Abstract
Pilot randomized placebo controlled chemoprevention trial in resected colorectal cancer patients demonstrated importance of synchronous adenomas and potential effects of oral calcium carbonate.
Background
Colorectal cancer is common worldwide and chemoprevention has the potential of reducing the number of individuals who may suffer and perish from this disease.
Methods
A randomized placebo controlled pilot study in colorectal cancer patients was performed using calcium carbonate as the test agent in a multi-institutional oncology study group.
Results
Two hundred twenty volunteers were randomized in the study. The primary goals of compliance, accrual, and toxicity monitoring are presented. Presence of multiple adenomas at study entry and subsequent development of metachronous adenomas were recorded and found to be associated with synchronous adenomas. The secondary endpoint of recurrent adenomas indicated lower rates of new adenoma in the volunteers randomized to the calcium group.
Conclusion
This pilot study indicates the feasibility of enrolling survivors of colorectal cancer as study volunteers in a colorectal neoplasm chemoprevention clinical trial and oral calcium continues to be a potentially effective drug in reducing colorectal adenomas.
Keywords: Calcium carbonate, Metachronous adenomas, Synchronous adenomas
Introduction
Prevention of colorectal carcinoma (CRC) has become a necessary and a realistic goal in the fight to reduce CRC deaths. Expenditures for needed prevention strategies are justified by the magnitude of the morbidity and mortality of this disease on societies worldwide.1 Although endoscopic screening can reduce the incidence of CRC, an effective primary prevention strategy, such as chemoprevention, can compliment screening by reducing the frequency of surveillance procedures. In addition, prevention of CRC is a realistic goal because recent studies in the dietary and physical activity contributions to the population incidence of CRC show promising outcomes.2
The adenoma represents the intermediate stage in the neoplastic progression to carcinoma. It carries a gradual risk for development of malignancy according to its size, histology, whether it is solitary or multiple, and its location in the large gut.3,4 Surgical removal of the adenoma decreases the rate of CRC.5 It is hypothesized that slowing the growth of the precursor lesion or inhibition of the adenoma-carcinoma progression by chemoprevention will eventually prevent or reduce the incidence of CRC.6
Placebo-controlled randomized trials have shown that calcium and nonsteroidal anti-inflammatory drugs can reduce the rates of new adenoma formation in the setting of sporadic adenomas,7,8–12 and in resected colon cancer patients.13 Higher calcium dose intake, in the range of 1200 to 1500 mg daily, was more likely to yield inhibitory effect on new adenomas.2,14,15 Patients with resected colon and rectal cancers have high rates of metachronous adenomas, especially among individuals with synchronous polyps.3 The rate of second primary carcinomas in this selected cancer survivor population exceeds the incidence rates of the primary CRC.16,17 In this group of individuals, recurrent adenomas occur in 35% of study subjects at 3 years of follow-up.18
The pilot chemoprevention study, presented here, used calcium as the intervention agent, with a calcium dose of 1800 mg daily intake, and a period of treatment for 5 years.
Methods
Patients from Southwest Oncology Group (SWOG) institutions were eligible for this study if they had stage 0, I, or II CRC, and the CRC had been resected within 550 days before registration. Ineligibility criteria were: patients younger than 18 years who had resections greater than 50% of the colon and rectum with history of renal stones and with history of hereditary nonpolyposis colon cancer syndrome.
After registration into the study, patients were entered into a 3-month run-in period on placebo to assess their compliance of study drug intake. Participants were considered compliant if they took between 80% and 120% of the placebo pills dispensed during this period. An additional entry requirement included a baseline colonoscopy performed within 180 days before the initial registration or during the run-in period. Either full colonoscopy reaching the cecum or barium enema with or without partial endoscopy was acceptable. Registered patients were randomized to 5 years of calcium carbonate (1800 mg/d in three 600-mg tablets) or 5 years of placebo (three tablets). Compliance to the assigned treatment was assessed at months 3, 6, and 12 months of the first year, every 6 months in year 2, and every year for years 3, 4, and 5 postrandomization. Follow-up colonoscopies to assess adenoma and CRC recurrence were required at 1, 3, and 5 years postrandomization. The 3-year adenoma recurrence rates were reported previously.18
There were three main objectives of this study: to assess the ability of the SWOG to enroll sufficient numbers of patients with early-stage CRC for the sake of a larger chemoprevention study of adenomas and/or new primary carcinomas; to monitor compliance to the study drug (calcium or placebo) and the follow-up colonoscopies and to estimate the dropout rate; and to assess toxicities related to calcium supplementation. A secondary objective was to assess the rate of new adenomas in the study and placebo groups.
