Abstract
There is a pressing clinical need for responsive neurostimulators, which sense a patient’s brain activity and deliver targeted electrical stimulation to suppress unwanted symptoms. This is particularly true in psychiatric illness, where symptoms can fluctuate throughout the day. Affective BCIs, which decode emotional experience from neural activity, are a candidate control signal for responsive stimulators targeting the limbic circuit. Present affective decoders, however, cannot yet distinguish pathologic from healthy emotional extremes. Indiscriminate stimulus delivery would reduce quality of life and may be actively harmful. We argue that the key to overcoming this limitation is to specifically decode volition, in particular the patient’s intention to experience emotional regulation. Those emotion-regulation signals already exist in prefrontal cortex (PFC), and could be extracted with relatively simple BCI algorithms. We describe preliminary data from an animal model of PFC-controlled limbic brain stimulation and discuss next steps for pre-clinical testing and possible translation.
Keywords: affect decoding, invasive BCI, prefrontal cortex, mental disorders, deep brain stimulation, hybrid BCI
1. Introduction and Rationale
1.1. The Clinical Need for Closed-Loop Neuromodulation
Decoding of emotion from the body’s electrical activity has been proposed for applications in neurofeedback, communication prostheses, and life-enhancing systems for healthy users [1-3]. These same technologies, however, may be useful for improving the efficacy of neurostimulation for treatment-resistant psychiatric disorders.
The need for affective monitoring is clearest in deep brain stimulation (DBS). Psychiatric DBS has been used at multiple targets [4,5], with preliminary success in treating depression and obsessive-compulsive disorder (OCD) [6-8]. Progress in psychiatric DBS, however, has been limited by its inherent open-loop nature. Present DBS systems deliver energy continuously at a pre-programmed frequency and amplitude, with parameter adjustments only during infrequent clinician visits. This has led, in our clinical experience, to more rapid depletion of device batteries (with a resulting need for battery-replacement surgeries and the associated pain/infection) and an increased side effect burden. Side effects in particular derive from present devices’ inability to match stimulation to a patient’s current affective state, brain activity, and therapeutic need.
A reliable brain-computer interface (BCI) that inferred emotional state from neural signals could enable a responsive, “closed loop” stimulator. In such a device, schematically illustrated in Figure 1, the BCI would continuously monitor affective state and adjust stimulus parameters to maintain the patient within healthy parameters. This monitoring and regulation of the limbic circuit is a natural function of the prefrontal cortex (PFC), and emerging evidence suggests that it is specifically disrupted in a variety of emotional disorders [9-11]. A combined closed-loop affective decoding and stimulation system would effectively be an “emotional prosthesis”, compensating for circuits that have become hypofunctional. Moreover, it would deliver electrical stimulation that was well-matched to the patient’s immediate need and level of distress. This would reduce the side effects of over-stimulation, alleviate residual symptoms that may relate to under-stimulation, and optimize power consumption for a longer battery life.
Figure 1.
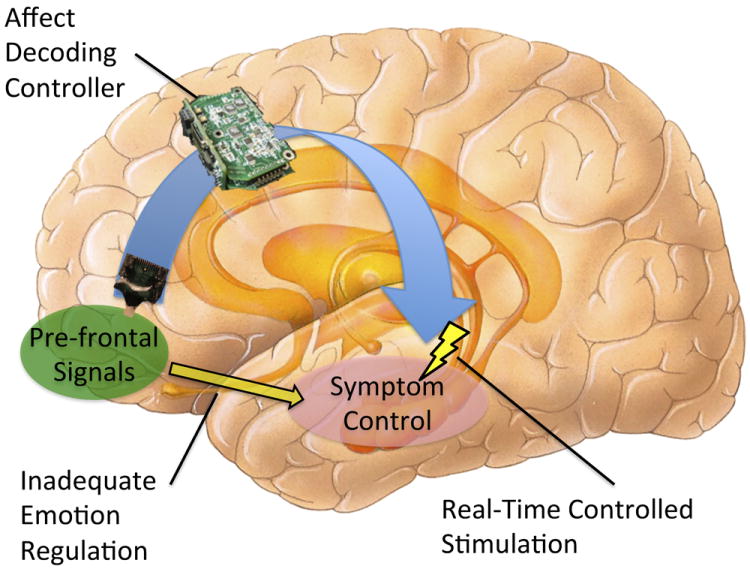
Schematic of closed-loop affective decoder and brain stimulator for psychiatric indications. Neural activity is monitored by a controller to infer the patient’s present affect. When continuous monitoring indicates that the system is moving into a pathological state, the controller adjusts parameters of an implanted deep brain stimulator (DBS) to counteract that trajectory. This compensates for an inherent deficit in emotional self-regulation. In the specific instance visualized here, the controller is monitoring prefrontal cortex and decoding the patient’s intention to activate the neurostimulator; the DBS is shown operating in the limbic circuit to modulate activity.
Atop this, many disorders have symptoms that rapidly flare and remit, on a timescale of minutes to hours. This is particularly common in the anxiety and trauma-related clusters[12]. Existing DBS strategies have been unable to effectively treat such fluctuations, because they occur on much shorter timescales than the infrequent clinical visits. A responsive closed-loop system could in theory detect and compensate for such flares. Not only would this improve the tolerability of DBS overall, it would allow these stimulators to help a clinical population that is currently poorly served. There is thus an opportunity for BCI, and affective BCI in particular, to address a set of disorders that cause as much disability as stroke, trauma, or any other motor dysfunction [13].
