Abstract
Severe asthma has been increasingly recognized as a heterogenous disease with varied clinical characteristics and pathophysiological processes. Patients with severe asthma suffer significant impairment in their daily life and impose a substantial burden on health care resources. The recent work of consortia groups has led to an improved definition of severe asthma as well as better characterization of the patients with severe disease. Different approaches, including unbiased cluster analyses, have been utilized to identify severe asthma phenotypes (subgroups) defined by their clinical characteristics and immune processes. Recognition of severe asthma phenotypes has assisted the development of targeted therapies by identifying patients more likely to respond to the specific agent. In this article, we discuss the evolution of our understanding of severe asthma and review the currently available therapies and promising drugs in development. In addition, we examine the role of bronchoscopy in severe asthma and the emerging evidence regarding bronchial thermoplasty.
Keywords: asthma, phenotype, omalizumab, bronchial thermoplasty
Asthma is a heterogenous syndrome defined by a combination of clinical symptoms along with reversible expiratory airflow limitation or bronchial hyperresponsiveness. The prevalence of the disease has been increasing and is estimated to affect more than 24 million people in the United States and around 300 million people worldwide.1,2 Despite guideline-based therapy, it is recognized that up to 50% of patients are not well controlled and 5% to 10% of patients suffer from a particularly severe disease that is often refractory to usual treatment.3,4 Severe asthmatics impose a significant burden on health care utilization through Emergency Department visits, hospitalizations, and ICU stays,4 and suffer from substantial levels of work, school, and daily activity impairment.5 The work of consortia groups, including the Severe Asthma Research Program (SARP) funded by the National Heart, Lung, and Blood Institute, has led to the recognition of different subgroups (phenotypes) of severe asthma that are characterized by varied clinical manifestations, pathophysiological mechanisms, and biomarkers. In this review, we describe the severe asthma phenotypes that have been proposed and the evidence supporting the role of patient-directed therapy.
DEFINING SEVERE ASTHMA
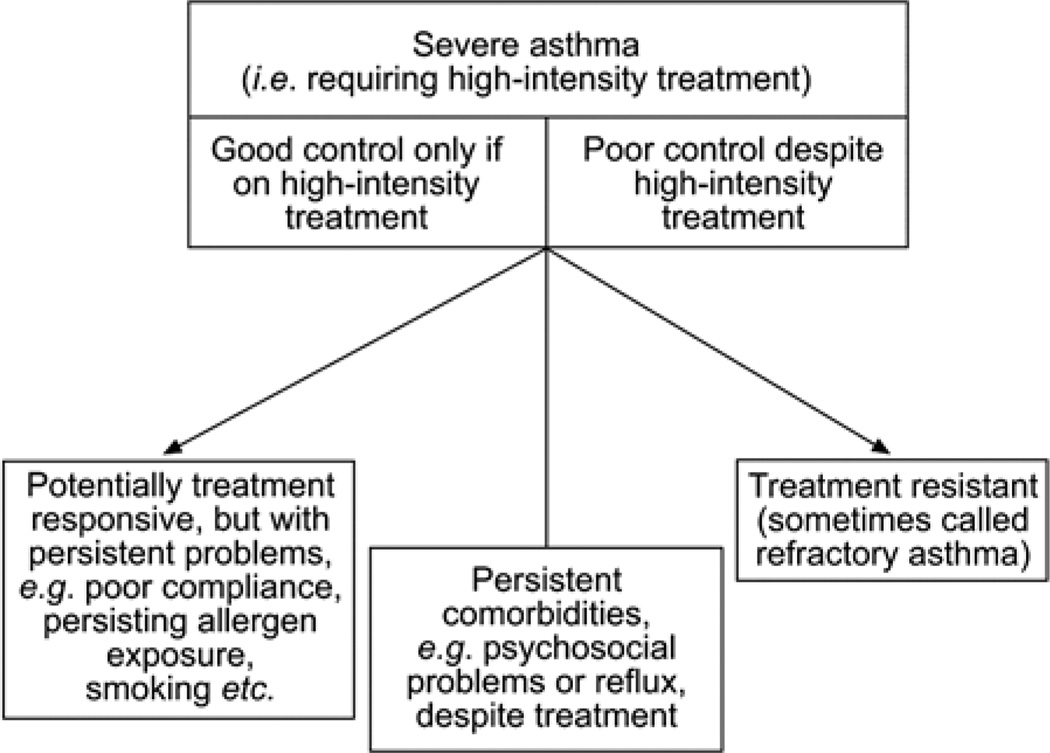
The concept of asthma severity has evolved over recent years. The initial severity classification published in the 1995 Global Initiative for Asthma guidelines separated asthma severity based on the patient’s clinical characteristics: symptoms, short-acting bronchodilator use, nocturnal symptoms, and peak expiratory flow or the percent predicted forced expiratory volume in 1 second (FEV1) before commencing treatment.6 This classification system of intermittent, mild persistent, moderate persistent, and severe persistent, however, does not allow for easy assessment of change with time and does not consider the intensity of therapy necessary to achieve control. Various terms have been used to describe severe asthmatics over the years, including “refractory asthma,” “difficult to control asthma,” and “irreversible asthma” that made it difficult to assess the true prevalence of patients with severe disease.7 In an effort to better understand the pathophysiological and clinical characteristics of this heterogenous group of patients, the American Thoracic Society (ATS) conducted a workshop on refractory asthma in 2000 and formed the SARP. The SARP committee released a consensus definition of severe or refractory asthma to include patients with persistent respiratory symptoms, frequent asthma exacerbations, airway obstruction despite high-dose inhaled corticosteroid use, or those who require high medication doses to maintain control of their asthma.7 The ENFUMOSA (European Network for Understanding Mechanisms of Severe Asthma) study group and the TENOR (The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens) study group have also identified severe asthmatics as those patients not only with poor control despite high-intensity treatment, but also patients who can only maintain good control while taking high-intensity treatment (Fig. 1).8 In addition, the most recent guidelines from the Expert Panel Report 3 of the National Heart, Lung, and Blood Institute support the concept that severity should indicate the intensity of treatment required to treat a patient’s asthma once the diagnosis has been confirmed, comorbidities treated, and inhaler technique and adherence have been optimized.9
FIGURE 1.
