Abstract
Despite suggestive early findings on the therapeutic use of hallucinogens in the treatment of substance use disorders, rigorous follow up has not been conducted. To determine the safety and feasibility of psilocybin as an adjunct to tobacco smoking cessation treatment we conducted an open-label pilot study administering moderate (20mg/70kg) and high (30mg/70kg) doses of psilocybin within a structured 15-week smoking cessation treatment protocol. Participants were 15 psychiatrically healthy nicotine-dependent smokers (10 males; mean age of 51 years), with a mean of 6 previous lifetime quit attempts, and smoking a mean of 19 cigarettes per day for a mean of 31 years at intake. Biomarkers assessing smoking status, and self-report measures of smoking behavior demonstrated that 12 of 15 participants (80%) showed seven-day point prevalence abstinence at 6-month follow-up. The observed smoking cessation rate substantially exceeds rates commonly reported for other behavioral and/or pharmacological therapies (typically <35%). Although the open-label design does not allow for definitive conclusions regarding the efficacy of psilocybin, these findings suggest psilocybin may be a potentially efficacious adjunct to current smoking cessation treatment models. The present study illustrates a framework for future research on the efficacy and mechanisms of hallucinogen-facilitated treatment of addiction.
Keywords: Hallucinogen, tobacco, smoking cessation, nicotine, addiction, psilocybin, psychedelic
Introduction
A promising research area from the 1950s through 1970s involved the therapeutic use of 5-HT2AR agonist hallucinogens in the treatment of drug dependence, including alcohol and opioid dependence (Chwelos et al., 1959; Hollister et al., 1969; Ludwig et al., 1969; Savage and McCabe, 1973; Smart et al., 1966). Some studies did not include the rigor and controls expected of modern clinical research, thus obscuring efficacy. However, a recent meta-analysis of randomized controlled trials found that LSD-facilitated treatment of alcoholism approximately doubled the success rates of control conditions at the first follow-up (Krebs and Johansen, 2012). Despite suggestive findings, this line of investigation was abandoned due to controversy surrounding the recreational use of hallucinogens and regulatory restrictions impeding subsequent research with 5-HT2AR agonists (Mangini, 1998; Nutt et al., 2013).
Recently, the 5-HT2AR agonist psilocybin was found to occasion mystical-type experiences with enduring personal meaning and spiritual significance in the majority of healthy volunteers (Griffiths et al., 2006). Moreover, at 14-month follow-up, 61% of volunteers associated these experiences with moderate to extreme positive behavior change (Griffiths et al., 2008). In another study, a moderate dose of psilocybin was found to significantly decrease anxiety and depression in advanced stage cancer patients (Grob et al., 2011). Results from these studies are atypical for pharmacotherapies in that positive effects were observed well beyond the time-course of acute drug effects. Although participants were not drug dependent, these results suggest the feasibility of a psilocybin-facilitated intervention for addiction treatment, consistent with findings that increased levels of spirituality are associated with improved outcomes in drug dependence recovery (Cole et al., 2006; Galanter, 2006; Piderman et al., 2007; Piedmont, 2004).
Smoking-related mortalities in the US are currently estimated at 480,000 annually (US Department of Health and Human Services, 2014), and 5 million annually worldwide (World Health Organization, 2011), highlighting the urgent need for novel treatments. Furthermore, most behavioral interventions and pharmacotherapies for smoking cessation exhibit only modest success rates at 6 months (typically <35%; Cahill et al., 2014; Mottillo et al., 2009). The present study was conducted as an open-label pilot study to determine the safety and feasibility (i.e., potential efficacy) of psilocybin-facilitated smoking cessation treatment. Although there is experimental evidence suggesting safety and efficacy of classic hallucinogens in the treatment of addiction, this is largely in relation to the use of LSD for treating alcoholism (Krebs and Johansen, 2012; Mangini, 1998). There has heretofore been no research on use of classic hallucinogens in the treatment of tobacco addiction. Therefore, this pilot study was conducted without a control condition as a first step to evaluate both the safety of the approach, and whether efficacy rates would be promising enough to warrant the investment of resources necessary for a randomized trial.
Methods
This study utilized a 15-week course of smoking cessation treatment, with psilocybin administration occurring in weeks 5, 7, and 13. This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board, and participants provided informed consent.
Participants
Participants were recruited through advertisements offering a novel treatment for smoking cessation involving psilocybin. Three hundred twenty-three individuals were telephone screened, and 27 of these underwent in-person medical and psychological screening. Individuals disqualified by phone were primarily seeking pay for participation, currently on medications, or did not meet minimum smoking requirements. Fifteen participants (10 males) were enrolled in the study. All of these individuals completed the study (Table 1). For inclusion in the study individuals needed to meet these criteria: smoke a minimum of 10 cigarettes per day, be healthy as determined via medical interview, electrocardiogram, blood and urinalysis laboratory tests, have multiple unsuccessful past quit attempts, and still desire to quit smoking. Furthermore, a Structured Clinical Interview for DSM-IV-TR (First et al., 2012) was performed at screening to exclude individuals with personal or family history of psychotic or bipolar disorders, and/or drug dependence (including alcohol; excluding nicotine) within the past 5 years. The study sample was relatively racially homogeneous, including 14 (93%) White individuals, and 1 Asian individual (7%). Ten participants reported minimal past use of hallucinogens with a mean of 8 (range 1–17) lifetime uses, with the most recent use being a mean of 27 years (range 7–43) before study intake. Five were hallucinogen-naïve. Participants received no monetary compensation.
Table 1.
Demographic and Smoking Characteristics, N=15
| Categories | Mean (SD) | Range |
|---|---|---|
| Sex a | 10 M, 5 F | |
| Age (years) | 51 (10.5) | 26–65 |
| Education b | ||
| Some college | 4 (26.7) | |
| Bachelor’s degree | 7 (46.7) | |
| Graduate degree | 4 (26.7) | |
| Cigarette dependence (FTCD) c | 5.3 (1.3) | 3–8 |
| Years smoking | 31 (9.9) | 10–49 |
| Previous quit attempts | 6 (3.6) | 2–12 |
| Cigarettes/day at intake | 19 (2.9) | 15–25 |
| Breath CO at intake | 30 (9.9) | 13–53 |
| Urine cotinine at intake | 1676 (594) | 841–3212 |
Mean (SD) do not apply to sex.
Mean (SD) do not apply to education. Educational achievement is represented as n (%) of total study sample.
FTCD=Fagerström Test for Cigarette Dependence. Possible scores on the FTCD range from 0 to 10, with higher scores indicating greater dependence.