1.2. Limits on Affective Decoding in the Presence of Psychopathology
Development of closed-loop emotional DBS has been blocked by a lack of accurate or feasible biomarkers. Three major challenges arise when considering existing affective BCIs as the sensing component of closed-loop DBS control.
First, many identified neural correlates of affective disorders cannot be continuously monitored in the community. Functional magnetic resonance imaging (fMRI) can provide deep insights into activity across the whole brain, and has been demonstrated for partial affective classification in real-time [14]. Similar results have been seen with near-infrared spectroscopy (NIRS), which also measures blood-oxygenation signals [15]. The former, however, requires bulky machines and is not compatible with implanted devices, and the latter has not yet been demonstrated in an online-decoding paradigm. Moreover, although NIRS can be reduced to a wearable/portable device, it requires an externally worn headset. Given the unfortunate persistence of stigma attached to patients with mental disorders, few would wear a visible display of their illness, even if it did control symptoms. This stigma is one of the reasons closed-loop DBS is so important – it would not be socially or clinically acceptable for a patient to frequently do something external, such as pushing a button or manipulating a patient controller, to trigger or adjust his/her neurostimulator on a moment-to-moment basis.
Second, even affective decoding modalities that support continuous recording may not function properly in the presence of psychiatric illness. Electrocorticography (ECOG) is a promising approach, as it can be implanted (and thus hidden) with relatively minimally invasive surgery. ECOG signals offer excellent temporal resolution and may be able to leverage decoders originally developed for electroencephalography (EEG). Non-invasive EEG has been a very successful approach in affective BCI, with some real-time decoding of emotional information [16,17]. Uncertainty arises because all successful EEG affective decoding has been demonstrated in healthy volunteers. Patients with mental illness, particularly those with treatment-resistant disorders, by definition do not have normal or healthy neurologic function. Furthermore, recent experiences with EEG in psychiatry suggest that measures that accurately decode healthy controls may not transfer to patients. EEG biomarkers that initially appeared to correlate with psychiatric symptoms and treatment response have often not held up under replication studies [18-21]. This is at least in part because psychiatric diagnosis focuses on syndromes and symptom clusters, not etiologies. There is a wide consensus that clinical diagnoses generally contain multiple neurologic entities, and that the same clinical phenotype might arise from diametrically opposite changes in the brain [22]. This may present a challenge for clinical translation of existing affective decoders.
Third, even if affective BCIs can function in the presence of clinical symptoms, they may not be able to adequately distinguish pathologic states. Newer affective BCI algorithms may yet be shown to accurately classify emotion even in the presence of abnormal neural circuit activity, but this is only part of the need. Psychiatric disorders are marked by extremes of the same emotions that occur in everyday normal life. The difference is not the degree or type of affect, but its appropriateness to the context. Post-traumatic stress disorder (PTSD) is one clear example. Patients with this disorder overgeneralize from a fearful event and experience high arousal and vigilance in contexts that are objectively safe [12]. It is likely possible for an affective BCI to detect high arousal in a patient with PTSD in uncontrolled real-world environments. It is less clear whether any algorithm could distinguish pathologic arousal (a “flashback” in a grocery store, confrontation with trauma cues) from healthy variance (riding a roller coaster, watching an exciting movie). These would be very difficult to differentiate solely on the basis of experienced affect, and yet the use of brain stimulation to neutralize the latter set of experiences would negatively impact the patient’s quality of life. In our clinical practice, we have repeatedly encountered patients who are fearful to accept any treatment because they fear it will render them “numb”; a stimulator that responds but does not discriminate could produce precisely that outcome.
This third difficulty is clear for the case of PTSD. In other disorders, such as depression, it could perhaps be circumvented by integrating the time course of emotion over days to weeks. Sadness will persist in a true depressive episode, whereas ordinary reactive emotion will abate as external stimuli change [12]. In practice, two complications limit that approach. First, it may not suffice to detect a clinical worsening only after the symptoms have been present for a prolonged period of time. Treatment-resistant mental illness is just that: a brain state that strongly resists transitions to a new point on the emotional landscape. By the time a patient is in a clear and verifiable clinical episode, it may well be too late, and the road back out may be long and arduous. The limited literature on patients with treatment-resistant illness and DBS suggests that relapses require weeks to months to subside once they are allowed to occur [7,8]. Thus, in an analogy to epilepsy, it may be important to detect when the patient is not yet experiencing pathologic emotion, but is in a trajectory towards it [23]. This is a much more difficult problem, as it involves determining not only the present affective state, but the likely end-point of the current affective trajectory.
These challenges combine to reveal a final complication: in a fully implanted system, onboard storage and computational resources are limited. It may not be possible to perform decoding and tracking over long periods of time. Thus, affective decoders are caught in a dilemma of temporal resolution: if they are tuned to respond to brief but intense events, they will over-react to natural and healthy emotional variation. If they instead focus only on detecting and compensating for long-term trends, sharp but short exacerbations will go uncorrected, decreasing patients’ quality of life and continuing the problems of existing open-loop DBS. In the very long run, these problems may be ameliorated by improvements in battery and processing technology. The path to that solution is, however, quite long; regulatory agencies require extensive review of all new technology components, meaning that any new battery could take a decade to reach clinical use even after being successfully demonstrated for non-implantable applications. Processors might be more easily upgraded, but increased processing power means increased heat, which cannot be readily dissipated inside the body. For the near future, methods for responsive decoding and stimulation must operate within the limits of current clinical technology [24].