A patient was considered compliant to the study drug if the amount taken was between 80% and 120% of dispensed and prescribed dose. For the purposes of analyzing the compliance, patients were included in this analysis if they had been followed-up for at least 2 years after randomization and if they had at least 6 months of compliance data over the course of the first 2 years on study after randomization. Compliance could not be calculated without dates of the current and previous visits, number of pills dispensed at the previous visit, and number of pills remaining in the current bottle. The patient was not included in the analysis if any of these values were missing.
We examined the frequency of follow-up colonoscopies among those participants with baseline adenomas and those without to assess if participants who had adenomas at the baseline colonoscopy were followed more closely for follow-up colonoscopies than participants who did not have adenomas at baseline. Estimating compliance proved challenging because patients may have had one or more follow-up colonoscopies outside of the required time frames around 1 year, 3 years, and 5 years postrandomization. For the purposes of estimation, a patient was considered compliant for the first required colonoscopy if he or she had at least one colonoscopy within 18 months after randomization. A patient was considered compliant for the second colonoscopy if he or she had at least one colonoscopy more than 18 and less than or equal to 42 months after randomization. A patient was considered compliant for the third colonoscopy if he or she had at least one colonoscopy more than 42 months and less than or equal to 66 months after randomization.
We created an ordinal variable for the number of colonoscopies obtained (0, 1, 2, or 3) and assessed whether it was associated with presence of baseline adenomas (Y/N) using the Fisher exact test. Additionally, for patients who had baseline adenomas, we used the Fisher exact test to assess whether the number of colonoscopies was associated with number of adenomas at baseline (≥4 versus 1–3).
Further endpoints of the study were to estimate the percentage of patients with an overall adenoma recurrence, the percentage with occurrence of second primary CRC on study, and the percentage of patients with primary locoregional or distant recurrence of the primary CRC on study. Overall adenoma recurrence was calculated as the percentage of randomized patients with at least one adenoma recurrence at any time during follow-up. Although this crude estimate does not account for the length of follow-up time, it was used primarily because of variability in compliance to the follow-up colonoscopy schedule between patients.
A logistic regression model with treatment arm as the only variable in the model was used to assess statistical significance for the main comparison of overall adenoma recurrence between the calcium and placebo arms, and an unadjusted odds ratio and 95% confidence interval (CI) was subsequently calculated. Additional logistic regression models (one for each factor measured at baseline) were used to assess statistical significance of the treatment arm comparison of overall adenoma recurrence adjusted for the baseline factor. Each model contained variables for treatment arm and for the baseline factor. Adjusted odds ratios and 95% CIs were calculated for the treatment effect in each of these models.
Results
Primary Endpoints
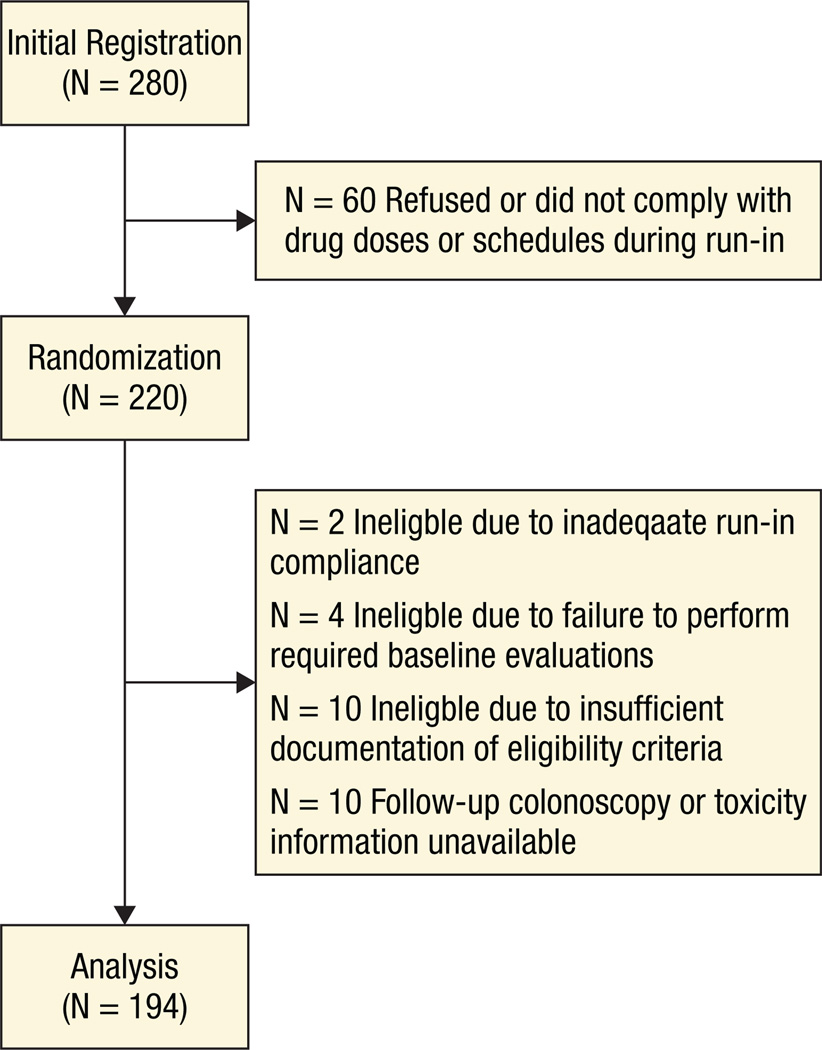
