Abstract
Tsetse flies (Diptera:Glossinidae) are vectors of African trypanosomes. Tsetse undergo viviparous reproductive biology, and depend on their obligate endosymbiont (genus Wigglesworthia) for the maintenance of fecundity and immune system development. Trypanosomes establish infections in the midgut and salivary glands of the fly. Tsetse’s resistance to trypanosome infection increases as a function of age. Among the factors that mediate resistance to parasites are antimicrobial peptides (AMPs) produced by the Immune deficiency (Imd) signaling pathway, peptidoglycan recognition protein (PGRP) LB, tsetse-EP protein and the integrity of the midgut peritrophic matrix (PM) barrier. The presence of obligate Wigglesworthia during larval development is essential for adult immune system maturation and PM development. Thus, Wigglesworthia prominently influences the vector competency of it’s tsetse host.
1. Tsetse Disease Vectors
1.1 Tsetse (Diptera: Glossinidae)
The genus Glossina contains 22 species within 3 subgenera; the fusca, palpalis, and morsitans species groups [1]. While all tsetse species are disease vectors, their ability to transmit pathogenic African trypanosomes varies, with the palpalis group including the most prolific human disease vector species. The different species complexes occupy varying ecological niches. Morsitans group taxa are adapted to relatively dry savannah habitats. Conversely, palpalis group flies tend to inhabit riverine and lacustrine habitats while the majority of fusca group flies can be found mainly in moist West African forests [2]. The host-specificity of the different species groups also vary, with the palpalis group flies displaying strong anthrophilic tendencies, while the others are more zoophilic in preference.
1.2 Trypanosomiasis
Two trypanosome species, Trypanosoma brucei rhodesiense and T. b. gambiense, are the causative agents of Human African trypanosomiasis (HAT). Trypanosoma b. rhodesiense occurs east of the Rift valley and causes acute human disease that is rapidly fatal if not properly treated. Trypanosoma b. gambiense occurs in west/central Africa, and infection with this parasite causes a chronic disease that results in eventual death if left untreated [3]. In addition to HAT, a non-human animal form of the disease called Animal African Trypanosomiasis (AAT; commonly referred to as nagana) is caused by T. b. brucei and the related trypanosomatids, T. congolense and T. vivax. AAT severely reduces the availability of meat and milk products in large regions of Africa by excluding cattle rearing from ten million square kilometers of grazable land [4]. This widely impacts land use practices by reducing the availability of animal labor for plowing and placing constraints on the use of mixed agriculture [5]. The Programme on African Animal Trypanosomiasis (PAAT) estimates that AAT causes approximately 3 million cattle deaths per year and requires farmers to administer approximately 35 million doses of costly trypanocidal drugs [6]. Economic losses in cattle production are estimated at US$ 1–1.2 billion and total agricultural losses caused by AAT are estimated at US$ 4.75 billion per year [7].
Trypanosomes were determined to cause HAT over a century ago, and since this time, several epidemics have plagued the African continent [8]. After the devastating epidemics that occurred between 1920–1940 subsided, HAT control programs in endemic countries were gradually eliminated during the post-independent 1960s. Regretfully, a steep rise in disease incidence occurred during the following 40 years. Estimating the true burden of HAT is difficult, as the disease afflicts the poorest and most neglected populations that live in remote and rural settings located beyond the reach of health care systems [9]. In 2008, mortality associated with HAT ranked ninth out of 25 among human infectious and parasitic diseases in Africa [10]. After intense international interventions, HAT cases in Africa have recently dropped below 10,000 for the first time in 50 years, signaling a possible end to the latest epidemic cycle as a major public health crisis [11].
1.3 Trypanosomiasis Control
HAT and AAT are both fatal if left untreated. Chemotherapy is expensive [12], and current treatments for late stage disease are complicated [13,14]. Mammalian vaccines are not available due to the antigenic variation capacity of trypanosomes. Active surveillance and treatment of patients are essential for effective disease control, but such programs can be too expensive to operate at times of low endemicity. Traps and targets can reduce local tsetse populations and thus disease transmission. However, they are not widely explored for HAT control due to problems in implementation and lack of effective attractants to improve their efficacy, particularly for human disease-transmitting tsetse species [15]. New genetic approaches that aim to reduce tsetse’s vector competence by blocking parasite transmission through the tsetse fly vector are of interest [16].
2. Unique Aspects of Tsetse Biology
Multiple aspects of tsetse’s physiology differentiate them from other insects. These distinctions include a diet consisting exclusively of vertebrate blood, the utilization of proline rather than sugars as an energy source, the nourishment and birthing of live offspring (viviparous reproduction) and their essential relationship with an obligate symbiont (Wigglesworthia) to maintain fecundity and for development of the immune system.
