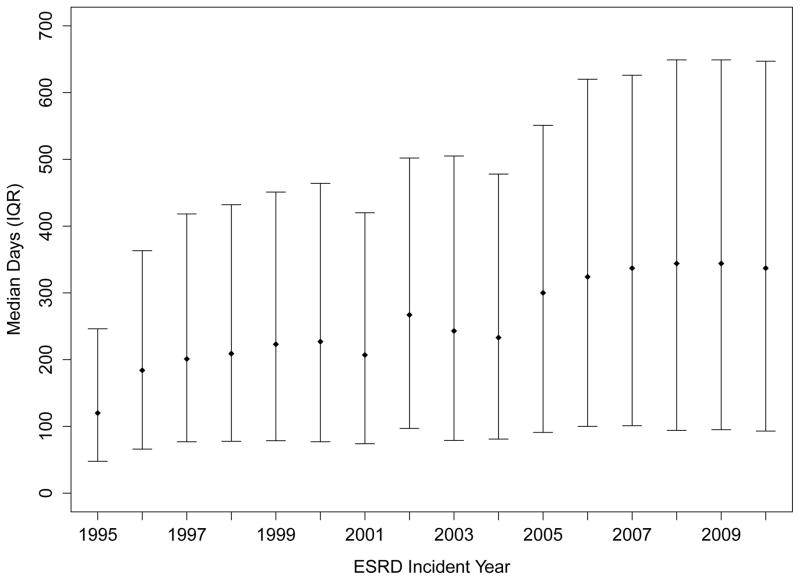
Use of ESAs increased from 3.2% in patients approaching ESRD in 1995 to 40.8% among those who did so in 2007; therafter, ESA use plateaued or decreased slightly to 35.0% in 2010 (Figure 1, Panel A). The proportion using darbepoetin alfa, after its FDA approval in 2001 and availability of a specific billing code in 2003, increased swiftly and exceeded 46% in 2007 (Figure 1, Panel A). Among patients who received an ESA, median time from first recorded ESA use to ESRD increased from 120 days in 1995 to 337 days in 2010 (Figure 1, Panel B).
Figure 1. Trends in Treatment with Erythropoiesis-Stimulating Agents Prior to End-Stage Renal Disease.
Panel A: Proportion of Patients Having Received Treatment with an Erythropoiesis-Stimulating Agent (any; epoetin alfa; darbepoetin alfa)
Panel B: Median Time from First Recorded Treatment with an Erythropoiesis-Stimulating Agent to End-Stage Renal Disease (in days)
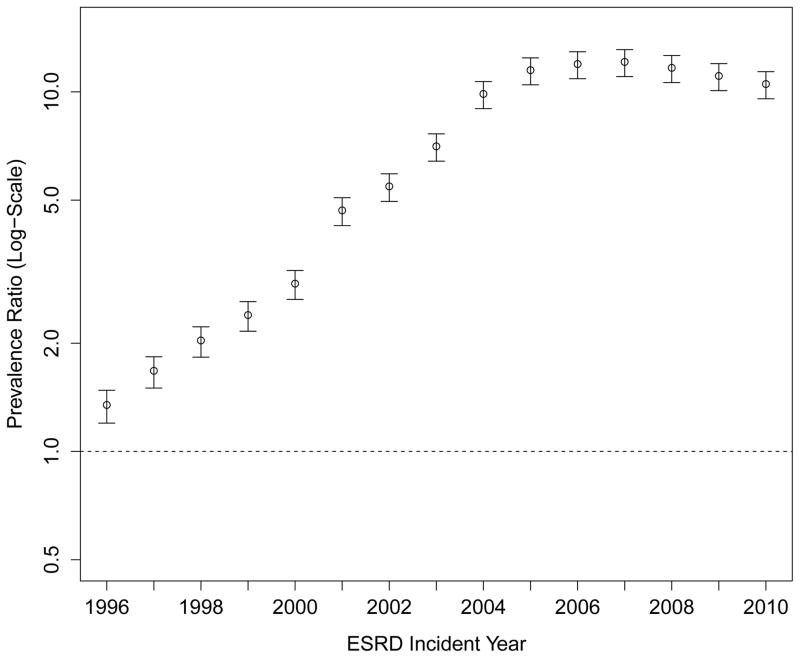
Panel C: Adjusted Prevalence Rates of Erythropoesis-Stimulating Agent Use Prior to End-Stage Renal Disease (Referent: 1995)
Multivariable model adjusted for age, sex, race, Medicaid (“dual”) eligibility, comorbid diabetes, estimated glomerular filtration rate and body mass index at end-stage renal disease.