Procedures
Participants were assigned to a team of two or three study staff who conducted treatment and oversaw psilocybin sessions. At least one member of each team was a doctoral level psychologist trained in delivering the study intervention and conducting psilocybin sessions. A Target Quit Date (TQD) was set to coincide with the first psilocybin session at week 5 of treatment. Participants attended four weekly meetings in which a manualized intervention consisting of cognitive-behavioral therapy (CBT) for smoking cessation and preparation for psilocybin administration were delivered (Table 2). On the TQD, participants were administered a moderate dose of psilocybin (20mg/70kg). Participants continued meeting weekly with study staff, and received another dose of psilocybin at week 7, and optionally again at week 13. Although the default dose for sessions 2 and 3 was a high dose (30mg/70kg), participants were permitted to repeat the moderate dose on these sessions.
Table 2.
Summary of Treatment Meetings and Content
| Meeting (duration) | CBT Modules | Psilocybin Session Related Topics |
|---|---|---|
| Prep. Mtg.a 1 (90 min) | Assign Target Quit Date (TQD) Sign contract to quit NURD b program Smoking diary introduction |
Introduction to overall approach Discuss childhood / early family life |
| Prep. Mtg. 2 (90 min) | Smoking diary review Reasons to quit vs. continue smoking Develop brief motivational statement WEST-D c program Health effects of smoking Smoking financial costs |
Discuss current and past relationships / romantic life |
| Prep. Mtg. 3 (90 min) | Smoking diary review Discuss previous quit attempts Weight gain and quitting smoking Withdrawal framed as “recovery” |
Discuss work, hobbies, and other important activities |
| Prep. Mtg. 4 (90 min) | Smoking diary review Dealing with urges after TQD Preparing to quit |
Discuss worldview (philosophical and/or spiritual beliefs) What to expect in psilocybin sessions |
| Psilocybin Ses.d (8 hrs) | - | - |
| Integration Mtg.e (1 hr) | - | Participant narrative of session |
| Support Mtg.f (45 min) | Support smoking abstinence Review CBT techniques |
Discuss session experiences |
Preparation meetings were held weekly for 4 weeks prior to TQD. Approximately half the duration of each meeting was spent conducting CBT, and half preparing for psilocybin sessions.
NURD program card was to be read each time a cigarette was smoked: “This cigarette is giving me No satisfaction; This is an Unpleasant experience; This cigarette is making me feel Rotten; I am losing the Desire to smoke.”
WEST-D program card was to be read each time participants noticed an urge to smoke: “What’s the trigger? Each time I feel like smoking, Stop, Think, Deprogram.”
Psilocybin sessions were introspective and contained no formal treatment content other than listening to a music program.
Integration meetings occurred the day after each psilocybin session. Participants’ experiences of the preceding day’s psilocybin session were discussed.
Support meetings were conducted weekly after TQD for 10 weeks.
The second and third sessions were intended as additional quit opportunities for those who failed to achieve abstinence after the first session. For those who did quit on the first session, the additional psilocybin sessions were meant to support motivation for long-term abstinence. This approach was informed by studies of hallucinogen-facilitated substance dependence treatment in which investigators described an extended, time-limited, post-session period (sometimes referred to as an “afterglow”) associated with decreased substance use, elevated mood and energy, decreased anxiety, and increased capacity for close interpersonal relationships (e.g., Bowen et al., 1970; Pahnke et al., 1970). As investigators have suggested, long-term abstinence may be enhanced by including multiple sessions, which may work by extending the “afterglow” period through the time of greatest relapse risk, or by increasing the probability of a transformative mystical experience (Grinspoon and Bakalar, 1979; Halpern 1996; Osmond et al., 1967; Savage and McCabe, 1973; Smart et al., 1966).
The doses and dosing sequence used in this study were informed by previous psilocybin dose-response research showing greater prevalence of mystical experience at 20mg/70kg and 30mg/70kg over other lower doses, and that ascending dose sequences produced significantly increased well-being, life satisfaction, and persisting positive mood at 1-month post-session as compared to descending dose sequences (Griffiths et al., 2011). Study staff met with participants the day after each psilocybin session, and weekly after the TQD to discuss session experiences and provide support for smoking cessation, for a total of 19 in-person meetings. A staff member made brief (<5 min.) daily phone calls to participants for two weeks after the TQD to provide encouragement for smoking abstinence. Participants were instructed to abstain from using any additional smoking cessation treatments (e.g., nicotine replacement) during the study.
Psilocybin sessions
Sessions were conducted as previously described (Griffiths et al., 2006, 2011), with the exception that participants repeated their brief motivational statement for smoking cessation before each psilocybin administration, and participated in a guided imagery exercise at the resolution of psilocybin effects on the first psilocybin session (see Cognitive-behavioral therapy section below). Sessions followed safety guidelines for human hallucinogen research (Johnson et al., 2008). Participant blood pressure and heart rate were monitored at ≤60 min. intervals, and at least one staff member was present throughout sessions. For each session a physician was on call, and rescue medications were available in case of adverse cardiovascular or psychological events (Johnson et al., 2008). During sessions, participants were encouraged to lie down on a couch and focus on their internal experience. Participants wore an eye mask and listened to a music program through headphones. During sessions, staff provided non-directive interpersonal support for managing psilocybin effects, but did not deliver smoking cessation specific content. After drug effects subsided, participants were asked to write an open-ended narrative describing their session for discussion with staff the following day.
Cognitive-behavioral therapy
In four weekly preparation meetings, participants received smoking cessation CBT (Marks, 1993; Perkins et al., 2007), largely based on the Quit For Life program (Table 2), shown to be effective in controlled studies (Marks and Sykes, 2002; Sykes and Marks, 2001). Sessions started with a brief (<10 min) “body-scan” meditation (Kabat-Zinn, 1990). In preparatory sessions participants developed their most important reasons to quit smoking into a brief motivational statement (e.g., “I want to be free, clean, and clear”). Study treatment also included two components of an effective group based smoking cessation therapy (Zernig et al., 2008). First, participants smelled a scented oil during preparatory and support meetings before each exercise. This oil was provided to the participant at the TQD and the participant was encouraged to smell it when experiencing cravings. Second, brief (<10 min) guided imagery exercises were conducted during preparatory and support meetings, and at the end of the first psilocybin session.