2.1 Viviparous Reproduction
Tsetse’s mode of reproduction is one of the fly’s most dramatic biological adaptations [17]. Rather than laying eggs, tsetse develop a single offspring per reproductive cycle, which is carried and nourished by the mother throughout embryonic and larval development. Female flies are limited to a maximum of 8–10 progeny due to the time and nutrient intensive nature of this process. Females birth fully developed larvae that burrow into the ground, pupate, spend ~30 days undergoing metamorphosis and eclose as adults. Nutritional requirements for larval and pupal development are supplied by the mother [18]. Each gonotrophic cycle a single egg is fertilized and undergoes embryonic development within the mother’s uterus. Embryos hatch into larva that undergo three developmental instars also within the uterus [19]. Nutrients are provided to the developing larva via a specialized accessory gland termed the milk gland that empties its secretions into the uterus (Figure 1). The milk constituents fulfill a variety of functions, including provision of amino acids, lipid emulsification, iron transport, assisting larval digestion and immunity [20–23]. Tsetse’s obligate symbiont Wigglesworthia is also transmitted to the feeding offspring via maternal milk [24,25] and colonizes the larval milk gland and gut bacteriome organs [26]. Interestingly, during each lactation cycle, transcription of the major milk proteins is tightly regulated by a transcription factor, LadyBird Late [27,28]. Each reproductive cycle generates significant oxidative stress for the mother, yet tsetse females can produce offspring for almost their entire lifespan. This is accomplished in part by mitigating oxidative stress with antioxidant enzymes during and after pregnancy to prevent oxidative damage [29].
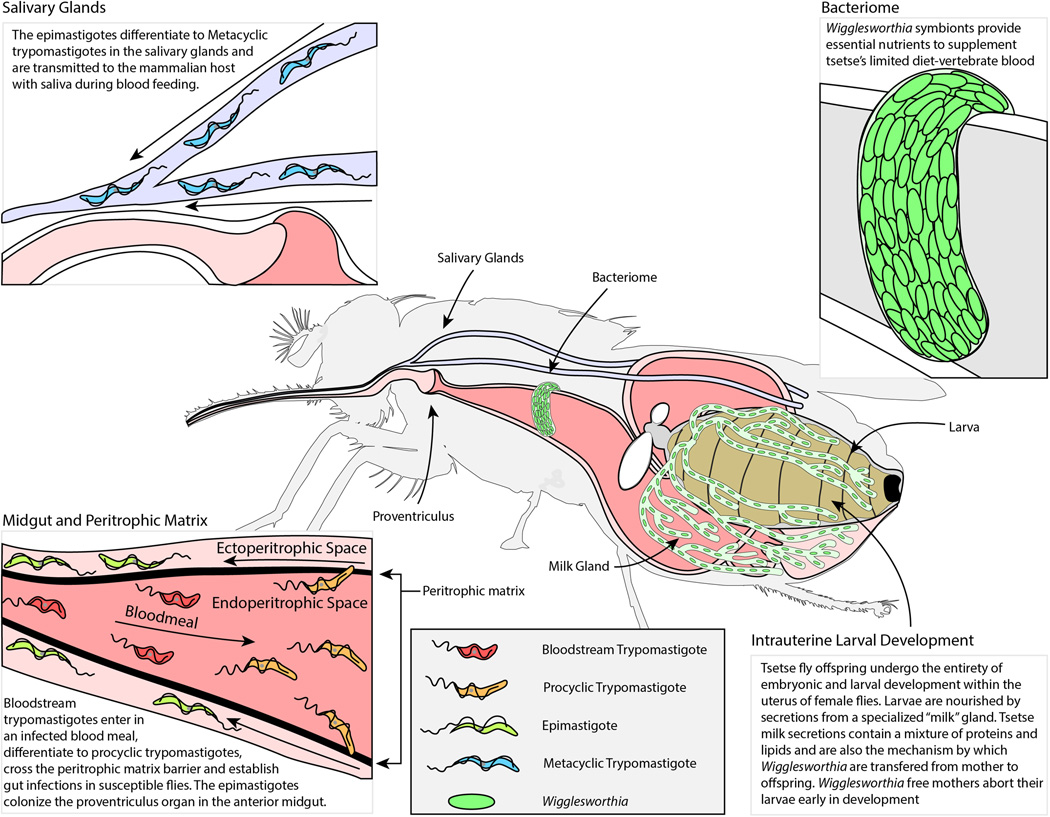
Figure 1.
Diagrammatic Representation of the Interactions between Tsetse, Trypanosomes and the Obligate Symbiont Wigglesworthia. This figure represents the life cycle and metamorphosis of trypanosomes within the tsetse beginning with their introduction via an infective blood meal, followed by their escape from the peritrophic matrix into the endoperitrophic space and subsequent invasion of the salivary glands for transfer to a vertebrate host. The diagram also illustrates the localization of the obligate endosymbiont Wigglesworthia in tsetse’s bacteriome in the anterior midgut as well as its transmission into tsetse’s intrauterine larva in milk secretions. The viviparous reproductive physiology is depicted with an intrauterine larva and the large network of milk gland tubules that provide nutrients.
