Abstract
Aims
High-density lipoprotein (HDL) is highly heterogeneous and the link of its subclasses to prognosis remains controversial. We aimed to rigorously examine the associations of HDL subclasses with prognosis in secondary prevention.
Methods and results
We collaboratively analysed data from two, complementary prospective cohorts: the TRIUMPH study of 2465 acute myocardial infarction patients, and the IHCS study of 2414 patients who underwent coronary angiography. All patients had baseline HDL subclassification by vertical-spin density gradient ultracentrifugation. Given non-linearity, we stratified by tertiles of HDL-C and its two major subclasses (HDL2-C, HDL3-C), then compared multivariable-adjusted hazard ratios for mortality and mortality/myocardial infarction. Patients were middle-aged to elderly (TRIUMPH: 58.2 ± 12.2 years; IHCS: 62.6 ± 12.6 years), and the majority were men (TRIUMPH: 68.0%; IHCS: 65.5%). IHCS had lower mean HDL-C levels (34.6 ± 10.1 mg/dL) compared with TRIUMPH (40 ± 10.6 mg/dL). HDL3-C accounted for >3/4 of HDL-C (mean HDL3-C/HDL-C 0.78 ± 0.05 in both cohorts). During 2 years of follow-up in TRIUMPH, 226 (9.2%) deaths occurred, while death/myocardial infarction occurred in 401 (16.6%) IHCS patients over 5 years. No independent associations with outcomes were observed for HDL-C or HDL2-C. In contrast, the lowest tertile of HDL3-C was independently associated with >50% higher risk in each cohort (TRIUMPH: with middle tertile as reference, fully adjusted HR for mortality of HDL3-C, 1.57; 95% CI, 1.13–2.18; IHCS: fully adjusted HR for mortality/myocardial infarction, 1.55; 95% CI, 1.20–2.00).
Conclusion
In secondary prevention, increased risk for long-term hard clinical events is associated with low HDL3-C, but not HDL2-C or HDL-C, highlighting the potential value of subclassifying HDL-C.
Keywords: Acute myocardial infarction, Coronary heart disease, Secondary prevention, Lipids, High-density lipoprotein cholesterol, HDL subclasses, HDL2, HDL3, Mortality
See page 10 for the editorial comment on this article (doi:10.1093/eurheartj/ehu306)
Introduction
Coronary heart disease (CHD) remains a leading cause of death and disability in Europe and the USA.1,2 Despite major advances in the treatment of atherogenic lipoproteins, substantial residual risk in CHD patients is fuelling intensive investigation into the pharmacomodulation of high-density lipoprotein metabolism.3 In this search for novel therapeutic options, raising the total circulating cholesterol concentration of high-density lipoprotein (HDL-C) has served as a central guide.
However, there is a growing movement to take a more granular approach to more fully capture the complex array of functions through which HDL may confer cardiovascular protection. These functions include cholesterol efflux from peripheral cells, reverse cholesterol transport (RCT), anti-inflammatory, antioxidative, anti-thrombotic, and pro-endothelial actions.4–6 Recent epidemiological work supports efforts to look beyond HDL-C, showing limited value of HDL-C for predicting vascular risk in CHD patients undergoing elective coronary artery bypass grafting7 or cardiovascular mortality in the setting of stable or unstable CHD.8
By ultracentrifugation, one can isolate HDL2-C and HDL3-C, or ‘large, buoyant’ and ‘small, dense’ HDL, respectively, and these variations in density of HDL are related to its functionality.5,6,9 It is often suggested that HDL2 is the ‘protective’ form of HDL, however, epidemiological data are conflicting with some studies showing that higher HDL2-C10,11 but others suggesting that higher HDL3-C12–15 levels are most strongly associated with lower risk. These studies varied in design, adjustment for confounders, and methods for HDL subclass separation and quantification.
To better understand the associations of HDL2-C and HDL3-C with clinical outcomes, we collaboratively analysed data from two complementary, prospective cohorts of secondary prevention patients in whom HDL-C was subclassified by a common method of ultracentrifugation: (i) the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patient's Health status (TRIUMPH) study and (ii) the Intermountain Heart Collaborative Study (IHCS) of patients undergoing coronary angiography.
Methods
TRIUMPH and IHCS studies
The TRIUMPH16 and IHCS17 prospective cohort studies each enrolled patients 18 years of age or older. The TRIUMPH study enrolled between 11 April 2005 and 31 December 2008 at 24 centres in the USA, while the IHCS study enrolled between 1 March 1999 and 5 November 2007 within the Intermountain Healthcare System (LDS Hospital: Salt Lake City, UT, USA; Intermountain Medical Center, Murray, UT, USA; and McKay Dee Hospital, Ogden, UT, USA). TRIUMPH participants were included on the basis of acute myocardial infarction (MI) and IHCS participants on the basis of coronary angiography for MI, unstable angina, or stable angina. Of eligible patients approached for participation, 74% of TRIUMPH and >95% of IHCS patients provided informed consent. All patients in this study provided informed consent and each enrolling site obtained Institutional Review Board approval.
Definitions of clinical presentations and coronary heart disease
In TRIUMPH, MI was defined as clinical features of ischaemia (e.g. prolonged ischaemic signs/symptoms, ST-segment changes in ≥2 contiguous leads on electrocardiogram) combined with cardiac biomarker elevation (troponin per local laboratory cutpoints) outside the setting of elective coronary revascularization. In IHCS, MI was defined similarly, and additionally, stable angina was defined as stable exertional symptoms only, and unstable angina as progressive symptoms or symptoms at rest only.
