Abstract
Idiopathic pneumonia syndrome (IPS) is an acute, non-infectious lung disorder associated with high morbidity and mortality following hematopoietic cell transplantation. Previous studies have suggested a role for TNFα in the pathogenesis of IPS. We report a multi-center phase II trial investigating a soluble TNF binding protein, etanercept (Enbrel®, Amgen) for the treatment of pediatric patients with IPS. Eligible patients were <18 years, within 120 days post-transplant, with radiographic evidence of a diffuse pneumonitis. All patients underwent a pre-therapy broncho-alveolor lavage (BAL) to establish the diagnosis of IPS. Systemic corticosteroids (2.0 mg/kg/day) plus etanercept (0.4 mg/kg twice weekly × 8 doses) were administered. Response was defined as survival and discontinuation of supplemental oxygen support by day 28 of study. Thirty-nine patients (median age 11y, range 1–17y) were enrolled, with 11 of 39 patients non-evaluable due to identification of pathogens from their pre-therapy BAL. In the remaining 28 patients, the median FiO2 at study entry was 45%, with 17 of 28 requiring mechanical ventilation. Complete responses were seen in 20 (71%) patients, with a median time to response 10 days (range 1–24). Response rates were higher for patients not requiring mechanical ventilation at study entry (100% vs. 53%,p=0.01). Overall survival at 28 days and 1-year post-therapy were 89% (95% CI:70–96) and 63% (95% CI:42–79) respectively. Plasma levels of pro-inflammatory cytokines were significantly increased at onset of therapy, subsequently decreasing in responding patients. The addition of etanercept to high dose corticosteroids was associated with high response rates and survival in children with IPS.
Keywords: Bone Marrow Transplantation, Idiopathic Pneumonia Syndrome, Etanercept
INTRODUCTION
Idiopathic Pneumonia Syndrome (IPS) describes an acute, non-infectious lung injury following hematopoietic cell transplantation (HCT). IPS responds poorly to conventional therapy, with mortality rates 50–80% within 28 days of diagnosis. [1–3] Criteria for IPS include symptoms of respiratory distress, plus radiographic evidence for diffuse alveolar injury in the absence of infection. [4] A recent update further categorized IPS by the primary anatomic site of cellular damage. [5] The incidence of IPS ranges from 2 to 12%, with a median onset 17–42 days post-HCT, and median time to death of 13 days from diagnosis. [1, 3, 5–8] Risk factors include acute graft versus host disease (GVHD) in both adult and pediatric HCT recipients, with a prior history of HCT or viral pneumonitis noted as additional risk factors in children. [9–11]
Pre-clinical studies have revealed that inflammatory cytokines play a role in the development of IPS. [5, 12–15] Specifically, TNFα contributes to endothelial cell injury and apoptosis, and directs leukocyte recruitment by regulating pulmonary chemokine expression. [12, 13, 15–18] Increased levels of TNFα and its soluble receptors have also been noted in the broncho-alveolar lavage (BAL) fluid of humans with IPS. [12–15, 19]
The management of IPS traditionally involves supplemental oxygen, systemic corticosteroids and advanced supportive care. Recent, limited institution, clinical trials using a soluble, dimeric, TNFα binding protein (etanercept, Enbrel®; Amgen, Thousand Oaks, CA), when given in combination with systemic corticosteroids have noted significant improvements in response rate and early survival for patients with IPS. [3, 6, 20] In collaboration with the Pediatric Blood and Marrow Transplant Consortium (PBMTC) and Children’s Oncology Group (COG), we have conducted a multi-center, phase II trial to determine whether the addition of etanercept to standard treatment would improve outcomes for children with IPS.
PATIENTS AND METHODS
Eligibility
Eligible patients were < 18 years old, received an allogeneic HCT within the prior 120 days, and met initial clinical and radiographic criteria for IPS. There were no exclusions to enrollment based on the underlying diagnosis, graft source, conditioning regimen, HLA match, or end-organ function. Patients with bacteremia within the prior 48 hours, CMV reactivation or CMV disease, mechanical ventilation > 7 days, or a history of tuberculosis, prior tuberculosis exposure, chronic active hepatitis B or C infections were ineligible. Patients receiving > 2.0 mg/kg/day methylprednisolone equivalent were ineligible. Written informed consent was required from all patients (or legal guardians). The trial was registered at ClinicalTrials.gov as NCT00309907.
Study Design
All patients underwent BAL at study entry to establish the diagnosis of IPS, including exclusion of infectious etiologies for the diffuse pneumonitis (Table 1). BAL samples were collected and subsequently sub-divided for assays outlined in Table 1. A clinical assessment of pulmonary dysfunction was obtained at study entry, recording the method of delivery and amount of supplemental oxygen. Other required observations at study entry included an echocardiogram (to exclude cardiogenic shock and pulmonary hypertension), chest x-ray (or CT scan), CMV PCR assay (whole blood or plasma) and blood cultures. C-reactive protein (CRP), serology and cytokine assays were performed at study enrollment and then weekly through day 28.
Table 1.
IPS diagnostic criteria:
|
Abbreviations: CXR, chest X Ray; CT, computed tomography; RSV, respiratory syncytial virus; HSV, herpes simplex virus; CMV, cytomegalovirus; PCR, polymerase chain reaction.
Key:
, quantitative bacterial culture ≥ 104 CFU/ml considered positive;
, per investigator discretion.