Measures
Biological markers of smoking abstinence
Two measures of recent smoking, exhaled carbon monoxide (CO) and urinary cotinine level (Benowitz et al., 2002), were assessed at intake, weekly throughout the intervention, and at 6-month follow-up. Breath CO was measured using a Bedfont Micro III Smokerlyzer (Haddonfield, NJ) to detect smoking over approximately the past 24 hours. Urine cotinine samples were collected and sent to an independent laboratory for analysis (Friends Medical Laboratory, Baltimore, MD) to detect smoking over approximately the previous six days. Urine cotinine levels of <200ng/mL, and breath CO of ≤6ppm were considered as biological verification of non-smoking status (Bramer and Kallungal, 2003; Javors et al., 2005; Middleton and Morice, 2000). A list of measures and their schedule of administration is summarized in Table 3.
Table 3.
Schedule of Measures Administered
| Measures | Time points assessed |
|---|---|
| Fagerström Test for Cigarette Dependence | Intake |
| Breath carbon monoxide | Intake, Weeks 2–15, 6-month follow-up |
| Urine cotinine | Intake, Weeks 2–15, 6-month follow-up |
| Timeline Follow-back | Intake, Weeks 2–15, 6-month follow-up |
| Questionnaire on Smoking Urges | Intake, Week 6, Weeks 8–15, 6-month follow-up |
| Smoking Abstinence Self-efficacy Scale | Intake, Week 6, Weeks 8–15, 6-month follow-up |
| Wisconsin Smoking Withdrawal Scale | Intake, Week 6, Weeks 8–15, 6-month follow-up |
| Visual Effects Questionnaire | Intake, 6-month follow-up |
| Post-session Headache Interview | Day after psilocybin sessions |
| Mysticism Scale | Intake, 1-week post psilocybin sessions 2 and 3 |
| States of Consciousness Questionnaire | Day of psilocybin sessions (7 hours after drug administration) |
| Persisting Effects Questionnaire | 1 week after every psilocybin session |
Timeline follow-back
Participants completed a smoking timeline follow-back (TLFB) assessment at each study meeting. The TLFB is a self-report calendar completed retrospectively by participants indicating the number of cigarettes smoked each day (Sobell and Sobell, 1992).
Fagerström Test for Cigarette Dependence
The Fagerström Test for Cigarette Dependence (FTCD; formerly Fagerström Test for Nicotine Dependence) is a 6-item questionnaire widely used to characterize the level of dependence of cigarette smokers (Fagerström, 2012; Heatherton et al., 1991). FTCD data were collected at intake.
Smoking cessation related measures
Three supplemental measures related to smoking cessation were administered at intake, weekly post-TQD until end of treatment (excluding psilocybin session weeks), and at 6-month follow-up. The Questionnaire on Smoking Urges (QSU) is a multidimensional assessment of smoking craving with demonstrated sensitivity to smoking cessation (Tiffany and Drobes, 1991). The Smoking Abstinence Self-Efficacy scale (SASE) provides a measure of smokers’ confidence to abstain from smoking in 20 hypothetical situations, and temptation to smoke in those situations, and has been found to correlate with smoking cessation outcomes (DiClemente and Prochaska, 1985; DiClemente et al., 1985). The Wisconsin Smoking Withdrawal Scale (WSWS) measures severity of smoking withdrawal and exhibits good validity and reliability in smoking cessation studies (Shiffman et al., 2004; Welsch et al., 1999).
Visual Effects Questionnaire
To probe for potential Hallucinogen Persisting Perception Disorder (Halpern and Pope, 2003), participants completed this 16-item questionnaire designed to assess occurrence, duration, and severity of visual disturbances (e.g., halos, strobe-like trails) at intake and 6-month follow-up.
Post-session Headache Interview
The first five participants had completed their psilocybin sessions when another study found that psilocybin increased headache occurrence (Johnson et al., 2012). Therefore, the final ten participants completed a staff-administered scale the day after each psilocybin session which assessed the occurrence, severity, and time-course of post-psilocybin headache.
Mysticism Scale
This 32-item questionnaire was designed to assess primary mystical experience across the lifetime (Hood, 1975), and has been employed extensively in research, demonstrating good validity, and cross-cultural reliability (Hood et al., 2001), as well as sensitivity to the effects of psilocybin (Griffiths et al., 2006, 2011). Participants completed the Mysticism scale at intake, 1-week post psilocybin session 2, and 1-week post session 3 (when applicable).
States of Consciousness Questionnaire (SOCQ)
This 100-item questionnaire has previously been used to characterize the subjective effects of psilocybin (Griffiths et al., 2006, 2011). For this study it was used to assess the occurrence of fearful or otherwise challenging experiences. Participants completed the SOCQ at the conclusion of each psilocybin session, approximately seven hours after psilocybin administration.
Persisting Effects Questionnaire
This 145-item questionnaire was designed to measure changes in attitudes, moods, behavior, and spiritual experience, and has demonstrated sensitivity to the intermediate and long-term effects of psilocybin (Griffiths et al., 2008, 2011). One hundred forty of the items were rated on a 6-point scale ranging from 0 (none) to 5 (extreme) in six categories: Attitudes about life (26 items); Attitudes about self (22 items); Mood changes (18 items); Relationships (18 items); Behavioral changes (2 items); Spirituality (43 items). Each category reflected positive and negative changes, resulting in 12 total subscales (Table 4). The questionnaire included five additional questions. The first three asked participants to rate the overall personal meaning, spiritual significance, and effects on well-being or life satisfaction attributed to their most recent psilocybin experience. The final two asked participants to endorse mechanisms by which they believed psilocybin had facilitated smoking cessation (including that psilocybin had not helped), and to rank order these items in terms of their importance (Table 5). This questionnaire was administered 1-week after each psilocybin session.
Table 4.
Persisting Effects Questionnaire Ratings at 1-week after Psilocybin Sessions*
| Subscale / Item | Mean (SEM) Session 1d | Mean (SEM) Session 2 | Mean (SEM) Session 3 |
|---|---|---|---|
| Positive attitudes about life | 49.3 (6.5) | 63.2 (4.2) | 69.7 (6.0) |
| Negative attitudes about life | 10.4 (3.2) | 6.3 (1.4) | 5.5 (1.4) |
| Positive attitudes about self | 42.2 (6.6) | 57.7 (5.2) | 65.3 (5.8) |
| Negative attitudes about self | 10.4 (2.4) | 6.1 (1.3) | 6.1 (1.3) |
| Positive mood changes | 34.6 (6.0) | 53.5 (5.8) | 62.0 (8.4) |
| Negative mood changes | 14.1 (5.4) | 4.4 (1.9) | 5.4 (4.5) |
| Altruistic/positive social effects | 34.9 (7.8) | 56.3 (6.0) | 62.2 (7.1) |
| Antisocial/negative social effects | 3.5 (1.7) | 2.8 (1.7) | 3.7 (2.2) |
| Positive behavior changes | 52.9 (9.3) | 65.3 (7.9) | 80.0 (4.9) |
| Negative behavior changes | 7.1 (5.0) | 0.0 (0.0) | 3.3 (2.3) |
| Increased spirituality | 40.0 (7.4) | 55.1 (6.0) | 60.5 (7.1) |
| Decreased spirituality | 3.4 (1.3) | 1.2 (0.6) | 1.0 (0.5) |
| How personally meaningful was the experience? (score range: 1–8) a | 5.4 (0.5) | 6.3 (0.2) | 6.3 (0.3) |
| How spiritually significant was the experience? (score range: 1–6) b | 3.4 (0.4) | 4.2 (0.2) | 4.4 (0.4) |
| Did the experience change your sense of well-being or life satisfaction? (score range: −3 to +3) c | 1.4 (0.5) | 2.5 (0.2) | 2.7 (0.3) |
Data are mean scores with 1 SEM shown in parentheses (N=15); data on attitudes, mood, altruistic/social effects, and behavior changes are expressed as percentage of maximum possible score; data for the final three questions are raw scores.