Severe asthma defined as the need for high-intensity treatment. This definition includes patients who require high-intensity controller therapy to maintain good control as well as patients with poor asthma control despite high-intensity treatment. Reproduced with permission of the European Respiratory Society.8 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
IDENTIFYING CLINICAL ASTHMA PHENOTYPES
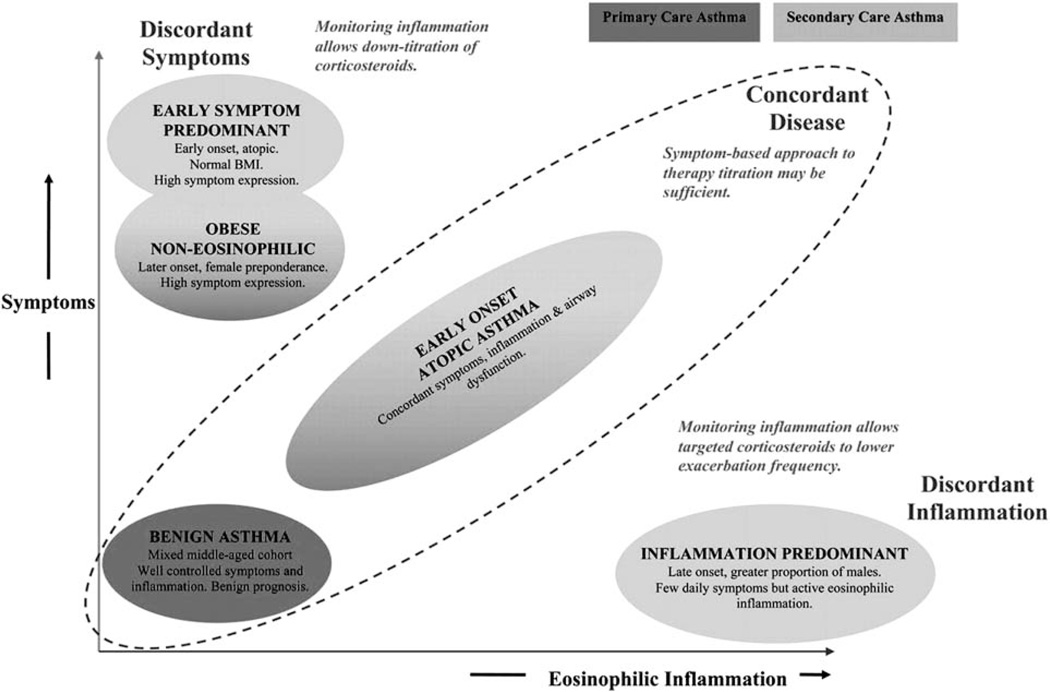
The heterogenous nature of asthma is easily recognized in patients with severe disease. These patients vary significantly in their clinical characteristics, triggers, and inflammatory processes. In an attempt to gain better insight into the pathophysiology of severe asthma, investigators have been working to identify specific subgroups, or phenotypes, to facilitate research exploring the mechanisms of the disease and ultimately improve therapies.10 Initial studies performed in asthmatics identified subtypes based on inflammatory patterns obtained from bronchoalveolar lavage and endobronchial biopsies. Wenzel et al11,12 identified neutrophilic inflammation not previously seen in mild asthmatics and found an association between clinical variables and different amounts of eosinophilic inflammation. Two large studies utilized an unbiased, statistical approach to identify subgroups of asthma with consistent patterns of disease. The first study was performed in the United Kingdom and utilized cluster analysis with variables reflective of symptoms, atopic status, eosinophilic inflammation, age at onset, BMI, and bronchodilator responsiveness of asthmatics recruited from primary care clinics and severe asthmatics treated by a respiratory specialist.13 They identified 3 clusters within the primary care population and 4 clusters within the severe asthma population with the obese, noneosinophilic group and the early-onset atopic group occurring in both patient groups. Figure 2 depicts their results when plotted according to their relative expression of symptoms and eosinophilic inflammation.13
FIGURE 2.
Clinical asthma phenotypes identified using cluster analysis in primary-care and secondary-care asthma populations. The clusters are plotted according to their relative expression of symptoms and inflammation. Reprinted with permission of the American Thoracic Society. Copyright © 2012 American Thoracic Society.13 Copyright American Thoracic Society, New York. All permission requests for this image should be made to the copyright holder.