When modeling temporal trends in pre-ESRD ESA use (Figure 1, Panel C; Supplemental Table S2), compared with 1995, patients initiating treatment for ESRD in 2010 were almost 10-times (PR: 9.9; 95% CI: 9.0 to 10.7) more likely to have received an ESA. The findings were similar after adjustment for demographic characteristics and for eGFR, BMI and the prevalence of diabetes at ESRD incidence (Table S2).
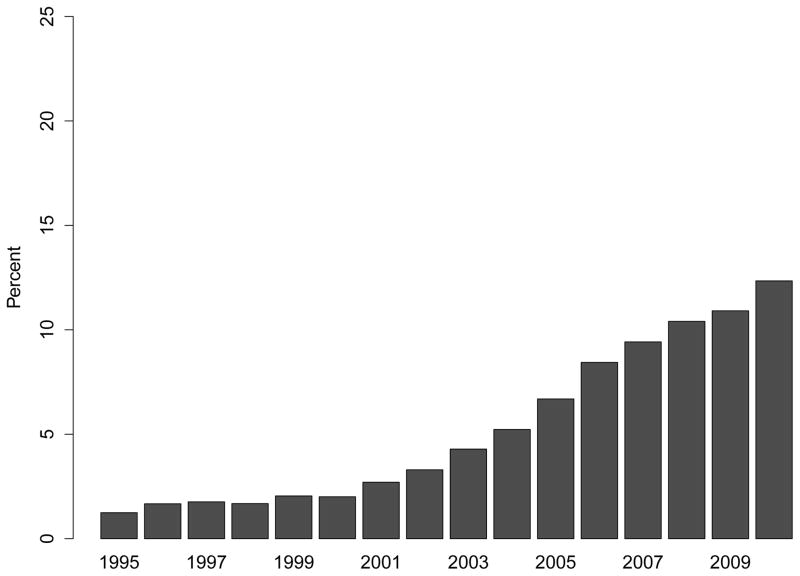
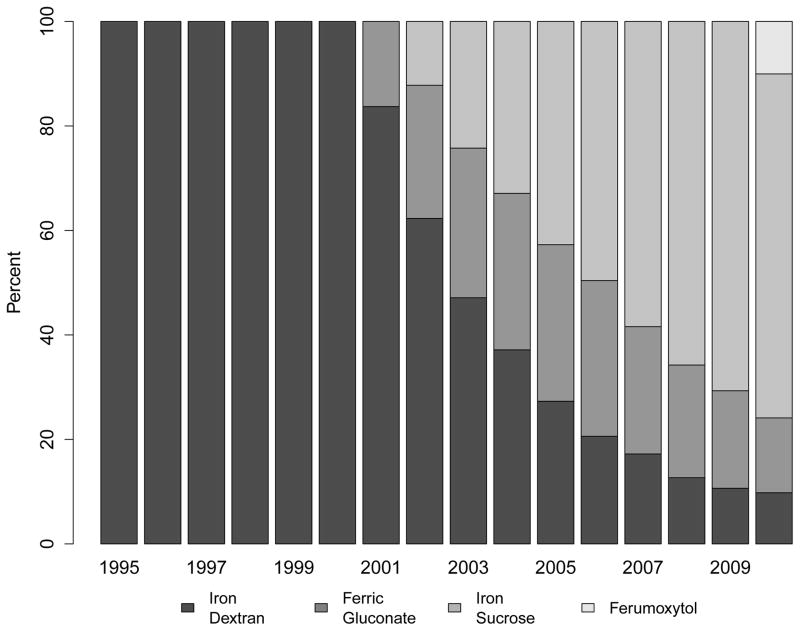
Use of intravenous iron supplementation was low while iron dextran remained the only treatment option (e.g., 1.3% in 1995) and increased rapidly once sodium ferric gluconate and iron sucrose became available (Figure 2, Panel A), which were approved by the FDA in 1999 and 2000, respectively. The specific intravenous iron formulation used in patients’ earliest recorded intravenous iron claim is shown in Figure 2, Panel B. Among patients initiating treatment for ESRD in 2010, 12.3% had received intravenous iron in the two years before ESRD treatment, a more than 9-fold increase over the 15-year study period (PR: 9.20; 95% CI: 8.0–10.6; Figure 2, Panel C; Table S3).
Figure 2. Trends in Treatment with Intravenous Iron Supplements Prior to End-Stage Renal Disease.
Panel A: Proportion of Patients Receiving Any Intravenous Iron
Panel B: Specific Intravenous Iron Formulation Used in the Earliest Available Claim (iron dextran; iron sucrose; ferrous gluconate; ferumoxytol)
Panel C: Adjusted Prevalence Ratios of Intravenous Iron Use Prior to End-Stage Renal Disease (Referent: 1995)
Multivariable model adjusted for age, sex, race, Medicaid (“dual”) eligibility, comorbid diabetes, estimated glomerular filtration rate and body mass index at end-stage renal disease.
