Abstract
Prior work has established the zebrafish embryo as an in vivo model for studying the biological effects of exposure to low doses of ionizing radiation. One of the known effects of radiation is to elevate the levels of reactive oxygen species (ROS) in tissue. However, ROS are also produced as byproducts of normal metabolism and, regardless of origin, ROS produce similar chemical damage to DNA. Here we use the zebrafish embryo model to investigate whether the effects of low-dose (0–1.5 Gy) radiation and endogenous ROS are mechanistically distinct. We increased levels of endogenous ROS by exposure to low concentrations of the quinone drug, menadione. Imaging studies in live embryos showed that exposure to 3 μM or higher concentrations of menadione dramatically increased ROS levels. This treatment was associated with a growth delay and morphologic abnormalities, which were partially or fully reversible. By contrast, exposure to low doses of ionizing radiation had no discernable effects on overall growth or morphology, although, there was an increase in TUNEL-positive apoptotic cells, consistent with the results of prior studies. Further studies showed that the combined effect of radiation and menadione exposure are greater than with either agent alone, and that attenuation of the expression of Ku80, a gene important for repair of radiation-induced DNA damage, had only a slight effect on menadione sensitivity. Together, results suggest that ionizing radiation and menadione affect the embryo by distinct mechanisms.
INTRODUCTION
Ionizing radiation produces reactive oxygen species both as the result of primary interaction with tissue and as a secondary consequence of biological injury (reviewed in refs. 1, 2). Acute and persistent oxidative stress are important components of radiation injury (3). However, deciphering the role of ROS in radiation injury is complicated because normal cellular processes release ROS even in the absence of radiation exposure. Mitochondrial energy metabolism and accompanying leakage from the electron transport chain release superoxide and other species in normal cells (4). Immune and inflammatory processes can also be a significant source of ROS (5). Regardless of their origin, ROS produce similar oxidative lesions in DNA. If ROS production is one of the major mechanisms of radiation injury, then increasing the levels of endogenous ROS should have many of the same biological effects as radiation exposure.
Prior work has established the zebrafish embryo as a model for studying effects of ionizing radiation exposure in an intact organism (6–8). We have previously developed a model in which pre-early gastrula embryo is exposed to low doses of ionizing radiation (7, 10). Exposure at this stage results in a linear, dose-dependent increase in the number of TUNEL-positive apoptotic cells, with no apparent threshold (8). Attenuation of expression of the DNA repair proteins, Ku70 and Ku80, increases the number of apoptotic cells, whereas attenuation of expression of the zebrafish homolog of tumor suppressor TP53 decreases apoptosis (7). Together, these data indicate that short-term cytotoxic effects of radiation in this system are attributable to DNA damage.
In the present study, we sought to address a simple question: does elevation of endogenous ROS produce the same or different effect as exposure to low doses of ionizing radiation in the zebrafish embryo model? To increase the levels of endogenous ROS, we exposed embryos to menadione (2-methyl-1,4-naphthoquinone). This compound undergoes an enzymatic one-electron reduction, using flavoproteins as the donor, to form a semiquinone radical. This rapidly converts O2 to a superoxide, which can produce more toxic ROS, including hydroxyl radicals (11). The reaction regenerates the quinone, which can then enter into a new cycle of ROS production. Menadione has been widely used in studies of oxidative stress, including studies in aquatic model organisms (12, 13).
We report here the results of direct comparison of menadione and radiation treatment in the zebrafish model. We also report the effects of the attenuation of DNA repair protein expression on sensitivity to these two stressors. Results suggest that the effects of endogenous ROS and radiation are mechanistically distinct in this model.
MATERIALS AND METHODS
Zebrafish Methods
Experiments were carried out according to protocol number BR09-10-259 approved by the Institutional Animal Care and Use Committee of Georgia Health Sciences University. The institution’s animal facilities and program are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and the United States Department of Agriculture. Experiments were performed using wild-type Tüebingen or brass zebrafish embryos and standard protocols for breeding and staging (14, 15). To minimize the effect of genetic variability, each experiment was performed with embryos from a single clutch, containing 200 or more embryos with a minimum of 20 embryos per experimental group. Each experiment was repeated with at least two clutches and each figure shows representative embryos from within an experimental group.
