Abstract
Surface functionalization of nanoparticles and host-guest properties of nanoassemblies are two critical features in the utilization of nanostructures in a variety of applications in materials, chemical, and biological nanotechnology. However, simultaneously incorporating these two features in one nanoparticle design is a rather challenging task. We have developed a simple and versatile nanoparticle platform that addresses this challenge. We have designed and characterized a polymer nanoparticle that provides the ability to encapsulate hydrophobic guest molecules and surface functionalization with a wide range of functional groups. In addition, we have also demonstrated a new and simple approach to tune the size of the nanoparticles.
Nanoparticles, polymer or otherwise, have had a significant impact on a variety of areas such as utilization in microelectronics, multiphase catalysis, sensing and therapeutics.1 For all of these applications, facile modulation of the nanoparticle surface is critical, in order to obtain appropriate interfacial properties. Similarly, the ability to encapsulate and release guest molecules within the nanoparticle interior is also required for applications such as sensing and therapeutics. A platform that affords both surface functionalization and guest encapsulation in a single nanoscopic scaffold is highly desirable. Nanoscale materials, such as metallic or semiconductor nanoparticles and dendrimers, are excellent scaffolds for displaying surface functional groups.2 For example, monolayer protection of gold nanoparticles is easily achieved with thiol-bearing molecules due to the high affinity of thiol moiety toward gold nanoparticles. However, these scaffolds generally lack features that allow for favorable non-covalent host-guest interactions. On the contrary, amphiphilic molecules readily self-assemble into nanoassemblies, such as micelles and liposomes, which can encapsulate guest molecules within their interior.3 Nevertheless, modifying their surface functional groups are challenging, because these modifications often result in change in the hydrophilic-lipophilic balance that is necessary for retaining the fidelity of the assembly. An impending intellectual challenge in this area is to capture the essence of surface functionalization capabilities available in dendrimers and metallic nanoparticles and combine them with the host-guest features presented in micelles and vesicles. Amphiphilic block copolymer micelles, which are cross-linked either at the core or at the shell, can potentially satisfy this requirement.4 While these architectures have had an impressive impact, these assemblies do require demanding polymer synthesis. Also, these assemblies are often achieved under rigorous processing conditions. We were interested in developing a simple approach for functionalizable polymer nanoparticles, where we stipulated that: (i) the precursor polymer is based on a random copolymer, which is synthetically accessible; (ii) the polymer self-assembles in a solvent, which can then be converted to nanoparticle in one step without the need for any additional processing; (iii) the nanoparticle contains a surface functional group, which can be further manipulated easily; (iv) the size of the nanoparticle is tunable; and (iv) the interior of the nanoparticle is capable of sequestering guest molecules.
In this paper, we report on the design, synthesis, characterization, and further functionalization of amine-functionalized polymeric nanoparticles that satisfy all these requirements. We targeted primary amines as the surface functional group, because its reactivity complements a wide range of functional groups such as alkyl halides, Michael acceptors, carboxylic acid, acid chlorides, activated esters, epoxides, anhydride and aldehydes. The basic premise behind our molecular design involves self-assembly of amphiphilic random copolymers. In the aqueous phase, the surface functional groups of such an assembly would be dictated by the hydrophilic moiety of the polymer. Therefore, we hypothesized the use of a primary amine based monomer as the hydrophilic moiety, combined with a reactive hydrophobic monomer as the crosslinkable moiety, will lead to a functionalizable polymer nanoparticle. The amphiphilic nature of the assembly should also allow for incorporation of guest molecules within the hydrophobic interior of the assembly prior to crosslinking. Accordingly, we targeted polymer 1, a poly(methacrylamide), derived from the co-polymerization of 2-aminoethylmethacylamide and 3-(9-methylcoumarinoxy)propylmethacrylamide. This co-polymer should self-assemble in to an amphiphilic aggregate, where the hydrophilic amino moieties are exposed to the aqueous phase, while the coumarin moieties are tucked in the hydrophobic interior. We will take advantage of the propensity of coumarins to undergo photochemically driven [2+2] cylcoaddition reaction5 to achieve the targeted polymer nanoparticles.