Study therapy (etanercept plus corticosteroids) was begun within 24 hours of the BAL, provided that required BAL fluid microbial stains (gram stain and fungal stain) were negative. The date therapy was initiated was defined as day 0 of study. Patients received etanercept (0.4 mg/kg/dose, maximum 25 mg) twice weekly over 4 weeks (total of 8 doses). The day 0 etanercept dose was administered intravenously to expedite attainment of maximum plasma levels. Subsequent doses were administered subcutaneously 72–96 hours apart. If, at any point following initiation of therapy, pre-therapy BAL fluid samples became positive for a pathogen, etanercept was discontinued, and not re-instituted. The patient was considered non-evaluable for response and replaced on-study, though still followed for toxicity and survival.
Corticosteroids were begun at 2 mg/kg/day (methylprednisolone equivalent) on day 0. Intravenous corticosteroids were required the first 3 days, with subsequent change to oral dosing permitted thereafter. No dose reduction was allowed through day 7, with subsequent taper as clinically indicated. Patients already receiving corticosteroids pre-study had dosing adjusted to 2 mg/kg/day on day 0. Other immunosuppressive agents were continued, without dosing adjustment, unless clinically indicated. Antimicrobial prophylaxis was given per local institutional practice.
Patients who developed sepsis syndrome, invasive fungal infections, disseminated viral infections, CMV reactivation (by PCR or antigenemia assay) or persistent bacteremia (> 72 hours on appropriate antimicrobial therapy) while undergoing study therapy were removed from study and not replaced. In each scenario, patients were followed for response, toxicity and survival. Patients who had not met the response criteria prior to the time of study removal were deemed non-responders.
Plasma biomarker analysis
Whole blood samples for cytokine assays were collected in heparinized tubes on day 0, then weekly through day 28. Frozen plasma samples were thawed and analyzed in batch using enzyme-linked immunosorbant (ELISA) assays for inflammatory cytokines, including TNFα, tumor necrosis factor receptor1 (TNFR1), TNFR2, IL-6, IL-8, sCD14, IFNγ, angiopoietin-2 (Ang-2), and lipopolysaccharide-binding protein (LPB). Plasma samples were also obtained from healthy controls (n=4), and allogeneic HCT recipients without complications (n=5). For each assay, samples were diluted and analyzed in replicate per manufacturer’s guidelines. Plasma samples from the transplant controls were obtained from a separate IRB approved study.
Statistical analysis
The primary study endpoint was response to therapy, defined as survival to day 28 of study plus complete discontinuation of supplemental oxygen support for > 72 consecutive hours. The time to response was defined as the first of three consecutive days off all supplemental oxygen. Secondary endpoints included day 56 survival, overall survival and toxicity assessment using CTCv.3.0 criteria (through 6/30/2011), then CTCv.4.0 thereafter. Patients were evaluable for response if they received at least one dose of etanercept, and their pre-therapy BAL studies remained negative for pathogen identification. Overall survival was computed using the Kaplan-Meier method, with survival defined from the time of study entry to the date of death or last contact. Statistical comparisons of plasma protein levels were performed using the non-parametric Mann-Whitney test. The study was designed to have 90% power and Type I error rate of 5% for detecting a 25% difference in response rates from 30% (historical controls) to 55%. The planned sample size was 40 patients evaluable for response. The protocol was approved by the COG, PBMTC and institutional review boards. A Data Safety Monitoring Board (DSMB) appointed by the COG, reviewed toxicity and response assessments.
RESULTS
Thirty-nine patients enrolled between 2006 and 2011 from 22 centers, with 28 patients evaluable for response assessment (Table 2). 11 patients enrolled and began study therapy, but were ultimately deemed non-evaluable due to identification of pathogens in their pre-therapy BAL (n=10), or corticosteroid dosing that exceeded study requirements (n=1).
Table 2.
Demographics.
| No. (%) | |
|---|---|
| Total enrolled | 39 |
| Total eligible | 28 |
| Age (years) | |
| Median | 14 |
| Mean | 11 |
| Range | 1–17 |
| Gender | |
| Female | 14 (50) |
| Male | 14 (50) |
| Primary disease | |
| ALL | 10 (36) |
| AML/MDS | 10 (36) |
| Lymphoma | 1 (3) |
| Non-malignant1 | 7 (25) |
| Stem cell source | |
| Peripheral stem cells | 3 (11) |
| Bone marrow | 14 (50) |
| Cord blood | 11 (39) |
| Conditioning Regimen | |
| TBI containing2 | 14 (50) |
| Non-TBI containing | 14 (50) |
| Myeloablative | 27 (96) |
| Non-myeloablative | 1 (4) |
| Donor | |
| Unrelated | 24 (86) |
| Related | 4 (14) |
| GVHD prophylaxis | |
| Calcineurin inhibitor3 | 22 (79) |
| Serotherapy4 | 15 (54) |
| No serotherapy | 13 (46) |
| Oxygen support (% FiO2 at entry) | |
| Median FiO2 | 45 |
| Mean FiO2 | 47 |
| Range | 25–100 |
| Oxygen support (method)5 | |
| Nasal cannula | 6 (21) |
| Face mask / Bipap6 | 5 (18) |
| Mechanical ventilation | 17 (61) |
Non-malignant diagnosis: aplastic anemia (1), hemoglobinopathy (2), immune-deficiency (4)
TBI, total body irradiation (1200 – 1320 cGy)
Tacrolimus or cyclosporine.
Serotherapy, includes anti-thymocyte globulin or anti-CD52 mono-clonal antibody.
Method of supplemental oxygen support at study entry.
Bipap, biphasic positive airway pressure.