2.2 Tsetse Symbiosis for Nutrient Supplementation
The diet of both the male and female tsetse is restricted to a single food resource: vertebrate blood. To supplement their diet, tsetse harbor the enteric endosymbiont, Wigglesworthia, which is a member of γ-proteobacteria. In tsetse’s gut, Wigglesworthia lives intracellularly in bacteriocytes that collectively form the bacteriome organ in the fly’s anterior midgut (Figure 1; [30]). The tsetse-Wigglesworthia symbiosis is ancient, as reflected in the concordant evolutionary history shared between organisms [31]. Although Wigglesworthia’s genome is dramatically reduced in size (about 700 kb; [32,33]), the bacterium has retained the ability to synthesize a plethora of B vitamins. Studies that utilize different antibiotic supplementation regimens to remove specific symbionts result in different fecundity outcomes. Fertile adults that received ampicillin-supplemented diets, which clears extracellular bacteria only, were not impaired in fecundity but gave birth to progeny that were free of Wigglesworthia [34]. In contrast, adults that received tetracycline-supplemented blood meals, which clears both intracellular and extracellular bacteria, became reproductively sterile likely due to the elimination of bacteriome-localized Wigglesworthia [34,35]. This sterility was partially recovered by supplementing the blood diet of these flies with micronutrients, particularly B vitamins [36] and yeast extract [37]. Wigglesworthia produced vitamin metabolites play a crucial role in proline homeostasis, and this amino acid is tsetse’s single energy source [38]. The Wigglesworthia produced co-factor pyridoxal phosphate (the active form of vitamin B6) is an essential co-factor for alanine-glyoxylate aminotransferase (AGAT), which catalyzes the conversion of alanine to proline in tsetse’s fat body. In the absence of AGAT (or Wigglesworthia), females are unable to maintain proline homeostasis and their fecundity decreases [38]. Interestingly, trypanosomes also utilize proline as their sole source of energy during their development in the tsetse host. Thus, Wigglesworthia produced vitamins are also indirectly essential for trypanosome fitness in tsetse [38] and may also play a yet unknown role in fly immunity.
2.3 Tsetse Symbiosis and Immune System Maturation
Wigglesworthia also exhibits an essential role in host immune functionality. Tsetse that undergo intrauterine larval development in the absence of obligate Wigglesworthia (GmmWgm−) present a severely compromised cellular immune system during adulthood. Specifically, GmmWgm− flies present a highly depleted population of hemocytes and loss of phagocytosis and melanization functions [39]. The immuno-compromised phenotype exhibited by GmmWgm− can be partially reversed when their moms are fed a diet supplemented with Wigglesworthia cell extracts [40]. As such, Wigglesworthia-derived molecule(s) may play a role to induce the maturation of cellular immune system during larvagenesis. Alternatively, the presence of Wigglesworthia (or its products) in pregnant females may induce a signaling cascade that stimulates immune system development in intrauterine larvae.
3. Tsetse-Trypanosome Biology
3.1 Trypanosome Transmission Dynamics in Tsetse
To survive in tsetse’s midgut, mammalian bloodstream form (BSF) adapted to survival in the midgut radically transform their metabolism [41] so that within several hours viable procyclic form trypanosomes (PF) that express a new surface coat (procyclin) become visible in the midgut and divide exponentially [42]. At around three days post infection, in a high proportion of the flies, the parasites are eliminated likely through the actions of host immunity proteins including antimicrobial peptides (AMPs) produced by the Immune deficiency (Imd) signaling pathway [43–45], Peptidoglycan Recognition Protein (PGRP)-LB [46] and tsetse-EP protein [47]. In susceptible flies, PF parasites continue to replicate, cross the chitinous gut peritrophic matrix (PM) that separates the gut lumen (the “endoperitropic space”) and its contents from immuno-reactive epithelial cells, and establish infections in the ectoperitrophic space of the midgut (Figure 1). Subsequently, PF parasites differentiate into epimastigote forms (EP) and accumulate around the proventriculus organ (Figure 1). In a proportion of flies, the EP parasites (T. brucei complex) depart the proventriculus, enter the foregut and invade the salivary glands (SG) of the fly through the mouthparts. In the salivary gland lumen, the EP parasites mature into mammalian infective metacyclic forms for transmission to the next mammalian host in fly saliva. A recent study that compared normal and parasitized SG transcriptomes revealed that transcripts for the most abundant putative secreted SG proteins with anti-hemostatic functions present in saliva were significantly reduced upon infection [48]. In contrast, expression of putative host proteins associated with immunity, stress, cell division and tissue remodeling were enriched in infected SG suggesting that parasite infections induce host immune and stress response(s) that likely results in tissue renewal [48]. The same study also identified novel parasite surface proteins that are expressed uniquely in the metacyclic stage of the parasite. Future characterization of the metacyclic proteins can reveal new candidate molecules that can be targeted for parasite control.
The host gut immune environment [43,45] as well as its nutritional status at the time of parasite acquisition [49] play important roles in determining the efficiency of parasite infection establishment. Tsetse’s symbiotic microfauna also contribute to the fly’s parasite resistance phenotype [50].