2. Hypothesis
2.1. Volition and Plasticity are Key Enablers for Affective BCI Control of Neurostimulation
Despite these challenges, we believe that affective BCI is a critical and valuable component in a closed-loop, symptom-responsive psychiatric DBS system. We propose that the key is adding two further components to the model: plasticity and volition.
Regarding plasticity, the general approach for building a BCI is to have participants perform a “predicate task”, such as hand movement, motor imagery, or emotional imagery in the case of affective BCI. From neural activity during this training period, a decoder is built, then deployed to classify new brain activity as it arises [14,17,25]. Recent work has shown that this process is not a simple matter of “cracking the neural code”. Instead, even as the BCI learns the mapping between brain signals and task variables, the brain itself is changing to better match the decoder [26,27]. In fact, it is possible to build an effective motor BCI without performing any decoding, simply by mapping neurons or other electrographic signals to degrees of freedom and allowing the user sufficient practice to adapt to the BCI [28-31]. Put another way, given sufficient motivation, the brain’s inherent capacity for plasticity will remap cortical signals to produce information that is optimized for the companion BCI. The implication for affective BCI is that patients may also be able to express emotional state through arbitrary neural signals, given sufficient learning time and motivation.
This highlights the second component of our hypothesis: volition. While arbitrary signals can be used to drive a BCI, that approach depends on the user being aware of the BCI and intending to control it. In the case of closed-loop psychiatric DBS, this implies a patient sensing that present stimulation parameters are not well-matched to his/her clinical needs, then choosing to alter the stimulation parameters by deliberately modulating specific aspects of brain activity (e.g. firing rates of specific neurons, power in certain bands of LFP/EEG/ECOG). Adding a volitionally controlled component resolves many of the limitations identified above. Affective decoding would not need to directly classify an emotion as pathologic vs. healthy-extreme; the patient could express his/her desire directly and efficiently through a BCI. The question of whether to optimize response to fast or slow time scales would become moot; stimulation should be adjusted when the patient explicitly requests the affective controller to do so. Heterogeneity of biomarkers within clinical disorders could also be controlled for, because the primary decoded variable would be the patient’s own intention to receive mood-altering neurostimulation.
A volitional component to the controller may even be critical for maintaining the patient’s overall well-being. Birbaumer [32] has reviewed an extended series of experiments in volitional control of physiologic signals, from autonomic functions to aspects of the EEG. He noted that chronically paralyzed rats and completely locked-in humans, both of whom had lost connection between their volitional desires and external outcomes, also became unable to exert control over their own neurologic functions. From this, he proposed that volition and outcomes contingently linked to volition are essential to maintain the basic components of thought and consciousness, and that without ongoing reinforcement, the capacity for intentional action can itself atrophy, a process that has been termed “extinction of thought”. This raises a potential concern for linking emotion-altering brain stimulation to a “passive BCI” that requires no intention on the patient’s part [2]. If, as in the examples above, extremes of emotion are regulated whether or not the patient desires it, his/her own capacity for autonomous self-regulation may be slowly worn away. Given that we believe this self-regulatory function to be at the heart of much psychopathology [9-11], a closed-loop system without a volitional component could, over time, actually worsen the underlying deficit. The patient would, in effect, learn helplessness in the face of an overriding neurostimulator.
2.2. Prefrontal Cortex is a Natural Source of Intentional Emotion Regulation Signals
Returning to the schematic of Figure 1, we have argued that the BCI controller should not only attempt to infer the patient’s emotional state, but should also decode volitionally controlled brain signals. This raises the question: signals from what brain region? We propose that prefrontal cortex (PFC) is an ideal choice. First, as noted above, PFC is a source of emotion regulation signals [9-11]. The desire to suppress or amplify emotional experiences is already contained in PFC activity, and that activity correlates directly with patients’ ability to succeed in emotion regulation. This suggests that it should be relatively simple to decode that intention, just as motor BCIs have succeeded in decoding movement intention from primary motor cortex. Second, PFC neurons are extremely flexible. There is a strong body of evidence that PFC neurons regularly re-tune themselves into new ensembles, encoding complex features of multiple tasks [33,34]. Given our hypothesis that plasticity is important for successful affective decoding and control, a BCI that decodes signals from highly plastic cortex is more likely to succeed, because the brain can more readily retune to communicate a clinical need.
Unifying the themes above, we propose that a simple but robust approach to affective BCI for closed-loop DBS is to record volitionally controlled signals from PFC, then use that activity as a reflection of the patient’s desire to adjust stimulation parameters. This would not directly decode emotion, but instead could be seen as decoding an intention towards emotional regulation. That signal is well known to exist in PFC based on neuro-imaging data [35,36]. An affective decoder, similar to those already demonstrated, could then classify the patient’s current emotional state and serve as a feedback signal for an adaptive stimulation algorithm. Alternatively, if a simpler strategy is required, the volitional PFC activity could itself be that feedback signal. This “direct control” BCI approach is known to be capable of decoding one or more degrees of freedom [31,37], which should be sufficient to control the parameters of most clinical brain stimulators. In the remainder of this paper, we present a rodent proof-of-concept study demonstrating this PFC-based affective BCI strategy, then discuss how it could be scaled and adapted to achieve the goal of closed-loop emotional brain stimulation.
