Abstract
Aims
The deacetylase sirtuin 1 (Sirt1) exerts beneficial effects on lipid metabolism, but its roles in plasma LDL-cholesterol regulation and atherosclerosis are controversial. Thus, we applied the pharmacological Sirt1 activator SRT3025 in a mouse model of atherosclerosis and in hepatocyte culture.
Methods and results
Apolipoprotein E-deficient (Apoe−/−) mice were fed a high-cholesterol diet (1.25% w/w) supplemented with SRT3025 (3.18 g kg−1 diet) for 12 weeks. In vitro, the drug activated wild-type Sirt1 protein, but not the activation-resistant Sirt1 mutant; in vivo, it increased deacetylation of hepatic p65 and skeletal muscle Foxo1. SRT3025 treatment decreased plasma levels of LDL-cholesterol and total cholesterol and reduced atherosclerosis. Drug treatment did not change mRNA expression of hepatic LDL receptor (Ldlr) and proprotein convertase subtilisin/kexin type 9 (Pcsk9), but increased their protein expression indicating post-translational effects. Consistent with hepatocyte Ldlr and Pcsk9 accumulation, we found reduced plasma levels of Pcsk9 after pharmacological Sirt1 activation. In vitro administration of SRT3025 to cultured AML12 hepatocytes attenuated Pcsk9 secretion and its binding to Ldlr, thereby reducing Pcsk9-mediated Ldlr degradation and increasing Ldlr expression and LDL uptake. Co-administration of exogenous Pcsk9 with SRT3025 blunted these effects. Sirt1 activation with SRT3025 in Ldlr−/− mice reduced neither plasma Pcsk9, nor LDL-cholesterol levels, nor atherosclerosis.
Conclusion
We identify reduction in Pcsk9 secretion as a novel effect of Sirt1 activity and uncover Ldlr as a prerequisite for Sirt1-mediated atheroprotection in mice. Pharmacological activation of Sirt1 appears promising to be tested in patients for its effects on plasma Pcsk9, LDL-cholesterol, and atherosclerosis.
Keywords: Sirt1, LDL-cholesterol, Pcsk9, LDL receptor, Atherogenesis
Translational perspective.
The deacetylase Sirt1 exerts beneficial effects on metabolic and inflammatory diseases. However, its effects on lipid metabolism and atherosclerosis remain controversial. Lowering plasma Pcsk9 emerges as a promising therapeutic strategy to lower plasma LDL-cholesterol. Unlike current antibody-based strategies that act on extracellular Pcsk9 activity, we found an alternative route to decrease Pcsk9-mediated Ldlr degradation: pharmacological Sirt1 activation reduced hepatic Pcsk9 secretion, increased Ldlr expression, and decreased plasma LDL-cholesterol and atherosclerosis in mice. Thus, Sirt1 activation appears as a promising approach to be tested for atheroprotection in patients.
Introduction
Atherosclerosis results from a complex interplay between innate and adaptive immunity involving modified LDL-cholesterol, activated endothelial cells, and monocyte-derived macrophages within the arterial wall. Sirt1 is a member of the sirtuin family of NAD+-dependent deacetylases.1 Studies on the role of Sirt1 in atherosclerosis have reported controversial effects.
Sirt1 has been shown to be atheroprotective in apolipoprotein E-deficient (Apoe−/−) mice2–4 whereas genetic overexpression of Sirt1 in LDL-receptor-deficient (Ldlr−/−) mice enhanced atherosclerosis.5 The role of Sirt1 in regulation of plasma LDL-cholesterol concentration, the key trigger of atherogenesis,6 remains incompletely understood. Hepatic deletion of Sirt1 in C57BL/6 mice fed a high-cholesterol diet induced mild hypercholesterolemia.7 Conversely, administration of a Sirt1 activating drug to elderly volunteers and cigarette smokers decreased plasma levels of total and LDL-cholesterol.8,9 These studies suggest that Sirt1 regulates plasma LDL-cholesterol.
Hepatic Ldlr clears LDL-cholesterol from the blood stream.10,11 Transcription of Ldlr is controlled by the sterol-responsive element binding protein 2 (Srebp2),12 while its turnover depends on proprotein convertase subtilisin/kexin type 9 (Pcsk9), a serine protease.13 Secreted Pcsk9 targets hepatic Ldlr for lysosomal degradation and thus prevents recycling of internalized Ldlr to the cell surface.14
We hypothesized that hepatic Ldlr mediates the effects of Sirt1 on plasma LDL-cholesterol levels and thus provides atheroprotection. To test this hypothesis, we fed Apoe−/− or Ldlr−/− mice a high-cholesterol diet supplemented with the novel Sirt1 activator SRT3025 or placebo, and investigated atherogenesis and lipid metabolism.
Methods
Detailed information is available in Supplementary material online.
Animals
Male Apoe−/− or Ldlr−/− mice on a pure C57BL/6J background were housed with a 12 h light–dark cycle and fed a high-cholesterol diet containing 1.25% cholesterol (D12108; Research Diets) supplemented with or without (SRT3025, 3.18 g kg−1 diet, provided by Sirtris, Cambridge, MA, USA) for 12 weeks starting at the age of 8 weeks. After this treatment period, mice were sacrificed (after overnight fasting), EDTA blood was taken and tissues were harvested. All experiments and animal care procedures were approved by the local veterinary authorities and carried out in accordance with our institutional guidelines.