A second analysis was performed with over 700 patients recruited for participation in the NIH-sponsored SARP.14 Despite statistical variations and the inclusion of different variables, the results were generally similar to those of the initial UK study. Table 1 displays a summary of the 5 clusters identified in the SARP study.14 Both studies found that the age of disease onset was a key differentiating factor with early-onset disease being associated with more atopic conditions over a range of severities and late-onset disease occurring more in women and being associated with eosinophilic inflammation and obesity. The UK study identified 2 severe allergic asthma clusters, whereas the SARP study distinguished 3 separate severe atopic asthma phenotypes (clusters 1, 2, and 4) that differed in baseline lung function, response to bronchodilators, medication requirements, health care utilization, and asthma symptoms and represented a continuum of increasing disease severity.14 Biomarker data including total immunoglobulin E (IgE), sputum eosinophil and neutrophil counts, fraction of nitric oxide in exhaled breath (FeNO), and bronchial responsiveness to methacholine were obtained in only a subset of patients, and therefore not included in the cluster analysis. A post hoc analysis of the subset of patients with biomarker data available revealed increased sputum neutrophils in cluster 5 and identified persistent airway eosinophilia in clusters 3, 4, and 5 despite receiving high doses of inhaled or oral corticosteroids.14 Although limited by the nature of the analysis, the biomarker data provide insight into potential pathobiologic mechanisms related to the severe asthma phenotypes. Together, these cluster analyses demonstrate the importance of disease heterogeneity in asthma and suggest different pathophysiological mechanisms that may have clinical therapeutic implications.
TABLE 1.
Characteristics of Asthma Patients by Cluster Analysis
| Cluster 1 (n = 110) |
Cluster 2 (n = 321) |
Cluster 3 (n = 59) |
Cluster 4 (n = 120) |
Cluster 5 (n = 116) |
|
|---|---|---|---|---|---|
| Demographics | Younger, predominantly female | Slightly older, predominantly female | Older woman (mean age 50) with high BMI | Equal men and women | Predominantly women |
| Age of asthma onset | Childhood onset | Childhood onset | Adult onset | Childhood onset, long disease duration | Early adult onset, long disease duration |
| Atopy status | Atopic disease | Atopic disease | Less atopic | Atopic disease | Less atopic |
| Health care utilization | Infrequent | Infrequent | Moderate | High | High |
| Controller medication use | 2 or fewer controller medications | Increased number of controllers | 50% with 3 or more controller meds | Majority with 3 or more asthma drugs | Majority with 3 or more asthma drugs |
| Postbronchodilator FEV1 (% predicted) | 113 | 94 | 84 | 76 | 58 |
Reprinted with permission of the American Thoracic Society. Copyright © 2013 American Thoracic Society.14 This modified table combines data from the original article available from http://www.atsjournals.org. Copyright American Thoracic Society, New York. All permission requests for this image should be made to the copyright holder.
CELLULAR INFLAMMATORY AND MOLECULAR PHENOTYPES
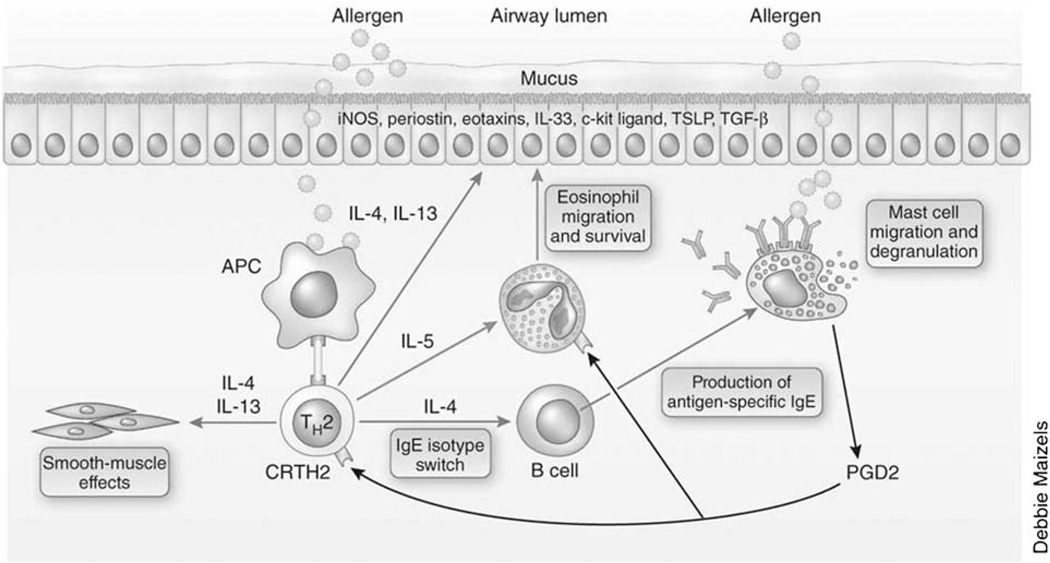
Asthma has classically been considered a T-helper type 2 (TH2) process that is linked to allergy and atopy, type I hypersensitivity reactions, eosinophilic inflammation, and response to corticosteroids.10 The initial approach to asthma classification focused on the type of cellular inflammation observed in analyses of sputum, bronchoalveolar lavage, and endobronchial biopsy specimens from asthmatics. An increased number of eosinophils was identified in the majority of patients and supported the focus of research activities on allergen-specific TH2 processes and the development of mouse models of allergic asthma.15,16 TH2 cells produce cytokines [interleukin (IL)-4, IL-5, and IL-13] that have been shown to regulate the allergicspecific synthesis of IgE and recruitment of eosinophils and stimulate epithelial and smooth muscle changes that contribute to the pathobiology of TH2-associated asthma.16 A hypothetical model depicting this pathway is illustrated in Figure 3.