Menadione and Radiation Exposure
Menadione was prepared as a stock solution in DMSO. At the early gastrula stage, approximately 6 h after fertilization, embryos were transferred to a container with the indicated concentrations of menadione and a final concentration of 1% DMSO in water. Control groups were exposed to DMSO alone. After 60 min, embryos were transferred to water and development was allowed to continue. Irradiation was performed, also at the early gastrula stage, using a Gammacell Exactor 137Cs irradiator (MDS Nordion, Ottawa, ON) at a dose rate of approximately 1 Gy/min. For irradiation, embryos (~1 mm diameter) were placed in petri dishes containing a small amount of water. Control groups were transported to the irradiator, but were not irradiated. Radiation doses were as follows: 0, 0.15, 0.50 and 1.5 Gy. Dosimetry was performed using thermoluminescent dosimetry devices (Landauer Inc., Glenwood, IL) placed in the irradiator on top of dishes containing the embryos. At 24 or 48 h after fertilization, embryos were examined for gross morphological abnormalities or processed to detect apoptotic cells by the TUNEL assay. In the groups that received both radiation and menadione, radiation was delivered first and was followed by exposure to menadione.
Imaging Methods
Gelatin sectioning of fixed zebrafish embryos was carried out as described previously (3). Embryos to be sectioned were fixed as in 3% paraformaldehyde, transferred to a solution of 30% sucrose in PBS and incubated at room temperature for 2 h with mixing. Embryos were transferred to PBS containing 15% gelatin for 6 h at 37°C, then transferred to PBS containing 25% gelatin and incubated overnight at 37°C. Single embryos were transferred in PBS containing 25% gelatin to aluminum foil molds and oriented. The gelatin was allowed to set at room temperature for 1 h, then at 4°C for 1 h and the embryos were stored at −20°C. The gelatin block was mounted on a cryostat chuck using OCT embedding medium (Bayer, Elkhart, IN), and trimmed and 14 mm sections were cut. Sections were placed on slides (Superfrost Plus, VWR, West Chester, PA) and were air dried overnight. Gelatin was removed from slides by incubating in distilled water at 50°C for 15 min. Sample were then mounted with a coverslip and aqueous mounting buffer. DNA fragmentation in apoptotic cells was detected by TUNEL assay as previously described (16). Dechorionated embryos were fixed in 4% cold paraformaldehyde in PBS for 6 h and dehydrated in a graded ethanol series (50%, 70%, 85%, 95%, 100%), followed by 20 min in acetone at −20°C. The embryos were further permeabilized by incubating in PBS with 0.5% Triton X-100 and 0.1% sodium citrate for 15 min and in 20 μg/ml Proteinase K for 10 min. The samples were refixed with 4% paraformaldehyde before incubation with terminal deoxynucleotidyl transferase solution according to the manufacturer’s instructions (In Situ Cell Death Detection Kit, Roche Applied Science). Detection of ROS in live embryos was performed using an Image-iT LIVE kit according to the vendor’s protocol (Molecular Probes, Inc., Eugene, OR).
Attenuation of Ku80 Expression by Morpholino Oligonucleotide Treatment
An antisense phosphorodiamidate morpholino oligonucleotide (MO), (5′-TGCTGGCTTCCGCTCGTGCCGAATT, Gene-Tools, LLC, Philomath, OR) was diluted to 5 μg/μl in 2× injection buffer and microinjected into the yolk of 1-cell embryos (7). Control embryos were injected with buffer alone.
Embryo Viability
Determination of embryo viability was made at 24 and 48 h after fertilization following treatment (menadione of IR) and compared to non-treated controls. Embryos were scored as either alive (presence of heartbeat) or dead.