In both studies, significant CHD was defined as the presence of one or more ≥70% obstructive lesions by coronary angiography. Angiograms were reviewed by the patient's cardiologist, and from those, patients were determined to have single-, double-, or triple-vessel disease as defined by the presence of a ≥70% stenosis in each major epicardial vessel, with left main stenosis of ≥50% counting as two vessels.
Lipid measurements
Serial laboratory testing was a voluntary substudy in TRIUMPH performed within a median of 1 day of discharge (25th–75th: 0–2 days). Blood samples in TRIUMPH were processed, serum separated, refrigerated, and sent by overnight mail in freezer packs to the core laboratory (Lenexa, KS, USA). Serum specimens were aliquoted, stored frozen at −70°C, and then sent to Atherotech (Birmingham, AL, USA) by overnight mail on dry ice. In IHCS, all patients consented to blood sampling at the time of coronary angiography. Samples were collected in EDTA and refrigerated at 4°C. Within 24 h, samples were centrifuged, plasma and DNA were separated and stored cryogenically, and then samples were later sent to Atherotech.
Cholesterol concentrations of lipoprotein classes [HDL-C, LDL-C, intermediate-density lipoprotein cholesterol (IDL-C), very-low-density lipoprotein cholesterol (VLDL-C), and lipoprotein(a) [Lp(a)] and subclasses (including HDL2-C and HDL3-C) were measured in the same laboratory by the Vertical Auto Profile (VAP) method (Atherotech).18–20 The VAP method separates lipoproteins based on their density using single vertical-spin density gradient ultracentrifugation, then quantifies cholesterol content using an enzymatic reaction and spectrophotometric absorbance.
The accuracy of the VAP procedure has been validated and is monitored on an ongoing basis by comparing its results with those obtained from the standard beta quantification procedure performed at the Core Laboratories for Clinical Studies at Washington University (St Louis, MO, USA) using split serum specimens. The following correlation coefficients (r) are typically obtained: total cholesterol, 0.99; HDL-C, 0.99; LDL-C, 0.98; VLDL-C, 0.98; IDL-C, 0.78; Lp(a)-C, 0.77; HDL2-C, 0.94, and HDL3-C, 0.91. Typical between-days coefficients of variation are: total cholesterol, 2.0%; HDL-C, 2.9%; LDL-C, 2.1%; VLDL-C, 2.8%; IDL-C, 8.2%; Lp(a)-C, 9.1%; HDL2-C, 9.2%, and HDL3-C, 2.5%.
Apolipoprotein A1 was measured in all TRIUMPH patients and 1478 IHCS patients by immunoassay (Abbott/Architect C8000; Abbott Park, IL, USA), calibrated against WHO/IFCC/CDC apolipoprotein A1 Reference Material SP1–01.21 Results from VAP and apolipoprotein testing were available for research purposes, but not to the treating providers.
Risk factor measurements
Research staff conducted detailed chart abstractions to capture baseline characteristics, including the sociodemographic and clinical parameters presented in Table 1. The methods of risk factor measurement have been previously described in TRIUMPH16 and IHCS.17
Table 1.
Baseline characteristics of TRIUMPH and IHCS
| TRIUMPH (n = 2465) | IHCS (n = 2414) | |
|---|---|---|
| Age | 58.2 (12.2) | 62.6 (12.6) |
| <65 years, (%) | 1771 (71.8) | 1284 (53.2) |
| ≥65 years, (%) | 694 (28.2) | 1130 (46.8) |
| Sex, (%) | ||
| Female | 789 (32.0) | 833 (34.5) |
| Male | 1676 (68.0) | 1581 (65.5) |
| Race, (%) | ||
| White/Caucasian | 1666/2461 (67.7) | 2162 (89.6) |
| Black/African-American | 629/2461 (25.6) | 22 (0.9) |
| Other | 166/2461 (6.7) | 230 (9.5) |
| Education, (%) | ||
| <High school | 503/2454 (20.5) | —a |
| High school/GED | 1476/2454 (60.1) | |
| College | 475/2454 (19.4) | |
| Insured, (%) | 1865/2414 (77.3) | — |
| Diabetes mellitus, (%) | 782 (31.7) | 490 (20.3) |
| Smoking status, (%) | ||
| Never | 670/2449 (27.4) (never or <100 cigarettes total) | 2009 (83.2) |
| Former | 757/2449 (30.9) (quit >30 days ago) | 405 (16.8) (current smoking or 10-pack-year history) |
| Current | 1022/2449 (41.7) (ongoing or quit <30 days ago) | |
| Alcohol use, (%) | ||
| No | 1040/2455 (42.2) | — |
| Yes | 1415/2455 (57.4) | — |
| AUDIT scores | 1.8 (2.5) | — |
| Body mass index | 29.8 (6.6) | 29.0 (7.6), n = 1668 |
| <25, (%) | 537/2333 (23.0) | 375 (22.5) |
| 25–<30, (%) | 807/2333 (34.6) | 714 (42.8) |
| ≥30, (%) | 989/2333 (42.4) | 579 (34.7) |
| Waist (cm) | 96.9 (14.2) | — |
| Systolic blood pressure, mmHg | 142.7 (29.9) | 145.6 (23.6), n = 2314 |