For the 28 evaluable patients, the median time to onset of study therapy was 20 days (range 6 – 119) post-HCT. The median duration of pre-therapy supplemental oxygen support was 2 days (range 1–20 days), with the median FiO2 45% (range 25–100%). 17 of 28 patients required mechanical ventilator support at day 0. Twenty-three (82%) patients received all eight etanercept doses. Five patients failed to complete etanercept dosing, due to infectious (n=2) or non-infectious (n=1) complications, or death (n=2).
Response and Survival
Early study closure was recommended by the DSMB, as the primary endpoint was achieved in 20 of 28 (71%) patients. One additional complete response occurred after day 28, bringing the overall response rate by day 56 to 75%. The median time to complete response was 10 days (range 1–24 days) requiring a median three doses (range 1–5) of etanercept. There were no differences in response by gender, recipient age, underlying diagnosis, graft source, HLA match, use of serotherapy for GVHD prophylaxis, or conditioning regimen (TBI vs. non-TBI) [data not shown]. Of note, two of the eight non-responders had a clear improvement in pulmonary status with study therapy, with both patients transitioned from mechanical ventilation to a nasal cannula during the course of study.
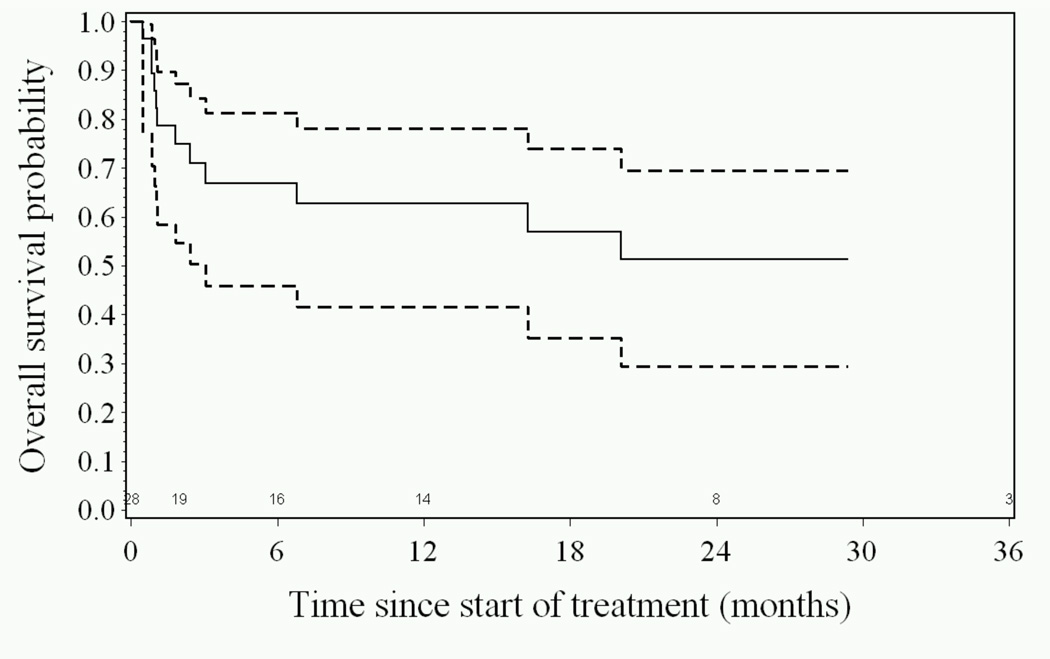
Patients requiring ≤ 45% FiO2 at study entry had higher response rates compared to patients requiring >45% FiO2 (87% vs 54%, p=0.05). Complete responses were seen in all 11 (100%) patients not requiring mechanical ventilation at study entry, compared to responses in 9 of 17 (53%) patients on ventilatory support (p=0.01). A trend toward improved response was seen in patients who initiated study therapy within 3 days of supplemental oxygen support (83% vs 50%, p=0.09). Compared to expected rates, overall survival (OS) was high, 89% (95% CI: 70–96) at day 28, 75% (95% CI: 55–87%) at day 56, and 63% (95% CI: 42–79%) at one year (Figure 1). Fourteen patients remain alive, 305 – 1761 days following initiation of study therapy. Eight of 17 patients (47%) requiring mechanical ventilation at study entry died within the initial 56 days of study. All 11 patients (100%) not requiring mechanical ventilation at study entry survived through day 56.
Figure 1.
Overall Survival (—) and 95% confidence intervals (---) as a function of time since initiation of study therapy. Kaplan-Meier plot.
Toxicity
Grade 3–5 organ toxicities occurred in eight of 28 patients between days 0 – 56. None of these toxicities [renal (n=2), neurologic (n=2), gastro-intestinal (n=2), cardiac (1) and hepatic (n=1)] were attributed to etanercept. Grade 3–5 infections occurred in seven patients, including CMV (n=2), HSV (n=2), virus – not specified (n=1), aspergillus (n=1), and bacterial enterocolitis (n=1). CMV viremia developed in two patients on 19 and 40 days of study respectively, one associated with CMV pneumonitis. Five of the seven grade 3–5 infections were pulmonary. No episodes of septicemia were observed during study or the observation period (day 0–56). Eight deaths occurred by day 56 and were secondary to progression of IPS (n=3), infectious pneumonitis (n=3), multi-organ failure (n=1), and cardiac arrhythmia (n=1), all 8 events occurring in the cohort of patients requiring mechanical ventilation at study entry.
Four patients exhibited grade 2–4 acute GVHD at study entry; grade 2 (n=2) and grade 3 (n-2). Two of the four patients had complete resolution of GVHD during study therapy, and two had no change in GVHD grading. One patient developed grade 2 GVHD while on study. The incidence and severity of chronic GVHD were not monitored.