Rating scale: 1=no more than routine, everyday experiences. 2=similar to meaningful experiences that occur on average once or more a week. 3=similar to meaningful experiences that occur on average once a month. 4=similar to meaningful experiences that occur on average once a year. 5=similar to meaningful experiences that occur on average once every 5 years. 6=among the 10 most meaningful experiences of my life. 7=among the 5 most meaningful experiences of my life. 8=the single most meaningful experience of my life.
Rating scale: 1=not at all. 2=slightly. 3=moderately. 4=very much. 5=among the 5 most spiritually significant experiences of my life. 6=the single most spiritually significant experience of my life.
Rating scale: −3=decreased very much. −2=decreased moderately. −1=decreased slightly. 0=no change. 1=increased slightly. 2=increased moderately. 3=increased very much.
One participant had incomplete Persisting Effects data for session 1 and was excluded from analysis.
Table 5.
Mechanisms Attributed to Psilocybin for Smoking Cessation at 3 weeks post-TQD*
| Mechanism a | No. Endorsed (%) | Mean Rank Order b |
|---|---|---|
| By changing how you prioritize values in life, so that reasons to smoke no longer outweighed reasons to quit | 10 (67.7%) | 1.9 |
| By changing the way you orient yourself concerning the future, such that you now act in your long term holistic benefit, rather than acting in response to immediate desire | 11 (73.3%) | 2.1 |
| By reframing your quitting and staying quit as a sacrament or spiritual task | 8 (53.3%) | 2.4 |
| By strengthening your belief that you have the ability to quit and stay quit | 11 (73.3%) | 3.2 |
| By reducing the amount of stress involved with quitting and staying quit | 7 (46.7%) | 3.6 |
| Not applicable. The sessions did not help me quit or stay quit. | 1 (6.7%) | |
| Other c | ||
| “By helping me understand that my true self is and always has been a non-smoker” –Participant 1 | 1 (6.7%) | |
| “By seeing the ‘so much more’ than smoking and how it is interconnected” –Participant 8 | 1 (6.7%) | |
| “Unclear as to the effect of the psilocybin session on quitting smoking” –Participant 9 | 1 (6.7%) | |
| “Gain[ed] distance in thinking” –Participant 11 | 1 (6.7%) | |
| “By revealing the real issues that I was using the smoking to mask” –Participant 15 | 1 (6.7%) |
TQD=Target Quit Date.
Participants were asked: “If you believe the most recent psilocybin session helped you quit smoking or remain quit, then how do you believe this effect was achieved? (Check all that apply).”
Participants were asked: “Please rank order the following items in terms of their importance to helping you quit smoking or remain quit.” Participants did not rank order Not applicable or Other items.
Five participants endorsed Other. Each wrote in an additional mechanism in his/her own words.
Data analysis
Safety data consisted of acute measures of cardiovascular function during psilocybin sessions, post-session States of Consciousness Questionnaire ratings of acute adverse psychological effects, next-day headache ratings, and Visual Effects Questionnaire data. Smoking cessation outcomes were assessed using Timeline Follow-back, and biomarker data (breath CO, urine cotinine). Changes in smoking between study intake and 6-month follow-up were examined with 2-tailed paired T-tests. For the Timeline Follow-back data, the T-test compared mean cigarettes per day between the 30 days prior to study intake and the 6 months after TQD. These T-tests were conducted for both the entire study sample (N=15) and the subsample of participants who continued smoking post-TQD (n=3).
Repeated measures ANOVA tested for changes in Questionnaire on Smoking Urges, Smoking Abstinence Self-Efficacy, and Wisconsin Smoking Withdrawal Scale scores across 10 time points from intake to 6-month follow-up, and tests for linear contrasts were performed to examine directionality of change over time. Lifetime Mysticism scale scores were compared between intake and 1-week post final psilocybin session using 2-tailed paired T-tests to assess effects of psilocybin administration on scores of mystical experience. Descriptive statistics were calculated to characterize Persisting Effects Questionnaire data regarding positive and negative effects across psilocybin sessions (Table 4), and mechanisms attributed to psilocybin for smoking cessation (Table 5).
Results
Safety data
Twelve participants completed three psilocybin sessions. Three participants did not undergo a third session, but completed the study. One participant chose a moderate dose on the second psilocybin session; all other participants chose the default recommended dosing sequence (moderate in first session and high in subsequent sessions). During the 42 psilocybin sessions (16 moderate, 26 high dose) conducted in the course of this study, no clinically significant adverse events requiring physician or pharmacologic intervention occurred. As expected from previous research (Griffiths et al., 2011), States of Consciousness Questionnaire data showed that one participant (7%) reported extreme ratings, and five others (33%) reported strong ratings of fear, fear of insanity, or feeling trapped at some time during a session. These episodes occurred in six participants (40%), and occurred during five moderate and five high dose sessions. They were readily managed by interpersonal support, and had resolved by the end of the sessions. Consistent with previous findings (Griffiths et al., 2006; Grob et al., 2011), blood pressure (BP) and heart rate (HR) were elevated during drug effects with peak values occurring on average between 1.5 and 2.5 hours post-psilocybin administration. Systolic BP showed a mean (SD; range) peak of 153 (11; 134–173) mmHg (compared with baseline: 125 [10; 105–153] mmHg). Diastolic BP showed a mean (SD; range) peak of 87 (11; 72–105) mmHg (compared with baseline: 71 [8; 55–89] mmHg). HR showed a mean (SD; range) peak of 87 (11; 66–120) beats/min (compared with baseline: 68 [9; 51–89] beats/min).