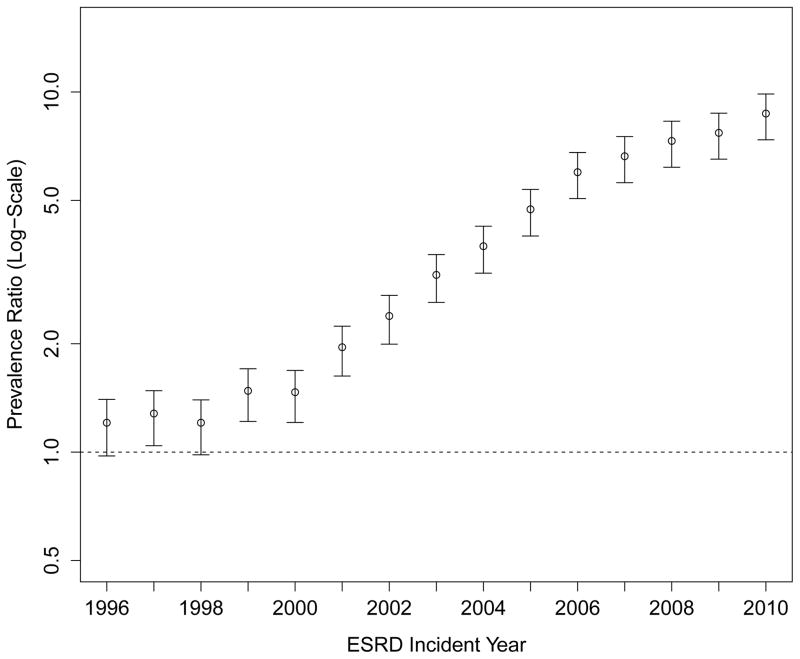
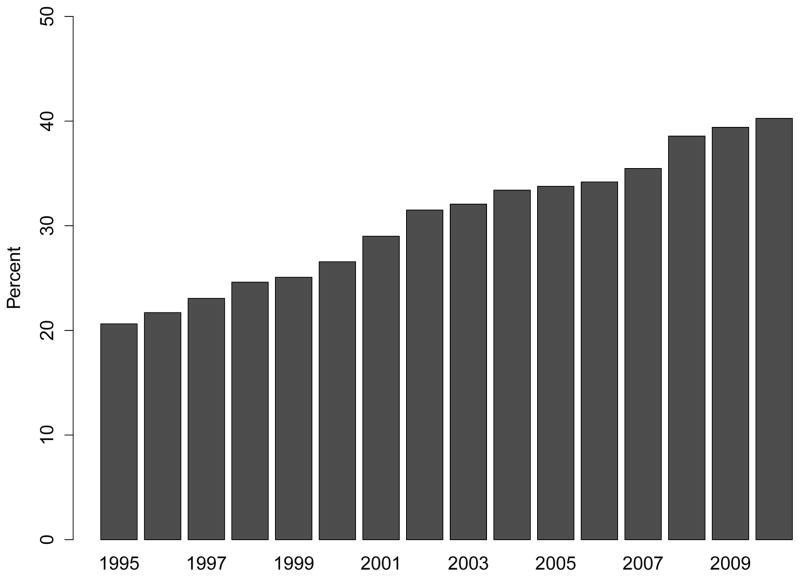
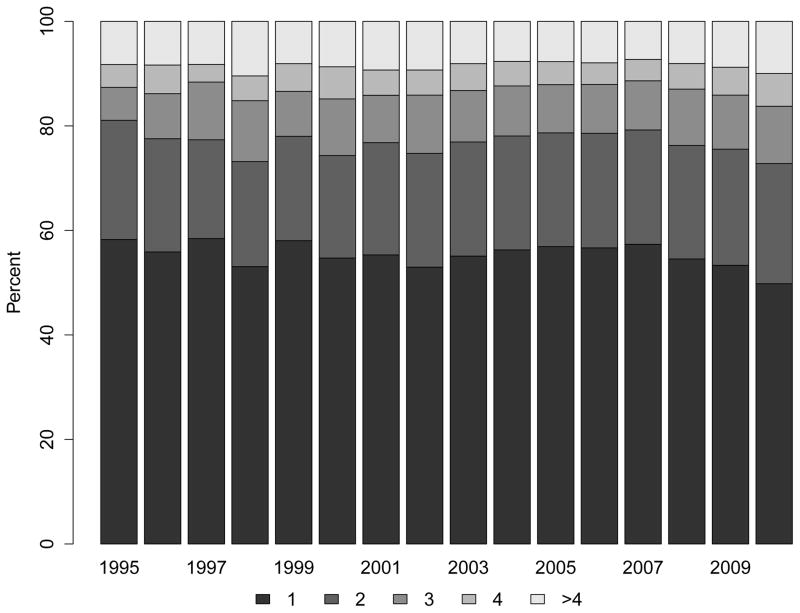
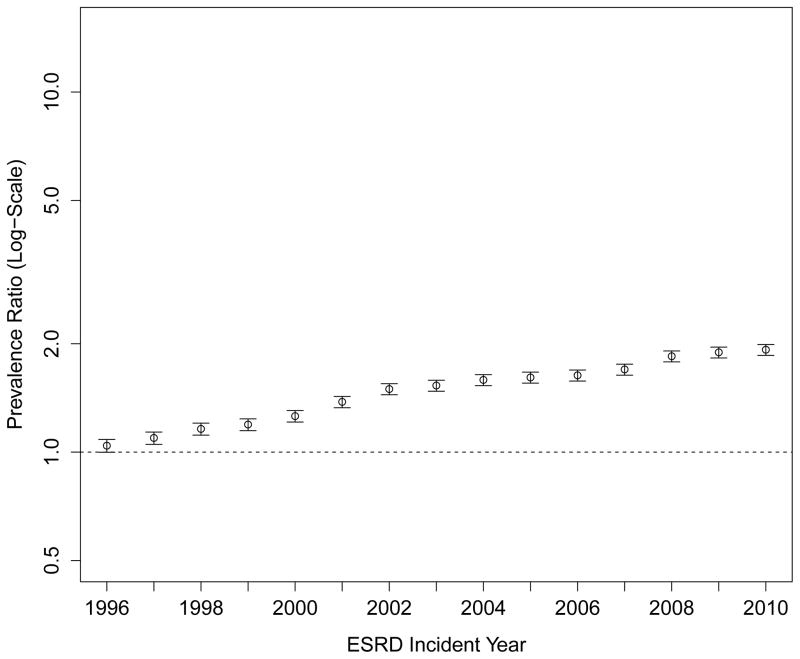
Despite more frequent use of ESAs and intravenous iron, the proportion of patients receiving at least one transfusion also increased considerably over time (Figure 3, Panel A). While 20.6% of patients reaching ESRD in 1995 had received at least one blood transfusion in the two years prior to their first ESRD treatment, the proportion increased steadily, reaching 40.3% among patients approaching ESRD in 2010. The PR comparing receipt of transfusion in patients approaching ESRD in 2010 vs. 1995 was 1.88 (95% CI: 1.82 to 1.95; Figure 3, Panel C; Table S4), and was unaffected by differences in case mix or multivariable adjustment. These findings were consistent with increases in the cumulative number of transfusion days (Figure 3, Panel B). Of note, the slope of the increase in any transfusion use or of number of transfusion days was statistically steeper after 2007 (when compared with 2003–2006; p<0.001 and p=0.04, respectively).