Sixty-two participating SWOG institutions initially registered 280 participants for the run-in portion of the study. Sixty patients did not continue on to randomization, mostly because of refusal or noncompliance to the study drug during the run-in period. Two hundred twenty participants were subsequently randomized to calcium carbonate or placebo. Sixteen patients were ineligible because of inadequate run-in compliance (n = 2), failure to perform required baseline evaluations (n = 4), and insufficient documentation of eligibility (n = 10). Follow-up colonoscopy and toxicity information was unavailable for 10 additional patients, leaving 194 patients for this analysis (95 on the calcium carbonate arm, 99 on the placebo arm). Figure 1 describes the flow of patients through the study. Baseline characteristics of these 194 patients are presented in Table 1.
Figure 1.
Patient Flow Diagram
Table 1.
Baseline Characteristics of Randomized Patients (n[%]) for Which Follow-Up Colonoscopy and Toxicity Information is Available (N = 194)
| Baseline Characteristic | Calcium Carbonate (n = 95) |
Placebo (n = 99) |
|---|---|---|
| Sex | ||
| Male | 57 (60) | 65 (66) |
| Female | 38 (40) | 34 (34) |
| Stratification Factors | ||
| Site of Primary CRC | ||
| Colon | 76 (80) | 80 (81) |
| Rectum | 19 (20) | 19 (19) |
| Stage of Primary CRC | ||
| 0 | 7 (7) | 4 (4) |
| I | 51 (54) | 49 (50) |
| II | 37 (39) | 46 (46) |
| Adenomas Present | ||
| Yes | 60 (63) | 59 (60) |
| No | 35 (37) | 40 (40) |
| Descriptive Factors | ||
| Synchronous Adenomas | ||
| ≥ 4 | 12 (13) | 16 (16) |
| 0–3 | 83 (87) | 83 (84) |
| Primary CRC confined to polyp | ||
| Yes | 23 (24) | 23 (23) |
| No | 72 (76) | 76 (77) |
| Time of resection of primary CRC before randomization | ||
| < 6 months | 63 (66) | 64 (65) |
| 6–18 months | 32 (34) | 35 (35) |
Eighty-four (88%) of the patients on the calcium arm and 91 (92%) of the patients on the placebo arm met the criteria to be assessed for compliance (follow-up at least 2 years, at least 6 months of compliance data without missing dates or pill counts). Sixty-six patients (79%) on the calcium arm took between 80% and 120% of their study pills for at least 6 months during the first 2 years after randomization. On the placebo arm, this proportion was slightly higher, at 81% (74 patients).
Estimates of compliance to the colonoscopy schedule are presented in Table 2. Included in the table are patients who deviated from the colonoscopy schedule. Two patients had at least three follow- up colonoscopies by 42 months (one on each arm), and three patients on the placebo arm had at least two colonoscopies by 18 months. There were an additional 17 patients on the calcium arm and 15 patients on the placebo arm that had more than one colonoscopy completed within the same time frame (eg, first follow-up colonoscopy not completed within 18 months after randomization but two follow-up colonoscopies completed between 18 and 42 months after randomization).
Table 2.
Percentages of Patients With at Least One Colonoscopy Occurring Within the Specified Timeframe, by Length of Follow-Up Time and Treatment Arm
| Length of Follow-Up Time on Study (Months) | |||||||
|---|---|---|---|---|---|---|---|
| Calcium Carbonate | Placebo | ||||||
| ≥ 18 (n = 95) |
≥ 42 (n = 87) |
≥ 66 (n = 70) |
≥ 18 (n = 96) |
≥ 42 (n = 86) |
≥ 66 (n = 72) |
||
| Time from Randomization (Months) | > 0 and ≤ 18 | 7% | 77% | 80% | 74% | 71% | 74% |
| > 18 and ≤ 42 | 53% | 54% | 48% | 46% | |||
| > 42 and ≤ 66 | 20% | 26% | |||||
In examining whether patients who had adenomas at baseline (n = 119) had differing numbers of follow-up colonoscopies (0, 1, 2, or 3) than patients who did not have adenomas at baseline (n = 75), and no statistically significant difference was found (P = .36). Among the patients who had adenomas at baseline, there was no evidence that those who had ≥ 4 adenomas at baseline had a greater number of follow-up colonoscopies than patients who had 1–3 adenomas at baseline (P = .76).