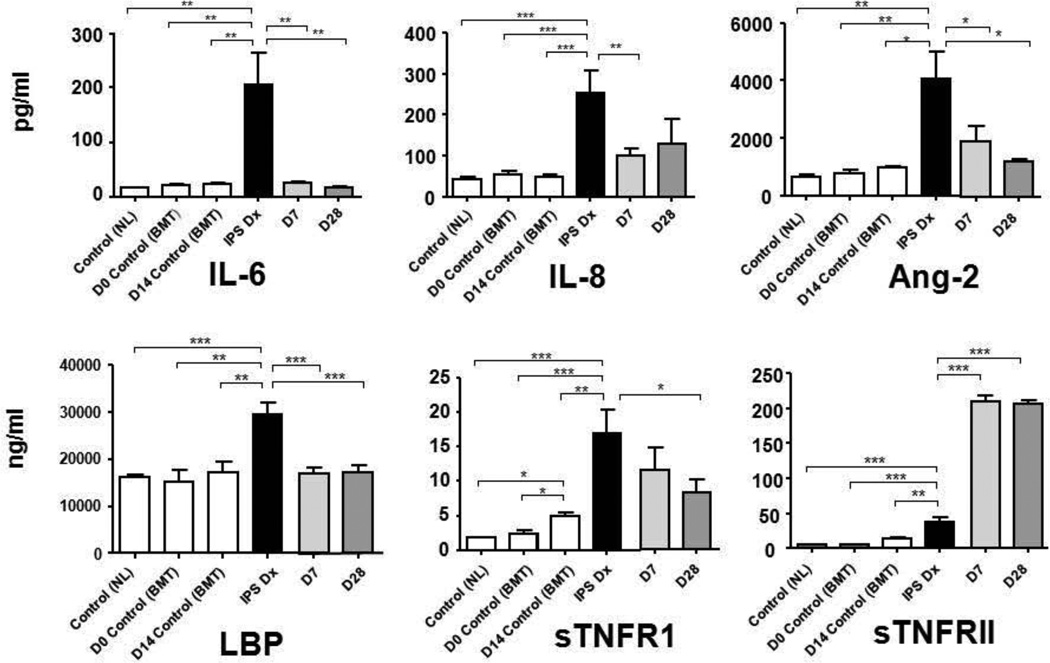
Plasma cytokine analysis
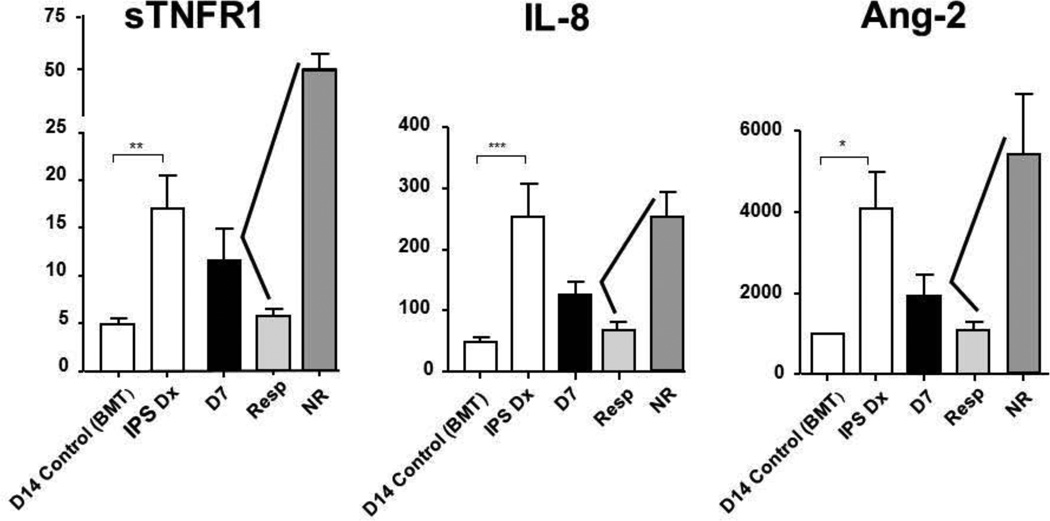
Plasma samples were available for biomarker analysis in 26 of 28 patients. Mean levels of TNFR1, a surrogate marker for TNFα, were significantly higher in patients at study entry, when compared to non-HCT and HCT controls (Figure 2). The diagnosis of IPS was also associated with significantly higher plasma levels of IL-6, IL-8, angiopoietin-2, LPB, and sTNFR2. Subsequent therapy for IPS led to reductions in day 7 and day 28 plasma levels of sTNFR1, IL-6, IL-8, angiopoietin-2, and LPB. By contrast, plasma levels of sTNFR2 increased during study therapy, indicating uptake of etanercept, a TNFR2 analogue. Levels of sTNFR1, angiopoietin-2 and IL-8 correlated well with response to therapy; mean plasma levels of all three cytokines declined by day 7 in responders, but increased in non-responders (Figure 3). The mean CRP level on day 0 was 28.1 ± 12.1, with levels decreased to 1.8 ± 0.6 in responders compared to 5.4 ± 3.3 in non-responders by day 7. Though mean CRP levels were lower in responders than non-responders at each time point (day 7, 14, 21, 28), none of the differences were significant.
Figure 2.
Plasma biomarkers in IPS.
Plasma protein levels were analyzed from patient samples collected at the time of IPS diagnosis (IPS Dx), day 7 (D7) and day 28 (D28) of study. Two control groups were included, (1) healthy, non-transplant patients [Control(NL)], and (2) allogeneic transplant recipients without complications [(Control(BMT)]. Plasma samples from Control(BMT) were analyzed at day 0 and day 14 post-transplant, the day 14 time point chosen to approximate the time of onset of IPS. Data are expressed as mean ± SEM. Significant differences between groups are shown as * p<0.05, ** p< 0.01, *** p<0.001
Figure 3.