Figure 3. Trends in Blood Transfusion Use Prior to End-Stage Renal Disease.
Panel A: Proportion of Patients Receiving Any Blood Transfusion
Panel B: Number of Blood Transfusion Days (1; 2; 3; 4+)
Note: Compared with 2003–2006, the trend lines for transfusion use or number of transfusion days accelerated significantly in 2007–2010 (P<0.001 and P=0.04, respectively).
Panel C: Adjusted Prevalence Ratios of Any Blood Transfusion Prior to End-Stage Renal Disease (Referent: 1995)
Multivariable model adjusted for age, sex, race, Medicaid (“dual”) eligibility, comorbid diabetes, estimated glomerular filtration rate and body mass index at end-stage renal disease.
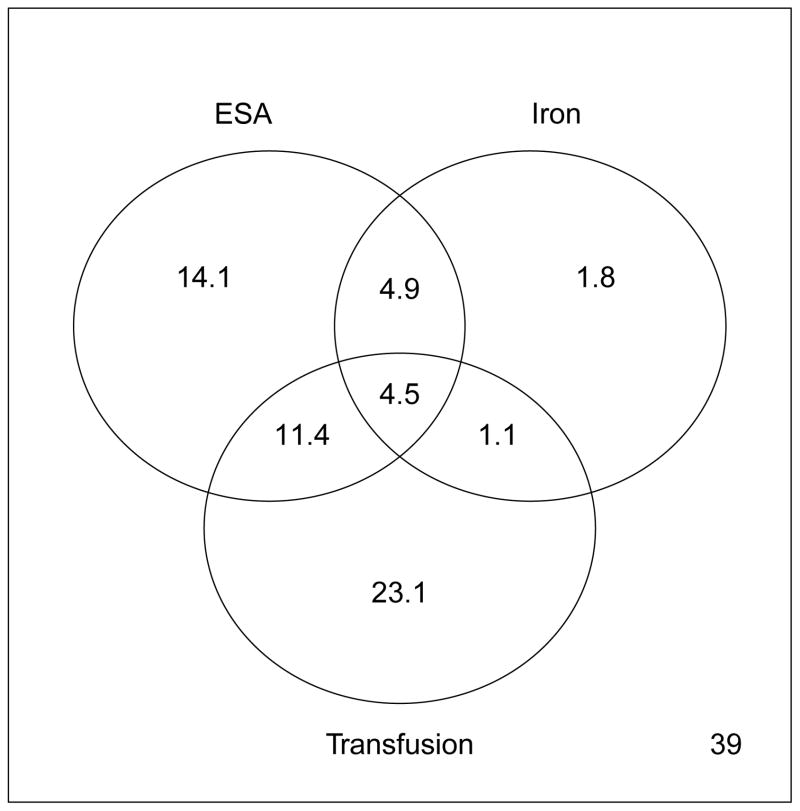
The proportions of all possible combinations of anemia treatments received prior to ESRD in 2010 are shown in Figure 4 (the corresponding can be gleaned from the Technical Appendix, Supplemental Figure S1).