| Diastolic blood pressure, mmHg | 83.2 (19.0) | 82.1 (13.2), n = 2313 |
| Physical activity, (%) | ||
| Mainly sedentary | 1091/2451 (44.5) | — |
| Mild exercise | 746/2451 (30.4) | |
| Moderate exercise | 517/2451 (21.1) | |
| Strenuous exercise | 97/2451 (4.0) | |
| Hypertension, (%) | 1630 (66.1) | 1420 (58.8) |
| Hyperlipidaemia, (%) | 1170 (47.5) | 1308 (54.2) |
| Family history of CHD, (%) | 1818 (74.7) | 1035 (42.9) |
| Renal failure, (%) | 176 (7.1) | 28 (1.2) |
| Heart failure, (%) | 214 (8.7) | 20 (0.8) |
| Ejection fraction | 48.7 (13.4) | 59.2 (15.3) |
| Left-ventricular systolic function, (%) | ||
| Normal | 1550 (62.9) | 1114 (46.1) |
| Mild impairment | 445 (18.1) | 542 (22.5) |
| Moderate impairment | 272 (11.0) | 388 (16.1) |
| Severe impairment | 197 (8.0) | 370 (15.3) |
| Prior myocardial infarction, (%) | 499 (20.2) | 167 (6.9) |
| Prior stroke, (%) | 118 (4.8) | 84 (3.5) |
| Presentation, (%) | ||
| Stable angina | N/A | 1438 (59.6) |
| Unstable angina | N/A | 658 (27.2) |
| Acute myocardial infarction | 2465 (100) | 318 (13.2) |
| Extent of angiographic CHD, (%) | ||
| 0-vessel | 195/2279 (8.6) | 1114 (46.1) |
| 1-vessel | 955/2279 (41.9) | 542 (22.5) |
| 2-vessel | 598/2279 (26.2) | 388 (16.1) |
| 3-vessel | 531/2279 (23.3) | 370 (15.3) |
| GRACE mortality risk score (6 m) | 98.9 (29.6) | — |
| Atrial fibrillation, (%) | 119 (4.8) | 245 (11.7), n = 2090 |
| Discharge medications, (%) | ||
| Statin | 2156 (87.5) | 1154 (47.8) |
| Other lipid medicationb | 336 (13.6) | 1227 (50.8) |
| Aspirin | 2333 (94.6) | — |
| Diabetes medication | 717 (29.1) | 303 (12.6) |
| Diuretic | 654 (26.5) | 546 (22.7) |
| ACE-I/ARB | 1839 (74.6) | 777 (32.3) |
| Beta-blocker | 2217 (89.9) | 605 (25.1) |
| Calcium channel blocker | 285 (11.6) | 308 (12.8) |
| Coumadin | 246 (10.0) | 178 (7.4) |
| Antidepressant | 295 (12.0) | 339 (14.3) |
Cells represent mean (SD) or n (%) with the denominators at the top of the table, unless otherwise specified within cells.
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; GED, general educational development; GRACE, Global Registry of Acute Coronary Events; N/A, not applicable.
a—, data not available or not complete.
bEzetimibe, bile acid sequestrants, fibrates, niacin, and fish oil.
Follow-up and outcome adjudication
In both studies, mortality was adjudicated using the Social Security Death Index. Mortality in IHCS was also adjudicated using Intermountain Healthcare's electronic health records and Utah State Health Department death certificates. In addition to mortality, IHCS but not TRIUMPH used electronic health records to adjudicate MI during follow-up, defined as hospitalization with a troponin I level ≥0.4 ng/mL or a discharge diagnosis of an MI (ICD-9 code 410).
Statistical analysis
Based on the rationale that TRIUMPH and IHCS had differences in eligibility criteria, covariates, and endpoints, we analysed the studies in parallel, rather than as pooled data, as each study was sufficiently powered independently. Following a peer-reviewed analysis plan, descriptive statistics were utilized to characterize the populations. Continuous variables with a Gaussian distribution were reported as mean (standard deviation) and their differences were assessed by independent t-tests. Continuous variables without a Gaussian distribution were reported as median (25th–75th percentile) with differences compared by the Wilcoxon rank-sum test. Categorical variables were reported as proportions and differences were compared using the χ2 method and Fisher's exact test.
To examine the associations of HDL parameters with outcomes, we used unadjusted Kaplan–Meier estimates, adjusted restricted cubic spline curves, and multivariable-adjusted Cox proportional hazards regression. Based on study-specific analysis protocols, TRIUMPH analyses were limited to the 2-year time point, while the IHCS protocol allowed for analyses at 1, 2, 3, 4, and 5 years. Findings were similar across time points in IHCS and the 1-, 3-, and 5-year results are presented. Sequentially adjusted models are detailed in Figure 1. Given non-linearity, we stratified patients by tertiles of HDL-C, HDL2-C, and HDL3-C. We examined for interactions with age/sex/race, and performed additional subgroup (HDL-C subclass combinations), and sensitivity analyses (those with angiographically proven CHD).
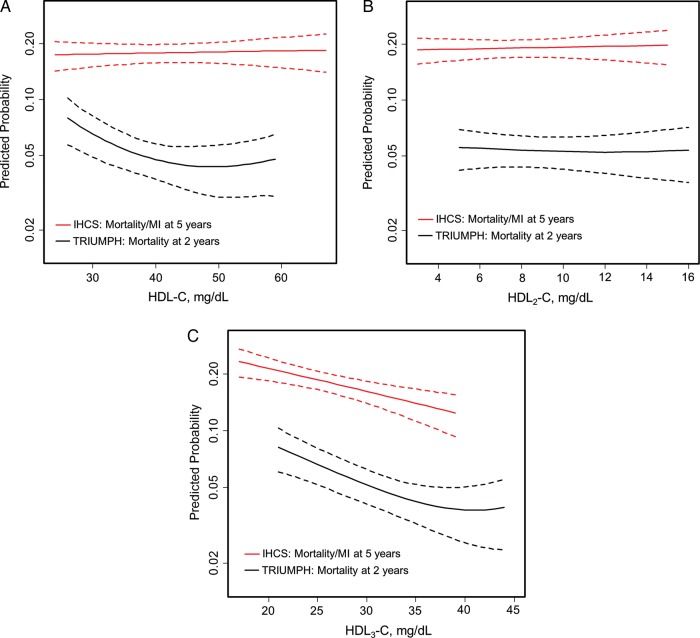
Figure 1.