FIGURE 3.
T-helper type 2 (TH2) immune processes in asthma. TH2 cells produce cytokines interleukin (IL)-4, IL-5, and IL-13 that stimulate allergic and eosinophilic inflammation and cause changes in the airway epithelium and smooth muscle that contribute to the pathobiology of asthma. Reprinted by permission from Macmillan Publishers Ltd. Copyright 2012.10 Copyright Nature Publishing Group, New Yok. All permission requests for this image should be made to the copyright holder.
Over the past decade, accumulating clinical and experimental observations have suggested that the pathobiology of asthma is more heterogenous and complex and includes several non-TH2 factors. These factors include T-helper type 1 (TH1) cells with production of IL-17 and interferon-γ along with the development of neutrophilic inflammation.16 Additional components of the innate immune response include IL-25 and IL-33 that have been shown to induce TH2 cytokines in the absence of TH2 cells and stimulate multiple effector cell types including mast cells and eosinophils.16 Increased recognition of the importance of nonallergic triggers such as air pollutants, viral infection, and obesity has further facilitated efforts to investigate the role of innate, nonallergic immune responses in the development of asthma.
BIOMARKERS OF AIRWAY INFLAMMATION
The recognition of asthma phenotypes and the association with significant variability in disease pathogenesis and response to therapy has fueled the search for biomarkers to help classify patients and guide therapeutic interventions. Initial efforts have focused primarily on measuring airway inflammation to allow for adjustment of anti-inflammatory medications with the goal of preventing exacerbations. Randomized, controlled trials have studied the utility of measuring induced sputum eosinophil counts in moderate to severe asthmatics and have found a reduction in the frequency and severity of asthma exacerbations when the inhaled corticosteroid dose is adjusted based on induced sputum eosinophil counts compared with the standard-of-care method based on symptoms, lung function, or rescue medication use.17–19 However, the routine use of sputum analysis is currently not recommended in clinical practice because of the expertise required to accurately perform the test and the associated cost.
An alternative biomarker of airway inflammation is the FeNO, which is a more reproducible measurement and easier to obtain than sputum analysis. FeNO levels have been shown to be increased in asthmatics and associated with eosinophilic airway inflammation.20 Although levels of FeNO are similar among severe and nonsevere asthmatics, those patients with severe asthma and increased FeNO have persistent eosinophilic airway inflammation despite therapy with inhaled and systemic steroids.21 Elevated FeNO in severe asthmatics also identifies a phenotype with the greatest degree of airflow obstruction and hyperinflation and most frequent use of emergency care.22 The 2011 ATS clinical practice guideline recommends that FeNO values >50 ppb in symptomatic patients can be used to identify eosinophilic inflammation that may respond to corticosteroids.23 Utilizing FeNO measurements to guide asthma management has been shown to allow for the safe reduction of maintenance doses of inhaled corticosteroids,24 but does not result in decreased exacerbations in the general asthma population.24,25 Further longitudinal studies evaluating the role of FeNO as a biomarker to guide management of severe and nonsevere asthma patients will be required before the technique is adopted into routine clinical practice.
ROLE OF BRONCHOSCOPY IN SEVERE ASTHMA
Safety
In efforts to better characterize the pathophysiology of severe asthma, investigative bronchoscopy was incorporated as a key component of the SARP. Previous studies utilizing bronchoscopy to obtain bronchoalveolar lavage samples and airway biopsies were primarily performed in patients with mild to moderate disease. Over a 6-year period, 143 subjects with severe asthma underwent investigative bronchoscopy that included airway sampling (mucosal biopsy, endobronchial brushing) along with bronchoalveolar lavage or transbronchial biopsy. Asthma exacerbations after bronchoscopy were rare, but did occur more commonly in severe asthmatics, whereas postprocedure reduction in FEV1 was similar among subjects with severe and nonsevere asthma.26 The results of the SARP study suggest that with proper precautions, bronchoscopy may be safely performed in patients with severe disease.
Bronchoscopy-directed Therapy
A recent study suggests that bronchoscopy may be a useful tool to identify asthma phenotypes and direct therapy.27 Fifty-eight patients meeting the ATS definition of severe asthma underwent bronchoscopy with visual scoring systems of the upper and lower airways along with bronchoalveolar lavage and endobronchial biopsy and brushing. Five phenotypes were generated based on bronchoscopic evaluation and included gastroesophageal reflux (GER), subacute bacterial infection, tissue eosinophilia, combination, and nonspecific. The largest number of subjects fit into the GER subtype and the visual supraglottic scoring system correlated well with formal GER testing (esophageal pH or impedance measurement). Targeted therapy included intense medical therapy of GER with one third of the patients requiring Nissen fundoplication for improved asthma control. The subacute bacterial infection phenotype was defined by either a positive culture or PCR for mycoplasma or chlamydophila from BAL or endobronchial sampling. Antimicrobial therapy was specific for the identified pathogen and continued for at least 2 weeks and often up to 6 months. The tissue eosinophilia phenotype was arbitrarily defined as ≥10 eosinophils per high power field and subjects that met this criterion were treated with omalizumab. As shown in Table 2, after 12 to 60 weeks of targeted therapy, a significant improvement in the asthma control test score and percent predicted FEV1 occurred in all phenotypes except the nonspecific subgroup.27 Although further multicenter studies are needed to confirm the findings of this study, the results suggest that bronchoscopic phenotyping of severe asthma may facilitate the use of targeted therapies that improve clinical outcomes.