RESULTS
Effects of Menadione Treatment on ROS Levels and Development
To investigate the effects of exposure to endogenous ROS in the zebrafish model, we treated pre-early gastrula embryos (6 h after fertilization) with 3 or 10 μM menadione in 1% DMSO. To confirm that this treatment resulted in elevated levels of ROS, we stained the embryos with 5- (and 6-) carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA), an indicator of intracellular ROS levels (17, 18). The dye undergoes oxidation to a green fluorescent form in the presence of high levels of endogenous ROS. Exposure of the pre-early gastrula embryo to 3 μM menadione at 6 h after fertilization led to a marked increase in green fluorescence at 24 h after fertilization, and exposure at 24 h after fertilization led to a similar increase at 48 h after fertilization (Fig. 1A). The results indicated that menadione treatment leads to persistent elevation of ROS. One percent DMSO alone did not have any adverse effect on the embryos.
FIG. 1.
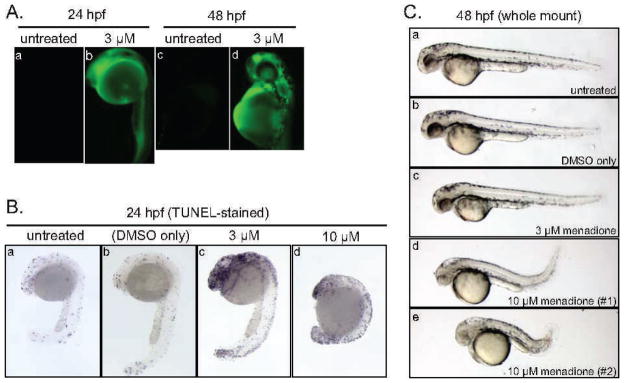
Effects of menadione on endogenous ROS levels and embryonic development. Panel A: Menadione treatment elevates ROS levels. Embryos were exposed to 3 μM menadione at 6 h after fertilization and stained with carboxy-H2DCFDA and imaged at 24 h after fertilization or exposed at 24 h after fertilization and stained and imaged at 48 h after fertilization. Fluorescence indicates ROS induction. Anterior is at the top left, posterior is to the bottom and the large sphere is the yolk. Panel B: Menadione treatment leads to developmental delay. Embryos were exposed to DMSO vehicle alone or to the indicated concentrations of menadione (3 μM or 10 μM) at 6 h after fertilization and was processed for TUNEL staining at 24 h after fertilization. Orientation is as in panel A. Note that the presence of some apoptotic cells is a feature of normal development. Developmental delay is particularly evident at 10 μM menadione where the embryos fail to develop normal head and tail regions and more resemble 16–18 h after fertilization embryos rather than 24 h after fertilization embryos. Panel C: Effects of menadione are partially reversible. Embryos were exposed to DMSO vehicle alone or to the indicated concentrations of menadione at 24 h after fertilization, and whole mount specimens were prepared at 48 h after fertilization. Note that sub-panels d and e show variability in the severity of the observed phenotype.
To investigate the biological effects of elevated endogenous ROS, we treated embryos with menadione at 6 h after fertilization and examined the embryo morphology and the amount of TUNEL-staining at 24 h after fertilization (Fig. 1B). Treatment with 3 μM menadione produced a mild developmental delay, relative to untreated or 1% DMSO-treated control embryos. Treatment with 10 μM menadione produced a more severe delay and in some experiments was lethal. We did not observe consistent changes in the pattern of TUNEL staining in control and treated embryos. In all cases, there was a modest and somewhat variable number of apoptotic cells, consistent with the pattern that is expected in normal development. Notably, treatment with 10 μM menadione, which produced severe developmental delay, had at most a modest effect on TUNEL staining.
To investigate whether the effects of menadione exposure were reversible, we exposed embryos at 6 h after fertilization (for 60 min), transferred them to normal conditions in water and allowed development to continue. Embryos that were exposed to 3 μM menadione typically showed complete recovery at 48 h after fertilization and were indistinguishable from embryos in the control groups. Embryos that were exposed 10 μM menadione showed a somewhat more variable pattern of recovery. Although some embryos remained severely affected or eventually died, others showed evidence of partial recovery (Fig. 1C).