3. Proof of Concept: Rodent PFC BCI with Limbic Stimulation
3.1. Overview
As proof of concept for our schema of PFC BCI decoding and closed-loop stimulation, we developed a model system in which rats could modulate single units within PFC to trigger fixed-parameter reinforcing brain stimulation. To increase the model’s relevance for our longer-term goal of responsive psychiatric neurostimulation, we chose a reinforcement site that is also a target for human psychiatric DBS trials. Four outbred rats were implanted with stimulating and recording electrodes, and all animals successfully learned to trigger the neurostimulator using PFC. In line with the plasticity framework proposed above, we found that animals were able to dissociate and specifically modulate the neurons involved in the BCI. The BCI algorithm, animal training, and performance are summarized below, and have been described in detail elsewhere [38].
3.2. Methods
A block diagram of the experimental setup and schematic of our affective BCI algorithm are shown in Figure 2. Briefly, adult female Long-Evans rats were implanted with arrays of recording electrodes in PFC (prelimbic and infralimbic cortices), and stimulating deep-brain electrodes. Stimulating electrodes targeted medial forebrain bundle (MFB), a structure within the reward pathway where electrical stimulation is known to be reinforcing. MFB is a target for human clinical trials in DBS for depression [5], highlighting its relevance as a stimulation site in this closed-loop testbed. For this work, however, we chose MFB solely for its reinforcing properties, and not as a candidate treatment site for human translation.
Figure 2.

Schematic of model system for testing PFC-based decoding in rodents.
(A), block diagram of tethered recording, decoding, and stimulating system. Modular components control each function, permitting modification of experimental setup for alternate decoders or stimulation schemes. As illustrated, neural data flow from animal in operant chamber, through dedicated on-line spike sorting and behavioural tagging, to a desktop PC for processing and storage. Based on neural signals, the PC controls the behavioural system and stimulator, which deliver neurofeedback and brain stimulation to animal.
(B), BCI algorithm schematic. Single recorded units from PFC are sorted online, after which spike rates are estimated and converted to an audio cursor for animal.
(C), BCI trials schematic. Animal is presented with auditory cues (“targets”, red, with cue period indicated in grey), initiated in a self-paced fashion by holding PFC activity at initial baseline (yellow). Moving the audio cursor to within a window of the cue (reaching a target) constitutes control of the BCI, and activates neurostimulation in the medial forebrain bundle (MFB). Failure to reach the target within a pre-set time leads to a brief time-out. Because stimulation is reinforcing in this paradigm, animal learns to use BCI to express desire for stimulation by acquiring targets when available (communicating an affect-regulation desire).
Our broad hypothesis is that affective dysregulation and a desire to activate or alter brain stimulation can be decoded from volitionally controlled PFC activity. For the initial proof of concept, we focused on a simpler question: can animals be trained to volitionally alter PFC activity in order to obtain a brain stimulation reward? Demonstrating basic PFC BCI control is essential for the next translational step, namely showing that animals or humans can use such a system to relieve psychic distress.
To demonstrate that rodents can and will learn to use an intention-decoding BCI to drive brain stimulation, we trained the animals to use an auditory BCI, following the methods of Gage, Marzullo, and Kipke [39,40]. As diagrammed in Figure 2B, activity of a single unit recorded from PFC was converted to an auditory cursor. The firing rate of the decoded unit controlled the frequency of a tone presented to the animal, implementing a paradigm similar to a neurofeedback system. To model the plasticity component of our hypothesis, the BCI was not trained or otherwise pre-tuned to neural firing modulation. Rather, at the start of each session a unit was selected and mapped into the BCI with fixed parameters. Animals were thus required to learn and adapt to the new BCI mapping each day to communicate their desire for neurostimulation.
Animals used the BCI in a series of self-paced trials, as shown in Figure 2C. Baseline firing of the selected PFC unit was measured at the start of each day, and dwelling the firing rate at this baseline initiated a new trial. For each trial, a tone was briefly played, and the animal had 5-10 seconds to modulate PFC firing rate to match her audio feedback cursor to the target. Targets were based on the standard deviation (SD) of the baseline firing rate, and always required the animal to elevate firing rate by at least 1.5 SD. Successful target acquisition triggered a phasic burst of MFB stimulation, whereas failure did not. That is, every time the animal successfully controlled PFC neural firing, electrical stimulation was delivered within her limbic circuit. Trials were followed by brief time-outs, after which a new trial became available for self-initiation. This is a preliminary demonstration of the concept that volitional PFC activity can be decoded via a BCI and used as a signal of desire to activate a neurostimulator. Importantly, we did not use a predicate task such as limb or eye movements; animals directly learned to control the BCI through repeated operant shaping.
Because the decoded unit and BCI parameters were set on a daily basis and turned to maintain an adequate operant reinforcement rate, it was important to verify that target acquisition was not occurring through stochastic, non-intentional fluctuations of neural activity. We performed two controls to confirm that animals’ neurostimulator use exceeded chance performance. First, 20% of trials were randomly designated “catch” trials, where no cue or audio BCI feedback cursor was given; successful target acquisition on such a trial would by definition be non-volitional. Second, for each testing session, we performed a bootstrap re-analysis of the firing rate data. The actual trials presented to the animal were randomly re-located across that session’s spiking record and the success rate calculated. By replicating that random shuffling 10,000 times for each testing session, we derived the distribution of target acquisition rates that could be expected by chance for that specific unit and session.