Adjusted spline curves of HDL parameters in association with predicted mortality and mortality/myocardial infarction. Restricted cubic spline curves are presented. The x-axis represents observed values for HDL parameters and the y-axis represents predicted mortality at 2 years in TRIUMPH and mortality/myocardial infarction at 5 years in IHCS after adjusting for all covariates in the fully adjusted model 3.*Dotted lines indicate the 95% confidence interval. (A) HDL-C, (B) HDL2-C, and (C) HDL3-C. *Results were consistent across models 1, 2, and 3; therefore, results from model 3 only are highlighted. Model 1 was unadjusted in both cohorts. Model 2 was adjusted for GRACE score in TRIUMPH and for age and sex in IHCS. TRIUMPH model 3 was adjusted for GRACE score, age, sex, race, insurance, education, tobacco use, diabetes mellitus, hypertension, AUDIT alcohol use scores, physical activity, body mass index, non-HDL-C, log-transformed triglycerides, statins, and non-statin lipid-modifying medications (including ezetimibe, bile acid sequestrants, fibrates, niacin, and fish oil), and site. IHCS model 3 was adjusted for age, sex, tobacco use, diabetes mellitus, hypertension, hyperlipidaemia, family history of CHD, renal failure, heart failure, prior MI, prior stroke, reason for angiography, angiographic CHD, non-HDL-C, and log-transformed triglycerides, statins, and non-statin lipid-modifying medications. Of note, the GRACE score is the Global Registry of Acute Coronary Events score, a composite score of the following components: age, heart rate, systolic blood pressure, creatinine, congestive heart failure, in-hospital percutaneous coronary intervention, in-hospital coronary artery bypass surgery, prior MI, ST-segment depression on electrocardiogram, and elevated cardiac biomarkers.
In TRIUMPH, the overall covariate missing rate was 9.7%, mainly due to missing information on race, insurance, education, tobacco use, AUDIT scores, physical activity, or body mass index. Data were assumed to be missing at random and were imputed using an imputation model that contained all of the variables from the multivariable model. For IHCS, all patients had values for all covariates used in the multivariable modelling.
Statistical analyses were coordinated across centres by the Lipoprotein Investigators Collaborative (LIC). TRIUMPH analyses were conducted with SAS version 9.2 (SAS Institute, Cary, NC, USA) and R version 2.7.2 (R Foundation for Statistical Computing, Vienna, Austria); IHCS analyses were conducted with SPSS version 15.0 (IBM, Chicago, IL, USA) and SAS version 9.3 (SAS Institute, Cary, NC, USA). Two-tailed P-values <0.05 were considered statistically significant.
Results
Baseline characteristics
The baseline sociodemographic and clinical characteristics of the TRIUMPH and IHCS cohorts are shown in Table 1. Patients were middle-aged to elderly, and more than two-thirds were men. Approximately, two-thirds of patients were white in TRIUMPH, whereas IHCS was predominantly white. About one-third of TRIUMPH patients and 20% of IHCS patients had diabetes mellitus. A minority of patients in both cohorts had heart failure. A discharge statin prescription was given to 87.5% in TRIUMPH, and nearly half of IHCS patients were discharged on a statin. There were relatively low rates of use of other lipid-modifying agents such as ezetimibe, fibrates, or niacin.
Baseline lipid profile and HDL-C subclasses
Baseline lipid profile and HDL-C subclass information are presented in Table 2. Mean baseline levels of total cholesterol, LDL-C, non-HDL-C, and triglycerides were below goal levels for high-risk patients as defined by NCEP ATP III. HDL-C levels were low (TRIUMPH: 40 ± 10.6 mg/dL; IHCS: 34.6 ± 10.1 mg/dL), and the ratio of total cholesterol to HDL-C was high (TRIUMPH: 4.1 ± 1.2; IHCS: 4.5 ± 2.0). HDL3-C accounted for more than three-fourths of HDL-C; the mean ratio of HDL3-C to HDL-C was 0.78 ± 0.05 in both cohorts. Apolipoprotein A1 was more tightly correlated with HDL3-C than HDL2-C (Supplementary material online, Table S1).
Table 2.
Baseline lipid parameters in TRIUMPH and IHCS
| TRIUMPH (n = 2465) | IHCS (n = 2414) | |
|---|---|---|
| Standard lipid profile | ||
| Total cholesterol, mg/dL | 156.2 (38.5) | 134.7 (33.5) |
| HDL-C, mg/dL | 40.0 (10.6) | 34.6 (10.1) |
| Triglycerides, mg/dL | 132.0 (100.0–179.0) | 130.0 (93.0–181.0) |
| LDL-C (direct), mg/dL | 95.4 (32.3) | 81.9 (28.8) |
| Non-HDL-C, mg/dL | 116.2 (36.4) | 100.1 (53.8) |
| TC/HDL-C | 4.1 (1.2) | 4.5 (2.0) |
| TG/HDL-C | 3.5 (2.4–5.0) | 3.6 (2.1–5.2) |
| HDL subclasses and ApoAI | ||
| HDL2-C, mg/dL | 9.0 (4.0) | 7.8 (3.8) |
| HDL3-C, mg/dL | 31.0 (7.3) | 26.8 (6.9) |
| HDL2-C/HDL3-C | 0.29 (0.09) | 0.28 (0.10) |
| HDL3-C/HDL-C | 0.78 (0.05) | 0.78 (0.05) |
| HDL2-C/HDL-C | 0.22 (0.05) | 0.22 (0.05) |
| ApoAI, mg/dL | 113.2 (25.3) | 106.8 (22.9) |
| HDL-C/ApoAI | 0.37 (0.81) | 0.30 (0.04) |
Mean (SD) or median (25th–75th percentile).