Visual Effects Questionnaire data showed no increase in the occurrence of clinically significant or bothersome visual effects, comparing intake and 6-month follow-up assessments.
Of the ten participants assessed for headache, eight reported at least one post-psilocybin headache with a mean (SD; range) duration of 5.8 (2.4; 2.0–9.5) hours, onset at a mean of 6.2 (2.1; 3.5–11.5) hours after psilocybin administration and mean severity rating of mild, or 2.6 (1.3; 1–5) on a scale from 1=minimal, to 6=excruciating. Five reported use of over-the-counter headache medication the evening following the session to alleviate symptoms. These were considered approved medications for use during the study, as there is no evidence to suggest they would affect smoking cessation outcomes.
Smoking cessation outcomes
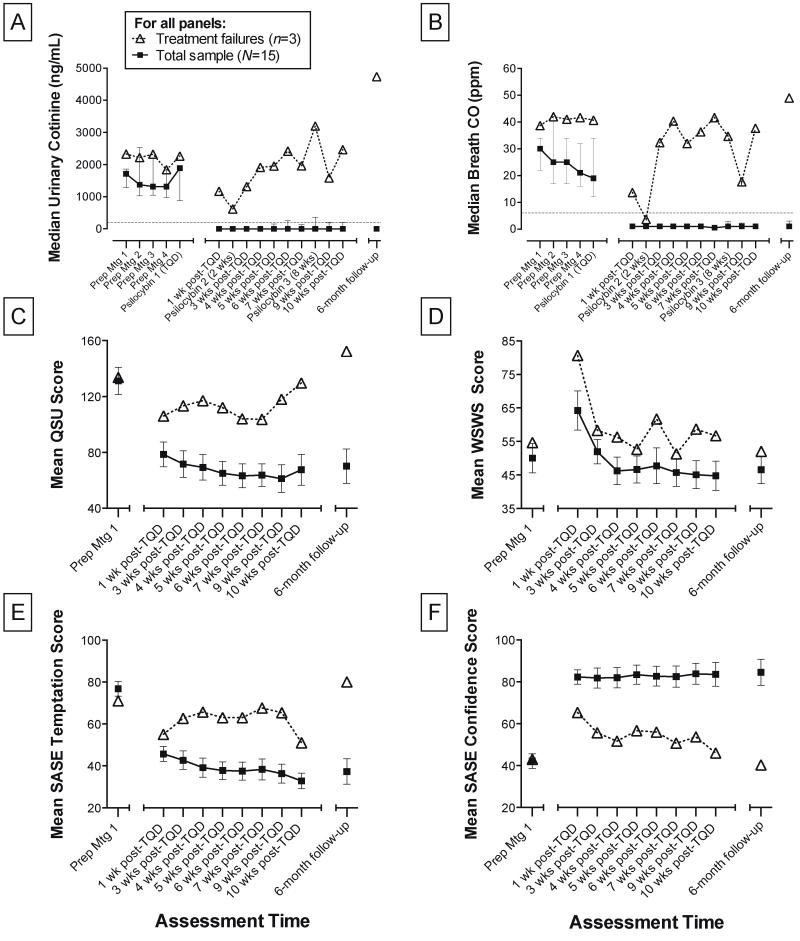
Based on the Timeline Follow-back and verified by CO and cotinine measures, 12 of 15 (80%) participants showed seven-day point prevalence abstinence at 6-month follow-up (Figure 1A–B). Eleven of these 12 self-reported quitting smoking on their TQD and demonstrated biologically verified smoking abstinence throughout the following 10 weeks of active treatment. One participant of these 12 reported quitting on the TQD and was biologically verified as abstinent at all attended meetings, but was unexpectedly required to leave the country for business, and was therefore unable to undergo a third psilocybin session, or provide CO and urine samples for weeks 6–10 post-TQD. Three of these 12 participants reported self-corrected lapses1 (consisting of 1, 4, and 48 cigarettes) during the 16-week period between end of treatment and 6-month follow-up. Another participant reported a relapse after 13 weeks of continuous abstinence, smoking an average of 5 cigarettes/day for 14 weeks (compared to a mean of 19 cigarettes/day at intake), but resumed smoking abstinence prior to 6-month follow-up, as biologically confirmed. This participant also reported use of nicotine replacement (lozenges and gum) during the relapse. No other participant reported use of smoking cessation medications throughout the study.
Figure 1.
Smoking cessation measures. Median (interquartile range) of urinary cotinine (A) and breath CO (B) at all time points are shown. Prep Mtg=Preparation Meeting. TQD=Target Quit Date. Threshold values for determining non-smoking status are indicated at dotted line (<200ng/mL cotinine; ≤6ppm breath CO). Mean (SEM) of Questionnaire on Smoking Urges (QSU) scores (C), Wisconsin Smoking Withdrawal Scale (WSWS) scores (D), Smoking Abstinence Self-Efficacy (SASE) temptation (E) and confidence (F) scores at Prep Mtg 1, at 1, 3, 4, 5, 6, 7, 9, and 10 weeks post-TQD, and at 6-month follow-up are shown. C, The QSU contains 32 items (e.g., “Smoking would make me feel happier now”), rated on a 7-point scale ranging from strongly disagree, to strongly agree (Range=32–224). D, The WSWS contains 28 items (e.g., “I have been tense or anxious”) rated on a 5-point scale ranging from strongly disagree, to strongly agree (Range=28–140). E, The SASE assesses temptation to smoke and confidence in smoking abstinence (F) in 20 hypothetical situations (e.g., “At a bar or cocktail lounge having a drink”) rated on a 5-point scale ranging from not at all, to extremely (Range=20–100).
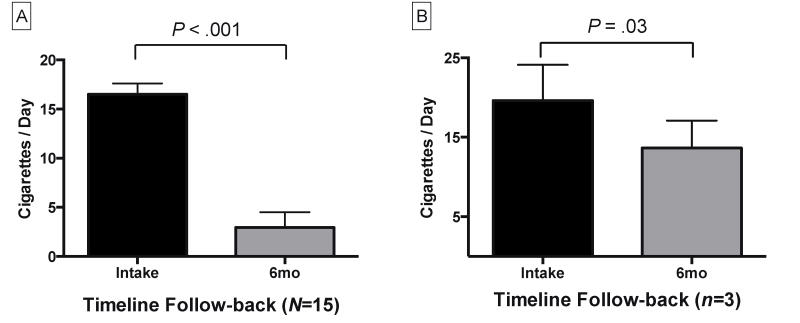
Two-tailed paired T-tests demonstrated significant reductions in self-reported daily smoking from intake to 6-month follow-up (TLFB; t14=11.1, P<.001) among the entire study sample (Figure 2A). Smoking biomarker data among the entire sample also demonstrated statistically significant reductions from intake to 6-month follow-up for breath CO levels (t14=3.8, P<.01), and urine cotinine (t14=2.3, P=.04).