3.3. Results
In this model system, all animals successfully learned to control the PFC BCI to trigger MFB stimulation. Figure 3 illustrates a session during which the animal successfully controlled the BCI and neurostimulator. Target acquisition rates were initially low as the animal learned the new decoder, then rapidly increased and were sustained for over 20 minutes (roughly 250 trials). During this core performance period, when the animal had learned the decoder and was actively attending to the BCI, target hit rates remained well above both on-line (catch trials) and off-line (bootstrap replication) measures of chance. We observed this pattern of a performance peak followed by a slow decline in all tested animals, with animals generally learning to control newly isolated PFC units after only 20-40 minutes of practice. 80% of tested sites in PFC were controllable, consistent with our hypothesis that arbitrary neurons can be used for affective decoding by exploiting neuroplasticity.
Figure 3.

Example of successful PFC BCI control for limbic stimulation. Solid blue line represents the target acquisition rate over a single testing session. Dashed line and horizontal solid line represent on-line (“catch”) and off-line (“bootstrap”) estimates of chance-level performance, respectively. BCI target acquisition, and thus successful delivery of reinforcing neurostimulation, rises to a peak above both measures of chance. Performance is sustained for over 20 minutes (nearly 1/3 of the session) before the performance declines, likely due to fatigue.
Control of PFC neurons in this BCI paradigm was highly specific. Figure 4 shows the averaged firing rates of multiple simultaneously recorded PFC units during two consecutive training days, time-locked to successful acquisition of a BCI target and delivery of reinforcing brain stimulation. The only substantial modulation in discharge rate occurred on the channel used for the BCI (arrow head). That channel, which was changed between these two sessions, shows a sharp rise into the target and equally sharp return to baseline after the onset of brain stimulation. The other, non-decoded channels show no average time-locked modulation. This provides further evidence that animals were able to specifically remap arbitrary PFC neurons to match the BCI decoder, establishing an “emotional communication channel” to indicate their intent to receive neurostimulation.
Figure 4.


Two examples of peri-stimulus discharge rates on single channels involved in the PFC BCI. Examples are taken from the same animal, on two successive days, during which different units were controlled. Layout of subfigures within each example reflects relative location of individual electrodes within the cortex. In each panel, the channel controlling the BCI (A, Channel 8 ; B, Channel 14) shows a sharp rise into the middle of the target (arrowhead) followed by equally sharp return to baseline once success is achieved. Other channels show little to no peri-event modulation, consistent with specific control of PFC unit selected for the BCI.
The peak-and-decline pattern of success highlights the importance of volition in this model. We attribute the eventual decline in performance to a decline in volitional capacity, potentially through fatigue and reward satiation. As the effort required to control the neurostimulator became greater than the present value of the reinforcing brain stimulation, the animal became less willing to expend effort in modulating PFC units to match the decoder. Intentional control of PFC units is also demonstrated by the channel-specific modulation of Figure 4. This is evidence that animals were executing a learned and specific skill to achieve control of the BCI.
4. Discussion and Generalizations
In a simplified model of a responsive closed-loop stimulation system, outbred rats were able to modulate PFC single units to control a BCI, using that BCI to trigger neurostimulation in a deep brain structure. To achieve this, the animals were required to actively re-tune PFC neurons (a goal-oriented neuroplastic process) to match the expectations of the decoding BCI. This offers a preliminary demonstration of our proposal for an “emotion regulation decoder” that focuses primarily on the patient’s intent to trigger emotion-modulating neurostimulation. In this case, animals used PFC activity to encode an appetitive/seeking signal, the desire to experience a reinforcing/rewarding stimulus. This is not a direct demonstration of a symptom-relieving responsive stimulator; the MFB stimulation used here was an operant reinforcer supporting proof-of-concept, not a treatment paradigm to be applied in humans. However, it did demonstrate the key point: even animals with an evolutionarily simple PFC can learn to operate a BCI solely for an intracranial emotional reward. We believe the same model can be generalized to a negative reinforcement, such as decoding the desire to have a negative-valence emotion removed.
This initial proof of concept demonstrates that a BCI decoding volitional activity from PFC can control a closed-loop psychiatric DBS, but also leaves open many questions for pre-clinical testing. We do not yet know if animals can learn to exercise the same kind of BCI control in high-arousal environments, such as an anxiogenic open field or a chamber associated with conditioned fear. That would be a direct test of the negative reinforcement hypothesis above, and an important next set of experiments. If animals can learn to modulate neural activity to communicate distress and obtain relief from that distress, human patients could almost certainly do likewise. As described in the Introduction, it also remains to be demonstrated that human psychiatric disorders do not in some way interfere with capacity to use a BCI. If that does not prove a barrier, it is likely that patients could learn to use this responsive system. Psychiatric patients frequently report that they recognize emotional symptoms as “not my real self” (ego-dystonic) and attempt to suppress them, clear evidence that they would be able to recognize the need to activate a stimulator [12]. Some are even able to learn new cognitive skills that enable active suppression of symptoms [41-43]. A responsive BCI-based stimulator would effectively amplify those skills and achieve what some patients are unable to do on their own. There are certainly some disorders, such as the manic phase of bipolar disorder, where symptoms are ego-syntonic and this volition-decoding approach would not work. The existence of those disorders, however, does not obviate the very large space of unipolar mood, obsessive-compulsive, anxious, and trauma-related disorders where the volitional approach is viable.