Unadjusted outcomes
Follow-up was 100% complete in both studies at 2 years. In addition, in IHCS, follow-up was 89 and 46% complete at 3 and 5 years, respectively. Mortality occurred in 226 (9.2%) TRIUMPH and 243 (10.1%) IHCS patients. A total of 208 (8.6%) IHCS patients were rehospitalized for MI. There were no significant differences in mortality rates between tertiles of HDL-C (Supplementary material online, Table S2). Within subclasses, there were also no differences between HDL2-C tertiles. However, for the lowest tertile of HDL3-C compared with the middle and highest tertiles, approximately two-fold higher rates of events were observed for mortality in TRIUMPH and mortality or MI in IHCS. This finding emerged by 1 year in IHCS, and remained consistent over time.
Adjusted outcomes
Findings were consistent across sequentially adjusted models in both TRIUMPH and IHCS (Supplementary material online, Table S3). Using fully adjusted models, Figure 1 shows restricted cubic spline curves examining the association of HDL parameters with outcomes. For HDL-C, there was a non-linear, weak U-shaped association with mortality in TRIUMPH, whereas in IHCS, the curve was flat and not statistically significant. Examining HDL-C subclasses, the association of HDL2-C with outcomes was flat and not statistically significant in either cohort. In contrast, HDL3-C was strongly inversely associated with outcomes in both cohorts up through levels of ∼40 mg/dL after which in TRIUMPH there was suggestion of non-linearity with modest upturning of the curve in U-shaped fashion (could be adversely affected by the small sample size at upper limits).
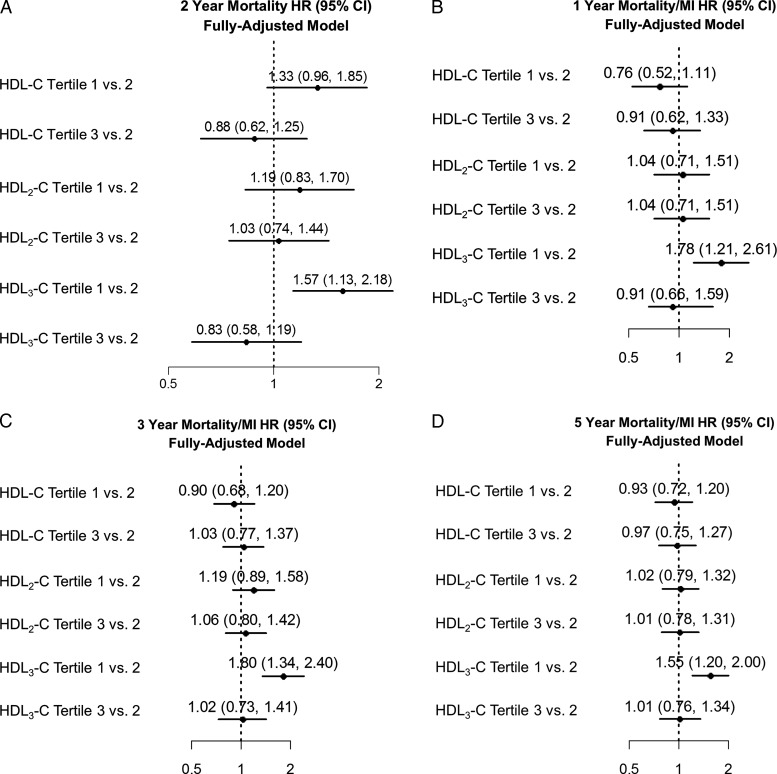
Figure 2 presents forest plots of point estimates and 95% confidence intervals for the relationship of HDL parameters with outcomes in fully adjusted models. In both cohorts, confidence intervals crossed unity for each HDL parameter with the exception of HDL3-C. For the lowest vs. middle tertile of HDL3-C (similar results for lowest vs. highest tertile of HDL3-C; Supplementary material online, Table S4), there was a higher risk of mortality (HR, 1.57; 95% CI, 1.13–2.18) in TRIUMPH and mortality or MI in IHCS (HR, 1.55; 95% CI, 1.20–2.00). These associations were only modestly attenuated by further adjustment for apolipoprotein A1 (TRIUMPH: HR, 1.42; 95% CI, 1.00–2.02 and IHCS: HR, 1.43; 95% CI, 1.05–1.96).
Figure 2.
Hazard ratios for mortality and mortality/myocardial infarction in association with hdl parameters. Models were adjusted for all covariates in the fully adjusted model 3. (A) TRIUMPH mortality at 2 years, (B) IHCS mortality/myocardial infarction at 1 year, (C) IHCS mortality/myocardial infarction at 3 years, and (D) IHCS mortality/myocardial infarction at 5 years.