Cell culture
AML12 mouse hepatoma cells were cultured in a 1:1 (v/v) mixture of DMEM and Ham's F12 medium supplemented with insulin (5 μg ml−1), transferrin (5 μg ml−1), selenium (5 ng ml−1), dexamethasone (40 ng ml−1), and 10% FBS (v/v). Where indicated, AML12 cells were exposed to 10 μM SRT3025 in 1% DMSO (v/v).
Statistics
All data are presented as means ± SD. Data distribution was assessed using the Kolmogorov–Smirnov test. Normally distributed data were compared by an unpaired two-tailed Student's t-test; for non-parametric data the Mann–Whitney test was used. Three or more groups were compared using a Kruskal–Wallis test followed by a Dunn's post-hoc comparison (non-parametric data). At least three independent experiments in triplicates were performed. Significance was accepted at P < 0.05. Analyses were done using Graphpad Prism (version 5.0d, 2010).
Results
SRT3025 reduces plasma cholesterol, inflammation, and atherosclerosis in Apoe−/− mice
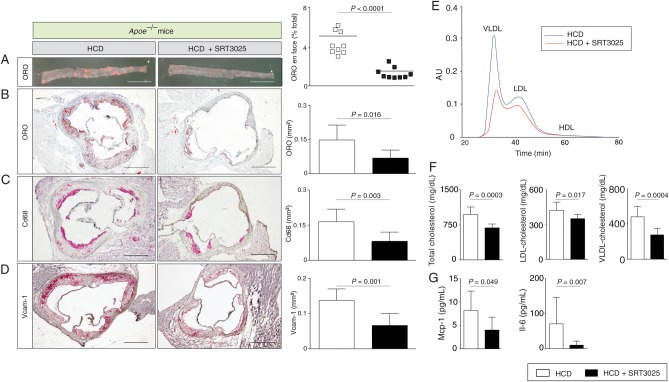
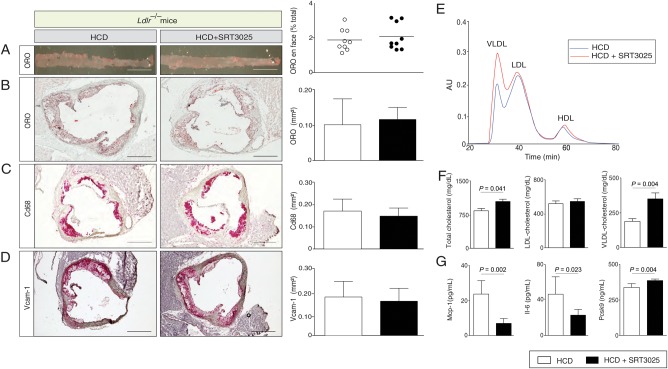
Histomorphometry of thoraco-abdominal aortae en face and cross-sections of aortic roots revealed a significant reduction in plaque size in SRT3025-treated Apoe−/− mice compared with placebo-treated controls (Figure 1A and B). Moreover, a marked reduction of Cd68-positive macrophages within the plaque and Vcam-1 expression in aortic roots was observed (Figure 1C and D). Interestingly, plasma levels of total-, LDL- and VLDL-cholesterol were significantly lower after SRT3025 treatment compared with placebo (Figure 1E and F). Triglycerides and HDL-cholesterol remained unchanged (Supplementary material online, Figure S1A). In addition, we observed reduced plasma levels of Mcp-1 and Il-6 (Figure 1G) and lower hepatic mRNA expression of these cytokines (Figure 3A).
Figure 1.
SRT3025 confers atheroprotection, reduces plasma cholesterol and systemic inflammation in Apoe−/− mice. Eight-week-old Apoe−/− mice were fed a high-cholesterol diet (1.25% w/w) supplemented with the Sirt1 activator SRT3025 (n = 9) or placebo (n = 9) for 12 weeks. Representative micrographs (left) and quantifications (right) of thoraco-abdominal aortae en face (A) and of aortic root cross sections (B–D) stained with ORO or immunohistochemically for macrophages (Cd68) or Vcam-1; (E) cholesterol distribution in the different lipoprotein subfractions separated by gel filtration chromatography; (F) Plasma total cholesterol, LDL-, and VLDL-cholesterol concentrations; (G) Plasma levels of Mcp-1 and Il-6. Scale bars in photomicrographs: 1 mm (A) and 500 μm (B–D). HCD, high-cholesterol diet; AU, arbitrary units; LDL, low-density lipoprotein; ORO, oil-red O; VLDL, very low-density lipoprotein; Vcam-1, vascular cell adhesion molecule 1; Mcp-1, monocyte chemoattractant protein 1; Il-6, interleukin 6.