3.2 Wigglesworthia Mediates Trypanosome Infection Outcomes in Tsetse
Replication of Wigglesworthia cells, and/or Wigglesworthia cell death, results in the release of peptidoglycan (PGN) into tsetse’s bacteriome environment. PGN is a potent inducer of the fly’s immune deficiency (IMD) signaling pathway, the end products of which are bacteria-damaging antimicrobial peptides (AMPs) [51]. To evade destructive tsetse immune responses, Wigglesworthia induces expression of the secreted host protein, PGRP-LB in the fly’s bacteriome organ [51]. Through its amidase activity, PGRP-LB degrades Wigglesworthia released PGN to prevent the expression of host immune effectors and thereby protects the tsetse-Wigglesworthia symbiosis [51]. Interestingly, PGRP-LB also exhibits anti-trypanosomal activity and can act as the first line of defense against trypanosome infection establishment [46]. Female tsetse that harbor trypanosome infections in their gut display heightened immunity, which in turn decreases host fecundity [52]. This phenotype is characterized by lengthened gonotrophic cycles and thus decreased cumulative reproductive output. Thus, in addition to stimulating host immune system development and providing vitamins absent from vertebrate blood, Wigglesworthia also protects its host from fecundity-reducing parasite infections.
3.3 Role of PM for Trypanosome Establishment
Tsetse’s gut is lined by a chitinous, sleeve-like PM structure that acts as a biophysical barrier that regulates pathogen infection outcomes, prevents pore forming microbial toxins from damaging midgut epithelial cells and alters the temporal kinetics of host immune responses [53–55].
Adult tsetse (≥ 8 days post-eclosion) that have fed multiple times are highly refractory to infection with trypanosomes. Conversely, newly eclosed adults (referred to as ‘teneral’) are highly susceptible to infection when their first blood meal contains infectious parasites [56]. Similarly, adults that are starved are also highly susceptible to infection [49]. The structural integrity of tsetse’s PM increases as a function of adult age post-pupal eclosion, and the higher parasite susceptibility presented by young adults has been largely attributed to the absence of a robust PM at this stage of host development [57,58]. In comparison to their wild-type counterparts, adult Wigglesworthia-free (GmmWgm−) flies (progeny of females receiving ampicillin-supplemented blood diet) are highly susceptible to trypanosome infection [34,51]. This phenotype may result from the fact that GmmWgm− individuals house a structurally compromised PM [59]. The absence of a robust PM significantly alters the dynamics of trypanosome infection establishment. Specifically, in the wild-type environment, tsetse’s immunoreactive midgut epithelium does not detect parasites until after they have completed differentiation to PF forms (~ 12 hours post-ingestion) and circumvented the PM midgut barrier (~ 2–3 days post-ingestion). However, in GmmWgm− gut, mammalian blood stream form (BSF) trypanosomes are detected by midgut cells immediately following ingestion. Early detection of BSF parasites by tsetse’s midgut epithelium results in a dysfunctional immune response that is most conspicuously characterized by the absence of induced attacin expression [60]. This gene, which is normally up-regulated following trypanosome challenge [59], encodes a potent trypanocidal AMP [43,44,60]. Experimental elimination of tsetse’s PM through a gene silencing strategy renders normally resistant adults susceptible to parasitism [60]. These findings imply that tsetse’s PM is not a physical impediment to infection establishment, but instead serves as a barrier that regulates the fly’s ability to immunologically detect and respond to the presence of these microbes. Additionally, tsetse’s immune system may differentially recognize distinct parasite forms and react less antagonistic against PF trypanosomes that have established successful midgut infections.
4. Conclusion
Tsetse vector African trypanosomes, which are the causative agents of deadly HAT and AAT. Tsetse give birth to live young and depend on obligate endosymbionts for the maintenance of fecundity and immune system development. Particularly the indirect contributions of obligate symbionts for gut PM development mediates parasite establishment. Recent technological advances in high-throughout sequencing methodologies and functional genomics have allowed us to obtain the whole genome sequence of the tsetse host [61] and better understand the molecular mechanisms that underlie tsetse’s unusual physiological characteristics. This information can now be exploited to develop novel control strategies aimed at reducing tsetse’s reproductive output and/or the fly’s competence as a vector of pathogenic trypanosomes. Towards this end a paratransgenic gene expression system has been developed using tsetse’s commensal symbiont Sodalis [16]. Expression of trypanocidal products in the gut in the symbiotic bacteria can modify the gut environment to reduce parasite transmission.
Highlights.