Interaction, subgroup, and sensitivity analyses
In IHCS, but not TRIUMPH, there was a significant interaction between age and HDL3-C in fully adjusted Cox models (Supplementary material online, Table S5). There were no other significant interactions between age/sex and HDL parameters in either cohort (Supplementary material online, Table S6). In TRIUMPH, there was no interaction between race and HDL parameters. When stratified by sex, only HDL3-C was significantly associated with outcomes in both cohorts (Supplementary material online, Table S6A and B).
Supplementary material online, Figure S1 presents forest plots of point estimates and 95% CIs for the relationship of combinations of HDL2-C and HDL3-C with outcomes in fully adjusted models. The signal for risk was concentrated in the subgroups in both the lowest tertile of HDL3-C and lowest or middle tertile of HDL2-C.
In a sensitivity analysis, the IHCS population was restricted to only those with angiographic CHD (n = 1300) and the same patterns were found, in that HDL3-C tertiles were strongly associated with outcomes, whereas they were not for HDL-C or HDL2-C (Supplementary material online, Table S7A and B).
Discussion
To our knowledge, this is the largest and most completely adjusted observational study seeking to define the independent associations of HDL-C and its two major subclasses with clinical outcomes in patients with established CHD. In this type of population, it is also the first to examine mortality as an endpoint in relation to HDL2-C vs. HDL3-C. Our results show higher mortality in MI patients and mortality or MI in patients undergoing angiography to be strongly associated with lower HDL3-C, but not with HDL2-C levels. The consistency of findings across two complementary, prospective studies of patients spanning the clinical spectrum of CHD enhances the generalizability of our findings. Importantly, the risk associated with low HDL3-C is minimally attenuated by adjustment for apolipoprotein A1, an HDL measure previously cited to be more predictive of HDL-associated risk than HDL-C.22
HDL2 vs. HDL3
High-density lipoprotein is a highly heterogeneous particle in terms of its size, density, charge, chemical composition, and functionality.5,9 These properties are differentially related to HDL2-C and HDL3-C, the less dense and denser subclasses. HDL3 is the major form of HDL as evidenced by the majority (75%) of HDL cholesterol residing in this subclass. HDL3 particles play a central role in RCT, extracting cholesterol from the periphery, and mature into HDL2 particles via progressive lipidation by lecithin:cholesteryl acyltransferase. Indeed, apolipoprotein A1 is considered the major structural HDL protein and is more tightly correlated with HDL3-C than HDL2-C in our data. The central positioning of HDL3 in RCT and greater contribution to total HDL-C may indicate that HDL3 assumes the majority of responsibility for HDL functions, such as RCT. One could speculate that low concentrations of HDL3 indicate inefficiency in this process, potentially explaining in part the findings of our study. In addition, the findings may be related to other HDL functions, such as antioxidative capacity, that may track more closely with HDL3.
HDL-C subclasses and risk
Studies as early as the 1960s suggested that measurement of HDL-C subclasses might add information in CHD risk prediction.23 Consistent with our findings, multiple studies have found that higher HDL3-C is most strongly associated with lower risk.12–14 However, other studies showed the opposite10,11 and, by extension, suggested that HDL2 is the more protective subclass. Working towards resolution of this subclass controversy is ever more important given the demonstration in our study and other contemporary secondary prevention studies7,8 that total HDL-C levels are not predictive of risk combined with difficulties in developing therapies targeting total HDL-C levels.24,25 The results of this study, along with parallel primary prevention analyses using the same methodology for HDL-C subclassification (manuscript submitted jointly), tip the balance of evidence to HDL3-C as the primary mediator or marker of risk (Supplementary material online, Table S8).
U-shaped curve
Mortality is an objective endpoint but subject to competing non-cardiovascular risks. Our data suggest that the relationship of HDL3-C levels with mortality in CHD patients may be modestly U-shaped. This is compatible with findings from the Livermore Cohort wherein the lowest HDL3 quartile was associated with shorter longevity, while linear increases in HDL3 were not.26 A U-shaped relationship between HDL-C and mortality has also been described in several prior studies.27–29 In a Veterans Administration Medical Center Population with low LDL-C, investigators observed a U-shaped relationship between HDL-C and 1-year all-cause mortality in unadjusted and adjusted analyses.27
This observation might in part reflect the disconnect between HDL-C levels and HDL function and might also reflect HDL as a marker for non-cardiovascular conditions that lead to mortality, such as diseases or accidents related to alcohol abuse. Nevertheless, adjustment for alcohol use failed to fully account for the weak U-shaped relationship in our study, as was also the case in the aforementioned Veterans Administration population.27 This leaves open the possible role of dysfunctional HDL30 or other mechanistic possibilities that are not yet well characterized. Transformation of HDLs to dysfunctional, pro-inflammatory forms appears to be an important determinant of cardiovascular risk, and more work is needed in this novel area of research.30
The question of causality
Mendelian randomization suggests that aggregate cholesterol content of HDL particles, or HDL-C, is not causal in CHD.31 However, potential anti-atherogenic functions attributed to HDL include RCT, reduction of inflammation in the arterial wall, inhibition of LDL oxidation, and improvement of endothelial function.5–7 Given that HDL functions vary by subclass, size, and composition of HDL,32 it may be informative to conduct Mendelian randomization studies mapped to HDL subclass or particle concentration measures if genes can be identified to allow for such analysis.