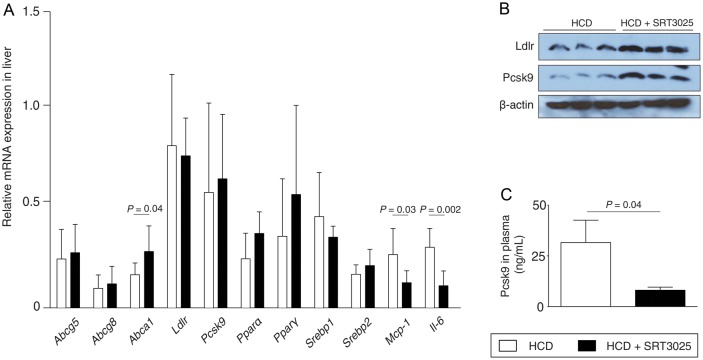
Figure 3.
SRT3025 increases hepatic Ldlr protein expression while decreasing plasma Pcsk9 in Apoe−/− mice. Eight week-old Apoe−/− mice were fed a high-cholesterol diet (1.25% w/w) supplemented with the Sirt1 activator SRT3025 (n = 9) or placebo (n = 9) for 12 weeks. (A) Relative mRNA expression levels of hepatic genes involved in cholesterol regulation. (B) Western blots of liver lysates for Ldlr, Pcsk9, and β-actin. (C) Plasma levels of Pcsk9. HCD, high-cholesterol diet; Pcsk9, proprotein convertase subtilisin/kexin type 9.
These findings indicate that SRT3025 administration to Apoe−/− mice provides atheroprotection and reduces plasma LDL-cholesterol and inflammation.
SRT3025 mimics Sirt1 activity in vitro and in Apoe−/− mice
SRT3025 concentration-dependently activated wild-type Sirt1, but failed to activate the activation-resistant Sirt1 mutant E230K in vitro (Figure 2A). Increased deacetylation of known Sirt1 targets upon SRT3025 treatment, hepatic p65 and forkhead transcription factor family O1 (Foxo1) in skeletal muscle (Figure 2B and C) indicate successful Sirt1 activation in vivo. SRT3025 prevented an increase in weight gain and epididymal white adipose tissue without affecting food intake, thereby mimicking a caloric restriction phenotype (Supplementary material online, Figure S1B–D). Pharmacokinetic analysis of the drug in Apoe−/− mice showed that the drug indeed reached target tissues (Supplementary material online, Table S1). Plasma protein levels of glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, and alkaline phosphatase were not different between drug- and placebo-treated mice (Supplementary material online, Figure S1E).
Figure 2.
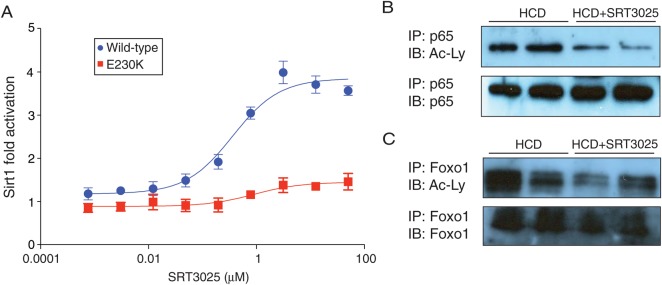
SRT3025 mimics Sirt1 activity in vitro and in Apoe−/− mice. (A) Concentration-response curve of SRT3025 on the activity of wild-type Sirt1 and activation-resistant mutant E230K in vitro. (B, C) Western blots for acetylation status of Sirt1 target proteins (B) p65 and (C) Foxo1 immunoprecipitated from nuclear extracts of liver and skeletal muscle, respectively, from Apoe−/− mice fed a high-cholesterol diet (1.25% w/w) supplemented with the Sirt1 activator SRT3025 or placebo for 12 weeks. HCD, high-cholesterol diet; Ac-Ly, anti-acetyl-lysine antibody.
SRT3025 increases hepatic Ldlr expression and Pcsk9 accumulation in Apoe−/− mice
Pharmacological Sirt1 activation increased liver expression of Abca1, but did not affect Abcg5, Abcg8, Ldlr, Pcsk9, Pparα, Pparγ, Srebp1, and Srebp2 (Figure 3A). Despite no changes in Ldlr and Pcsk9 mRNA expression, both were markedly increased at the protein level (Figure 3A and B). This intracellular accumulation of Pcsk9 protein with no change in mRNA suggests a disturbed transport and/or secretion of the protein. In agreement with this, we found reduced plasma Pcsk9 protein levels following pharmacological Sirt1 activation in Apoe−/− mice compared with placebo-treated controls (Figure 3C).