To realize the synthesis of the targeted polymer 1, we first synthesized the precursor random copolymer, which contains 30% of N-Boc-aminoethylmethacrylamide and 70% of 3-(9-methylcoumarinoxy)propylmethacrylamide; this polymer was prepared by reversible addition-fragmentation chain transfer (RAFT) polymerization. The Boc group was deprotected using trifluoroacetic acid in dichloromethane to yield the random copolymer 1. Our design requires that this amphiphilic polymer form a nanoscale aggregate, which can be photochemically trapped to form a surface functionalized nanoparticle. Indeed, this polymer with 70:30 monomer ratio exhibits the right hydrophilic-lipophilic balance required to form the amphiphilic assemblies. An aqueous solution of this polymer forms an aggregate of ~22 nm at 1 mg/mL concentration, as discerned by dynamic light scattering (DLS). This solution was then irradiated at 365 nm for 10 minutes to generate the crosslinked nanoparticle. Several features of this reaction are noteworthy: (i) the intensity of the absorption peak centered at 320 nm, which corresponds to the coumarin moiety, reduces within this irradiation time – confirming the photochemical reaction of the coumarin moiety; (ii) the size of the nanoparticle is the same as the aggregate, suggesting that the coumarin dimerization process is exclusively intra-aggregate – note that inter-aggregate reactions would result higher nanoparticle sizes; (iii) there is no discernible nanoaggregate of the uncrosslinked polymer in 10% water in DMSO, while the cross-linked nanoparticle’s size is slightly increased in this solvent mixture (Figure S1)6 – this swelling feature further confirms the crosslinked nature of the nanoparticle. The degree of swelling should inversely vary with the degree of crosslinking. It is known that irradiation of coumarin dimers at 250 nm causes it to revert to the monomer.5 This reaction is often not complete because of the photo-stationary state between the monomer and the dimer at this irradiation wavelength. Therefore, this reaction should cause the crosslink density to lower. Accordingly, crosslinked nanoparticle in 10% water in DMSO was irradiataed at 250 nm for 30 minutes. DLS study of this solution indeed showed a further increase in size (Figure S1)6.
Since these nanoparticles are formed from amphiphilic assemblies in aqueous solutions, we envisaged the possibility of these nanoparticles being hosts for hydrophobic guest molecules. Indeed, we were able to encapsulate hydrophobic dye molecules, such as 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) or 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) using the polymer aggregates successfully and guest molecules were retained in the interiors of nanoparticles after photoinduced crosslinking.6
To investigate the versatility of the amine functionality as a handle for surface functionalization of the nanoparticles, we tested a wide range of functional groups and characterized these modifications with different techniques. First, we reacted amines with an activated ester and a cyclic anhydride to provide amides with complementary surface characteristics. Reaction of the amine nanoparticles with a peg-2000 NHS ester should convert the positively charged surface of the polymer nanoparticle to a charge neutral surface, while the reaction with succinic anhydride should convert the charge to negative. Zeta potential measurements of the reactants and the products indeed confirmed such surface charge modification (Figure 2a). Second, the nanoparticles were modified by a NHS ester (of lauric acid) and an isocyanate (dodecyl). Both modifications would change the surface nanoparticles from hydrophilic to hydrophobic. Evaluation of the nanoparticle surface hydrophobicity by contact angle showed that the unmodified nanoparticles exhibited a contact angle of 33°, while the modified nanoparticles have a contact angle of 103° and 98°, respectively (Figure 2b). To confirm that the contact angle change after modification was due to hydrophobicity change rather than surface roughness difference, atomic force microscopy (AFM) was used to evaluate the surface roughness of these nanoparticles. AFM images of these nanoparticles showed a similar mean roughness (Ra) value of ~9 nm6. Next, we sought to monitor the surface functionalization by FTIR. To this end, the nanoparticles were reacted with the NHS-ester of azidoacetic acid. The FTIR spectrum of modified nanoparticles showed the appearance of a peak at 2100 cm-1, characteristic of the azido group, with the concurrent disappearance of the NHS ester peaks at 1812 cm-1 and 1783 cm-1 (Figure 2c).