Limitations
First, while we performed extensive bivariate comparisons of relevant factors in our well-characterized samples and used adjusted multivariable modelling, unmeasured confounders are possible in this observational analysis. Secondly, a one-time lipid profile at the time of MI or coronary angiography may not represent levels of HDL-C parameters over a lifetime and does not address the relationship of changes in HDL-C parameters with outcomes. Third, HDL-C subclassification by ultracentrifugation is one method of measurement among a heterogeneous set of available technologies for more granular characterization of HDL.32 Nuclear magnetic resonance spectroscopy and 2D gel electrophoresis provide information on additional subfractions, and there may be discernible differences between these technologies in risk associations. Further research is necessary to compare these techniques with ultracentrifugation, delineate which subfractions are prone to change functionality (and when), while examining their clinical implications.30 Fourth, while all-cause mortality is a reliable and important hard endpoint, competing non-cardiovascular risks may contribute. We also did not study cardiovascular endpoints other than recurrent MI, such as angina or revascularization. Finally, we address the question of prognosis, not management of HDL, wherein there is currently clinical uncertainty, and novel approaches are needed.33,34
Conclusion
These contemporary, rigorouslyadjusted analyses, supplemented with prior biological, epidemiological, and clinical trial evidence, appear to implicate the HDL3 density subclass as being the primary marker or mediator of risk. In secondary prevention populations, these data provide evidence that, by ultracentrifugation, a worse prognosis is associated with low HDL3-C, but not HDL2-C or HDL-C. Our results highlight the value of subclassifying HDL for risk stratification and provide a strong impetus for further research leveraging technologies that characterize HDL at a more granular level and map this information to clinical outcomes.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the National Heart, Lung, and Blood Institute (P50 HL 077113). S.S.M. and P.H.J. are supported by the Pollin Cardiovascular Prevention Fellowship and by NIH training grants (T32HL07024 and T32HL007227, respectively). S.S.M. is also supported by the Marie-Josée and Henry R. Kravis endowed fellowship.
Conflict of interest: None for A.A.K., H.T.M., M.J.B., P.H.J., J.B.M., J.L.A., S.K., Y.L. S.S.M. and S.R.J.: listed as co-inventors on a pending patent filed by Johns Hopkins University for a method of low-density lipoprotein cholesterol estimation. S.R.J.: Medical advisory board for Atherotech. K.R.K.: Atherotech Research Director; receives royalty from the University of Alabama in Birmingham. P.P.T.: consultancy for Amgen, AstraZeneca, Atherotech, Boehringer-Ingelheim, GSK, Kowa, Liposcience, and Merck; speakers bureau for Amarin, Amgen, AstraZeneca, Genzyme, Kowa, and Merck. J.A.S.: Research grants from Amgen, Bristol-Myers Squibb/Sanofi-Aventis, Eli Lilly, Medtronic; consultancy for Amgen, Novartis, St Jude Medical, United Healthcare.
References
- 1.Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe: epidemiologic update. Eur Heart J. 2013;34:3028–3034. doi: 10.1093/eurheartj/eht356. [DOI] [PubMed] [Google Scholar]
- 2.Gillespie CD, Wigington C, Hong Y Centers for Disease Control and Prevention (CDC) Coronary heart disease and stroke deaths - United States, 2009. MMWR Surveill Summ. 2013;62(Suppl. 3):157–160. [PubMed] [Google Scholar]
- 3.Toth PP. Pharmacomodulation of high-density lipoprotein metabolism as a therapeutic intervention for atherosclerotic disease. Curr Cardiol Rep. 2010;12:481–487. doi: 10.1007/s11886-010-0136-3. [DOI] [PubMed] [Google Scholar]
- 4.Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D'Agostino RB, Sr, Davidson MH, Davidson WS, Heinecke JW, Karas RH, Kontush A, Krauss RM, Miller M, Rader DJ. High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol. 2013;7:484–525. doi: 10.1016/j.jacl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Assmann G, Gotto AM. HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109:III8–III14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 6.Davidson WS, Silva RA, Chantepie S, Lagor WR, Chapman MJ, Kontush A. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler Thromb Vasc Biol. 2009;29:870–876. doi: 10.1161/ATVBAHA.109.186031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angeloni E, Paneni F, Landmesser U, Benedetto U, Melina G, Lüscher TF, Volpe M, Sinatra R, Cosentino F. Lack of protective role of HDL-C in patients with coronary artery disease undergoing elective coronary artery bypass grafting. Eur Heart J. 2013;34:3557–3562. doi: 10.1093/eurheartj/eht163. [DOI] [PubMed] [Google Scholar]
- 8.Silbernagel G, Schöttker B, Appelbaum S, Scharnagl H, Kleber ME, Grammer TB, Ritsch A, Mons U, Holleczek B, Goliasch G, Niessner A, Boehm BO, Schnabel RB, Brenner H, Blankenberg S, Landmesser U, März W. High-density lipoprotein cholesterol, coronary artery disease, and cardiovascular mortality. Eur Heart J. 2013;34:3563–3571. doi: 10.1093/eurheartj/eht343. [DOI] [PubMed] [Google Scholar]
- 9.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salonen JT, Salonen R, Seppanen K, Rauramaa R, Tuomilehto J. HDL, HDL2, and HDL3 subfractions, and the risk of acute myocardial infarction. A prospective population study in eastern Finnish men. Circulation. 1991;84:129–139. doi: 10.1161/01.cir.84.1.129. [DOI] [PubMed] [Google Scholar]