SRT3025 increases Ldlr expression and Pcsk9 accumulation in AML12 hepatocytes
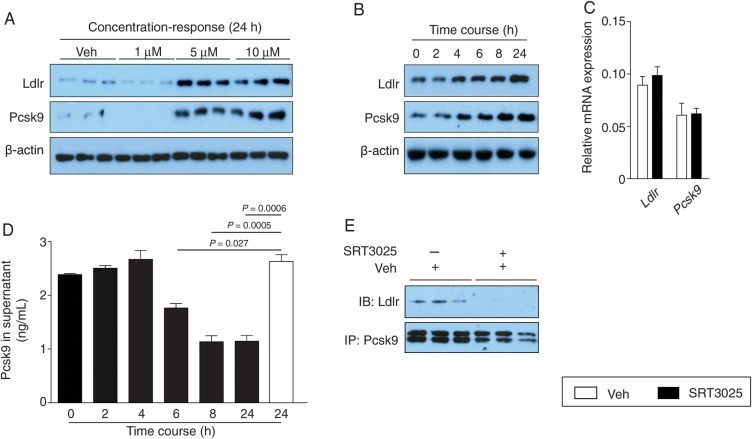
To delineate the mechanisms by which SRT3025 affects Ldlr protein expression, we performed in vitro experiments administering SRT3025 to mouse hepatoma AML12 cells. We observed a concentration- and time-dependent increase in Ldlr and Pcsk9 protein expression upon SRT3025 administration in cell lysates (Figure 4A and B). As observed in vivo, mRNA levels of Ldlr and Pcsk9 were not altered by SRT3025 (Figure 4C), indicating that post-translational effects cause the changes in protein expression.
Figure 4.
SRT3025 increases Ldlr expression in AML12 hepatocytes and decreases Pcsk9 in the supernatant. Western blots of Ldlr, Pcsk9, and β-actin in cultured AML12 cells (A) treated with SRT3025 at indicated concentrations for 24 h and (B) incubated with 10 μM SRT3025 for the times indicated. (C) Relative mRNA expression levels of Ldlr and Pcsk9 in AML12 cells after incubation with 10 μM SRT3025 for 24 h. (D) Pcsk9 protein levels in the supernatant of AML12 cells incubated with 10 μM SRT3025 for the times indicated. (E) Pcsk9 immunoprecipiated from AML12 cells incubated with vehicle (Veh, DMSO) or 10 μM SRT3025, and blotted for Ldlr and Pcsk9. Pcsk9, proprotein convertase subtilisin/kexin type 9.
Incubation of AML12 cells with 10 μM SRT3025 was associated with a time-dependent decrease in Pcsk9 secretion into the supernatant (Figure 4D). Moreover, co-immunoprecipitation of endogenous Pcsk9 and Ldlr after 24 h incubation with 10 μM SRT3025 revealed that Pcsk9 binding to Ldlr was impaired after SRT3025 treatment compared with vehicle control (Figure 4E). Thus, the question arises whether the increase in hepatic Ldlr protein expression upon Sirt1 activation is related to limited extracellular availability of Pcsk9 and/or a defective degradation of internalized Ldlr.
SRT3025 impairs Pcsk9-dependent degradation of Ldlr in AML12 hepatocytes
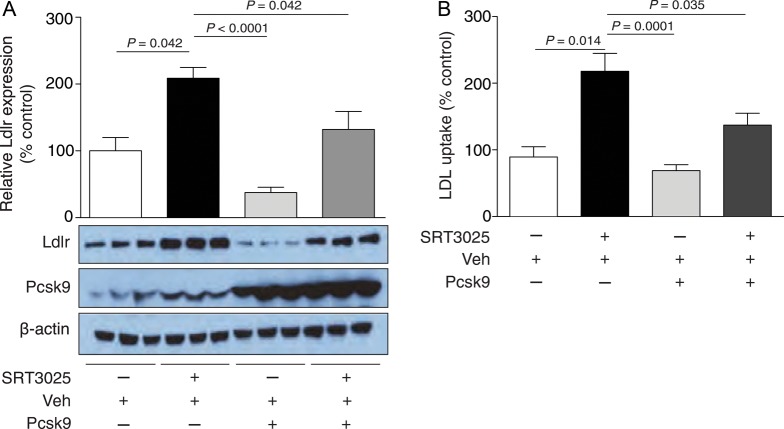
To address the above question, we stimulated AML12 cells with 10 μM SRT3025 for 24 h and compensated for the decrease in extracellular Pcsk9 by adding exogenous active Pcsk9 protein. Co-administration of Pcsk9 [3 ng ml−1, based upon concentrations measured in the supernatants of untreated AML12 (Figure 4D)] and SRT3025 to AML12 hepatocytes attenuated the drug-dependent increase in Ldlr protein expression (Figure 5A). These data indicate that the internalization and degradation process of Ldlr is not defective and that limited extracellular availability of Pcsk9 contributes to the increase in Ldlr protein expression.
Figure 5.
Exogenous Pcsk9 prevents SRT3025-induced increase in Ldlr expression and activity in AML12 hepatocytes. (A) Western blot and corresponding quantifications of AML12 cells treated with vehicle (Veh, DMSO) or 10 μM SRT3025 and incubated with or without Pcsk9 active protein (3 ng ml−1) for 1 h. (B) BODIPY-labelled LDL uptake in AML12 cells incubated for 24 h with 10 μM SRT3025 or Veh and incubated with or without Pcsk9 active protein (3 ng ml−1) for 1 h. Fluorescence intensity and western blot quantifications are given as percentage of Veh control. BODIPY, 4,4-difluoro-3a,4a-diaza-s-indacene; AU, arbitrary units; Pcsk9, proprotein convertase subtilisin/kexin type 9.