Figure 2.
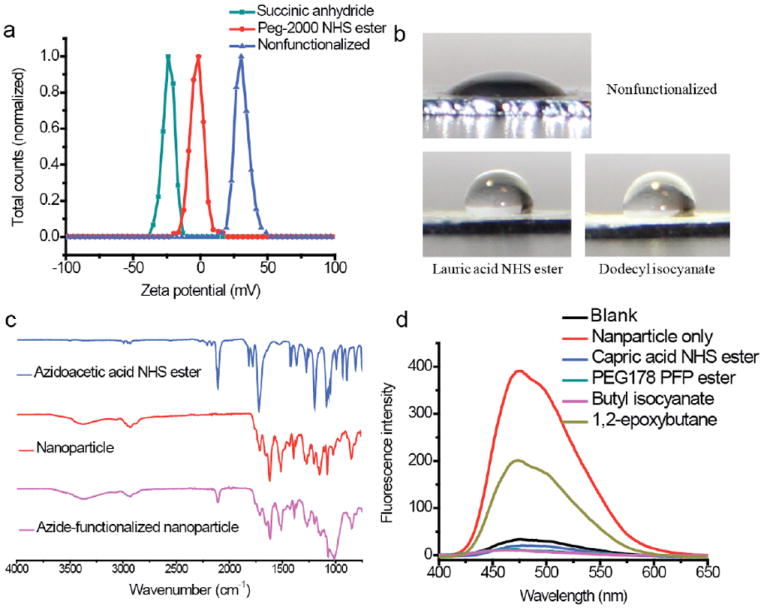
(a) Surface charges of nanoparticles by zeta potential (b) Contact angle measurements of unmodified nanoparticles (top) and nanoparticles modified by lauric acid NHS ester (bottom left) and dodecyl isocyanate (bottom right). (c) IR spectra of azidoacetic acid NHS ester (top), unmodified nanoparticles (middle), and nanoparticles functionalized with azidoacetic acid NHS ester (bottom). (d) Fluorescence emission intensity of nanoparticles treated with excess fluorescamine after reacting with different functional groups.
The techniques outlined above, including the IR, do not directly analyze the conversion of the amino moiety on the nanoparticle surface. Therefore, we used the well-established fluorescamine assay,7 in which selective reaction of primary amines with fluorescamine provides a fluorescent derivative. The relative fluorescence can then be used to ascertain the extent of surface functionalization. Accordingly, nanoparticles were first reacted with molecules with different aminereactive functional groups (pentafluorophenol (PFP) ester, NHS ester, epoxide, and isocyanate). The extent of functionalization was analyzed by comparing the fluorescence from the functionalized amine nanoparticles and the unreacted amine nanoparticle. The fluorescence from three of the functionalized nanoparticles (PFP ester, NHS ester, and isocyanate) was found to be similar to that found with the negative control, suggesting that the reaction is quantitative in these cases (Figure 2d). The reason for the inefficiency of the epoxide ring opening reaction is not clear.
Next, we sought to investigate the possibility of tuning the nanoparticle sizes. While variations such as polymer MW, concentration, and monomer ratio could afford different aggregate sizes, we were interested in a simpler variation with the same polymer. We envisioned that the pH of the solution and the ensuing variation the hydrophilic-lipophilic balance of the polymer could afford polymer aggregates with different sizes. To test our hypothesis, aqueous solutions of polymer 1 at different pH were prepared. The polymer precipitates out at pH~9, consistent with the pKa of amine groups. We also observed that the aggregate sizes were not significantly different between pH 3.0 and 6.5. Interestingly, the greatest size differences were observed with subtle pH changes between 7.0 and 8.5, indicating that subtle changes in the degree of protonation of the amines lead to significant size differences. This is presumably due to the difference in hydrophilic lipophilic balance of the polymer at these pHs. We have utilized these differences to systematically tune the size of the nanoparticles by photochemically locking these aggregates, as shown in Figure 3a.
Figure 3.