Figure 2.
Smoking self-report data. Mean (SEM) of Timeline Follow-back data at intake and 6-month follow-up for the entire study sample, N=15 (A), and for participants who tested positive for smoking at 6-month follow-up, n=3 (B). 6mo=6-month follow-up. Results shown are for 2-tailed paired T-tests comparing average daily smoking.
Three participants tested positive for smoking at 6-month follow-up, and reported periods of 4, 11, and 22 days of smoking abstinence post-TQD, with two showing >6 days biologically verified continuous abstinence. These individuals ultimately resumed daily smoking. However, a 2-tailed paired T-test analysis of Timeline Follow-back data for these three participants revealed significantly reduced smoking post-TQD (Figure 2B), with a reported average of 20 cigarettes/day before TQD, and an average of 14 cigarettes/day afterwards (t2=5.3, P=.03). Two-tailed paired T-tests found no significant differences in these three participants’ smoking biomarker data from intake to 6-month follow-up for breath CO or urine cotinine levels.
Repeated measures one-way ANOVA of the entire study sample (N=15) showed significant differences across time points for Smoking Abstinence Self-Efficacy confidence (F2,34=24.9, P<.001), Smoking Abstinence Self-Efficacy temptation (F3,43=18.5, P<.001), Questionnaire on Smoking Urges (F3,39=12.7, P<.001), and Wisconsin Smoking Withdrawal Scale scores (F4,46=4.0, P=.009). Post-hoc testing for linear contrast found significantly increased confidence to abstain (SASE; P<.001) from intake to 6-month follow-up, as well as significantly decreased craving (QSU; P<.001) and temptation to smoke (SASE; P<.001) across all time points. Withdrawal scores (WSWS) fluctuated over time, peaking at 1-week post-TQD, and decreasing significantly through 6-month follow-up (P<.001). One participant had incomplete Wisconsin Smoking Withdrawal Scale data, and was excluded from analysis. Figure 1(C–F) illustrates mean scores on these measures.
Persisting effects
Lifetime Mysticism scale scores indicated a significant increase in mystical experience from intake to 1-week post final psilocybin session (difference M=+54; t14=3.5, P=0.004). Participants reported greater positive than negative effects across all Persisting Effects Questionnaire subscales, with a mean (SD) score of 5.3% (3.6) across negative subscales, and a mean (SD) score of 55.8% (12.1) across positive subscales (scores expressed as percentage of maximum possible score; Table 4).
Participants attributed substantial personal meaning to their psilocybin experiences, with 13 (87%) rating at least one psilocybin session among the 10 most meaningful experiences of their lives. Eleven (73%) rated at least one psilocybin session among the 5 most spiritually significant experiences of their lives, and 13 (87%) reported that their personal well-being or life satisfaction had increased very much as a result of at least one psilocybin session.
Participants were asked to rate several mechanisms by which psilocybin may have helped in quitting smoking at 3-weeks post-TQD (Table 5). The most commonly endorsed items (paraphrased) included: changing orientation toward the future, so that long-term benefits outweighed immediate desires (73%); strengthening participants’ belief in their ability to quit (73%); and changing life priorities/values, such that smoking was no longer more important than quitting (68%). Only one participant (7%), who exhibited the shortest duration of abstinence (4 days) among the study sample, responded that psilocybin had not helped in smoking cessation.
Discussion
This is the first study to provide preliminary data on the safety and feasibility of psilocybin as an adjunct to smoking cessation treatment. The present results are consistent with previous studies examining 5-HT2AR agonists in the treatment of drug dependence (Chwelos et al., 1959; Hollister et al., 1969; Krebs and Johansen, 2012; Savage and McCabe, 1973), suggesting both safety and feasibility, which are discussed in turn.
Our results show promise regarding the safety of psilocybin as an adjunct to smoking cessation treatment. Adverse effects associated with psilocybin consisted of modest acute increases in blood pressure, heart rate, dysphoric subjective effects (e.g., anxiety, fear; <7 hours), and headaches (<24 hours). Consistent with previous research administering psilocybin in controlled settings, these effects were readily managed (Griffiths et al., 2006, 2011; Grob et al., 2011; Johnson et al., 2008, 2012). Such time-limited effects stand in contrast to adverse effects (e.g., nausea, insomnia, abnormal dreams) associated with approved smoking cessation medications requiring daily administration (e.g., bupropion, varenicline; Gonzales et al., 2006; Jorenby et al., 2006). An advantage of the temporally confined adverse effects of psilocybin is that staff and medical personnel can readily respond with appropriate support and treatment.
Results of the present pilot study also support the feasibility of the approach, as 80% of participants were abstinent at 6-month follow-up. Results should be interpreted with caution given the small N and open-label design. Therefore, no definitive conclusions can be drawn about the causal role of psilocybin per se. However, abstinence rates were substantially higher than typical. For example, when paired with 12 brief weekly counseling meetings, pharmacotherapies have shown seven-day point prevalence abstinence rates of 24.9% to 26.3% (bupropion) and 33.5% to 35.2% (varenicline) at approximately 6 months post-TQD (Gonzales et al., 2006; Jorenby et al., 2006). Furthermore, a randomized controlled trial of the Quit for Life CBT program that provided the primary foundation for the manualized intervention used in this study found a 17.2% abstinence rate at 6-month follow-up (Sykes and Marks, 2001), although participants in our study received substantially more contact with study staff.
Our study provided higher levels of psychosocial support than typical in smoking cessation treatment. However, efficacy rates were higher than observed in studies utilizing similarly extensive CBT-based support. For example, in two trials of extended smoking cessation treatments using a combination of bupropion, nicotine replacement, and CBT ranging from 5 to 12 months in duration, participants showed 45% to 59% seven-day point prevalence abstinence at approximately 6 months (Hall et al., 2009; Killen et al., 2008). Another issue is the relative racial homogeneity and high education levels of the present study sample, which may have impacted the outcomes. Future studies would benefit from more diverse samples.
The study design was unable to discern differential benefits of moderate dose (20mg/70 kg) and high dose (30mg/70kg) sessions. All participants who quit smoking (n=12) did so after their initial moderate dose session, and those who did not quit (n=3) were unable to do so even after their subsequent high dose sessions.