We describe tripartite interactions between tsetse, trypanosome and symbionts
Tsetse undergo intrauterine larvagenesis and lactate (viviparous reproduction)
Fecundity relies on obligate symbiont for diet supplementation and proline synthesis
Symbiont presence during larval growth influences adult immune and gut development
Gut peritrophic matrix barrier integrity affects trypanosome transmission dynamics
Acknowledgements
We are grateful for the funds provided by NIH GM069449, AI051584 and AI081774 and the Ambrose Monell Foundation and Li Foundation awards to SA, AI062680 and AI101456 to BLW and GM077964 to GMA. We are also grateful to our laboratory colleagues whose studies we refer to in this review including Drs. Jingwen Wang, Changyun Hu, Zhengrong Hao, Joshua Benoit, Veronika Michalkova, Huang Hu, Amy Savage, Michelle Maltz, Roshan Pais, Uzma Alam, Cory Brelsfoard, Xin Zhao and Suleyman Yildirim as well as for technical help to Ms. Claudia Lohs, Michelle O’Neill and Yineng Wu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krafsur ES. Tsetse flies: genetics, evolution, and role as vectors. Infect Genet Evol. 2009;9:124–141. doi: 10.1016/j.meegid.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers D, Robinson T. Tsetse distribution. In: Maudlin I, Holmes P, Miles M, editors. The Trypanosomiases. CAB International; 2004. pp. 139–179. [Google Scholar]
- 3.Welburn SC, Fevre EM, Coleman PG, Odiit M, Maudlin I. Sleeping sickness: a tale of two diseases. Trends Parasitol. 2001;17:19–24. doi: 10.1016/s1471-4922(00)01839-0. [DOI] [PubMed] [Google Scholar]
- 4.Steelman CD. Effects of external and internal arthropod parasites on domestic livestock production. Annu Rev Entomol. 1976;21:155–178. doi: 10.1146/annurev.en.21.010176.001103. [DOI] [PubMed] [Google Scholar]
- 5.Jordan AM. Trypanosomiasis control and African rural development. Longman, London. 1986 [Google Scholar]
- 6.Kabayo JP. Aiming to eliminate tsetse from Africa. Trends Parasitol. 2002;18:473–475. doi: 10.1016/s1471-4922(02)02371-1. [DOI] [PubMed] [Google Scholar]
- 7.Budd LT. In: Tsetse and Trypanosomosis Research and Development since 1980: an Economic Analysis. Development DfI, editor. Vol. 2. UK: 1999. [Google Scholar]
- 8.Headrick DR. Sleeping sickness epidemics and colonial responses in East and Central Africa, 1900–1940. PLoS Negl Trop Dis. 2014;8:e2772. doi: 10.1371/journal.pntd.0002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matemba LE, Fevre EM, Kibona SN, Picozzi K, Cleaveland S, Shaw AP, Welburn SC. Quantifying the burden of rhodesiense sleeping sickness in Urambo District, Tanzania. PLoS Negl Trop Dis. 2010;4:e868. doi: 10.1371/journal.pntd.0000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fevre EM, Odiit M, Coleman PG, Woolhouse ME, Welburn SC. Estimating the burden of rhodesiense sleeping sickness during an outbreak in Serere, eastern Uganda. BMC Public Health. 2008;8:96. doi: 10.1186/1471-2458-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simarro PP, Diarra A, Ruiz Postigo JA, Franco JR, Jannin JG. The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000–2009: the way forward. PLoS Negl Trop Dis. 2011;5:e1007. doi: 10.1371/journal.pntd.0001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simarro PP, Franco J, Diarra A, Postigo JA, Jannin J. Update on field use of the available drugs for the chemotherapy of human African trypanosomiasis. Parasitology. 2012;139:842–846. doi: 10.1017/S0031182012000169. [DOI] [PubMed] [Google Scholar]
- 13.Jannin J, Cattand P. Treatment and control of human African trypanosomiasis. Curr Opin Infect Dis. 2004;17:565–571. doi: 10.1097/00001432-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Nok AJ. Arsenicals (melarsoprol), pentamidine and suramin in the treatment of human African trypanosomiasis. Parasitol Res. 2003;90:71–79. doi: 10.1007/s00436-002-0799-9. [DOI] [PubMed] [Google Scholar]
- 15.Joja LL, Okoli UA. Trapping the vector: community action to curb sleeping sickness in southern Sudan. Am J Public Health. 2001;91:1583–1585. doi: 10.2105/ajph.91.10.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aksoy S, Weiss B, Attardo G. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. Advances in experimental medicine and biology. 2008;627:35–48. doi: 10.1007/978-0-387-78225-6_3. [DOI] [PubMed] [Google Scholar]
- 17.Tobe SS. Reproductive physiology of Glossina. Annu Rev Entomol. 1978;23:283–307. doi: 10.1146/annurev.en.23.010178.001435. [DOI] [PubMed] [Google Scholar]
- 18.Denlinger DL, Ma WC. Dynamics of the pregnancy cycle in the tsetse Glossina morsitans. Journal of Insect Physiology. 1974;20:1015–1026. doi: 10.1016/0022-1910(74)90143-7. [DOI] [PubMed] [Google Scholar]