Fluorescence analysis revealed an increase in labelled LDL uptake upon 10 μM SRT3025 treatment compared with vehicle controls (Figure 5B), suggesting a functional relevance of the SRT3025-dependent increase in Ldlr expression. Moreover, co-administration of exogenous active Pcsk9 also attenuated the drug-induced increase in LDL uptake (Figure 5B), consistent with the observed attenuation of Ldlr protein expression (Figure 5A).
These data indicate that SRT3025 decreases hepatic Pcsk9 release, impairs Pcsk9 binding to hepatic Ldlr, and thereby prevents hepatic Ldlr degradation. These events result in increased hepatic Ldlr expression and enhanced LDL-cholesterol plasma clearance.
Increased Ldlr protein expression in AML12 hepatocytes is mediated by Sirt1
To assess whether this increase in Ldlr protein expression is Sirt1-dependent, Sirt1 knockdown in AML12 cells was performed in the presence of 10 μM SRT3025. In contrast to control scramble siRNA-treated cells, cells transfected with Sirt1 siRNA did not show an increase in Ldlr expression upon SRT3025 addition (Figure 6A). Conversely, genetic overexpression of Sirt1 increased Ldlr protein expression in AML12 cells compared with control transfection (Figure 6B). Thus, Sirt1 is required for SRT3025-induced increase in Ldlr protein expression.
Figure 6.
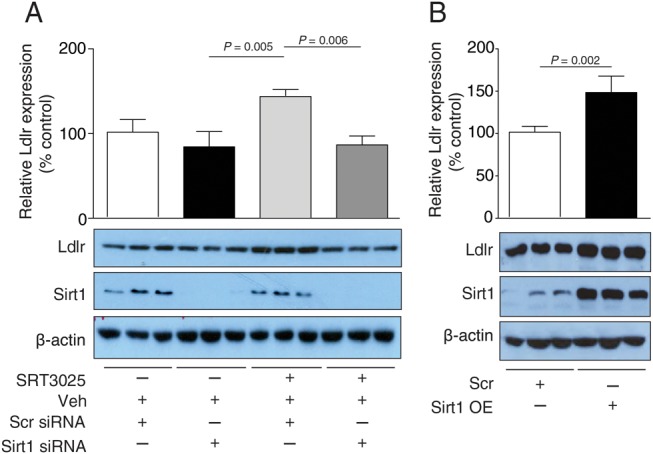
Sirt1 knockdown reduces and Sirt1 overexpression enhances SRT3025-induced increase in Ldlr expression in AML12 hepatocytes. (A) Western blots of Ldlr, Sirt1, and β-actin with corresponding quantifications of AML12 cell lysates following transfection with Sirt1 siRNA or scrambled siRNA for 24 h and incubated with 10 μM SRT3025 or vehicle (Veh, DMSO) for additional 24 h. (B) Western blots of Ldlr, Sirt1, and β-actin with corresponding quantifications of AML12 cell lysates following Sirt1 overexpression plasmid or scramble control for 24 h and incubated with 10 μM SRT3025 or Veh for additional 24 h. Western blot quantifications are given as a percentage of Veh control. Scr, scramble plasmid; Sirt1 OE, Sirt1 overexpression plasmid.
Genetic deletion of Ldlr abolishes atheroprotective effects of SRT3025 in vivo
Given the critical role of hepatic Ldlr in the clearance of plasma cholesterol and our observation of increased hepatic Ldlr expression in SRT3025-treated Apoe−/− mice, we investigated whether Ldlr accounts for the atheroprotective effects of SRT3025. For that purpose, similar experiments were performed in Ldlr−/− mice. Plaque analyses of thoraco-abdominal aortae en face and cross-sections of aortic roots revealed no difference in the extent of atherosclerosis between drug- and placebo-treated mice (Figure 7A and B). Furthermore, the number of plaque-resident macrophages (Cd68) and Vcam-1 expression in aortic roots did not differ between the groups (Figure 7C and D).
Figure 7.
Genetic deletion of Ldlr abolishes anti-atherosclerotic effects of SRT3025 in vivo. Eight-week-old Ldlr−/− mice were fed a high-cholesterol diet (1.25% w/w) supplemented with the Sirt1 activator SRT3025 (n = 9) or placebo (n = 9) for 12 weeks and fasted for 12 h before blood was drawn and aortae were explanted. Representative micrographs (left) and quantifications (right) of thoraco-abdominal aortae en face (A) and of aortic root cross sections (B–D) stained with oil-red O (ORO) or immunohistochemically for macrophages (CD68) or Vcam-1; (E) cholesterol distribution in the different lipoprotein subfractions separated by gel filtration chromatography; (F) plasma total cholesterol, LDL-, and VLDL-cholesterol concentrations; (G) plasma levels of Mcp-1, Il-6, and Pcsk9. Scale bars in photomicrographs = 1 mm (A) and 500 μm (B–D). HCD, high-cholesterol diet; AU, arbitrary units; LDL, low-density lipoprotein; ORO, oil-red O; VLDL, very low-density lipoprotein; Vcam-1, vascular cell adhesion molecule 1; Mcp-1, monocyte chemoattractant protein 1; Il-6, interleukin 6; Pcsk9, proprotein convertase subtilisin/kexin type 9.