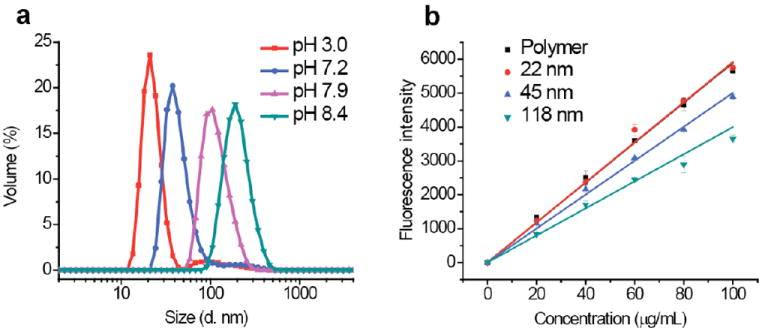
(a) Size distribution of nanoparticles cross-linked at different pHs in water. The DLS measurements were all done at pH 3. (b) Percentage of amine available for functionalization on different nanoparticle sizes accessed by fluorescamine assay.
Although the fluorescamine assay showed that all the accessible amines can be utilized for surface decoration, it is important to investigate the percentage of amines in the polymer nanoparticle that are inherently accessible. We hypothesized that smaller nanoparticles would have higher percentage of accessible amines, since some of these moieties might be buried in the interior of larger particles. To test our hypothesis, fluorescamine assay was carried out in 1:3 water/DMSO mixture, in which no aggregation was observed for the non-crosslinked polymer. Therefore, the fluorescence generated from the uncrosslinked polymer is a true indicator of the amine moieties available in the polymer. Evaluation of nanoparticles of different sizes, using this as the standard, indicated that nearly all the amine moieties seem to be available at a particle size of 22 nm. However, only 85% and 65% of the amine moieties were available for functionalization in 45 nm and 118 nm particles respectively (Figure 3b). This supports the expectation that the smaller surface area of larger nanoparticles will lead to decreased availability of surface functionalities.
In summary, we have designed and characterized a versatile polymer nanoparticle platform that: (i) displays a versatile functional group on its surface, which can be further manipulated with a variety of complementary reactive moieties; (ii) is capable of non-covalently binding hydrophobic guest molecules; (iii) afford size tunability by simply altering the pH at which the nanoparticle is synthesized; (iv) has a very high percentage of the accessible surface moieties at smaller sizes. Overall, the simplicity and versatility of the surface functionalizable soft nanoparticles with host-guest capabilities will have implications in a variety of applications from materials to biology. Incorporating stimuli-responsive characteristics and expanding this method to a broader range of functional groups are among the current foci in our laboratory.
Supplementary Material
Figure 1.
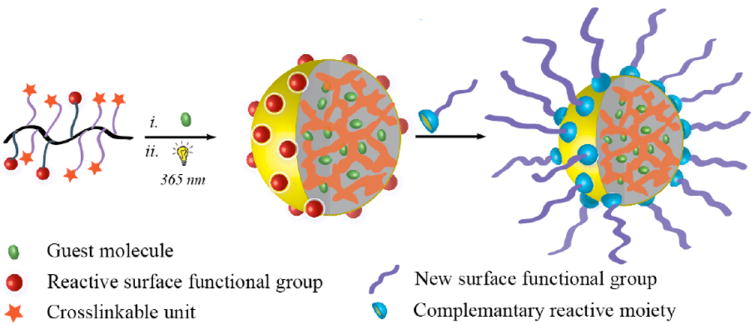
Schematic representation of the polymer nanoparticle with surface functionalization and guest binding abilities.
Scheme 1.
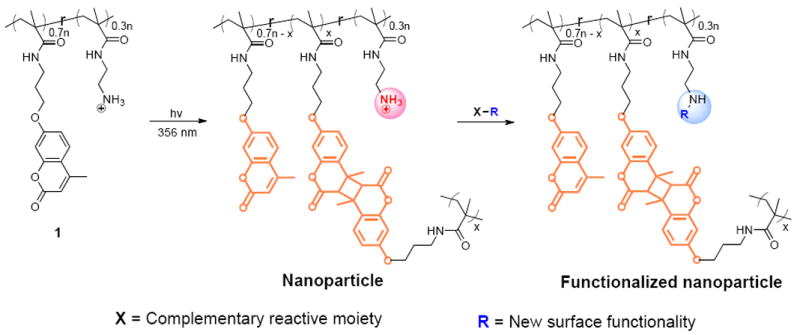
Synthesis of polymer precursor and the nanoparticle.