- 19.Attardo GM, Lohs C, Heddi A, Alam UH, Yildirim S, Aksoy S. Analysis of milk gland structure and function in Glossina morsitans: Milk protein production, symbiont populations and fecundity. Journal of Insect Physiology. 2008;54:1236–1242. doi: 10.1016/j.jinsphys.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guz N, Attardo GM, Wu Y, Aksoy S. Molecular aspects of transferrin expression in the tsetse fly (Glossina morsitans morsitans) J Insect Physiol. 2007;53:715–723. doi: 10.1016/j.jinsphys.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benoit JB, Attardo GM, Michalkova V, Takac P, Bohova J, Aksoy S. Sphingomyelinase activity in mother's milk is essential for juvenile development: a case from lactating tsetse flies. Biol Reprod. 2012;87(17):11–10. doi: 10.1095/biolreprod.112.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumann AA, Benoit JB, Michalkova V, Mireji PO, Attardo GM, Moulton JK, Wilson TG, Aksoy S. Juvenile hormone and insulin suppress lipolysis between periods of lactation during tsetse fly pregnancy. Mol Cell Endocrinol. 2013;372:30–41. doi: 10.1016/j.mce.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attardo GM, Benoit JB, Michalkova V, Yang G, Roller L, Bohova J, Takac P, Aksoy S. Analysis of lipolysis underlying lactation in the tsetse fly, Glossina morsitans. Insect Biochem Mol Biol. 2012;42:360–370. doi: 10.1016/j.ibmb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attardo GM, Lohs C, Heddi A, Alam UH, Yildirim S, Aksoy S. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J Insect Physiol. 2008;54:1236–1242. doi: 10.1016/j.jinsphys.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denlinger DL, Ma WC. Maternal nutritive secretions as possible channels for vertical transmission of microorganisms in insects: the tsetse fly example. Ann N Y Acad Sci. 1975;266:162–165. doi: 10.1111/j.1749-6632.1975.tb35097.x. [DOI] [PubMed] [Google Scholar]
- 26.Balmand S, Lohs C, Aksoy S, Heddi A. Tissue distribution and transmission routes for the tsetse fly endosymbionts. J Inverteb Path. 2013;112(Suppl):S116–S122. doi: 10.1016/j.jip.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benoit JB, Attardo GM, Michalkova V, Krause TB, Bohova J, Zhang Q, Baumann AA, Mireji PO, Takac P, Denlinger DL, et al. A Novel Highly Divergent Protein Family Identified from a Viviparous Insect by RNA-seq Analysis: A Potential Target for Tsetse Fly-Specific Abortifacients. PLoS genetics. 2014;10:e1003874. doi: 10.1371/journal.pgen.1003874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Attardo GM, Benoit JB, Michalkova V, Patrick KR, Krause TB, Aksoy S. The homeodomain protein ladybird late regulates synthesis of milk proteins during pregnancy in the tsetse fly (Glossina morsitans) PLoS Negl Trop Dis. 2014;8:e2645. doi: 10.1371/journal.pntd.0002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michalkova V, Benoit JB, Attardo GM, Medlock J, Aksoy S. Amelioration of reproduction-associated oxidative stress in a viviparous insect is critical to prevent reproductive senescence. PLoS One. 2014;9:e87554. doi: 10.1371/journal.pone.0087554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aksoy S. Wigglesworthia gen. nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbionts of tsetse flies. Int J Syst Bacteriol. 1995;45:848–851. doi: 10.1099/00207713-45-4-848. [DOI] [PubMed] [Google Scholar]
- 31.Chen X, Li S, Aksoy S. Concordant evolution of a symbiont with its host insect species: molecular phylogeny of genus Glossina and its bacteriome-associated endosymbiont, Wigglesworthia glossinidia. J Mol Evol. 1999;48:49–58. doi: 10.1007/pl00006444. [DOI] [PubMed] [Google Scholar]
- 32.Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. Genome sequence of the endocellular obligate symbiont of tsetse flies Wigglesworthia glossinidia. Nature genetics. 2002;32:402–407. doi: 10.1038/ng986. [DOI] [PubMed] [Google Scholar]
- 33.Rio RV, Symula RE, Wang J, Lohs C, Wu YN, Snyder AK, Bjornson RD, Oshima K, Biehl BS, Perna NT, et al. Insight into the transmission biology and species-specific functional capabilities of tsetse (Diptera: glossinidae) obligate symbiont Wigglesworthia. MBio. 2012;3 doi: 10.1128/mBio.00240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pais R, Lohs C, Wu Y, Wang J, Aksoy S. The obligate mutualist Wigglesworthia glossinidia influences reproduction, digestion, and immunity processes of its host, the tsetse fly. Appl Environ Microbiol. 2008;74:5965–5974. doi: 10.1128/AEM.00741-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nogge G. Sterility in tsetse flies (Glossina morsitans Westwood) caused by loss of symbionts. Experientia. 1976;32:995–996. doi: 10.1007/BF01933932. [DOI] [PubMed] [Google Scholar]
- 36.Nogge G. Significance of Symbionts for the Maintenance of An Optimal Nutritional State for Successful Reproduction in Hematophagous Arthropods. Parasitology. 1981;82:101–104. [Google Scholar]
- 37.Alam U, Medlock J, Brelsfoard C, Pais R, Lohs C, Balmand S, Carnogursky J, Heddi A, Takac P, Galvani A, et al. Wolbachia symbiont infections induce strong cytoplasmic incompatibility in the tsetse fly Glossina morsitans. PLoS Pathog. 2011;7:e1002415. doi: 10.1371/journal.ppat.1002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michalkova V, Benoit JB, Weiss BL, Attardo GM, Aksoy S. Obligate symbiont-generated vitamin B6 is critical to maintain proline homeostasis and fecundity in tsetse flies. Appl Environ Microbiol. 2014 doi: 10.1128/AEM.01150-14. in press. * Wigglesworthia genome encodes various vitamin B products, which are low in the single vertebrate blood diet of tsetse. One of these, pyridoxal phosphate (vitamin B6), is the co-factor for the enzyme alanine-glyoxylate aminotransferase (AGAT), which is essential in proline biosynthesis. In the absence of Wigglesworthia and when AGAT levels are experimentally reduced, tsetse fecundity is lost through compromised proline levels. Trypanosome also use proline as the sole energy source and parasitized flies how lower hemolymph proline levels.