Toxicity grades were assessed using the National Cancer Institute (NCI) Common Toxicity Criteria v2.0, and this information is presented in Table 3. On the calcium arm, grade 4 hypercalcemia was reported for one patient. Three other patients experienced grade 3 toxicities. These toxicities included diarrhea, constipation, and an increase in bilirubin levels. On the placebo arm, one patient experienced grade 3 diarrhea. Eight patients on the calcium arm and 10 patients on the placebo arm experienced grade 2 toxicities. On both arms, the most common grade 2 toxicity was constipation.
Table 3.
Number of Patients With a Given Type and Grade of Adverse Eventa
| Calcium Carbonate | Placebo | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 95) | (n = 99) | |||||||||||||
| Grade | Grade | |||||||||||||
| Adverse Event | Unk | 0 | 1 | 2 | 3 | 4 | 5 | Unk | 0 | 1 | 2 | 3 | 4 | 5 |
| Allergy/hypersensitivity | 0 | 98 | 0 | 1 | 0 | 0 | 0 | 0 | 101 | 0 | 0 | 0 | 0 | 0 |
| Bilirubin increase | 0 | 94 | 4 | 0 | 1 | 0 | 0 | 0 | 92 | 8 | 1 | 0 | 0 | 0 |
| Constipation/bowel obstruction | 0 | 80 | 14 | 4 | 1 | 0 | 0 | 0 | 83 | 11 | 7 | 0 | 0 | 0 |
| Creatinine increase | 0 | 96 | 3 | 0 | 0 | 0 | 0 | 0 | 95 | 5 | 1 | 0 | 0 | 0 |
| Diarrhea without colostomy | 1 | 95 | 1 | 1 | 1 | 0 | 0 | 0 | 100 | 0 | 0 | 1 | 0 | 0 |
| Dyspnea | 0 | 98 | 0 | 1 | 0 | 0 | 0 | 0 | 101 | 0 | 0 | 0 | 0 | 0 |
| Hypercalcemia | 0 | 97 | 1 | 0 | 0 | 1 | 0 | 0 | 99 | 2 | 0 | 0 | 0 | 0 |
| Nausea | 1 | 96 | 1 | 1 | 0 | 0 | 0 | 0 | 99 | 1 | 1 | 0 | 0 | 0 |
| Proteinuria | 0 | 99 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 1 | 0 | 0 | 0 |
| SGOT (AST) increase | 0 | 99 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 1 | 0 | 0 | 0 |
| SGPT (ALT) increase | 0 | 99 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 1 | 0 | 0 | 0 |
| Weight gain | 0 | 98 | 0 | 1 | 0 | 0 | 0 | 0 | 101 | 0 | 0 | 0 | 0 | 0 |
| Maximum Grade Any Adverse Event (N) | 2 | 65 | 20 | 8 | 3 | 1 | 0 | 0 | 61 | 29 | 10 | 1 | 0 | 0 |
Adverse events unlikely or not related to treatment excluded adverse events with no entries for grades 2 to 5 have been suppressed.
Secondary Endpoints
Forty-two of 95 patients (44%) of the patients on the calcium arm had an adenoma recurrence any time during follow-up compared with 62 of the 99 patients (63%) on the placebo arm (P = .01). The odds of having an adenoma recurrence at any time during follow-up for those taking calcium were 53% lower than the odds of an adenoma recurrence in those taking placebo (odds ratio [OR] = 0.473, 95% CI,0.266, 0.840).
Overall adenoma recurrence percentages by baseline stratification/descriptive factors are presented in Table 4. Adjusted for each of the baseline factors, the overall adenoma percentage was observed to be higher in patients on the placebo arm than in patients on the calcium arm. Adjusted for treatment arm, the overall adenoma recurrence percentages were significantly different between patients who had synchronous adenomas at baseline and those who did not (P = .006). A significant difference was also found in overall adenoma percentages in patients who had 4 or more synchronous adenomas at baseline and patients who had 0–3 synchronous adenomas at baseline (P = .009).
Table 4.