Figure 4. Venn Diagrams of Utilization among Anemia Treatments prior to End-Stage Renal Disease (2010).
ESA – erythropoiesis-stimulating agents. All numbers correspond to proportion of patients who received the corresponding anemia treatment(s) in the two years prior to reaching end-stage renal disease. The number in the bottom right indicates the proportion of patients who did not receive any of the three treatments.
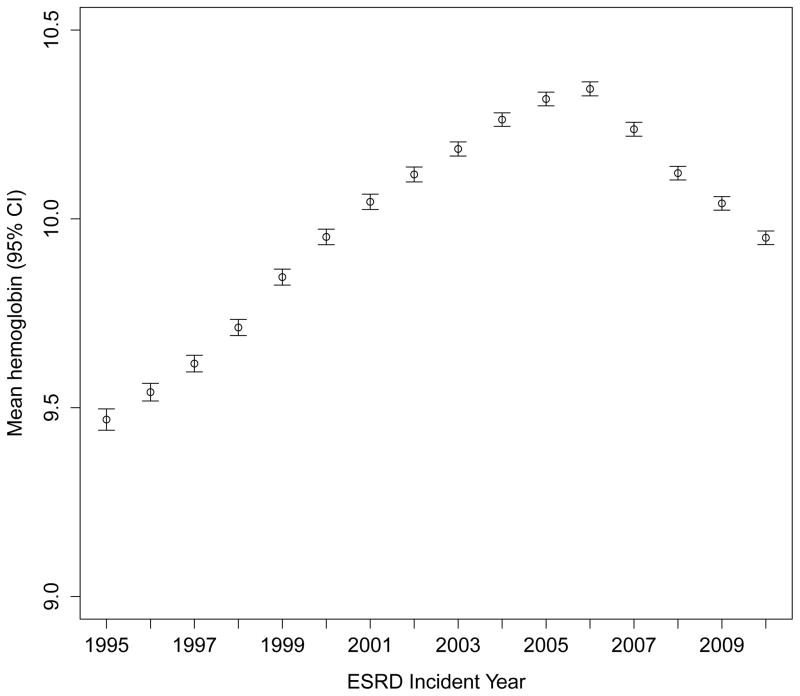
During these marked changes in the use of ESAs, IV iron and transfusion, mean hemoglobin concentrations were 9.5 g/dL in 1995, increased to a peak of 10.3 g/dL in 2006, and then declined to 9.9 g/dL in 2010 (Figure 5). The corresponding fractions of patients with hemoglobin concentrations <8.0 g/dL (the upper value of the 7–8 g/dL range suggested by the American Association of Blood Banks for hospitalized patients with relevant symptoms or pre-existing cardiovascular disease18) were 17.3%, 5.5%, and 7.0%.
Figure 5. Mean hemoglobin concentration at start of therapy for end-stage renal disease.
Hemoglobin concentration in g/dL. ESRD – end-stage renal disease.