One potential concern is the use of an abused drug (psilocybin) in the treatment of dependence on another drug (tobacco). However, 5-HT2AR agonists do not engender compulsive drug seeking (National Institute on Drug Abuse, 2001, 2005), consistent with evidence that they are not reliably self-administered in animals (Fantegrossi et al., 2004; Griffiths et al., 1980; Poling and Bryceland, 1979). Furthermore, the observation that two participants voluntarily declined a third psilocybin session suggests a lack of psilocybin seeking in the context of the present study.
Several plausible mechanisms of hallucinogen-facilitated treatments have been proposed (Bogenschutz and Pommy, 2012; Carhart-Harris et al., 2012, 2014; Ross, 2012; Vollenweider and Kometer, 2010). However, the mechanistic role of psilocybin in smoking cessation remains unclear. Participant responses in the present study suggest that increased temporal horizon, increased self-efficacy, and altered life priorities may be involved. The present results regarding tobacco addiction, combined with previous studies showing efficacy of 5-HT2AR agonists for treatment of alcoholism and opioid dependence (Chwelos et al., 1959; Hollister et al., 1969; Krebs and Johansen, 2012; Savage and McCabe, 1973), suggest higher-order psychological and/or biological mechanisms related to addiction are involved. This contrasts with conventional pharmacotherapies for drug dependence, which are typically specific to the pharmacology of a particular drug class. Valuable directions for future research include qualitative study of participants’ accounts regarding potential psychological mechanisms, and neuroimaging studies to inform biological mechanisms.
This study is the first to examine a 5-HT2AR agonist in the treatment of tobacco addiction, and illustrates a viable framework for psilocybin-based addiction treatment interventions. An estimated 5 million worldwide deaths per year are caused by tobacco use, and those numbers are projected to rise to over 8 million deaths annually by 2030 (World Health Organization, 2011). Given the global scope of smoking-related mortality, and the modest success rates of approved smoking cessation treatments (Cahill et al., 2014; Gonzales et al., 2006; Jorenby et al., 2006; Mottillo et al., 2009), the novel approach presented here warrants further investigation with a randomized controlled trial.
Acknowledgments
Funding/Support: Support for Dr. Griffiths was provided in part by NIDA Grant R01DA003889.
Amanda Feilding of the Beckley Foundation provided initial encouragement and funding for this research, with continued funding provided by Heffter Research Institute. Support for Dr. Garcia-Romeu was provided by National Institute on Drug Abuse Grant T32DA07209. Margaret Klinedinst, BS, Patrick Johnson, PhD, Matthew Bradstreet, PhD, Fred Reinholdt, MA, Samantha Gebhart, BS, Grant Glatfelter, BS assisted in data collection. Annie Umbricht, MD, and Leticia Nanda, CRNP provided medical screening and coverage. William A. Richards, S.T.M., PhD provided valuable clinical consultation. George Bigelow, PhD and August Holtyn, PhD provided comments on the manuscript.
Footnotes
Smoking lapses were defined as any discrete instances of smoking (even a puff of a cigarette) post-TQD, while relapses were defined as smoking on seven or more consecutive days after TQD (Hughes et al., 2003).
Previous Presentation: Portions of these findings were presented at the 2013 meeting of the College on Problems of Drug Dependence and the 2010 American Psychological Association Convention.
Financial Disclosure: Dr. Griffiths is on the board of directors of the Heffter Research Institute, a funding sponsor of the study.
References
- Benowitz NL, Jacob P, III, Ahijevych K, et al. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Pommy JM. Therapeutic mechanisms of classic hallucinogens in the treatment of addictions: From indirect evidence to testable hypotheses. Drug Test Anal. 2012;4(7–8):543–555. doi: 10.1002/dta.1376. [DOI] [PubMed] [Google Scholar]
- Bowen WT, Soskin RA, Chotlos JW. Lysergic acid diethylamide as a variable in the hospital treatment of alcoholism: a follow-up study. J Nerv Ment Dis. 1970;150:111–118. doi: 10.1097/00005053-197002000-00003. [DOI] [PubMed] [Google Scholar]
- Bramer SL, Kallungal BA. Clinical considerations in study designs that use cotinine as a biomarker. Biomarkers. 2003;8(3–4):187–203. doi: 10.1080/13547500310012545. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stevens S, Lancaster T. Pharmacological Treatments for Smoking Cessation. JAMA. 2014;311(2):193–194. doi: 10.1001/jama.2013.283787. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci. 2012;109(6):2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Tagliazucchi E, et al. The entropic brain: A theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Human Neurosci. 2014;8(20):1–20. doi: 10.3389/fnhum.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwelos N, Blewett DB, Smith CM, et al. Use of d-lysergic acid diethylamide in the treatment of alcoholism. Quart J Stud Alcohol. 1959;20:577–590. [PubMed] [Google Scholar]
- Cole C, Crum RM, Ford DE. Associations between spirituality and substance abuse symptoms in the Baltimore Epidemiologic Catchment Area follow-up, 1993–1996. J Addict Dis. 2006;25(4):125–132. doi: 10.1300/J069v25n04_12. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Prochaska JO. Processes and stages of change: Coping and competence in smoking behavior change. In: Shiffman S, Wills TA, editors. Coping and Substance Abuse. New York, NY: Academic Press; 1985. pp. 319–343. [Google Scholar]
- DiClemente CC, Prochaska JO, Gibertini M. Self-efficacy and the stages of self-change of smoking. Cognit Ther Res. 1985;9(2):181–200. [Google Scholar]
- Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerström Test for Cigarette Dependence. Nicotine Tob Res. 2012;14(1):75–78. doi: 10.1093/ntr/ntr137. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woods JH, Winger G. Transient reinforcing effects of phenylisopropylamine and indolealkylamine hallucinogens in rhesus monkeys. Behav Pharmacol. 2004;15(2):149–157. doi: 10.1097/00008877-200403000-00007. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Pub; 2012. [Google Scholar]
- Galanter M. Spirituality and addiction: A research and clinical perspective. Am J Addict. 2006;15(4):286–292. doi: 10.1080/10550490600754325. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Henningfield JE. Similarities in animal and human drug-taking behavior. Adv Subst Abuse. 1980;1:1–90. [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl) 2011;218(4):649–665. doi: 10.1007/s00213-011-2358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, Johnson MW, et al. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol. 2008;22(6):621–632. doi: 10.1177/0269881108094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, et al. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl) 2006;187(3):268–283. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Grinspoon L, Bakalar JB. Psychedelic drugs reconsidered. New York: Basic Books; 1979. [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, et al. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Arch Gen Psychiatry. 2011;68(1):71–78. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Muñoz RF, et al. Extended treatment of older cigarette smokers. Addiction. 2009;104(6):1043–1052. doi: 10.1111/j.1360-0443.2009.02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern JH. The use of hallucinogens in the treatment of addiction. Addict Res Theory. 1996;4(2):177–189. [Google Scholar]