Even if this proof of concept successfully translates to a clinical device, is not known how the brain might respond in the long run to continued interaction with a responsive, BCI-based stimulator. There is a potential for positive effects, particularly with the PFC-based controller described here. Work using similar operant paradigms has shown that artificial coupling of activity between two brain sites or between brain and spinal cord can induce long-term increases in functional connectivity [44,45]. One could speculate that long-term use of a BCI-controlled responsive stimulator could boost PFC’s ability to regulate limbic circuits, strengthening key brain pathways for long-term symptom relief.
On the other hand, the system might also cause habituation – over time, stimulation might become less effective, requiring the user to trigger the BCI with increasing frequency to gain the same clinical effect. We see this in our clinical DBS experience, where patients often require upward “dose” adjustments to maintain benefit. However, if anything, that effect should be ameliorated by responsive stimulation. Responsive stimulation, especially coupled to an emotion-monitoring aBCI, should occur infrequently and on no predictable schedule. This should reduce or eliminate habituation, although that remains a hypothesis to be tested. A second concern, depending on stimulator target, might be addiction. Animals are sometimes reported to forego needed food or engage in dangerous tasks to obtain MFB stimulation [46-48]. This is one of the reasons the current proof of concept does not support clinical translation at the specific MFB target; any putative clinical target would need to be carefully studied to rule out addictive potential. We derive some reassurance from the fact that animals in our experiment did stop triggering the stimulator after 1-2 hours of use, and thus we did not observe continuous, compulsive, addiction-like behaviour.
Those limitations notwithstanding, there remain substantial advantages to implementing a BCI that focuses on decoding regulatory intention. Chief among these is simplicity. The system described here was able to recover a meaningful control signal from a single prefrontal unit. When we consider design constraints for implantable, battery-powered systems (i.e., all known DBS), computationally simpler BCIs are much more easily implemented on that limited hardware.
The PFC BCI approach also produces useful signals with recordings from only one brain region. We used single-unit potentials in our proof of concept, but this is not obligatory. In the motor domain, good results have been obtained with relatively focal recordings of LFP and ECOG, both of which are technically less challenging and perhaps more stable over time [29,30,49,50]. By contrast, the most successful EEG decoder required 124 channels spread across the cranium [17]. That degree of spatial coverage is not readily achievable in an implantable system, nor are even the most sophisticated clinical DBS systems capable of processing that many channels without substantial power and heat dissipation [24].
We also cannot ignore the substantial successes and advantages of recently demonstrated BCIs that directly decode emotion. Those passive systems could, in theory, be effortless to use [2]. This returns to the concern originally raised by Birbaumer [32] about self-efficacy, but that concern must be balanced against the very real problem of fatigue in intentional, active BCIs. Most BCIs that depend on volitionally modulated activity require substantial effort, and users can fatigue [32,51]. We may have observed a similar effect in our rodent study, where the animals’ performance eventually declined after a period of intensive, continuous use. This may not fully reflect the performance of an eventual human system, as we required the animals to activate the BCI-controlled stimulator multiple times per minute. An actual patient using such a system would likely only need to alter stimulation parameters every few hours. Nevertheless, fatigue and difficulty may impact initial training as well as ongoing use. It is also clear that different recording channels may have substantially different difficulties, as evidenced by the fact that 20% of our recorded units could not support BCI control.
We therefore suggest two lines of future affective BCI research that could enable closed-loop emotional DBS. Both are focused on leveraging the success of existing affective decoders, while also adding the key components of volition and plasticity. First, there is value in investigating affective BCIs that function with lower-dimensionality data. The approach we highlight above, decoding of volitionally modulated PFC activity, is one step in this direction. This could be generalized to patients using multiple separate PFC signals as “emotion communication channels”. Usable emotion and intention related signals may also exist outside of PFC, either in cortex or deep structures. For instance, since emotion is classically associated with visceral experiences, emotional experience may be reflected in somatosensory cortex, an area that is already known to support BCI [52,53].
Equally valuable, however, would be investigation of whether existing aBCIs can produce adequate results on smaller feature sets. There is certainly evidence from motor BCIs that a large number of source signals can be dropped from a decoder without substantially impairing performance [54-56]. Similar analyses would not be difficult to perform with existing emotion classifiers, and might yield a substantial reduction in computational complexity or hardware requirements. This has been attempted by one group, although with less than ideal results in their particular BCI framework [57]. Such studies might even be feasible using existing datasets, in an approach similar to off-line cross-validation.
Second, at least for the purpose of closed-loop brain stimulation, it would be desirable to implement the “hybrid BCI” strategy in the emotional domain. Hybrid BCIs, in which multiple physiologic signals or decoding strategies are fused together to improve control of a system, have recently attracted attention in motor control [58]. There has been preliminary evidence for this strategy’s efficacy in both invasive and non-invasive implementations [59,60]. An emotional hybrid BCI could be synthesized from an algorithm that estimated emotional state from numerous signals, combined with one that estimated clinical need/intention from relatively few. The more complex algorithm could thus run at a lower frequency, conserving power and computational effort. It would provide a much lower temporal resolution of the patient’s emotional state, but could still capture essential features (e.g., overall valence) that would then inform adjustment of stimulation parameters once the high-frequency, low-complexity BCI detected a clinical need. Conversely, adding the active intentional component would address concerns under Birbaumer’s [32] “extinction of thought” model.