Plasma biomarker levels of IPS at the time of diagnosis and on day 7 of study therapy, in responders and non-responders.
Plasma protein levels were analyzed from patient samples collected at the time of IPS diagnosis (IPS Dx), and on day 7 (D7) of study. Day 7 levels are then subdivided by those patients that responded (Resp) or did not-respond (NR) to study therapy. Control samples were obtained on day 14 post-transplant in allogeneic transplant recipients without complications [D14 Control (BMT)]. Data are expressed as mean ± SEM. Significant differences between groups are shown as: * p<0.05, ** p< 0.01, *** p<0.001
Non-evaluable patients
Eleven patients were deemed non-evaluable for response assessment. In 10 cases, pathogens were identified from the pre-therapy BAL fluid cultures (or PCR assays) a median 2 days (range 1–10 days) following initiation of study treatment. Viruses accounted for nine of the 10 abnormal results, with CMV (n=5) the most common virus identified. Two patients had already achieved a complete response to study therapy by the time CMV was detected in the pre-study BAL fluid, but given the abnormal BAL results, both patients were deemed non-evaluable for response assessment.
DISCUSSION
Improved therapy options for IPS are desperately needed, given the historically poor outcomes in both children and adults. Response rates of 18 to 30%, with day 28 survival < 50% have been reported when patients were treated with high dose corticosteroids and supportive care measures. [1–3, 20] In our current study, treatment with systemic corticosteroids and etanercept resulted in complete response rates of 71%, with day 28 survival 89% and 1-year survival 63%. Three other pediatric studies have confirmed that IPS remains an important complication in children, with cumulative incidence rates ranging from 6.7% – 11.8% post-HCT in children. [9, 10, 21] In all three studies, transplant-related mortality remained unacceptably high (>50%) and overall survival poor. [9, 10, 21] Keates-Baleeiro (2006) examined 11 pediatric patients treated for IPS with systemic corticosteroids (n=6), in which infliximab (n=2) or etanercept (n=3) were added for worsening pulmonary status. Though overall response rates were high in this report, the median survival was only 150 days. [21] Sano (2014) noted a median survival of only nine days from IPS onset to death (range, 0 to 183 days) in children managed with corticosteroids and supportive care, with 100% mortality for those patients who required mechanical ventilation during therapy. [10]
Early recognition of the disorder, prior to the development of severe pulmonary dysfunction, is key in the management of IPS in pediatric patients. Treatment responses were optimal when children were treated at lower baseline FiO2 values (≤ 45%), or prior to the requirement for mechanical ventilation. Day 56 survival was 100% in this subset of patients (n=11) underscoring the importance of initiating therapy prior to the development of severe lung injury. Although outcomes were inferior in patients requiring mechanical ventilation at study entry, survival was still significantly higher than observed in other pediatric published reports. [10]
Within this context, the current trial recommended that patients began treatment within 24 hours of performing the on-study BAL, provided that BAL fluid special stains were negative. We found no evidence that the early initiation of etanercept therapy increased therapy-related toxicity, even in patients later identified with pathogens on their pre-therapy BAL. Interestingly, two patients with CMV pneumonitis had a complete response to study therapy prior to initiation of anti-CMV treatment. Although only two patients, such results suggest that TNF inhibition may play a potential role in CMV therapy. Elevated levels of TNFα have been reported in neonates with CMV pneumonitis, but not previously reported in HCT recipients. [22]
A strength of our current study was the application of strict diagnostic criteria, with the requirement for BAL and echocardiogram in all subjects. In addition, a strict response definition was used, in which patient survival with “complete” discontinuation of all supplemental oxygen by day 28 were required. Clinical improvement rates in affected patients may have actually been higher, as significant reductions in supplemental oxygen support were noted in two additional study subjects by day 28, both subjects deemed non-responders.
Comparison with adult IPS trial
The results of this trial warrant comparison with a parallel IPS study recently conducted in adults by the BMT CTN. [8] Both trials had uniform eligibility (excluding age), dosing schedules for both etanercept and corticosteroids, and response assessments. The BMT CTN trial was a randomized, placebo controlled trial of corticosteroids ± etanercept. There were no significant differences in response and survival based upon treatment assignment (etanercept or placebo). Response rates were 65% for the entire cohort, similar to the 71% response rate seen in our pediatric IPS study. However, in marked contrast to the dramatic survival rates noted in our pediatric IPS study, 1-year OS was extremely poor (<25%) for adults in both arms of the BMT CTN study.