- 39.Weiss BL, Wang J, Aksoy S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011;9:e1000619. doi: 10.1371/journal.pbio.1000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiss BL, Maltz M, Aksoy S. Obligate symbionts activate immune system development in the tsetse fly. J Immunol. 2012;188:3395–3403. doi: 10.4049/jimmunol.1103691. ** Tsetse that undergo larval development in the absence of Wigglesworthia, exhibit a compromised immune system during adulthood that is characterized by the absence of phagocytic hemocytes and atypical expression of immunity-related genes. These flies quickly succumb to infection with normally nonpathogenic bacteria. The susceptible phenotype exhibited by such adults can be reversed when they receive hemocytes transplanted from wild-type donor flies prior to infection or when their mothers are fed a diet supplemented with Wigglesworthia cell extracts. These finding indicate that molecular components of Wigglesworthia exhibit immunostimulatory activity during development. Results provide evidence of an important physiological adaptation that further anchors the obligate symbiosis between tsetse and Wigglesworthia.
- 41.Dean S, Marchetti R, Kirk K, Matthews KR. A surface transporter family conveys the trypanosome differentiation signal. Nature. 2009;459:213–217. doi: 10.1038/nature07997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson W, Bailey M. The development of Trypanosoma brucei within the tsetse fly midgut observed using green fluorescent trypanosomes. Kinetoplastid Biol Dis. 2003;2:1. doi: 10.1186/1475-9292-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu C, Aksoy S. Innate immune responses regulate trypanosome parasite infection of the tsetse fly Glossina morsitans morsitans. Mol Microbiol. 2006;60:1194–1204. doi: 10.1111/j.1365-2958.2006.05180.x. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, Aksoy S. An antimicrobial peptide with trypanocidal activity characterized from Glossina morsitans morsitans. Insect Biochem Mol Biol. 2005;35:105–115. doi: 10.1016/j.ibmb.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Hao Z, Kasumba I, Lehane MJ, Gibson WC, Kwon J, Aksoy S. Tsetse immune responses and trypanosome transmission: implications for the development of tsetse-based strategies to reduce trypanosomiasis. Proc Natl Acad Sci U S A. 2001;98:12648–12653. doi: 10.1073/pnas.221363798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Aksoy S. PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse's offspring. Proc Natl Acad Sci U S A. 2012;109:10552–10557. doi: 10.1073/pnas.1116431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haines LR, Lehane SM, Pearson TW, Lehane MJ. Tsetse EP protein protects the fly midgut from trypanosome establishment. PLoS pathogens. 2010;6:e1000793. doi: 10.1371/journal.ppat.1000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Telleria EL, Benoit JB, Zhao X, Savage AF, Regmi S, e Silva TL, O'Neill M, Aksoy S. Insights into the trypanosome-host interactions revealed through transcriptomic analysis of parasitized tsetse fly salivary glands. PLoS Negl Trop Dis. 2014;8:e2649. doi: 10.1371/journal.pntd.0002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kubi C, van den Abbeele J, R DED, Marcotty T, Dorny P, van den Bossche P. The effect of starvation on the susceptibility of teneral and non-teneral tsetse flies to trypanosome infection. Med Vet Entomol. 2006;20:388–392. doi: 10.1111/j.1365-2915.2006.00644.x. [DOI] [PubMed] [Google Scholar]
- 50.Weiss B, Aksoy S. Microbiome influences on insect host vector competence. Trends Parasitol. 2011;27:514–522. doi: 10.1016/j.pt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang J, Wu Y, Yang G, Aksoy S. Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proc Natl Acad Sci U S A. 2009;106:12133–12138. doi: 10.1073/pnas.0901226106. ** Tsetse Peptidoglycan Recognition Protein (PGRP-LB) is expressed in adult bacterium and is a major component of the milk that nourishes the developing progeny. The amidase activity associated with PGRP-LB scavenges PGN and prevents the induction of symbiont damaging Imd pathway effectors. Experimental reduction of PGRP-LB diminishes female fecundity and damages Wigglesworthia in the milk through induction of antimicrobial peptides. Larvae that receive less maternal PGRP-LB give rise to adults with fewer Wigglesworthia and hyperimmune responses. Such adults also suffer dysregulated immunity, with higher trypanosome densities in parasitized adults. recPGRP-LB has antimicrobial and antitrypanosomal activities that may regulate symbiosis and impact immunity. Thus, PGRP-LB plays a pivotal role in host’s fitness by protecting symbiosis and controlling parasite infections in adults.