TABLE 2.
Effects of Targeted Therapy After Bronchoscopic Phenotyping of Severe Asthma Patients
| Gastroesophageal Reflux |
Subacute Bacterial Infection |
Tissue Eosinophilia |
Combination | Nonspecific | |
|---|---|---|---|---|---|
| ACT score prebronchoscopy | 10.0 ± 3.4 | 12.8 ± 3.7 | 9.5 ± 3.5 | 11.7 ± 4.3 | 15.0 ± 4.9 |
| ACT score postbronchoscopy | 17.5 ± 4.3* | 19.9 ± 3.7* | 17.0 ± 5.6† | 19.2 ± 3.8* | 17.5 ± 3.4NS |
| FEV1 % predicted prebronchoscopy | 61.5 ± 16.1 | 63.8 ± 18.3 | 46.8 ± 11.8 | 53.0 ± 13.4 | 59.6 ± 24.8 |
| FEV1 % predicted postbronchoscopy | 75.9 ± 15.3‡ | 79.0 ± 14.5‡ | 76.6 ± 5.5§ | 67.5 ± 13.8‡ | 68.5 ± 21.5NS |
Prebronchoscopy data were obtained while patients were on standard therapy and postbronchoscopy values were measured after 12 to 60 weeks of targeted phenotype-specific therapy.
ACT indicates asthma control test; NS, not significant.
P ≤ 0.0005,
≤ 0.02,
≤ 0.0004, and
≤ 0.003.
Adapted from Good et al.27 Adaptations are themselves works protected by copyright. So in order to publish this adaptation, authorization must be obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
TARGETED MOLECULAR THERAPY
Anti-IgE Therapy
The key role of IgE in the pathogenesis of allergic asthma has led to the development of therapeutic agents targeting the IgE-mediated immunologic pathways. Omalizumab is a recombinant humanized monoclonal antibody that binds circulating IgE, blocking the interaction of IgE to the high affinity receptor for IgE of mast cells and basophils and inhibiting cell activation and mediator release.28 Omalizumab is currently the only available anti-IgE therapy in clinical use for patients with allergic asthma and elevated serum levels of IgE. A recent systematic review including 8 trials and 3429 patients with moderate to severe allergic asthma showed that patients receiving omalizumab were more likely to reduce their dose or discontinue inhaled glucocorticoid therapy and were less likely to experience an asthma exacerbation or be hospitalized for asthma.28 Only a minority of these patients, however, were treated with both long-acting β2-agonists (LABA) and inhaled corticosteroids which have become the standard-of-care for treatment of severe asthma that is not well controlled with single controller therapy.9 A randomized controlled trial conducted in patients with inadequately controlled severe asthma who were receiving high-dose inhaled corticosteroid and LABAs was conducted at multiple sites in the United States and Canada and showed that 48 weeks of omalizumab resulted in a 25% reduction in the incident rate of asthma exacerbations compared with placebo.29 On the basis of these trials, omalizumab should be considered as adjunctive therapy in patients with uncontrolled severe allergic asthma already receiving recommended controller therapy. Severe asthma patients without the phenotypic characteristics of allergic asthma and elevated IgE levels are unlikely to benefit from omalizumab therapy.
Anti-IL-5 Therapy
IL-5 is a proinflammatory cytokine that functions as a key TH2 mediator in the maturation, recruitment, and activation of eosinophils. Inhibition of IL-5 has been shown to reduce the number of blood and sputum eosinophils in asthma patients,30 however, initial studies in patients who were not selected according to their phenotype failed to show a benefit in reducing the signs and symptoms of asthma.30,31 In contrast, the monoclonal IL-5 antibody mepolizumab was effective in reducing exacerbations and improving asthma quality of life questionnaire (AQLQ) scores in patients with severe asthma with increased sputum eosinophil counts.32 Mepolizumab was also shown to decrease daily prednisone dose when given to patients with severe prednisone-dependent asthma and increased sputum eosinophil counts.33 The results of these 2 small proof-of-concept studies led to a larger multicenter, double-blind, placebo-controlled trial with 621 patients with severe asthma and evidence of eosinophilic inflammation by increased sputum or peripheral blood eosinophil count or increased FeNO.34 Patients were assigned to placebo or one of 3 doses of mepalizumab for 1 year. All 3 doses of mepalizumab resulted in significantly decreased number of exacerbations without change in pulmonary function, asthma control scores, or AQLQ.34 A different monoclonal IL-5 antibody, reslizumab, was shown to improve lung function and asthma symptoms in severe asthmatics with persistent sputum eosinophilia.35 Treatment with reslizumab resulted in a trend toward decreased exacerbations that may have been limited by the short study duration (13 wk).35 Together, these studies support a future role for anti-IL-5 therapy in the subtype of severe asthmatics with persistent eosinophilic inflammation and recurrent exacerbations despite maximal controller therapy. The mixed results with the effect of anti-IL-5 therapy on lung function and asthma quality of life and symptom scores highlights the complex pathobiology of severe asthma and suggests that multiple processes may need to be targeted for comprehensive therapy.36
Anti-IL-13 Therapy
IL-13 is secreted predominantly by activated TH2 cells and is a key mediator in allergic inflammation with effects on eosinophils, basophils, and mast cells as well as respiratory epithelial cells and smooth muscle cells.37 Among its many effector functions, IL-13 has been shown to induce bronchial epithelial cells to secrete periostin, a matricellular protein that has paracrine effects on fibroblasts and autocrine effects on epithelial cells that may contribute to mechanisms of airway remodeling in asthma38 (Fig. 3). Anti-IL-13 therapy, by a monoclonal IL-13 antibody lebrikuzumab significantly improves lung function (prebronchodilator FEV1) in patients with uncontrolled asthma despite inhaled corticosteroids.39 Notably, the improvement in FEV1 was found through post hoc analysis to be greatest in patients with high serum periostin levels, suggesting that periostin may be used as a biomarker to identify patients likely to respond to anti-IL-13 therapy.39 In addition, a recently published phase 2 study of the IL-13 antibody, tralokinumab, showed improved lung function in moderate to severe asthma with a suggestion of an enhanced response in patients with elevated IL-13 levels in their sputum.40 Together, these studies highlight the potential role for specific molecular-targeted therapy in selected severe asthma phenotypes and support further investigation with anti-IL-13 therapy in larger clinical trials.