Several differences between the two studies may account for these findings. Over 40% of patients in the BMT CTN trial received a reduced intensity regimen, compared with only 4% in the pediatric IPS trial. Cytokine production, including TNFα, is diminished following reduced intensity regimens. [23] Hence, it is possible that IPS developing in this context may initially be more responsive to corticosteroids alone. Cytokine analysis of BAL fluid and plasma from patients with IPS following reduced conditioning regimen are pending from the BMT CTN IPS trial, and will help address this issue. Whereas adults in the BMT CTN trial received almost exclusively peripheral blood stem cells as the donor source, the vast majority of children in the pediatric IPS trial received marrow (50 %) or cord blood (39%). Steroid responsive peri-engraftment syndrome has been reported following cord blood transplantation, with peri-engraftment respiratory distress syndrome (PERDS) recognized as a subset of IPS. [24–26]
Interpretation of both studies is influenced by patient numbers. The pediatric IPS trial terminated early when it successfully met an efficacy stopping rule. In contrast, a major limitation of the BMT CTN IPS trial was its early termination due to poor accrual, with only 34 patients (out of a targeted 120) randomized. [8] Thus, the BMT CTN trial was drastically under-powered to draw any definitive conclusions. In addition, protocol adherence significantly impacted the BMT CTN trial; 37% of patients on the etanercept arm received ≤ 2 etanercept doses, in several cases due to physician / patient discretion to discontinue “blinded” therapy. By comparison, over 80% of patients in the pediatric IPS trial received all 8 scheduled etanercept doses, irrespective of therapy response. Hence, whereas compliance was high on the “open label”, phase II pediatric trial, compliance (and ultimately enrollment) on the phase III adult trial was significantly lower. Bronchoscopy was mandated (for diagnostic purposes) in both the pediatric and BMT CTN IPS trials. Critics have contended that the requirement for bronchoscopy may have also excluded the worst IPS cases from enrollment in the current trials, due to physician reluctance to perform a BAL in ventilated patients. The results from the pediatric IPS trial are thus even more impressive, as 17 of 28 (61%) patients required mechanical ventilation at study entry. The ability to ever perform the definitive phase III trial of etanercept in children with IPS is highly unlikely.
Biomarker studies
A key finding in our pediatric study was the association of plasma biomarkers with response, providing further evidence that inflammatory cytokines contribute to the pathogenesis of IPS. Consistent with our previous work in animal models and humans [6], elevations in pro-inflammatory cytokines, including TNFRI, TNFRII, IL-6, IL-8, and LPB, a component of the LPS cascade, were present on day 0. Increased levels of angiopoietin-2 (Ang-2) have not been previously reported in HCT patients with IPS. Angiopoietin (Ang)-1 and Ang-2 are peptide ligands for the receptor tyrosine kinase, Tie-2, that is expressed on the surface of endothelial cells (ECs) and are known to regulate vascular integrity [27]. Ang-2 sensitizes ECs to TNFα and regulates TNFα-induced adhesion molecule expression, findings that directly support preclinical data generated using murine IPS models [28]. Plasma levels of Ang-2 are increased in patients with acute respiratory distress syndrome and steroid refractory GVHD [29–32]. We found that Ang-2 levels in the plasma of patients with IPS were significantly elevated compared to controls. Importantly, levels of Ang-2 returned to baseline in IPS patients who responded to therapy, but continued to rise in patients who progressed.
While results of this pediatric phase II trial are encouraging, not all patients with IPS respond to anti-TNFα therapy. The reasons for this remain undetermined and limit the optimization of care. We recently analyzed the plasma proteome of patients with IPS and identified distinct similarities between IPS in humans and animal models, identifying a set of IPS-associated proteins that could predict at time of HCT which subjects would: 1) progress to IPS and 2) respond to etanercept therapy. [33] Hence, patients who are “hot-wired” to respond to immunologic stress with high levels of TNFα secretion may be more likely to respond to medications like etanercept while others may require alternative, novel strategies. For example, prior studies by our group suggest that other pro-inflammatory cytokines and chemokines, including IL-6 and MCP-1 are also markedly elevated at the onset of IPS. [3, 6] Therapeutic agents targeting MCP-1 and the IL-6 receptor are now available for clinical use, and could be considered as single agents, or in combination with etanercept and corticosteroids.
In addition, an effort to categorize patients with IPS based upon the presumed anatomic site of primary injury, in conjunction with mechanistic insights gained in the laboratory may lead to the use of other promising, non-cross reactive therapeutic or preventive agents [5]. For example, it is conceivable that approaches to maintain endothelial cell (EC) integrity may be effective at preventing or treating IPS. The administration of molecules that function as survival factors for ECs has been successful in preventing endothelial damage and mortality from septic shock and radiation injury. Similarly, ongoing studies examining the role of surfactant replacement therapy might prove useful in overcoming the effects of epithelial injury and dysfunction. Finally, since IPS develops and progresses to respiratory failure despite conventional immune suppression, it is possible that novel strategies directed toward inhibiting pathways of leukocyte recruitment to the lung may serve as future adjuncts to standard therapy. Such strategies have been successful in early phase studies for GVHD prevention. [34]
Etanercept administration
Questions remain regarding the optimal route and administration schedule for etanercept in the management of IPS. The current trial used a 4 week course of therapy, with a median time to response 10 days (3 doses of etanercept). Based upon results from the current trial, a shorter course of therapy, which could reduce the risks of opportunistic infections, may be warranted. While “recurrent IPS” was not an issue in responders, whether this was because TNFα neutralization was continued for 4 weeks remains to be determined. Ideally, therapy would be guided by “real time” monitoring of cytokine levels, with such technology currently under development at centers. In addition, the current study mandated that the first etanercept dose be given intravenously (IV), with subsequent dosing via subcutaneous (SQ) administration. Intravenous dosing was safe in our study, and not associated with infusion related events. Prior pharmaco-kinetic data noted a rapid attainment of Cmax following IV administration of etanercept, in comparison to SQ dosing. [35] Given the critical nature of affected patients, the importance of rapid attainment of Cmax is evident, and IV etanercept dosing remains recommended at initiation of IPS therapy.
In conclusion, etanercept in combination with corticosteroids was associated with high response rates and overall survival in children with IPS. Therapy was well tolerated with an acceptable toxicity profile. Plasma biomarker studies further support the role for inflammatory cytokines in the pathogenesis of IPS, with reductions in biomarker levels coinciding with clinical response to therapy. Collectively, these results represent the culmination of a translational research endeavor that used established animal models, encouraging early phase clinical data and two collaborative pediatric consortium to bring novel insights into the pathophysiology of a lethal clinical disorder, from the laboratory to the clinic.