- 11.Lamarche B, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 1997;17:1098–1105. doi: 10.1161/01.atv.17.6.1098. [DOI] [PubMed] [Google Scholar]
- 12.Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med. 1991;325:373–381. doi: 10.1056/NEJM199108083250601. [DOI] [PubMed] [Google Scholar]
- 13.Sweetnam PM, Bolton CH, Yarnell JW, Bainton D, Baker IA, Elwood PC, Miller NE. Associations of the HDL2 and HDL3 cholesterol subfractions with the development of ischemic heart disease in British men. The Caerphilly and Speedwell Collaborative Heart Disease Studies. Circulation. 1994;90:769–774. doi: 10.1161/01.cir.90.2.769. [DOI] [PubMed] [Google Scholar]
- 14.Yu S, Yarnell JW, Sweetnam P, Bolton CH. High density lipoprotein subfractions and the risk of coronary heart disease: 9-years follow-up in the Caerphilly Study. Atherosclerosis. 2003;166:331–338. doi: 10.1016/s0021-9150(02)00361-1. [DOI] [PubMed] [Google Scholar]
- 15.Parish S, Offer A, Clarke R, Hopewell JC, Hill MR, Otvos JD, Armitage J, Collins R. Lipids and lipoproteins and risk of different vascular events in the MRC/BHF Heart Protection Study. Circulation. 2012;125:2469–2478. doi: 10.1161/CIRCULATIONAHA.111.073684. [DOI] [PubMed] [Google Scholar]
- 16.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH): Design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor GS, Muhlestein JB, Wagner GS, Bair TL, Li P, Anderson JL. Implementation of a computerized cardiovascular information system in a private hospital setting. Am Heart J. 1998;136:792–803. doi: 10.1016/s0002-8703(98)70123-1. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni KR. Cholesterol profile measurement by Vertical Auto Profile method. Clin Lab Med. 2006;26:787–802. doi: 10.1016/j.cll.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni KR, Garber DW, Marcovina SM, Segrest JP. Quantification of cholesterol in all lipoprotein classes by the VAP-II method. J Lipid Res. 1994;35:159–168. [PubMed] [Google Scholar]
- 20.Kulkarni KR, Marcovina SM, Krauss RM, Garber DW, Glasscock AM, Segrest JP. Quantification of HDL2 and HDL3 cholesterol by the Vertical Auto Profile-II (VAP-II) methodology. J Lipid Res. 1997;38:2353–2364. [PubMed] [Google Scholar]
- 21.Marcovina SM, Albers JJ, Kennedy H, Mei JV, Henderson LO, Hannon WH. International Federation of Clinical Chemistry standardization project for measurements of apolipoproteins A-I and B. IV. Comparability of apolipoprotein B values by use of international reference material . Clin Chem. 1994;40:586–592. [PubMed] [Google Scholar]
- 22.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, Kazmi K, Yusuf S. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): A case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 23.Gofman JW, Young W, Tandy R. Ischemic heart disease, atherosclerosis, and longevity. Circulation. 1966;34:679–697. doi: 10.1161/01.cir.34.4.679. [DOI] [PubMed] [Google Scholar]
- 24.Landmesser U, von Eckardstein A, Kastelein J, Deanfield J, Lüscher TF. Increasing high-density lipoprotein cholesterol by cholesteryl ester transfer protein-inhibition: a rocky road and lessons learned? The early demise of the dal-HEART programme. Eur Heart J. 2012;33:1712–1715. doi: 10.1093/eurheartj/ehs182. [DOI] [PubMed] [Google Scholar]
- 25.HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34:1279–1291. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams PT. Low high-density lipoprotein 3 reduces the odds of men surviving to age 85 during 53-year follow-up. J Am Geriatr Soc. 2012;60:430–436. doi: 10.1111/j.1532-5415.2011.03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.deGoma EM, Leeper NJ, Heidenreich PA. Clinical significance of high-density lipoprotein cholesterol in patients with low low-density lipoprotein cholesterol. J Am Coll Cardiol. 2008;51:49–55. doi: 10.1016/j.jacc.2007.07.086. [DOI] [PubMed] [Google Scholar]
- 28.Stensvold I, Urdal P, Thurmer H, Tverdal A, Lund-Larsen PG, Foss OP. High-density lipoprotein cholesterol and coronary, cardiovascular and all cause mortality among middle-aged Norwegian men and women. Eur Heart J. 1992;13:1155–1163. doi: 10.1093/oxfordjournals.eurheartj.a060331. [DOI] [PubMed] [Google Scholar]
- 29.Paunio M, Heinonen OP, Virtamo J, Klag MJ, Manninen V, Albanes D, Comstock GW. HDL cholesterol and mortality in Finnish men with special reference to alcohol intake. Circulation. 1994;90:2909–2918. doi: 10.1161/01.cir.90.6.2909. [DOI] [PubMed] [Google Scholar]
- 30.Otocka-Kmiecik A, Mikhailidis DP, Nicholls SJ, Davidson M, Rysz J, Banach M. Dysfunctional HDL: a novel important diagnostic and therapeutic target in cardiovascular disease? Prog Lipid Res. 2012;51:314–324. doi: 10.1016/j.plipres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Charakida M, Masi S, Deanfield JE. The Year in Cardiology 2012: focus on cardiovascular disease prevention. Eur Heart J. 2013;34:314–317. doi: 10.1093/eurheartj/ehs429. [DOI] [PubMed] [Google Scholar]
- 32.Rizzo M, Otvos JD, Nikolic D, Montalto G, Toth PP, Banach M. Subfractions and subpopulations of HDL: An update. Curr Med Chem. 2014 doi: 10.2174/0929867321666140414103455. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Toth PP, Barylski M, Nikolic D, Rizzo M, Montalto G, Banach M. Should low high-density lipoprotein cholesterol (HDL-C) be treated? Best Pract Res Clin Endocrinol Metab. 2014;28:353–368. doi: 10.1016/j.beem.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D European Association for Cardiovascular Prevention & Rehabilitation, ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]