Overall Adenoma Recurrence Percentages for Select Baseline Characteristics by Treatment Arm
| Baseline Characteristic |
Calcium Carbonate % (N/Total) (Arm Total: 95) |
Placebo % (N/Total) (Arm Total: 99) |
OR of Calcium Versus Placeboa |
95% CI for OR* | Treatment P Valuea |
Baseline Characteristics P Valueb |
|---|---|---|---|---|---|---|
| Site of Primary CRC | ||||||
| Colon | 45% (34/76) | 64% (51/80) | 0.473 | (0.266, 0.841) | 0.011 | 0.632 |
| Rectum | 42% (8/19) | 58% (11/19) | ||||
| Stage of Primary CRC | ||||||
| 0 | 57% (4/7) | 100% (4/4) | 0.462 | (0.258, 0.827) | 0.009 | 0.170 |
| I | 37% (19/51) | 59% (29/49) | ||||
| II | 51% (19/37) | 63% (29/46) | ||||
| Age | ||||||
| < 60 y | 42% (10/24) | 64% (18/28) | 0.472 | (0.265, 0.841) | 0.011 | 0.999 |
| ≥ 60 and < 70 y | 49% (16/33) | 58% (15/26) | ||||
| ≥ 70 y | 42% (16/38) | 64% (29/45) | ||||
| Sex | ||||||
| Female | 45% (17/38) | 62% (21/34) | 0.473 | (0.266, 0.841) | 0.011 | 0.978 |
| Male | 44% (25/57) | 63% (41/65) | ||||
| Adenomas Present | ||||||
| Yes | 53% (32/60) | 71% (42/59) | 0.437 | (0.241, 0.793) | 0.006 | 0.002 |
| No | 29% (10/35) | 50% (20/40) | ||||
| Synchronous Adenomas (SA) | ||||||
| ≥ 4 SAs | 75% (9/12) | 88% (14/16) | 0.448 | (0.245, 0.818) | 0.009 | 0.001 |
| 0–3 SAs | 40% (33/83) | 58% (48/83) |
Abbreviations: CI = confidence interval; CRC = colorectal cancer; OR = odds ratio.
Adjusted for baseline characteristic.
Adjusted for treatment arm.
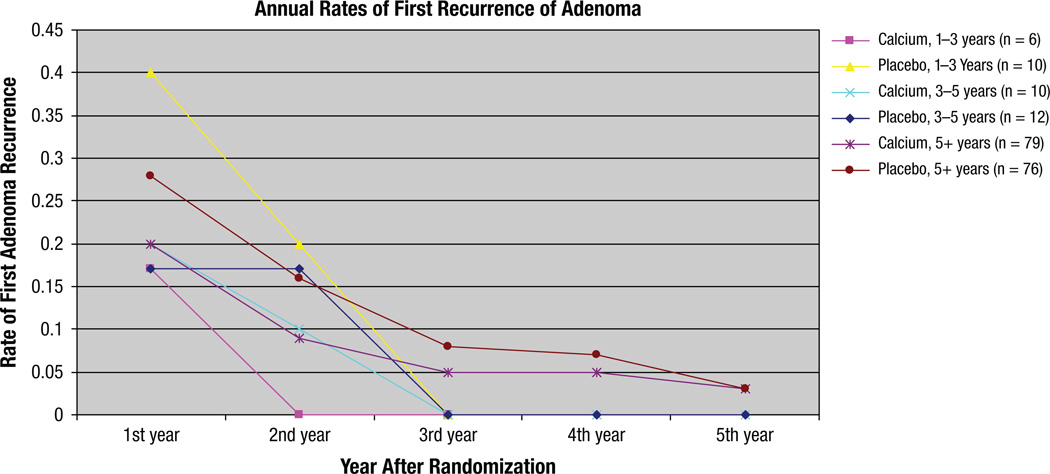
Timing of the first recurrence of adenoma is described in Figure 2. Among the placebo patients who were followed-up for 1 to 3 years, 40% had their first adenoma recurrence 1 year after randomization, compared with 17% of calcium patients. In patients followed-up for 3 to 5 years, 17% of placebo patients had their first adenoma recurrence in year 1 compared with 20% of calcium patients. In patients followed longer than 5 years, 28% of placebo patients had their first adenoma recurrence in the first year, compared with 20% of calcium patients. This difference is steady through the second year, then decreases, indicating that the action of calcium may have occurred within the first 2 years after randomization.
Figure 2.