HIGHLIGHTS.
Etanercept plus steroids is associated with high response rates in pediatric patients with IPS.
Therapy is optimized if initiated prior to the development of severe lung injury.
Plasma biomarkers are associated with response, supporting a role for TNF inhibition in IPS therapy.
ACKNOWLEDGMENTS
We would like to thank the staff and administration from the Pediatric Blood and Marrow Transplant Consortium (PBMTC) and Children’s Oncology Group (COG) for their collaborative efforts in this study. In particular, we would like to thank Meera Raman and Christopher Corral from the COG and Robin Ryan from the Children’s Mercy Hospital and Laura Hancock from the PBMTC for their combined work in clinical trial coordination. Etanercept was supplied by Immunex Corporation, a wholly owned subsidiary of Amgen Inc.
Grant Support: This study was supported in part by N01 HC-45220/HHSN268200425220C and U10 CA098543, The Leukemia and Lymphoma Society (KRC) and the Burroughs Welcome Fund (KRC). PBMTC activities were also supported by 2U01HL069254 and the St. Baldrick’s Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no financial disclosures or conflicts of interest to disclose.
REFERENCES
- 1.Kantrow SP, Hackman RC, Boeckh M, Myerson D, Crawford SW. Idiopathic pneumonia syndrome: Changing spectrum of lung injury after marrow transplantation. Transplantation. 1997;63:1079–1086. doi: 10.1097/00007890-199704270-00006. [DOI] [PubMed] [Google Scholar]
- 2.Crawford SW, Hackman RC. Clinical course of idiopathic pneumonia after bone marrow transplantation. Am Rev Respir Dis. 1993;147:1393–1400. doi: 10.1164/ajrccm/147.6_Pt_1.1393. [DOI] [PubMed] [Google Scholar]
- 3.Yanik G, Hellerstedt B, Custer J, Hutchinson R, Kwon D, Ferrara JL, et al. Etanercept (Enbrel) administration for idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2002;8:395–400. doi: 10.1053/bbmt.2002.v8.pm12171486. [DOI] [PubMed] [Google Scholar]
- 4.Clark JG, Hansen JA, Hertz MI, Parkman R, Jensen L, Peavy HH. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. The American Review of Respiratory Disease. 1993;147:1601–1606. doi: 10.1164/ajrccm/147.6_Pt_1.1601. [DOI] [PubMed] [Google Scholar]
- 5.Panoskaltsis-Mortari A, Griese M, Madtes DK, Belperio JA, Haddad IY, Folz RJ, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanik GA, Ho VT, Levine JE, White ES, Braun T, Antin JH, et al. The impact of soluble tumor necrosis factor receptor etanercept on the treatment of idiopathic pneumonia syndrome after allogeneic hematopoietic stem cell transplantation. Blood. 2008;112:3073–3081. doi: 10.1182/blood-2008-03-143412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuda T, Hackman RC, Guthrie KA, Sandmaier BM, Boeckh M, Maris MB, et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood. 2003;102:2777–2785. doi: 10.1182/blood-2003-05-1597. [DOI] [PubMed] [Google Scholar]
- 8.Yanik GA, Horowitz MM, Weisdorf DJ, Logan BR, Ho VT, Soiffer RJ, et al. Randomized, double-blind, placebo-controlled trial of soluble tumor necrosis factor receptor: enbrel (etanercept) for the treatment of idiopathic pneumonia syndrome after allogeneic stem cell transplantation: blood and marrow transplant clinical trials network protocol. Biol Blood Marrow Transplant. 2014;20:858–864. doi: 10.1016/j.bbmt.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi H, Takahashi Y, Watanabe N, Doisaki S, Muramatsu H, Hama A, et al. Incidence, Clinical Features, and Risk Factors of Idiopathic Pneumonia Syndrome Following Hematopoietic Stem Cell Transplantation in Children. Pediatr Blood Cancer. 2012;58:780–784. doi: 10.1002/pbc.23298. [DOI] [PubMed] [Google Scholar]
- 10.Sano H, Kobayashi R, Iguchi A, Suzuki D, Kishimoto K, Yasuda K, et al. Risk factor analysis of idiopathic pneumonia syndrome after allogeneic hematopoietic SCT in children. Bone Marrow Transplant. 2013 doi: 10.1038/bmt.2013.123. [DOI] [PubMed] [Google Scholar]
- 11.Versluys AB, Rossen JW, van Ewijk B, Schuurman R, Bierings MB, Boelens JJ. Strong association between respiratory viral infection early after hematopoietic stem cell transplantation and the development of life-threatening acute and chronic alloimmune lung syndromes. Biol Blood Marrow Transplant. 2010;16:782–791. doi: 10.1016/j.bbmt.2009.12.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark JG, Madtes DK, Martin TR, Hackman RC, Farrand AL, Crawford SW. Idiopathic pneumonia after bone marrow transplantation: cytokine activation and lipopolysaccharide amplification in the bronchoalveolar compartment. Critical Care Medicine. 1999;27:1800–1806. doi: 10.1097/00003246-199909000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Cooke KR, Hill GR, Gerbitz A, Kobzik L, Martin TR, Crawford JM, et al. Tumor necrosis factor-alpha neutralization reduces lung injury after experimental allogeneic bone marrow transplantation. Transplantation. 2000;70:272–279. doi: 10.1097/00007890-200007270-00006. [DOI] [PubMed] [Google Scholar]
- 14.Cooke KR, Hill GR, Gerbitz A, Kobzik L, Martin TR, Crawford JM, et al. Hyporesponsiveness of donor cells to lipopolysaccharide stimulation reduces the severity of experimental idiopathic pneumonia syndrome: potential role for a gut-lung axis of inflammation. J Immunol. 2000;165:6612–6619. doi: 10.4049/jimmunol.165.11.6612. [DOI] [PubMed] [Google Scholar]