Effects of Radiation and Menadione are Phenotypically and Mechanistically Distinct
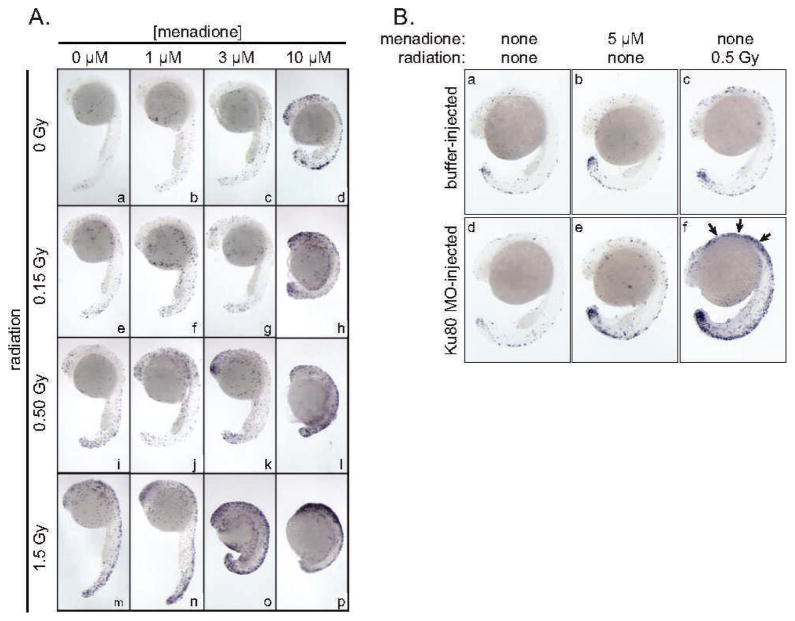
We next performed an experiment to directly compare the effect of low-dose (0–0.5 Gy) radiation exposure and menadione treatment. To assure that results within the experiment were comparable, all embryos were from the same clutch. Embryos were irradiated or exposed to menadione at 6 h after fertilization. The morphology and number of apoptotic cells, as determined by TUNEL staining, were examined at 24 h after fertilization as in Fig. 1. Consistent with prior work (8), low-dose radiation exposure was associated with a modest, dose-dependent increase in TUNEL staining with no apparent effect on embryo morphology. By contrast, menadione exposure was associated primarily with developmental delay (Fig. 2A). The effects of radiation and menadione are clearly distinct. Radiation exposure exacerbated the effects of menadione and vice versa, as evidenced by the heightened developmental effects of 3 μM menadione in embryos that were also exposed to radiation (in Fig. 2A compare sub-panels k and o with panel c) and the increased TUNEL-staining in embryos that were exposed to radiation and to menadione (Fig. 2A compare sub-panels o and p to panel m).
FIG. 2.
Panel A: Direct comparison of the effects of menadione and ionizing radiation and effects of combination treatment. Embryos received the indicated dose of 137Cs γ radiation, menadione or both at the pre-early gastrula stage (6 h after fertilization), and were processed for TUNEL staining at 24 h after fertilization. Orientation of embryos is the same as in Fig. 1. Panel B: Microinjection of Ku80 antisense MO affects sensitivity to radiation but not to menadione. Embryos were injected at the single-cell stage with ~5 ng of Ku80 MO (sub-panels d, e and f) or with the same volume of buffer only (sub-panels a, b and c). Arrows denote increase apoptosis in Ku80 MO-injected, irradiated embryo.
The zebrafish embryo model affords the ability to transiently attenuate gene expression by microinjection of antisense morpholino oligonucleotides (MOs). We have previously shown that microinjection of a Ku80 MO sensitizes embryos to radiation and that the effects can be reversed by co-injection of an antisense-resistant Ku80 mRNA (7). To test the effect of attenuation of Ku80 expression on menadione sensitivity, we injected single-cell embryos with 5 ng Ku80 MO as described in ref. (7). Embryos received either the indicated dose of 137Cs γ radiation or menadione at the pre-early gastrula (6 h after fertilization) stage, were allowed to continue development until 24 h after fertilization, and were processed to detect apoptotic cell death by the TUNEL assay. Microinjection of the Ku80 MO had no effect on menadione-induced developmental delay (Fig. 2B, compare sib-panels b and e), but increased sensitivity to radiation, as expected (Fig. 2B, panels c and f). In a control experiment, microinjection of Ku80 MO had no effect on its own in the absence of other treatments (Fig. 2B, panels a and d). The finding that the Ku80 MO sensitized embryos to low-dose radiation but not to menadione provides further evidence that these two stressors work by different mechanisms.