Annual Rates of First Recurrence of Adenoma, by Treatment Arm and Length of Follow-Up on Study
Among the 60 patients on the calcium arm who had adenomas at baseline, 27 (45%) had advanced adenomas defined as adenoma 1 cm or larger or containing villous/tubulo-villous or atypical histology at baseline. Twelve of these 27 patients with advanced adenomas (44%) had an adenoma recurrence in follow-up, and 2 of those were advanced. Six other patients with nonadvanced adenomas at baseline had advanced adenomas at follow-up, and three patients with no adenomas at baseline had an advanced adenoma at follow-up (11 patients with advanced adenomas were found during follow-up period). Among the 59 patients on the placebo arm who had adenomas at baseline, 22 (37%) had advanced adenomas at baseline. Sixteen of these 22 (73%) patients had an adenoma recurrence at follow-up, and 6 of those were advanced. Two other patients with nonadvanced adenomas at baseline had advanced adenomas at follow-up, and 3 patients with no adenomas at baseline had an advanced adenoma at follow-up (11 patients with advanced adenomas, in total, found during follow-up period). In the subset of advanced adenomas at baseline (27 patients in the calcium arm and 22 in the placebo arm) 12 new adenomas were found in the calcium-treated group (44%) and 16 in the placebo group (73%).
Recurrence of the primary CRC was recorded for six patients on the calcium arm (6%). Three of these recurrences were locoregional and three were distant. On the placebo arm, a primary CRC recurrence was recorded for 10 patients (10%). Five of these recurrences were locoregional and five were distant.
Among the six patients on the calcium arm with primary CRC recurrence, three patients had an adenoma recurrence as well, none of which were advanced. In the 10 patients on the placebo arm with primary CRC recurrence, 7 patients had an adenoma recurrence as well, and two of these patients had advanced adenomas.
Twelve patients on the calcium arm (13%) and five patients on the placebo arm (5%) had second primary cancers reported at follow-up. Four of these were second primary CRC (three on the calcium arm, one on the placebo arm). The other second primary cancers reported included prostate,4 breast,1 squamous-cell carcinoma (skin),2 melanoma, 1 chondrosarcoma,1 myelodysplastic syndrome,1 urothelial transitional-cell carcinoma,1 gastrointestinal stromal tumor,1 and prostate and basal-cell carcinoma.1
Discussion
This colorectal chemoprevention study was launched in the SWOG, a multi-institutional study group, with the goal of examining the effects of oral calcium on development of new adenomas in patients with resected CRCs. Although the investigators in SWOG had extensive experience in conducting therapeutic oncology studies, conducting a chemoprevention clinical study was a novel direction and it was believed that a pilot study design was the prudent approach which could lead into a larger phase III trial.
The results on the endpoints of compliance to the intake of oral agent and to the colonoscopic procedures, and on the endpoints of toxicities, were similar to previous reported studies.7,13
Multiple colorectal lesions, namely synchronous adenomas to the incident carcinoma, were identified at study entry colonoscopy and at follow-up. The presence of synchronous adenomas increases the risk of developing a new adenoma during the study. This trend was also reported at 3-year of follow-up examinations.18 The increased numbers of observed events, both in patients with single and multiple colorectal lesions, was higher than the assumed rate of 30% in 5 years and was higher than reported in Sandler’s study.13 These increased rates of adenoma recurrence allowed the observations in the secondary endpoint with greater statistical power, namely, the effects of the treatment agent, calcium carbonate, on the rate of recurrent adenomas. As observed in this pilot study, patients on calcium carbonate developed fewer recurrent adenomas in the colon and rectum compared with patients on placebo, shown in Table 4.
There is only one other chemoprevention trial in colorectal neoplasms using resected colon and rectal cancer patients as subjects.8 Other reported trials were conducted that studied individuals who had sporadic adenomas.7,8–10,12 In this particular trial, aspirin was used as the test agent. The dose of 325 mg, taken daily for 3 years, was found efficacious in reducing the new adenoma development rate from 27% to 17%.13 The rate of synchronous adenoma was not evaluated. The rate of sporadic adenoma in unselected population is approximately 30% at age 60 years and increases with age.10
Chemoprevention trials with single agents, such as calcium or nonsteroidal anti-inflammatory drugs, demonstrated inhibitory effects on new adenomas in the order of 8% to 22%. A combination of two agents, dufluoromethylorithine and sulindac, used in the trial reported by Meyskens12 showed an impressive 70% inhibitory effect, and up to 95% inhibitory effects on advanced and multiple adenomas.