Discussion
We studied treatment patterns for anemia, a common complication of advanced CKD, in a national registry of older patients approaching ESRD in the U.S. between 1995 and 2010. We found that the use and duration of ESA treatment prior to ESRD increased considerably during that era (at least until 2007) along with increasing use of intravenous iron, which jointly contributed to significantly, but modestly higher hemoglobin concentrations by the time patients reached ESRD. However, almost twice as many patients received blood transfusions prior to reaching ESRD, even when accounting for changes in case mix and comorbidity over time. Thus, the main objective of ESA treatment that gave rise to its original approval – transfusion avoidance – was not consistently met at the population level. These findings are in contrast with declining transfusion trends in older patients without diagnosed CKD, older patients with diagnosed CKD (mostly of lesser stages) as well as in patients of any age receiving dialysis in whom transfusion rates declined between 1992 and 2004 or 2005.8, 19 The observed doubling over time of the proportion of older patients approaching ESRD who received a transfusion may be a consequence of an upward drift in practitioners’ hemoglobin transfusion thresholds over the 15 years we studied, which was previously shown in patients receiving dialysis,8 or a function of increasing attention to the correction of anemia or responsiveness to patients’ reported symptoms. Our findings are also compatible with a study of younger patients with CKD insured by United Healthcare (Ingenix database) in whom patients who transitioned to ESRD in a given year experienced a 2.5-fold increase in transfusion rates between 2002 and 2008.20
Transfusion avoidance is an important clinical goal, especially in patients with advanced CKD or ESRD. These patients are particularly prone to hyperkalemia and fluid overload resulting in heart failure, both known complications of blood transfusions. Further, repeated transfusions can lead to iron overload (in extreme cases hemosiderosis), which was a challenging clinical problem in the ESRD population before the approval of epoetin alfa in 1989.21 In addition, exposure to blood transfusion markedly increases the likelihood of allosensitization in patients eligible for kidney transplantation.22 In addition to increasing the odds of a positive crossmatch, disqualifying potential donors,23 allosensitization is associated with accelerated graft loss, even among patients lucky enough to crossmatch negative and receive a kidney.24 With waiting times for kidney transplantation exceeding life expectancy in most geographic regions for most middle aged and older patients with ESRD, we can ill afford the provision of unnecessary transfusions, as doing so will virtually assure that these patients live out their lives on dialysis.
While transfusion avoidance provided the formal reason for the approval and use of ESAs, the enthusiastic adoption and use of ESAs may have been mostly grounded in the expectation of other clinical benefits. As stated in the introduction to the 1997 KDOQI anemia guidelines, “effective treatment of the anemia of [CKD] improves survival, decreases mortality, and increases quality of life.”7 The citations listed in support of that statement or in the guideline on target hemoglobin (recommending a target range of 11–12 g/dL) referred to either studies of (unproven) surrogate endpoints (e.g. left ventricular hypertrophy, exercise capacity), or to observational studies associating hemoglobin concentration with outcomes whose ability to control for confounding of inherent characteristics that differed between individuals who achieved a higher vs. a relatively lower hemoglobin concentration was limited. In 2006, revised KDOQI anemia guidelines changed their language indicating that “in patients with CKD, [hemoglobin] should be 11.0 g/dL or greater. (MODERATELY STRONG RECOMMENDATION)”25 In addition, without providing any convincing evidence, a new recommendation suggested that “there is insufficient evidence to recommend routinely maintaining [hemoglobin] levels at 13.0 g/dL or greater in ESA-treated patients,”25 which was widely interpreted as an expansion to a 11–13 g/dL hemoglobin target range. Thus, throughout this era ESA utilization was driven by expectation of benefit that was unproven in any quality trials of hard study endpoints.
Our study shows that initiation of ESA treatment prior to ESRD reached a peak in 2007 and then plateaued or even declined, with hemoglobin concentrations at ESRD also declining during that time. This reversion in the long term upward trend reflected providers’ reactions to the sobering results from three relatively large randomized trials in patients with moderate to advanced (non-dialysis requiring) CKD, published in late 200610, 11 and 2009,12 respectively, one of which failed to demonstrate any benefit10 and the other two found significant (albeit distinct) cardiovascular risks from more aggressive ESA treatment.11, 12 Interestingly, it appears that the rate of transfusion prior to ESRD accelerated after 2007, compared with the slope prior to 2007. This change probably indicates the dueling effects of increasingly lenient transfusion thresholds while less aggressive ESA treatment provided less of a hemoglobin reserve to these patients. Apparently, providers (nephrologists, internists, hospitalists) consider hemoglobin the single most important factor in their decisions to transfuse, with clinical factors such as functional status and cardiovascular comorbidities playing a much lesser role.26 In patients on dialysis, increases between 2006 and 2010 in the proportion of patients whose hemoglobin concentration was below 10 g/dL have been linked to increased transfusion rates during the same time period, which was independent of any other recorded clinical factors.27
Although we found a more than 10-fold increase in the use of intravenous iron over the 15 years of study, the proportion of patients who received it remained small at roughly 10% in 2010. The modest use of IV iron in CKD contrasts starkly with the almost universal use of intravenous iron in ESA-treated patients on dialysis, where >95% receive IV iron within the first 6 months after initiating dialysis.28 Most clinicians prefer oral iron supplementation in patients with non-dialysis requiring CKD despite evidence that intestinal iron absorption is poor in these patients.28 Intravenous iron is far less convenient in patients with non-dialysis requiring CKD than in patients with ESRD, particularly those on hemodialysis, since venous cannulation and often, admission to an infusion center is required. Moreover, several sessions are required to give “repletion” doses of IV iron for patients with frank iron deficiency, and these repeated venous cannulations may compromise creation of arteriovenous fistulas or grafts.