- 15.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr, Crawford JM, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 16.Hildebrandt GC, Olkiewicz KM, Corrion LA, Chang Y, Clouthier SG, Liu C, et al. Donor-derived TNF-alpha regulates pulmonary chemokine expression and the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood. 2004;104:586–593. doi: 10.1182/blood-2003-12-4259. [DOI] [PubMed] [Google Scholar]
- 17.Panoskaltsis-Mortari A, Taylor PA, Yaegar TM, Wangensteen OD, Bitterman PB, Ingbar DH, et al. The critical early proinflammatory events associated with idiopathic pneumonia syndrome in irradiated murine allogenic recipients are due to donor T cell infusion and potentiated by cyclophoshamide. Journal of Clinical Investigation. 1997;100:1015–1027. doi: 10.1172/JCI119612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark JG, Madtes DK, Hackman RC, Chen W, Cheever MA, Martin PJ. Lung injury induced by alloreactive Th1 cells is characterized by host-derived mononuclear cell inflammation and activation of alveolar macrophages. J Immunol. 1998;161:1913–1920. [PubMed] [Google Scholar]
- 19.Hildebrandt GC, Duffner UA, Olkiewicz KM, Corrion LA, Willmarth NE, Williams DL, et al. A critical role for CCR2/MCP-1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood. 2004;103:2417–2426. doi: 10.1182/blood-2003-08-2708. [DOI] [PubMed] [Google Scholar]
- 20.Tizon R, Frey N, Heitjan DF, Tan KS, Goldstein SC, Hexner EO, et al. High-dose corticosteroids with or without etanercept for the treatment of idiopathic pneumonia syndrome after allo-SCT. Bone Marrow Transplant. 2012;47:1332–1337. doi: 10.1038/bmt.2011.260. [DOI] [PubMed] [Google Scholar]
- 21.Keates-Baleeiro J, Moore P, Koyama T, Manes B, Calder C, Frangoul H. Incidence and outcome of idiopathic pneumonia syndrome in pediatric stem cell transplant recipients. Bone Marrow Transplant. 2006;38:285–289. doi: 10.1038/sj.bmt.1705436. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Ji J, Li M, Chen Y, Chen F, Chen H, et al. Detection of serum Th1 and Th2 cytokines and its significance in neonates with cytomegalovirus pneumonia. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2007:361–363. [PubMed] [Google Scholar]
- 23.Mohty M, Blaise D, Faucher C, Vey N, Bouabdallah R, Stoppa A-M, et al. Inflammatory cytokines and acute graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation. Blood. 2005;106:4407–4411. doi: 10.1182/blood-2005-07-2919. [DOI] [PubMed] [Google Scholar]
- 24.Brownback KR, Simpson SQ, McGuirk JP, Lin TL, Abhyankar S, Ganguly S, et al. Pulmonary manifestations of the pre-engraftment syndrome after umbilical cord blood transplantation. Ann Hematol. 2014;93:847–854. doi: 10.1007/s00277-013-1981-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanda J, Kaynar L, Kanda Y, Prasad VK, Parikh SH, Lan L, et al. Pre-engraftment syndrome after myeloablative dual umbilical cord blood transplantation: risk factors and response to treatment. Bone Marrow Transplant. 2013;48:926–931. doi: 10.1038/bmt.2012.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frangoul H, Wang L, Harrell FE, Jr, Ho R, Domm J. Preengraftment syndrome after unrelated cord blood transplant is a strong predictor of acute and chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1485–1488. doi: 10.1016/j.bbmt.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Cooke KRJA, Ho V. The contribution of endothelial activation and injury to end-organ toxicity following allogeneic hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation. 2008;14:23–32. doi: 10.1016/j.bbmt.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Gerbitz A, Nickoloff BJ, Olkiewicz K, Willmarth NE, Hildebrandt G, Liu C, et al. A role for tumor necrosis factor-alpha-mediated endothelial apoptosis in the development of experimental idiopathic pneumonia syndrome. Transplantation. 2004;78:494–502. doi: 10.1097/01.tp.0000128839.13674.02. [DOI] [PubMed] [Google Scholar]
- 29.Luft T, Dietrich S, Falk C, Conzelmann M, Hess M, Benner A, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118:1685–1692. doi: 10.1182/blood-2011-02-334821. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher DC, Parikh SM, Balonov K, Miller A, Gautam S, Talmor D, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29:656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63:903–909. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 33.Schlatzer DM, Dazard JE, Ewing RM, Ilchenko S, Tomcheko SE, Eid S, et al. Human biomarker discovery and predictive models for disease progression for idiopathic pneumonia syndrome following allogeneic stem cell transplantation. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.015479. M111015479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reshef R, Luger SM, Hexner EO, Loren AW, Frey NV, Nasta SD, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wee S, Pascual M, Eason J, et al. Biological Effects and Fate of a Soluble, Dimeric, 80-kDa Tumor Necrosis Factor Receptor in Renal Transplant Patients who receive OKT3 Therapy. Transplantation. 1997;63:570–577. doi: 10.1097/00007890-199702270-00015. [DOI] [PubMed] [Google Scholar]