Antileukotriene Agents
Leukotrienes [cysteinyl leukotrienes (cysLTs) and leukotriene B4 (LTB4)] are synthesized in various types of leukocytes and released locally into tissue as well as plasma and contribute to the pathogenesis of asthma in various forms.41 cysLTs are recognized for their ability to induce smooth muscle contraction and protracted bronchoconstriction, mucous secretion, and airway edema formation, whereas LTB4 is best known as a leukocyte chemoattractant, recruiting nearly all subgroups of lymphocytes.41 Currently available antileukotriene therapy includes zileuton, a 5-lipoxygenase inhibitor that prevents the formation of both cysLTs and LTB4, and the cystLT1 receptor antagonists, montelukast and zafirlukast. Studies support the recommendation of adding LABAs over antileukotriene therapy in most patients not controlled with inhaled corticosteroids.9 However, the subgroup of patients with severe asthma and aspirin-insensitivity may benefit from antileukotriene therapy. Patients with aspirin-intolerant asthma were found to have significantly higher levels of leukotrienes in exhaled breath condensate than patients with aspirin-tolerant asthma.42 Studies have shown that the addition of zileuton43 or montelukast44 to inhaled or oral glucocorticoid therapy in patients with aspirin sensitivity and asthma resulted in improved lung function and nasal dysfunction43 as well as improved asthma symptoms and decreased exacerbations.44 Leukotriene modifiers may also have selective benefit in smoking asthmatics based on a study showing montelukast, but not inhaled corticosteroids, improved peak expiratory flow in mild asthmatics who smoked.45
BRONCHIAL THERMOPLASTY (BT)
BT is a new FDA-approved treatment of patients with severe asthma that aims to reduce the airway smooth muscle mass in attempt to diminish bronchial constriction and reduce asthma symptoms. BT is performed by delivering controlled thermal energy to the airway walls during a series of 3 bronchoscopies. Airway smooth muscle is believed to be the main effector of bronchoconstriction in response to various stimuli and can also promote inflammation by producing cytokines (IL-4, leukotriene B4) and interacting with associated mast cells.46 Initial experiments in canines showed reduced airway hyperresponsiveness and persistent airway smooth muscle reduction in BT-treated airways for up to 3 years after the procedure.47 The results of small clinical studies led to the Asthma Intervention Research 2 trial that utilized a multicenter, randomized, double-blind, sham-controlled format to investigate the impact of BT on lung function, AQLQ scores, exacerbations, and health care utilization in nearly 300 patients with severe asthma.48 Although there was a statistically significant improvement in the primary outcome of change in AQLQ, it failed to reach the clinically meaningful change of 0.5 or greater. However, there was a significant reduction in several meaningful secondary outcomes including severe exacerbations, emergency department visits, and days missed from work or school. Short-term adverse events were higher in the BT group and included hospitalization, primarily for worsening of asthma. Although the results of Asthma Intervention Research 2 trial are encouraging, the application of BT in severe asthma is currently limited by a lack of understanding of the mechanism of action, long-term safety and efficacy data, and cost.46 In addition, unlike the targeted biologic therapies, there are currently no disease characteristics to help predict which subtype of severe asthma patients are more likely to benefit from BT. Ongoing studies are addressing these concerns and may help to solidify a role for BT in the treatment of severe asthma.
FUTURE DIRECTIONS
Antineutrophil Strategies
There are currently only modest data to support a specific neutrophilic asthma phenotype.10 In a post hoc analysis, sputum neutrophilia was greatest in a SARP cluster of individuals with adult onset and severely obstructed asthma and the highest systemic corticosteroid use.14 It is unclear if the increased numbers of neutrophils in this population are reflective of a unique inflammatory pattern, a marker of unrecognized infection, or the consequence of high-dose corticosteroids.49 Therapy targeting the neutrophilic asthma phenotype has been limited to antimicrobial therapy following isolation of a pathogen (typically mycoplasma or chlamydophila pneumonia), which has shown to improve lung function and asthma symptoms.27 Recently, strategies to inhibit neutrophilic inflammation have begun to emerge. IL-8 is a potent neutrophil chemoattractant that binds the chemokine receptor CXCR2 to exert its effect. A novel small molecule drug, SCH 527123, binds CXCR2 receptors and was shown to reduce sputum neutrophilia in a small study of severe asthma patients with >40% sputum neutrophils at study onset.49 There was a trend toward decreased exacerbations and improved symptom scores that requires further investigation.49 T-helper type 17 (TH17) cells and the production of IL-17 has been linked with neutrophilia and has generated interest as potential therapeutic targets in severe asthma. The phosphodiesterase 4 inhibitor roflumilast is effective against neutrophilic inflammation and has also generated interest as a potential therapy in severe asthma.50 Finally, LTB4 is recognized to be a chemoattractant of neutrophils along with mast cells and T cells.50 Inhibiting LTB4 through lipoxygenase inhibitors or LTB4 receptor antagonists may prove to be beneficial in severe asthmatics. Additional studies are warranted to improve our current understanding of a neutrophil-predominant severe asthma phenotype and to facilitate clinical studies with molecular-targeted therapy.