Perhaps the most striking of our findings is the steady increase in transfusion despite the broad and consistent use of pharmaceutical agents effective at raising hemoglobin concentrations. Ironically, these agents were approved with the goal to reduce dependence on blood transfusion, mainly in the ESRD population where the need for such a therapy was clearly needed. Indeed, in the ESRD population transfusions have markedly declined since the introduction of ESAs.8 For a variety of reasons, the treatment paradigm for anemia management in dialysis patients was extended to the pre-ESRD population until evidence from randomized trials challenged these practices. As our study demonstrates, the goal of transfusion avoidance has not been consistently met in CKD patients not on dialysis, perhaps at least in part by increasingly liberal transfusion practices or sicker patients approaching dialysis., Furthermore, the use of transfusion in the ESA era was likely driven more by a hemoglobin target than by the prevalence of symptomatic anemia. Given these other factors, the increasing use of transfusion was not necessarily a failure of ESAs. While clinicians may individualize transfusion practices based on patients’ underlying conditions, including attempting to balance risks associated with uncorrected anemia and those associated with transfusion (as outlined above), clinical practice guidelines, including those from the American Association of Blood Banks suggest that transfusion may be used far too liberally in this population.18
There are certain limitations of our study that require consideration. We studied a survivor cohort of patients who reached and started treatment for ESRD; patients with advanced CKD who died from other causes before reaching ESRD, or who reached ESRD and declined dialysis or transplantation were not captured in the database. Our cohort was further restricted to older patients who possessed Medicare fee-for-service prior to reaching ESRD, since information on drug use and transfusion was only available for patients who had qualified for Medicare on age or disability grounds prior to reaching ESRD. Generalizability to younger patients reaching ESRD is uncertain. The data we had available covered only two years prior to treatment for ESRD, so we cannot rule out any additional use of ESAs, IV iron and transfusion earlier in patients’ lifetimes. We were unable to define and examine trends in the hemoglobin concentration that gave rise to initiation of ESA or intravenous iron therapy (or to blood transfusion) as this information was not required to be submitted to Medicare with the corresponding billing claims. Since all transfusions given on any single day are subsumed in a single procedure code, we were only able to study transfusion days, but could not ascertain the actual number of transfusions given. New anemia medications approved during the study period had variably short periods of availability without a dedicated HCPCS code. However, these time periods were short (few weeks to months) and the degree of under-ascertainment is likely small given their new introduction into clinical practice. Finally, the strongest response to the accumulating safety concerns with ESA use by the FDA did not occur until mid-2011, when ESA labels were revised to no longer include a target hemoglobin range and suggested that ESA initiation be considered only in patients whose hemoglobin concentrations were below 10 g/dL.29 We were unable to study the impact of this FDA action due to the lag time in availability of data.
In conclusion, over 15 years of study, we found a pronounced trend towards more aggressive treatment of anemia in older patients approaching ESRD, which included ESA, intravenous iron, and broad based use of red blood cell transfusions. All of these were weakly reflected in an increase in hemoglobin concentrations at the time of onset of ESRD. While ESA use plateaued and ensuing hemoglobin concentrations declined after 2007, following safety concerns about ESA use in patients with CKD, transfusion use appeared to increase even more rapidly. In light of the costs of anemia treatments and the safety concerns of currently available anemia treatments, identification of safe, effective, and economical anemia treatment strategies in patients with CKD and anemia need to be identified.
Supplementary Material
Acknowledgments
This work was supported by grant 1R01DK090181 from the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) to Dr. Winkelmayer. The manuscript was reviewed and approved for publication by an officer of the NIDDK. Data reported herein were supplied by the United States Renal Data System (USRDS). Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government. Dr. Winkelmayer had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Winkelmayer reports having served as a scientific advisor to Amgen, Bayer, GlaxoSmithKline, and Keryx, and on data safety monitoring boards for Medgenics and Medtronics. Dr. Chertow serves on the Board of Directors of Satellite Healthcare and on the Scientific Advisory Board for DaVita Clinical Research, and has served as an advisor to Amgen and Keryx. Dr. Brookhart has received investigator-initiated research funding from Amgen and has served as a scientific advisor to Amgen and Merck. None of the remaining authors have anything to disclose.
Footnotes
An earlier version of this work was presented on November 2nd 2012 at the 2012 Kidney Week of the American Society of Nephrology, San Diego, CA.