The combination of two BCI models also may address concerns that patients could become “addicted” to the neurostimulator and begin activating it solely for recreational/hedonic goals. This is commonly seen with certain psychiatric medications, particularly stimulants and benzodiazepines. In Parkinsons disease, the leading indication for DBS, there is a known “dopamine dysregulation syndrome” that may worsen with brain stimulation [61,62]. The overall brain activity of a patient seeking brain stimulation for pleasure would likely reflect a very different emotional content than activity of a patient seeking stimulation for symptom relief. An aBCI could readily distinguish the two, making addictive behaviors futile.
5. Conclusion
We have highlighted the need for psychiatric neurostimulators, particularly implanted deep-brain stimulators, that are able to sense and rapidly respond to changes in a patient’s clinical state. These responsive, closed-loop systems would expand the number of disorders treatable with an exciting but presently limited technology. Recent developments in affective BCI are directly relevant to the development of such stimulators, and neurostimulation may represent an important new application of these BCIs. However, limitations imposed by DBS hardware and clinical factors prevent the direct translation of existing affective decoders as the sensing component of a closed-loop psychiatric DBS.
Therefore, we have proposed monitoring of volitionally modulated PFC signals (from single-unit to EEG-related potentials) as a strategy for obtaining meaningful data on a patient’s clinical need with minimal computational complexity. We have further presented preliminary evidence in rodents that such PFC BCIs are feasible and that animals can use them to obtain brain stimulation. The volitional PFC strategy could prove a useful complement to present emotional decoding approaches, either as the sole control component for a responsive DBS system or in a hybrid BCI. This approach may be useful in a wide variety of disorders, and it merits further investigation and discussion within the affective BCI community.
Acknowledgments
This work was supported by a seed grant from the Center for Sensorimotor Neural Engineering (National Science Foundation EEC-1028725) to ASW, as well as grants from the National Institute for Neurological Disorders and Stroke (R01 NS066357) and a DARPA Young FacultyAward (D12AP00251) to CTM.
Biographies
Dr. Widge received his PhD in Robotics in 2007 and the MD in 2008 from Carnegie Mellon University and the University of Pittsburgh, respectively. He completed psychiatric residency and advanced research training at the University of Washington, Seattle, WA, USA. He is currently a Research Fellow in the Department of Psychiatry at Massachusetts General Hospital, where he holds a dual appointment at the Massachusetts Institute of Technology as the Picower Clinical Neuroscience Fellow. He has served on the Board of Trustees of the American Psychiatric Association and has published research in the areas of neural electrode development, computational simulations of nanotechnology, and neuroscience education. His current research focus is closed-loop responsive brain stimulation systems.
Dr. Dougherty earned his MD in 1993 at the University of Illinois in Chicago, IL, USA. He then completed psychiatric residency at Massachusetts General Hospital in Boston, MA, where he served as a Chief Resident and subsequently a Clinical Fellow. Post-residency, he completed the MSc in Clinical Investigation through the Harvard Clinical Investigators Training Program and joined the Department of Psychiatry as a faculty member. He now directs the Division of Neurotherapeutics, which oversees multiple neuromodulation treatments, including DBS, rTMS, VNS, ECT, and tDCS. Clinically, he has deep expertise in depression and anxiety disorders, and lectures frequently worldwide on the treatment of depression and obsessive-compulsive disorder. He balances his productive research program against continued clinical work as a lead psychopharmacologist at the Obsessive-Compulsive Disorders Institute of McLean Hospital in Belmont, MA.
Dr. Moritz received his PhD in Integrative Biology from the University of California at Berkeley, CA in 2003. He trained as a postdoctoral fellow at the University of Colorado (Boulder, CO, USA) and University of Washington (Seattle, WA, USA) before joining the University of Washington faculty as a jointly appointed Professor in Physiology & Biophysics and Rehabilitation Medicine. He is a founding member of the University’s Center for Sensorimotor Neural Engineering and has published extensively in the use of BCI and related technologies for motor rehabilitation. He has committed substantial time to education on neuroscience and neurotechnology, including regular outreach to K-12 students, public presentations and museum exhibits.