Addressing Corticosteroid Resistance
Some patients with severe asthma have a poor response to glucocorticoid therapy but few patients are believed to be completely resistant.50 Recent work has led to an improved understanding of the cellular and molecular functions involved in the anti-inflammatory effects of glucocorticoids in the treatment of asthma and has elicited possible mechanisms to account for glucocorticoid resistance.51 Activated glucocorticoid receptor within a cell typically recruits histone deacetylase-2 (HDAC2) to the activated inflammatory gene complex, resulting in effective suppression of inflammatory genes within the nucleus. A reduction in HDAC2 protein expression and activity has been found in smoking asthmatics and patients with severe asthma, possibly secondary to oxidative stress.51 Novel therapeutic strategies aimed at increasing HDAC2 expression and activity may prove beneficial in the treatment of the subtype of glucocorticoid-resistant severe asthma patients.
Inhibiting TH2 Cytokines
In addition to the monoclonal antibodies targeting IL-4, IL-5, and IL-13 that were discussed previously, alternate agents targeting specific components of the TH2 immune process are being developed and tested in asthma patients. A mutated form of IL-4 that blocks IL-4 receptor α, the common receptor for IL-4 and IL-13, along with an antibody against the IL-5 receptor α and a blocking IL-9 antibody have shown encouraging preliminary results and are currently being studied in larger clinical trials.50
CONCLUSIONS
Severe asthma is a heterogenous disease with a range of clinical characteristics and various pathophysiological mechanisms. Patients with severe asthma require high-intensity controller therapy and often continue to have uncontrolled disease with a high symptom burden and significant health care utilization. The recognition of severe asthma phenotypes has allowed for improved characterization of patients and increased focus on patient-directed therapies. Omalizumab, a monoclonal antibody directed against IgE, is an example of phenotype-specific therapy and has shown clinical benefit in the subgroup of patients with severe allergic asthma and elevated IgE levels. Monoclonal antibodies against IL-5 and IL-13 have also shown encouraging results in the appropriate subgroup of severe asthma patients. Additional molecular agents targeting both TH2 and TH1 processes are in various states of development and may play a significant role in the future care of severe asthma. A recent single-center study suggests that bronchoscopy can assist in phenotyping and enhance the patient-directed therapy of severe asthma patients. Early results with BT are also encouraging, however, long-term efficacy data and characterization of the subgroup of patients most likely to benefit are still needed. Moving forward, the evolution of severe asthma phenotypes with incorporation of biomarkers, genetic polymorphisms, and specific therapy response will lead to improved characterization of the disease that should translate into improved care of severe asthma patients.
ACKNOWLEDGMENT
David Kamp acknowledges support from a VA Merit Award and NIEHS-R01ESO20357.
Footnotes
Disclosure: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Akinbami LJ, Moorman JE. Asthma prevalence, health care use, and mortality: United States, 2005–2009. National Health Statistics Report. 2011 [PubMed] [Google Scholar]
- 2.Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126:926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The gaining optimal asthma controL study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 4.Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunology. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Blanc PD, Hayden ML, et al. Assessing productivity loss and activity impairment in severe or difficult-to-treat asthma. Value Health. 2011;11:231–239. doi: 10.1111/j.1524-4733.2007.00229.x. [DOI] [PubMed] [Google Scholar]
- 6.Global Initiative for Asthma. Asthma management and Prevention. 1995 NIH Publication number 95-3659A. [Google Scholar]
- 7.Wenzel SE, Fahy JV, Irvin C, et al. Proceedings of the ATS Workshop on Refractory Asthma: current understanding, recommendations, and unanswered questions. Am J Respir Crit Care Med. 2000;162:2341–2351. doi: 10.1164/ajrccm.162.6.ats9-00. [DOI] [PubMed] [Google Scholar]
- 8.Taylor DR, Bateman L-P, Boushey HA, et al. A new perspective on concepts of asthma severity and control. Eur Respir J. 2008;32:545–554. doi: 10.1183/09031936.00155307. [DOI] [PubMed] [Google Scholar]
- 9.National Heart, Lung, and Blood Institute; National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007 [Google Scholar]
- 10.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel SE, Szefler SJ, Leung DY, et al. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156:737–743. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 12.Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 13.Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore WC, Meyers DA, Wenzel SE, et al. Identification of asthma phenotypes using cluster analysis in the severe asthma research program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhakta NR, Woodruff PG. Human asthma phenotypes: from the clinic, to cytokines, and back again. Immunol Rev. 2011;242:220–232. doi: 10.1111/j.1600-065X.2011.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11:577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomized controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 18.Chlumsky J, Stritz I, Terl M, et al. Strategy aimed at reduction of sputum eosinophils decreases exacerbation rate in patients with asthma. J Int Med Res. 2006;34:129–139. doi: 10.1177/147323000603400202. [DOI] [PubMed] [Google Scholar]