Plasma lipids showed an increase in total and VLDL-cholesterol in SRT3025-treated Ldlr−/− mice compared with placebo-treated controls (Figure 7E and F), whereas plasma LDL-cholesterol, HDL-cholesterol, and triglycerides were unaffected by drug treatment (Figure 7F and Supplementary material online, Figure S2A). As in Apoe−/− mice, SRT3025 prevented an increase in weight gain and epididymal white adipose tissue without affecting food intake (Supplementary material online, Figure S2B–D), and lowered plasma levels of pro-inflammatory cytokines Mcp-1 and Il-6 (Figure 7G). Compared with Apoe−/− mice, baseline plasma Pcsk9 levels in Ldlr−/− mice were about 20-fold higher (Figure 7G). SRT3025 induced a minimal but significant increase in plasma Pcsk9 levels in Ldlr−/− mice (Figure 7G). Pharmacological Sirt1 activation decreased the expression of Pparα, but did not affect Abcg5, Abcg8, Abca1, Pcsk9, Pparγ, Srebp1, Srebp2, Mcp-1 and Il-6 (Supplementary material online, Figure S2E).
Discussion
We demonstrate that pharmacological Sirt1 activation using SRT3025 attenuated Pcsk9 secretion from murine hepatocytes in vitro and lowered plasma levels of Pcsk9 in atherosclerosis-prone Apoe−/− mice in vivo. As a consequence, hepatocyte Ldlr expression and activity were increased leading to a decrease in plasma LDL-cholesterol and atherosclerotic plaques in Apoe−/− mice. None of these variables were changed in Ldlr−/− mice despite a similar decrease in systemic inflammation. Thus, our findings identify reduced Pcsk9 secretion and increased Ldlr expression as novel downstream effects of Sirt1 activity and highlight the potential of pharmacological Sirt1 activation as a novel anti-atherosclerotic strategy.
SRT3025 was found to activate wild-type Sirt1 protein but failed to activate the E230K mutant, an activation-resistant Sirt1 protein (due to a mutation at position 230). Thus, similar to previously described Sirt1 activators,15 SRT3025 acts by allosteric binding to Sirt1 at the lysine site 230, which is located at its catalytic core. Analyses of the acetylation status of the Sirt1 target Foxo1 in skeletal muscle and p65 in the liver show that SRT3025 increases deacetylation of Sirt1 target proteins in vivo. Furthermore, the increase in Ldlr protein expression in AML12 cells in vitro in response to stimulation with the Sirt1 activator was time- and concentration- dependent and could be attenuated by knockdown of Sirt1, while genetic Sirt1 overexpression mimicked the effects of SRT3025. These data indicate that the SRT3025-associated changes in Ldlr expression are Sirt1-dependent.
Endothelial-specific overexpression of Sirt1 in Apoe−/− mice was reported to protect against atherosclerosis by increasing endothelial eNOS activity.2 Furthermore, using partial Sirt1 deletion in Apoe−/− mice, we have shown that Sirt1-induced inhibition of NFκB impairs endothelial expression of the macrophage scavenger receptor Lox-1, and thus attenuates foam cell formation.3 Although these findings address beneficial effects of Sirt1 in a particular setting of atherosclerosis, they provide limited insight into the effects of Sirt1 on cholesterol metabolism. Interestingly, hepatic deletion of Sirt1 in C57BL/6 mice fed a high-cholesterol diet was associated with a mild increase in plasma LDL-cholesterol,7 suggesting that hepatic Sirt1 regulates plasma LDL-cholesterol. In line with these findings, Apoe−/− mice treated with SRT3025 showed an increase in hepatic Ldlr expression accompanied by a significant reduction in plasma LDL-cholesterol levels. Moreover, the extent of aortic atherosclerosis and the plasma levels of LDL-cholesterol were unchanged in Ldlr−/− mice, highlighting the importance of Ldlr for the atheroprotective and lipid-lowering effects of Sirt1. Of note, we observed a similar reduction in plasma levels of pro-inflammatory cytokines in both Apoe−/− and Ldlr−/− mice, whereas a reduction in plaque formation was only observed in Apoe−/− mice with endogenous Ldlr. Therefore, our data suggest that not the reduction in inflammation, which still occurs in the absence of Ldlr, but the decrease in plasma LDL-cholesterol accounted for reducing atherosclerosis in Apoe−/− mice treated with SRT3025. Moreover, since we observed a reduction in weight gain in both Apoe−/− and Ldlr−/− mice despite unaltered food intake, the atherosclerotic phenotype is also independent of changes in body weight. Taken together, we demonstrate that the increased expression of hepatic Ldlr is essential for the cholesterol-lowering effects of SRT3025.
In line with our findings, clinical studies with another Sirt1 activator have shown a reduction in plasma total cholesterol and LDL-cholesterol.8,9 The presently observed reduction in VLDL-cholesterol in mice has yet to be assessed in patients. Furthermore, constitutive Sirt1 overexpression in Ldlr−/− mice was associated with increased atherosclerosis.5 Pharmacological Sirt1 activation using an oral Sirt1 activator and constitutive genetic Sirt1 overexpression are likely to differ in efficiency and may exert differential effects on genes involved in lipid metabolism. Moreover, a difference in NAD+ availability between both models may affect Sirt1 activity.16 It remains to be seen whether constitutive Sirt1 overexpression is associated with enhanced hepatic expression of Ldlr in Apoe−/− mice.