Embryo Viability
Determination of embryo viability was made at 24 and 48 h after fertilization following treatment with menadione or radiation, and was compared to nontreated controls. Embryos were scored as either alive (presence of heartbeat) or dead (absence of heartbeat). With the exception of treatment with 10 μM menadione, where we observed an increase in embryonic death of 2–5%, we did not observe an increase in embryonic death in all other treatments.
DISCUSSION
ROS are omnipresent in cells and tissues as the result of normal oxidative metabolism and other physiological processes. At the same time, it is known that many of the effects of ionizing radiation on cells and tissues are attributable to ROS chemistry. These facts support a hypothesis that endogenous ROS and low-dose radiation exposure may affect organisms in the same way. Moreover, the hypothesis predicts that, as dose decreases, the distinctive effects of radiation might vanish against the natural background of endogenous ROS.
Experiments here were designed to test this hypothesis in an intact vertebrate organism. The zebrafish embryo affords a feasible model to address this question. Because zebrafish embryos are small and develop outside the mother, introduction of the pharmacologic agent, menadione, into the embryo-rearing medium results in elevated ROS throughout the organism. Because they are optically transparent, it is possible to monitor ROS induction in the living embryos using a redox-sensitive fluorescent dye. We used the zebrafish embryo system to directly compare the biological effects of menadione and radiation exposure, and we found that the phenotypes associated with the two treatments are different. In addition, a genetic manipulation that increases sensitivity to radiation has no effect on sensitivity to menadione. Results do not support the hypothesis that endogenous ROS and low-dose radiation exposure affect the organism in the same way. Rather, they suggest that these stressors work by different mechanisms.
Menadione treatment results in minimal cytotoxicity and is at least partially reversible, which suggests that they are mediated at least in part through signaling events. It may be that oxidative DNA damage leads to transient cell cycle delay, or alternatively that menadione affects redox-sensitive cell signaling proteins. One note of caution is that we cannot rule out the possibility that menadione works by mechanisms in addition to ROS. In mammalian cells, menadione increases phosphorylaton of epidermal growth factor receptor, possibly via ROS-dependent inhibition of a receptor tyrosine kinase (19), or alternatively by menadione-mediated alkylation of the phosphatase active site (20). The effect of menadione on mammalian EGFR is seen primarily at concentrations of 25 μM and above (21), which are substantially higher than the 3–10 μM used here.
It is noteworthy that although ROS produce similar chemical damage regardless of their source, the spatial distribution of damage differs. Radiation-induced ROS originate along discrete radiation tracks and are capable of producing distinctive clustered damage to DNA and other substrates, whereas endogenous ROS are distributed more uniformly. It should be possible to address the mechanism of menadione and radiation more directly in the future, using tools available in the zebrafish model. In particular, it will be of interest to identify genes that modulate the effects of endogenous ROS on the embryo and to determine whether these overlap with genes that affect the radiation response.
CONCLUSION
Menadione (an inducer of endogenous ROS) and ionizing radiation affect the zebrafish embryo by distinct mechanisms.
Acknowledgments
We thank Dr. B. Yuan and the MCG Transgenic Zebrafish Core Facility for embryo production. Support for this work was provided by grant awards to WSD (DE-FG02-03ER63649 from the U.S. Department of Energy Low Dose Radiation Research Program) and DJK (R01 DC006140 from the National Institutes of Health).