- 52.Hu C, Rio RV, Medlock J, Haines LR, Nayduch D, Savage AF, Guz N, Attardo GM, Pearson TW, Galvani AP, et al. Infections with immunogenic trypanosomes reduce tsetse reproductive fitness: potential impact of different parasite strains on vector population structure. PLoS Negl Trop Dis. 2008;2:e192. doi: 10.1371/journal.pntd.0000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehane MJ. Peritrophic matrix structure and function. Annu Rev Entomol. 1997;42:525–550. doi: 10.1146/annurev.ento.42.1.525. [DOI] [PubMed] [Google Scholar]
- 54.Kuraishi T, Binggeli O, Opota O, Buchon N, Lemaitre B. Genetic evidence for a protective role of the peritrophic matrix against intestinal bacterial infection in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2011;108:15966–15971. doi: 10.1073/pnas.1105994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hegedus D, Erlandson M, Gillott C, Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annu Rev Entomol. 2009;54:285–302. doi: 10.1146/annurev.ento.54.110807.090559. [DOI] [PubMed] [Google Scholar]
- 56.Welburn SC, Maudlin I. The nature of the teneral state in Glossina and its role in the acquisition of trypanosome infection in tsetse. Annals of Tropical Medicine and Parasitology. 1992;86:529–536. doi: 10.1080/00034983.1992.11812703. [DOI] [PubMed] [Google Scholar]
- 57.Walshe DP, Lehane MJ, Haines LR. Post eclosion age predicts the prevalence of midgut trypanosome infections in Glossina. PLoS One. 2011;6:e26984. doi: 10.1371/journal.pone.0026984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haines LR. Examining the tsetse teneral phenomenon and permissiveness to trypanosome infection. Front Cell Infect Microbiol. 2013;3:84. doi: 10.3389/fcimb.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weiss BL, Wang J, Maltz MA, Wu Y, Aksoy S. Trypanosome infection establishment in the tsetse fly gut is influenced by microbiome-regulated host immune barriers. PLoS Pathogens. 2013;9:e1003318. doi: 10.1371/journal.ppat.1003318. ** Results show that following parasite challenge, young susceptible tsetse present a highly attenuated immune response while mature refractory flies express higher levels of genes associated with humoral (attacin and pgrp-lb) and epithelial (inducible nitric oxide synthase and dual oxidase) immunity. Adults that undergo larval development in the absence of their obligate symbiont show high susceptibility to trypanosome infections despite strong immune responses. Results show that such adults however lack structurally robust PM, which regulates the timing of host immune induction following parasite challenge.
- 60. Weiss BL, Savage AF, Griffith BC, Wu Y, Aksoy S. The Peritrophic Matrix Mediates Differential Infection Outcomes in the Tsetse Fly Gut following Challenge with Commensal, Pathogenic, and Parasitic Microbes. J Immunol. 2014;193:773–782. doi: 10.4049/jimmunol.1400163. ** The structurally robust PM was eliminated through RNA interference-approach. In such flies, bacteria Enterobacter and Serratia proliferation was impeded because these flies expressed the antimicrobial peptide gene, attacin, earlier in the infection process than did wild type flies. After challenge with trypanosomes, attacin expression was latent in tsetse that lacked an intact PM, and these flies were highly susceptible to parasite infection. Results suggest that Imd signaling pathway effectors, as opposed to reactive oxygen intermediates, serve as the first line of defense in tsetse's gut after the ingestion of exogenous microorganisms. Furthermore, tsetse's PM is not a physical impediment to infection establishment, but instead serves as a barrier that regulates the fly's ability to immunologically detect and respond to the presence of these microbes.
- 61. Initiative IGG. Genome sequence of the tsetse fly (Glossina morsitans): vector of African trypanosomiasis. Science. 2014;344:380–386. doi: 10.1126/science.1249656. * The whole genome sequence data from the tsetse species Glossina morsitans is presented. Particularly a reduction in the host pattern recognition proteins (PGRPs) are noted in relation to closely related other Diptera. This reduction may reflect tsetse’s evolutionary association with obligate microbes and restricted exposure to a wide array of microbes as a strict blood feeder.