Acknowledgments
Support from Army Research Office and National Science Foundation (through the MRSEC and the Center for Hierarchical manufacturing) are acknowledged.
Footnotes
Supporting Information
Synthetic procedures and characterizations of the nanoparticle. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Schmid G, editor. Nanoparticles: From Theory to Application. Wiley-VCH; Essen: 2004. [Google Scholar]; (b) Zhang J, Wang Z-l, Liu J, Chen S, Liu G-y. Self-Assembled Nanostructures; Nanostructure Science and Technology Series. Springer; 2002. [Google Scholar]; (c) Rotello VM, editor. Nanoparticles: Building Blocks for Nanotechnology. Springer; 2003. [Google Scholar]; (d) Daniel M-C, Astruc D. Chem Rev. 2004;104:293. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 2.(a) Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Angew Chem Int Ed. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Saha K, Agasti SS, Kim C, Li X, Rotello VM. Chem Rev. 2012;112:2739–2779. doi: 10.1021/cr2001178. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Khandare J, Calderon M, Dagi NM, Haag R. Chem Soc Rev. 2012;41:2824–2848. doi: 10.1039/c1cs15242d. [DOI] [PubMed] [Google Scholar]; (d) Grayson SM, Fréchet JM. J Chem Rev. 2001;101:3819–3867. doi: 10.1021/cr990116h. [DOI] [PubMed] [Google Scholar]; (e) Arima H, Motoyama K, Higashi T. Pharmaceutics. 2012;4:130–148. doi: 10.3390/pharmaceutics4010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Harada A, Kataoka K. Progress in Poly Sci. 2006;31:949–982. [Google Scholar]; (b) O’Reilly RK, Hawker CJ, Wooley KL. Chem Soc Rev. 2006;35:1068–1083. doi: 10.1039/b514858h. [DOI] [PubMed] [Google Scholar]; (c) Zhu Z, Sukhishvili SA. J Mat Chem. 2012;22:7667–7671. [Google Scholar]; (d) Owen SC, Chan DPY, Shoichet MS. Nano Today. 2012;7:53–65. [Google Scholar]; (e) Sawant RR, Torchilin VP. Soft Matter. 2010;6:4026–4044. [Google Scholar]; (f) Micheli M-Rita, Bova R, Magini A, Polidoro M, Emiliani C. Recent Patents on CNS Drug Discovery. 2012;7:71–86. doi: 10.2174/157488912798842241. [DOI] [PubMed] [Google Scholar]
- 4.(a) Van Nostrum CF. Soft Matter. 2011;7:3246–3259. [Google Scholar]; (b) Elsabahy M, Wooley KL. Chem Soc Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Bell CA, Smith SV, Whittaker MR, Whittaker AK, Gahan LR, Monteiro M. J Adv Mater. 2006;18:582–586. [Google Scholar]
- 5.(a) Muthuramu K, Ramamurthy V. J Org Chem. 1982;47:3976. [Google Scholar]; (b) Gnanaguru K, Ramasubbu N, Venkatesan K, Ramamurthy V. J Org Chem. 1985;50:2337–2346. [Google Scholar]; (c) He J, Tremblay L, Lacelle S, Zhao Y. Soft Matter. 2011;7:2380–2386. [Google Scholar]; (d) Trenor SR, Shultz AR, Love BJ, Long TE. Chem Rev. 2004;104:3059–3078. doi: 10.1021/cr030037c. [DOI] [PubMed] [Google Scholar]; (e) Raghupathi KR, Azagarsamy MA, Thayumanavan S. Chem Eur J. 2011;17:11752–11760. doi: 10.1002/chem.201101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.See Supporting Information for details.
- 7.Udenfriend S, Stein S, Böhlen P, Dairman W, Leimgruber W, Weigele M. Science. 1972;178:871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