Ldlr plays a critical role in the regulation of plasma LDL-cholesterol levels,10,11 a key determinant of atherogenesis.17 Mice overexpressing hepatic Ldlr have reduced plasma LDL-cholesterol levels and are protected from plaque formation, whereas Ldlr-deficient mice exhibit increased levels of plasma LDL- and VLDL-cholesterol and are prone to atherosclerosis when exposed to a high-cholesterol diet.4,18 We show that Ldlr protein expression is increased despite no change in Ldlr mRNA, indicating a post-translational mechanism. Indeed, degradation of Ldlr protein in liver cells is regulated via secreted Pcsk9, a serine protease that predominantly originates from the liver.14,19 By binding to the EGF-A domain of Ldlr, Pcsk9 targets this receptor for lysosomal degradation rather than for recycling to the cell surface.20,21 Ldlr was reported to be the main route of Pcsk9 clearance.22 In agreement with this, we found markedly increased plasma Pcsk9 levels in mice lacking Ldlr.
In our study, both SRT3025 treatment and Sirt1 overexpression increased Ldlr protein expression in AML12 cells, while reducing the concentration of Pcsk9 in the supernatant and lowering the amount of Pcsk9 bound to Ldlr, despite no change in Pcsk9 transcription. Notably, exogenous addition of active Pcsk9 to AML12 cells treated with SRT3025 decreased Ldlr protein expression and reduced LDL uptake by the cells. These data indicate that Ldlr internalization and degradation are not disrupted by SRT3025 treatment and can be re-induced upon exogenous addition of Pcsk9. Moreover, these data demonstrate that it is the limited extracellular availability of Pcsk9 that contributes to the increase in Ldlr protein expression, suggesting that plasma Pcsk9 levels are an important determinant of hepatic Ldlr protein surface expression in our model. The mechanisms by which Sirt1 affects Pcsk9 secretion remain to be investigated. Yet, the specific investigation of Pcsk9-dependent alterations on atherosclerosis may be hampered by reciprocal changes in Ldlr.22
Conclusions and perspectives
Our study describes a novel mechanism that causally links Sirt1 to the metabolism of LDL-cholesterol in vitro and in vivo. We demonstrate that Sirt1 activation increases hepatic Ldlr protein expression through a reduction in Pcsk9-mediated Ldlr degradation leading to decreased plaque formation.
Of note, statin therapy not only increases hepatic Ldlr, but also plasma Pcsk9 activity, thus limiting its effect on LDL-cholesterol.23 Antibody-induced inhibition of Pcsk9 binding to Ldlr is currently investigated as a very promising therapy to reduce plasma LDL-cholesterol and atherosclerosis in patients.24,25 Our study demonstrates a Sirt1-dependent alternative route to target Pcsk9 activity in a mouse model. Thus, a Sirt1 activator combined with a statin may confer the advantage of lowering plasma Pcsk9 activity. Future studies are needed to translate the effects of pharmacological Sirt1 activation to patients.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was funded by the Swiss National Science Foundation (to K.S., J.A., T.F.L., C.M.M.), the EU Ideas program (to J.A.; ERC-2008-AdG-23138), the University Research Priority Program Integrative Human Physiology at the University of Zurich (to M.M., T.F.L., C.M.M.), Matching Funds by the University of Zurich (to S.W., C.M.M.), the Swiss Heart Foundation (to C.M.M., L.J.v.T.), Sirtris (to C.M.M.) and the Zurich Heart House – Foundation for Cardiovascular Research, Switzerland. SRT3025 was provided by Sirtris Pharmaceuticals, a GSK company. Funding to pay the Open Access publication charges for this article was provided by Zurich Heart House – Foundation for Cardiovascular Research, Switzerland.
Conflict of interest: D.A.S. is a consultant to GlaxoSmithKline (GSK), J.L.E. an employee at GSK. V.S. and J.L.E. were employees of Sirtris Pharmaceuticals, a GSK company. B.S. is a member of the Institut Universitaire de France. T.F.L. received research grant by Sirtis (now GSK) to the institution. J.E. is an employee of GSK. V.S. reports personal fees from GlaxoSmithKline Inc., during the conduct of the study; personal fees from GlaxoSmithKline Inc, outside the submitted work; D.S. reports personal fees from GlaxoSmithKline, during the conduct of the study; personal fees from Ovascience, and MetroBiotech, grants from Caudalie and NovoNordisk, outside the submitted work; In addition, D.S. is also an inventor on patents on molecules to activate sirtuins and increase survival of mammalian cells with royalties paid by GlaxoSmithKline.
Acknowledgements
We thank George Vlasuk, PhD for his valuable advice.