References
- 1.Suzuki T, Gandhi P, Lin HY, Crawford DR. Mammalian resistance to oxidative stress: a comparative analysis. Gene Expr. 2002;10:179–191. doi: 10.3727/000000002783992442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 3.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–9. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 4.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Rad Biol Med. 2009;47:333–43. doi: 10.1016/j.freeradbiomed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek LA, et al. Nitric oxide and redox mechanisms in the immune response. J Leuko Biol. 2011;89:873–91. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAleer MF, Davidson C, Davidson WR, Yentzer B, Farber SA, Rodeck U, et al. Novel use of zebrafish as a vertebrate model to screen radiation protectors and sensitizers. Int J Radiat Oncol Biol Phys. 2005;61:10–3. doi: 10.1016/j.ijrobp.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Bladen CL, Lam WK, Dynan WS, Kozlowski DJ. DNA damage response and Ku80 function in the vertebrate embryo. Nucleic Acids Res. 2005;33:3002–10. doi: 10.1093/nar/gki613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bladen CL, Flowers MA, Miyake K, Podolsky RH, Barrett JT, Kozlowski DJ, et al. Quantification of ionizing radiation-induced cell death in situ in a vertebrate embryo. Radiat Res. 2007;168:149–57. doi: 10.1667/RR0803.1. [DOI] [PubMed] [Google Scholar]
- 9.Amatruda JF, Shepard JL, Stern HM, Zon JL. Zebrafish as a cancer model system. Cancer Cell. 2002;1:229–31. doi: 10.1016/s1535-6108(02)00052-1. [DOI] [PubMed] [Google Scholar]
- 10.Bladen CL, Navarre S, Dynan WS, Kozlowski DJ. Expression of the Ku70 subunit (XRCC6) and protection from low dose ionizing radiation during zebrafish embryogenesis. Neurosci Lett. 2007;422:97–102. doi: 10.1016/j.neulet.2007.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thor H, Smith MT, Hartzell P, Bellomo G, Jewell SA, Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982;257:12419–25. [PubMed] [Google Scholar]
- 12.Meyer JN, Smith JD, Winston GW, Di Giulio RT. Antioxidant defenses in killifish (Fundulus heteroclitus) exposed to contaminated sediments and model prooxidants: short-term and heritable responses. Aquat Toxicol. 2003;65:377–95. doi: 10.1016/j.aquatox.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Stephensen E, Sturve J, Forlin L. Effects of redox cycling compounds on glutathione content and activity of glutathione-related enzymes in rainbow trout liver. Comparative biochemistry and physiology. Toxicol Pharmacol. 2002;133:435–42. doi: 10.1016/s1532-0456(02)00129-1. [DOI] [PubMed] [Google Scholar]
- 14.Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio) University of Oregon Press; Eugene (OR): 1995. [Google Scholar]
- 15.Kimmel CB, Ballard DW, Kimmel SR, Ullman B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 16.Kozlowski DJ, Whitfield TT, Hukriede NA, Lam WK, Weinberg ES. The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev Biol. 2005;277:27–41. doi: 10.1016/j.ydbio.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Konorev EA, Zhang H, Joseph J, Kennedy MC, Kalyanaraman B. Bicarbonate exacerbates oxidative injury induced by antitumor antibiotic doxorubicin in cardiomyocytes. Am J Physiol. 2000;279:2424–30. doi: 10.1152/ajpheart.2000.279.5.H2424. [DOI] [PubMed] [Google Scholar]
- 18.Maurer BJ, Metelitsa LS, Seeger RC, Cabot MC, Reynolds CP. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)- retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–46. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 19.Blanchetot C, Tertoolen LG, den Hertog J. Regulation of receptor protein-tyrosine phosphatase alpha by oxidative stress. EMBO J. 2001;15:493. doi: 10.1093/emboj/21.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelmohsen K, Gerber PA, von Montfort C, Sies H, Klotz LO. Epidermal growth factor receptor is a common mediator of quinone-induced signaling leading to phosphorylation of connexin-43: role of glutathione and tyrosine phosphatases. JBC. 2003;278:38360. doi: 10.1074/jbc.M306785200. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Soler R, Zou Y, Li T, Ling YH. The phosphatase inhibitor menadione (vitamin K3) protects cells from EGFR inhibition by erlotinib and cetuximab. Clin Cancer Res. 2011;17(21):6766–77. doi: 10.1158/1078-0432.CCR-11-0545. [DOI] [PubMed] [Google Scholar]