There is only one randomized CRC prevention study reporting CRC rather than adenomas as the endpoint. This study, the Women’s Health Study (WHS), an 8-year investigation using 1,000 mg of calcium and 400 mU vitamin D taken as a daily oral dose for the duration of the study,17 showed no differences in CRC rates in the study and control individuals. Colonoscopy was not required and individuals undergoing colonoscopies by choice, which was the prevailing screening recommendation, was 28%. These findings have cast doubts on the role of calcium in colorectal chemoprevention. However, in this cohort of women, half of the volunteers were randomized to a combination drug of estrogen and progesterone, which in the same study (reported in a separate analysis19) were found to develop fewer CRCs. A potential interaction between estrogen therapy and calcium intake may also obscure the effects of calcium on possible colorectal carcinogenesis.20
The chemoprevention trials reviewed in this discussion were fairly mature, placebo-controlled, randomized clinical studies with designs based on a large body of preclinical data. The cell kinetics in the colorectal mucosa have been elegantly studied and described by Lipkin. 21 The colorectal mucosal crypt cells show an orderly rate of maturation and migration toward the luminal surface, some undergoing apoptosis, with eventual balance in the total cell population, providing an uniform mucosal surface. An excess in cell population, reflecting hyperplasia, results in folds and uneven surface in the mucosa, forming first aberrant crypt foci which, if persistent, may lead to mucosal microadenomas and polyps. Luminal calcium concentrations are capable of regulating the proliferative rate of the crypt cells through activation of calcium cell surface receptors, which in turn signal intracellular pathways involved with proliferation, differentiation, and apoptosis.21 On a historical note, one of the first randomized trials using calcium was performed by Hofstad.22,23
Endoscopic surveillance of colorectal adenomas and surgical excision or ablation can reduce the rate of CRC.5 These methods constitute the gold standard for screening and prevention. Although these procedures can potentially prevent the majority of CRC, several problems exist that complicate its implementation. The precursor of the CRC can occur as a flat adenoma, which can be missed or incompletely removed by endoscopy. This fact may explain the higher miss-rate of lesions in the right colon segment.24 Currently less than half of the individuals who could benefit from screening endoscopic procedures actually comply with the publicized recommendations.25 In addition, chemoprevention can reduce the cost of screening and prevention by decreasing the frequency of the endoscopic procedures. Emerging data show that combined endoscopic screening and chemoprevention lower the frequency of adenoma and advanced adenoma compared with endoscopic screening alone.26
The reported toxicity spectrum was acceptable. Constipation, being the most common reported toxicity, was found nearly equally distributed in both calcium and placebo groups. No increased numbers of renal stones were found in contrast to the WHS study.17 The rate of recurrent or new adenomas encountered was highest in the first 3 years and decreased to a rate of less than 5% per year at 5 years (Figure 2), thus justifying a 3-year rather than 5-year trial, as found in the majority of the reported chemoprevention trials of colorectal adenomas.
The ultimate goal is to determine whether a calcium-based chemoprevention intervention can decrease the numbers of new primary CRCs. This pilot study would suggest that future chemoprevention trials employing survivors of CRC are feasible and that combination agents, as suggested by the Meyskens’ trial,12 should be tested. This pilot study also indicates that oral calcium should be considered as one of the combination agents in a larger phase III clinical trial. A subset of high-risk individuals (ie, individuals with previous CRC and synchronous adenoma) could be enrolled in a trial in which the endpoints of adenoma and possibly carcinoma can be studied. The second primary CRC rate in this group could be as high as 1% per year.27 At this rate of new CRC, a study could be performed with 1000 to 2000 volunteers, within the budget and size range of current trials. Another potential advantage of using colorectal canceCRC patient volunteers as subjects in a large chemoprevention trial is the potential side benefit of the chemoprevention agent on suppressing cancer recurrences.28 In this pilot study there were fewer recurrences in the calcium arm compared with placebo arm (6 versus 10), equally distributed among locoregional and distant recurrent disease. Clearly, these numbers are too small to reach any conclusions, however, they would support further observations in a larger study.
Clinical Practice Points
Colorectal cancer show multifocal presentation of pre-cancerous luminal lesions, with potential prognostic significance and criteria.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA20319, CA58658, CA67663, CA35431, CA67575, CA63844, CA27057, CA45377, CA46368, CA35261, CA63845, CA46113, CA76429, CA13612, CA35178, CA42777, CA74647, CA45807, CA63850, CA28862, CA12644, CA35262, CA22433, CA76447, CA58415, CA12213, CA35192, CA14028, CA45450, CA58416, CA37981, CA35090, CA35281, CA45560, CA52757, CA04919, CA35176, CA52772, CA46441, CA58723, CA52654, CA68183.