References
- 1.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang QJ, Wang Z, Chen HZ, Zhou S, Zheng W, Liu G, Wei YS, Cai H, Liu DP, Liang CC. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein S, Lohmann C, Schafer N, Hofmann J, Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Boren J, McBurney MW, Landmesser U, Luscher TF, Matter CM. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur Heart J. 2010;31:2301–2309. doi: 10.1093/eurheartj/ehq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorenne I, Kumar S, Gray K, Figg N, Yu H, Mercer J, Bennett M. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation. 2013;127:386–396. doi: 10.1161/CIRCULATIONAHA.112.124404. [DOI] [PubMed] [Google Scholar]
- 5.Qiang L, Lin HV, Kim-Muller JY, Welch CL, Gu W, Accili D. Proatherogenic abnormalities of lipid metabolism in SirT1 transgenic mice are mediated through Creb deacetylation. Cell Metab. 2011;14:758–767. doi: 10.1016/j.cmet.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002;8:1211–1217. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 7.Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–338. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Libri V, Brown AP, Gambarota G, Haddad J, Shields GS, Dawes H, Pinato DJ, Hoffman E, Elliot PJ, Vlasuk GP, Jacobson E, Wilkins MR, Matthews PM. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS One. 2012;7:e51395. doi: 10.1371/journal.pone.0051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatasubramanian S, Noh RM, Daga S, Langrish JP, Joshi NV, Mills NL, Hoffmann E, Jacobson EW, Vlasuk GP, Waterhouse BR, Lang NN, Newby DE. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J Am Heart Assoc. 2013;2:e000042. doi: 10.1161/JAHA.113.000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietschy JM, Spady DK. Regulation of low density lipoprotein uptake and degradation in different animals species. Agents Actions Suppl. 1984;16:177–190. doi: 10.1007/978-3-0348-7235-5_21. [DOI] [PubMed] [Google Scholar]
- 11.Osono Y, Woollett LA, Herz J, Dietschy JM. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J Clin Invest. 1995;95:1124–1132. doi: 10.1172/JCI117760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudhof TC, Russell DW, Brown MS, Goldstein JL. 42 bp element from LDL receptor gene confers end-product repression by sterols when inserted into viral TK promoter. Cell. 1987;48:1061–1069. doi: 10.1016/0092-8674(87)90713-6. [DOI] [PubMed] [Google Scholar]
- 13.Attie AD, Seidah NG. Dual regulation of the LDL receptor—some clarity and new questions. Cell Metab. 2005;1:290–292. doi: 10.1016/j.cmet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J, Tremblay M, Jacques H, Jin W, Davignon J, Seidah NG, Prat A. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 2008;48:646–654. doi: 10.1002/hep.22354. [DOI] [PubMed] [Google Scholar]
- 15.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, Lee JE, E SY, Lamming DW, Pentelute BL, Schuman ER, Stevens LA, Ling AJ, Armour SM, Michan S, Zhao H, Jiang Y, Sweitzer SM, Blum CA, Disch JS, Ng PY, Howitz KT, Rolo AP, Hamuro Y, Moss J, Perni RB, Ellis JL, Vlasuk GP, Sinclair DA. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 17.Raal FJ, Pilcher GJ, Waisberg R, Buthelezi EP, Veller MG, Joffe BI. Low-density lipoprotein cholesterol bulk is the pivotal determinant of atherosclerosis in familial hypercholesterolemia. Am J Cardiol. 1999;83:1330–1333. doi: 10.1016/s0002-9149(99)00095-8. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo Surdo P, Bottomley MJ, Calzetta A, Settembre EC, Cirillo A, Pandit S, Ni YG, Hubbard B, Sitlani A, Carfi A. Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep. 2011;12:1300–1305. doi: 10.1038/embor.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, Anderson NN, Ho YK, Hammer RE, Horton JD. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest. 2006;116:2995–3005. doi: 10.1172/JCI29383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavori H, Fan D, Blakemore JL, Yancey PG, Ding L, Linton MF, Fazio S. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: evidence for a reciprocal regulation. Circulation. 2013;127:2403–2413. doi: 10.1161/CIRCULATIONAHA.113.001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welder G, Zineh I, Pacanowski MA, Troutt JS, Cao G, Konrad RJ. High-dose atorvastatin causes a rapid sustained increase in human serum PCSK9 and disrupts its correlation with LDL cholesterol. J Lipid Res. 2010;51:2714–2721. doi: 10.1194/jlr.M008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan JC, Piper DE, Cao Q, Liu D, King C, Wang W, Tang J, Liu Q, Higbee J, Xia Z, Di Y, Shetterly S, Arimura Z, Salomonis H, Romanow WG, Thibault ST, Zhang R, Cao P, Yang XP, Yu T, Lu M, Retter MW, Kwon G, Henne K, Pan O, Tsai MM, Fuchslocher B, Yang E, Zhou L, Lee KJ, Daris M, Sheng J, Wang Y, Shen WD, Yeh WC, Emery M, Walker NP, Shan B, Schwarz M, Jackson SM. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci USA. 2009;106:9820–9825. doi: 10.1073/pnas.0903849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling H, Burns TL, Hilleman DE. Novel strategies for managing dyslipidemia: treatment beyond statins. Postgrad Med. 2012;124:43–54. doi: 10.3810/pgm.2012.11.2612. [DOI] [PubMed] [Google Scholar]