- 19.Jayaram L, Pizzichini MM, Cook RJ, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27:483–494. doi: 10.1183/09031936.06.00137704. [DOI] [PubMed] [Google Scholar]
- 20.Berry MA, Shaw DE, Green RH, et al. The use of exhaled nitric oxide concentration to identify eosinophilic airway inflammation: an observational study in adults with asthma. Clin Exp Allergy. 2005;35:1175–1179. doi: 10.1111/j.1365-2222.2005.02314.x. [DOI] [PubMed] [Google Scholar]
- 21.Silkoff PE, Lent AM, Busacker AA, et al. Exhaled nitric oxide identifies the persistent eosinophilic phenotype in severe refractory asthma. J Allergy Clin Immunol. 2005;116:1249–1255. doi: 10.1016/j.jaci.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 22.Dweik RA, Sorkness RL, Wenzel S, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181:1033–1041. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dweik RA, Boggs PB, Erzurum SC, et al. An Official ATS Clinical Practice Guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith AD, Cowen JO, Brassett KP, et al. Use of exhaled nitric oxide measurements to guide treatment in chronic asthma. N Engl J Med. 2005;352:2163–2173. doi: 10.1056/NEJMoa043596. [DOI] [PubMed] [Google Scholar]
- 25.Shaw DE, Berry MA, Thomas M, et al. The use of exhaled nitric oxide to guide asthma management: a randomized controlled trial. Am J Respir Crit Care Med. 2007;176:231–237. doi: 10.1164/rccm.200610-1427OC. [DOI] [PubMed] [Google Scholar]
- 26.Moore WC, Evans MD, Bleecker ER, et al. Safety of investigative bronchoscopy in the Severe Asthma Research Program. J Allergy Clin Immunol. 2011;128:328–336. doi: 10.1016/j.jaci.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Good JT, Kolakowski CA, Groshong SD, et al. Refractory asthma: importance of bronchoscopy to identify phenotypes and direct therapy. Chest. 2012;141:599–606. doi: 10.1378/chest.11-0741. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139:28–35. doi: 10.1378/chest.10-1194. [DOI] [PubMed] [Google Scholar]
- 29.Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154:573–582. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 30.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyperresponsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 31.Flood-Page P, Swenson C, Faiferman I, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176:1062–1071. doi: 10.1164/rccm.200701-085OC. [DOI] [PubMed] [Google Scholar]
- 32.Nair P, Pizzichini MM, Kjarsgaard M, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 33.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–659. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 35.Castro M, Mathur S, Hargreave F, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–1132. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto S, Bel EH. Targeting IL-5 in severe asthma: a DREAM come true? Lancet. 2012;38:626–627. doi: 10.1016/S0140-6736(12)61132-5. [DOI] [PubMed] [Google Scholar]
- 37.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 38.Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma down-stream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 39.Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 40.Piper E, Brightling C, Niven R, et al. A phase 2 placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur Respir J. 2012;41:330–338. doi: 10.1183/09031936.00223411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters-Golden M, Henderseon WR. Leukotrienes. N Engl J Med. 2007;357:1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 42.Antczak A, Montuschi P, Kharitonov S, et al. Increased exhaled cysteinyl leukotrienes and 8-isoprostane in aspirin-induced asthma. Am J Respir Crit Care Med. 2002;166:301–306. doi: 10.1164/rccm.2101021. [DOI] [PubMed] [Google Scholar]
- 43.Dahlen B, Nizankowska E, Szczeklik A, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med. 1998;157:1187–1194. doi: 10.1164/ajrccm.157.4.9707089. [DOI] [PubMed] [Google Scholar]
- 44.Dahlen SE, Malmstrom K, Nizankowska E, et al. Improvement of aspirin-intolerant asthma by montelukast, a leukotriene-antagonist: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2002;165:9–14. doi: 10.1164/ajrccm.165.1.2010080. [DOI] [PubMed] [Google Scholar]
- 45.Lazarus SC, Chinchilli VM, Rollings NJ, et al. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007;175:783–790. doi: 10.1164/rccm.200511-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wahidi MM, Kraft M. Bronchial thermoplasty for severe asthma. Am J Respir Crit Care Med. 2012;185:709–714. doi: 10.1164/rccm.201105-0883CI. [DOI] [PubMed] [Google Scholar]
- 47.Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Apply Physiol. 2004;97:1946–1953. doi: 10.1152/japplphysiol.01282.2003. [DOI] [PubMed] [Google Scholar]
- 48.Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma. Am J Respir Crit Care Med. 2010;181:116–124. doi: 10.1164/rccm.200903-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nair P, Gaga M, Zervas E, et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy. 2012;42:1097–1103. doi: 10.1111/j.1365-2222.2012.04014.x. [DOI] [PubMed] [Google Scholar]
- 50.Barnes PJ. Severe asthma: advances in current management and future therapy. J Allergy Clin Immunol. 2012;129:48–59. doi: 10.1016/j.jaci.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Barnes PJ. Biochemical basis of asthma therapy. J Biol Chem. 2011;286:32899–32905. doi: 10.1074/jbc.R110.206466. [DOI] [PMC free article] [PubMed] [Google Scholar]