References
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Martinez ME. Primary prevention of colorectal cancer: lifestyle, nutrition, exercise. Recent Results Cancer Res. 2005;166:177–211. doi: 10.1007/3-540-26980-0_13. [DOI] [PubMed] [Google Scholar]
- 3.Chu DZJ, Giacco J, Martin RG, et al. The significance of synchronous polyps and adenocarcinoma in the colorectum. Cancer. 1986;57:445–450. doi: 10.1002/1097-0142(19860201)57:3<445::aid-cncr2820570307>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Leslie A, Carey FA, Pratt NR, et al. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002;89:845–860. doi: 10.1046/j.1365-2168.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 5.Thiis-Eversen E, Hoff GS, Sauar J, et al. Population based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol. 1999;34:414–420. doi: 10.1080/003655299750026443. [DOI] [PubMed] [Google Scholar]
- 6.Jankowski JA, Hawk ET. A methodologic analysis of chemoprevention and cancer prevention strategies for gastrointestinal cancer. Nat Clin Pract Gastroenterol Hepatol. 2006;3:101–111. doi: 10.1038/ncpgasthep0412. [DOI] [PubMed] [Google Scholar]
- 7.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 8.Bonithon-Koop C, Kronborg O, Giacosa A, et al. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomized intervention trial. European Cancer Prevention Organisation Study Group. Lancet. 2000;356:1300–1306. doi: 10.1016/s0140-6736(00)02813-0. [DOI] [PubMed] [Google Scholar]
- 9.Wallace K, Baron JA, Cole BF, et al. Effect of calcium supplementation on the risk of large bowel polyps. J Natl Cancer Inst. 2004;96:921–925. doi: 10.1093/jnci/djh165. [DOI] [PubMed] [Google Scholar]
- 10.Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 11.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 12.Meyskens FL, McLaren CE, Pelot D, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled double-blind trial. Cancer Prev Res (Phila Pa) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 14.Peters U, Chatterjee N, McGlynn KA, et al. Calcium intake and colorectal adenoma in a US colorectal cancer early detection program. Am J Clin Nutr. 2004;80:1358–1365. doi: 10.1093/ajcn/80.5.1358. [DOI] [PubMed] [Google Scholar]
- 15.Das A, Chak A, Cooper GS. Temporal trend in relative risk of second primary colorectal cancer. Am J Gastroenterol. 2006;101:1342–1347. doi: 10.1111/j.1572-0241.2006.00580.x. [DOI] [PubMed] [Google Scholar]
- 16.Green RJ, Metlay JP, Propert K, et al. Surveillance for second primary colorectal cancer after adjuvant chemotherapy: an analysis of Intergroup 0089. Ann Intern Med. 2002;136:261–269. doi: 10.7326/0003-4819-136-4-200202190-00005. [DOI] [PubMed] [Google Scholar]
- 17.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Women’s health initiative investigators: calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 18.Chu DZJ, Chansky K, Alberts DS, et al. Adenoma recurrences after resection of colorectal carcinoma: results from the Southwest Oncology Group 9041 calcium chemoprevention pilot trial. Ann Surg Oncol. 2003;10:870–875. doi: 10.1245/aso.2003.03.037. [DOI] [PubMed] [Google Scholar]
- 19.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 20.Ding EL, Mehta S, Fawzi WW, et al. Interaction of estrogen therapy with calcium and vitamin D supplementation on colorectal cancer risk: reanalysis of Women’s Health Initiative randomized trial. Int J Cancer. 2008;122:1690–1694. doi: 10.1002/ijc.23311. [DOI] [PubMed] [Google Scholar]
- 21.Lipkin M. Early development of cancer chemoprevention clinical trials: studies of dietary calcium as a chemopreventive agent for human subjects. Eur J Cancer Prev. 2002;11(suppl 2):S65–S70. [PubMed] [Google Scholar]
- 22.Hofstad B, Vatn MH, Andersen SN, et al. The relationship between faecal bile acid profile with or without supplementation with calcium and antioxidants on recurrence and growth of colorectal polyps. Eur J Cancer Prev. 1998;7:287–294. doi: 10.1097/00008469-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hofstad B, Almendingen K, Vatn M, et al. Growth and recurrence of colorectal polyps: a double-blind 3-year intervention with calcium and antioxidants. Digestion. 1998;59:148–156. doi: 10.1159/000007480. [DOI] [PubMed] [Google Scholar]
- 24.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 25.Meissner HI, Breen N, Klabunde CN, et al. Patterns of colorectal cancer screening uptake among men and women in the U.S. Cancer Epidemiol Biomarkers Prev. 2006;15:389–394. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 26.Cole BF, Logan RF, Halabi S, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101:256–266. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soreide K, Gudlaugsson E, Skaland I, et al. Metachronous cancer development in patients with sporadic adenomas – multivariate risk model with independent and combined value of hTERT and surviving. Int J Colorectal Dis. 2008;23:389–400. doi: 10.1007/s00384-007-0424-6. [DOI] [PubMed] [Google Scholar]
- 28.Holmes MD, Chen WY, Li L, et al. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]