- Halpern JH, Pope HG. Hallucinogen persisting perception disorder: what do we know after 50 years? Drug Alcohol Depend. 2003;69(2):109–119. doi: 10.1016/s0376-8716(02)00306-x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Shelton J, Krieger G. A controlled comparison of lysergic acid diethylamide (LSD) and dextroamphetamine in alcoholics. Am J Psychiatry. 1969;125(10):1352–1357. doi: 10.1176/ajp.125.10.1352. [DOI] [PubMed] [Google Scholar]
- Hood RW. The construction and preliminary validation of a measure of reported mystical experience. J Sci Study Relig. 1975;14(1):29–41. [Google Scholar]
- Hood RW, Ghorbani N, Watson PJ, et al. Dimensions of the mysticism scale: Confirming the three-factor structure in the United States and Iran. J Sci Study Relig. 2001;40(4):691–705. [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, et al. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005;100(2):159–167. doi: 10.1111/j.1360-0443.2004.00957.x. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Richards WA, Griffiths RR. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603–620. doi: 10.1177/0269881108093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Sewell RA, Griffiths RR. Psilocybin dose-dependently causes delayed, transient headaches in healthy volunteers. Drug Alcohol Depend. 2012;123(1):132–140. doi: 10.1016/j.drugalcdep.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. New York: Delacorte; 1990. [Google Scholar]
- Killen JD, Fortmann SP, Schatzberg AF, et al. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction. 2008;103(8):1381–1390. doi: 10.1111/j.1360-0443.2008.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs TS, Johansen PØ. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharm. 2012;26(7):994–1002. doi: 10.1177/0269881112439253. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Levine J, Stark L, et al. A clinical study of LSD treatment in alcoholism. Am J Psychiatry. 1969;126(1):59–69. doi: 10.1176/ajp.126.1.59. [DOI] [PubMed] [Google Scholar]
- Mangini M. Treatment of alcoholism using psychedelic drugs: a review of the program of research. J Psychoactive Drugs. 1998;30(4):381–418. doi: 10.1080/02791072.1998.10399714. [DOI] [PubMed] [Google Scholar]
- Marks DF. The QUIT FOR LIFE Programme: An easier way to stop smoking and not start again. Leicester, UK: British Psychological Society; 1993. [Google Scholar]
- Marks DF, Sykes CM. Randomized controlled trial of cognitive behavioural therapy for smokers living in a deprived area of London: outcome at one-year follow-up. Psychol Health Med. 2002;7(1):17–24. [Google Scholar]
- Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117(3):758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- Mottillo S, Filion KB, Bélisle P, et al. Behavioural interventions for smoking cessation: a meta-analysis of randomized controlled trials. Eur Heart J. 2009;30(6):718–730. doi: 10.1093/eurheartj/ehn552. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Hallucinogens and Dissociative Drugs. 2001 Mar01–4209 National Institute on Drug Abuse research report series, NIH Publication. [Google Scholar]
- National Institute on Drug Abuse. LSD NIDA Infofacts. National Institute on Drug Abuse; Rockville, MD: 2005. Feb, [Google Scholar]
- Nutt DJ, King LA, Nichols DE. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nat Rev Neurosci. 2013;14(8):577–585. doi: 10.1038/nrn3530. [DOI] [PubMed] [Google Scholar]
- Osmond H, Albahary R, Cheek F, Sarett M. Some problems in the use of LSD 25 in the treatment of alcoholism. In: Abramson HA, editor. The use of LSD in psychotherapy and alcoholism. Indianapolis, IN: Bobbs-Merrill; 1967. pp. 434–457. [Google Scholar]
- Pahnke WN, Kurland AA, Unger S, et al. The experimental use of psychedelic (LSD) psychotherapy. JAMA. 1970;212(11):1856–1863. [PubMed] [Google Scholar]
- Perkins KA, Conklin CA, Levine MD. Cognitive-behavorial therapy for smoking cessation: A practical guidebook to the most effective treatments. New York: Routledge; 2007. [Google Scholar]
- Piderman KM, Schneekloth TD, Pankratz VS, et al. Spirituality in alcoholics during treatment. Am J Addict. 2007;16(3):232–237. doi: 10.1080/10550490701375616. [DOI] [PubMed] [Google Scholar]
- Piedmont RL. Spiritual transcendence as a predictor of psychosocial outcome from an outpatient substance abuse program. Psychol Addict Behav. 2004;18(3):213–222. doi: 10.1037/0893-164X.18.3.213. [DOI] [PubMed] [Google Scholar]
- Poling A, Bryceland J. Voluntary drug self-administration by nonhumans: A review. J Psychoactive Drugs. 1979;11(3):185–190. doi: 10.1080/02791072.1979.10472103. [DOI] [PubMed] [Google Scholar]
- Ross S. Serotonergic hallucinogens and emerging targets for addiction pharmacotherapies. Psychiatr Clin North Am. 2012;35(2):357–374. doi: 10.1016/j.psc.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Savage C, McCabe OL. Residential psychedelic (LSD) therapy for the narcotic addict: a controlled study. Arch Gen Psychiatry. 1973;28(6):808–814. doi: 10.1001/archpsyc.1973.01750360040005. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West RJ, Gilbert DG. Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res. 2004;6(4):599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Smart RG, Storm T, Baker EFW, et al. A controlled study of lysergid in the treatment of alcoholism: The effects on drinking behavior. Quart J Stud Alcohol. 1966;27:469–485. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption. Rockville, MD: Humana Press; 1992. pp. 207–224. [Google Scholar]
- Sykes CM, Marks DF. Effectiveness of a cognitive behaviour therapy self-help programme for smokers in London, UK. Health Promot Int. 2001;16(3):255–260. doi: 10.1093/heapro/16.3.255. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress: a Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [PubMed] [Google Scholar]
- Vollenweider FX, Kometer M. The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat Rev Neurosci. 2010;11(9):642–651. doi: 10.1038/nrn2884. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, et al. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic, 2011: warning about the dangers of tobacco. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- Zernig G, Wallner R, Grohs U, et al. A randomized trial of short psychotherapy versus sustained-release bupropion for smoking cessation. Addiction. 2008;103(12):2024–2031. doi: 10.1111/j.1360-0443.2008.02348.x. [DOI] [PubMed] [Google Scholar]