References
- 1.Eschbach JW, Adamson JW. Anemia of end-stage renal disease (ESRD) Kidney Int. 1985;28:1–5. doi: 10.1038/ki.1985.109. [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol. 2002;13:504–510. doi: 10.1681/ASN.V132504. [DOI] [PubMed] [Google Scholar]
- 3.Zipf RE., Jr Fatal anaphylaxis after intravenous iron dextran. Journal of forensic sciences. 1975;20:326–333. [PubMed] [Google Scholar]
- 4.Novey HS, Pahl M, Haydik I, Vaziri ND. Immunologic studies of anaphylaxis to iron dextran in patients on renal dialysis. Annals of allergy. 1994;72:224–228. [PubMed] [Google Scholar]
- 5.Buchwald D, Argyres S, Easterling RE, Oelshlegel FJ, Jr, Brewer GJ, Schoomaker EB, Abbrecht PH, Williams GW, Weller JM. Effect of nandrolone decanoate on the anemia of chronic hemodialysis patients. Nephron. 1977;18:232–238. doi: 10.1159/000180834. [DOI] [PubMed] [Google Scholar]
- 6.Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haley NR, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000. doi: 10.7326/0003-4819-111-12-992. [DOI] [PubMed] [Google Scholar]
- 7.NKF-DOQI clinical practice guidelines for the treatment of anemia of chronic renal failure. National Kidney Foundation-Dialysis Outcomes Quality Initiative. Am J Kidney Dis. 1997;30:S192–240. [PubMed] [Google Scholar]
- 8.Ibrahim HN, Ishani A, Foley RN, Guo H, Liu J, Collins AJ. Temporal trends in red blood transfusion among US dialysis patients, 1992–2005. Am J Kidney Dis. 2008;52:1115–1121. doi: 10.1053/j.ajkd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 10.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 11.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 13.Ishida JH, McCulloch CE, Dudley RA, Grimes BA, Johansen KL. Dialysis facility profit status and compliance with a black box warning. JAMA internal medicine. 2013;173:1152–1153. doi: 10.1001/jamainternmed.2013.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang TI, Shilane D, Kazi DS, Montez-Rath ME, Hlatky MA, Winkelmayer WC. Multivessel coronary artery bypass grafting versus percutaneous coronary intervention in ESRD. J Am Soc Nephrol. 2012;23:2042–2049. doi: 10.1681/ASN.2012060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 16.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. Journal of clinical pharmacy and therapeutics. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 17.Winkelmayer WC, Liu J, Chertow GM, Tamura MK. Predialysis nephrology care of older patients approaching end-stage renal disease. Arch Intern Med. 2011;171:1371–1378. doi: 10.1001/archinternmed.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim HN, Ishani A, Guo H, Gilbertson DT. Blood transfusion use in non-dialysis-dependent chronic kidney disease patients aged 65 years and older. Nephrol Dial Transplant. 2009;24:3138–3143. doi: 10.1093/ndt/gfp213. [DOI] [PubMed] [Google Scholar]
- 20.Gill KS, Muntner P, Lafayette RA, Petersen J, Fink JC, Gilbertson DT, Bradbury BD. Red blood cell transfusion use in patients with chronic kidney disease. Nephrol Dial Transplant. 2013;28:1504–1515. doi: 10.1093/ndt/gfs580. [DOI] [PubMed] [Google Scholar]
- 21.Hakim RM, Stivelman JC, Schulman G, Fosburg M, Wolfe L, Imber MJ, Lazarus JM. Iron overload and mobilization in long-term hemodialysis patients. Am J Kidney Dis. 1987;10:293–299. doi: 10.1016/s0272-6386(87)80025-2. [DOI] [PubMed] [Google Scholar]
- 22.Yabu JM, Anderson MW, Kim D, Bradbury BD, Lou CD, Petersen J, Rossert J, Chertow GM, Tyan DB. Sensitization from transfusion in patients awaiting primary kidney transplant. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cecka JM. Calculated PRA (CPRA): the new measure of sensitization for transplant candidates. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:26–29. doi: 10.1111/j.1600-6143.2009.02927.x. [DOI] [PubMed] [Google Scholar]
- 24.Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398–1406. doi: 10.1681/ASN.2009101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kdoqi National Kidney F. II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006;47:S16–85. doi: 10.1053/j.ajkd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Whitman CB, Shreay S, Gitlin M, van Oijen MG, Spiegel BM. Clinical Factors and the Decision to Transfuse Chronic Dialysis Patients. Clin J Am Soc Nephrol. 2013 doi: 10.2215/CJN.00160113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbertson DT, Monda KL, Bradbury BD, Collins AJ. RBC Transfusions Among Hemodialysis Patients (1999–2010): Influence of Hemoglobin Concentrations Below 10 g/dL. Am J Kidney Dis. 2013 doi: 10.1053/j.ajkd.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 28.United States Renal Data System. USRDS 2010 Annual Data Report: Atlas of End-Stage Renal Disease and Chronic Kidney Disease in the United States, Volume 2: Atlas ESRD, Chapter 5: Clinical Indicators and Preventive Health. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. pp. 287–200. [Google Scholar]
- 29.U.S. Food and Drug Administration. FDA Drug Safety Communication: Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.