References
- 1.Nijboer F, Carmien SP, Leon E, Morin FO, Koene RA, Hoffmann U. Affective brain-computer interfaces: Psychophysiological markers of emotion in healthy persons and in persons with amyotrophic lateral sclerosis. 3rd International Conference on Affective Computing and Intelligent Interaction and Workshops, 2009 ACII 2009; 2009. pp. 1–11. [Google Scholar]
- 2.Brouwer A-M, van Erp J, Heylen D, Jensen O, Poel M. Effortless Passive BCIs for Healthy Users. In: Stephanidis C, Antona M, editors. Universal Access in Human-Computer Interaction Design Methods, Tools, and Interaction Techniques for eInclusion [Internet] Springer; Berlin Heidelberg: 2013. [2013 Oct 30]. pp. 615–22. Available from: http://link.springer.com/chapter/10.1007/978-3-642-39188-0_66. [Google Scholar]
- 3.Nijholt A, Heylen DKJ. [2013 Oct 30];Editorial (to: Special Issue on Affective Brain-Computer Interfaces) [Internet] 2013 Available from: http://eprints.eemcs.utwente.nl/17755/
- 4.Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-Term Effects of Nucleus Accumbens Deep Brain Stimulation in Treatment-Resistant Depression: Evidence for Sustained Efficacy. Neuropsychopharmacology. 2012;37(9):1975–85. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlaepfer TE, Bewernick BH, Kayser S, Mädler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013 Jun 15;73(12):1204–12. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 6.Malone DA, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009 Feb 15;65(4):267–75. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, Wint D, Craighead MC, Kozarsky J, Chismar R, Moreines JL, Mewes K, Posse PR, Gutman DA, Mayberg HS. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012 Jan 2;69(2):150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, Lozano AM. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168(5):502–10. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 9.Etkin A. Functional Neuroimaging of Major Depressive Disorder: A Meta-Analysis and New Integration of Baseline Activation and Neural Response Data. Am J Psychiatry. 2012 Apr 25;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012 Jan;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Vizueta N, Rudie JD, Townsend JD, Torrisi S, Moody TD, Bookheimer SY, Altshuler LL. Regional fMRI Hypoactivation and Altered Functional Connectivity During Emotion Processing in Nonmedicated Depressed Patients With Bipolar II Disorder. Am J Psychiatry. 2012 Aug 1;169(8):831–40. doi: 10.1176/appi.ajp.2012.11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM) 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 13.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382(9904):1575–86. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 14.Sitaram R, Lee S, Ruiz S, Rana M, Veit R, Birbaumer N. Real-time support vector classification and feedback of multiple emotional brain states. NeuroImage. 2011 May 15;56(2):753–65. doi: 10.1016/j.neuroimage.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Moghimi S, Kushki A, Power S, Guerguerian AM, Chau T. Automatic detection of a prefrontal cortical response to emotionally rated music using multi-channel near-infrared spectroscopy. J Neural Eng. 2012 Apr 1;9(2):026022. doi: 10.1088/1741-2560/9/2/026022. [DOI] [PubMed] [Google Scholar]
- 16.Kim M-K, Kim M, Oh E, Kim S-P. A Review on the Computational Methods for Emotional State Estimation from the Human EEG. [2013 Oct 28];Comput Math Methods Med [Internet] 2013 Mar 24; doi: 10.1155/2013/573734. Available from: http://www.hindawi.com/journals/cmmm/2013/573734/abs/ [DOI] [PMC free article] [PubMed]
- 17.Kothe CA, Makeig S, Onton JA. Emotion_ Recognition_ from_ EEG_ During _Self Paced_ Emotional Imagery. Geneva, Switzerland: 2013. [2013 Oct 28]. Available from: http://sccn.ucsd.edu/~scott/pdf/ABCI_Kothe_Onton_Makeig_13cam.pdf. [Google Scholar]
- 18.Ward MP, Irazoqui PP. Evolving refractory major depressive disorder diagnostic and treatment paradigms: toward closed-loop therapeutics. Front Neuroengineering [Internet] 2010;3 doi: 10.3389/fneng.2010.00007. Available from: www.frontiersin.org/neuroscience/neuroengineering/paper/10.3389/fneng.2010.00007/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nesse RM, Stein DJ. Towards a genuinely medical model for psychiatric nosology. BMC Med. 2012 Jan 13;10(1):5. doi: 10.1186/1741-7015-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Widge AS, Avery DH, Zarkowski P. Baseline and treatment-emergent EEG biomarkers of antidepressant medication response do not predict response to repetitive transcranial magnetic stimulation. Brain Stimulat. 2013;6(6):929–31. doi: 10.1016/j.brs.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLoughlin G, Makeig S, Tsuang M. In search of biomarkers in psychiatry: EEG-based measures of brain function. Am J Med Genet B Neuropsychiatr Genet. 2014 doi: 10.1002/ajmg.b.32208. to appear. [DOI] [PubMed] [Google Scholar]
- 22.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013 May 14;11(1):126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011 Sep 27;77(13):1295–304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 24.Afshar P, Khambhati A, Carlson D, Dani S, Lazarewicz M, Cong P, Denison T. A translational platform for prototyping closed-loop neuromodulation systems. Front Neural Circuits. 2013;6:117. doi: 10.3389/fncir.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kragel PA, LaBar KS. Multivariate pattern classification reveals autonomic and experiential representations of discrete emotions. Emotion. 2013;13(4):681–90. doi: 10.1037/a0031820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganguly K, Dimitrov DF, Wallis JD, Carmena JM. Reversible large-scale modification of cortical networks during neuroprosthetic control. Nat Neurosci. 2011;14(5):662–9. doi: 10.1038/nn.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koralek AC, Jin X, Ii JDL, Costa RM, Carmena JM. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature. 2012 Mar 4;483(7389):331–5. doi: 10.1038/nature10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moritz CT, Perlmutter SI, Fetz EE. Direct control of paralysed muscles by cortical neurons. Nature. 2008;456:639–43. doi: 10.1038/nature07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schalk G, Miller KJ, Anderson NR, Wilson JA, Smyth MD, Ojemann JG, Moran DW, Wolpaw JR, Leuthardt EC. Two-Dimensional Movement Control Using Electrocorticographic Signals in Humans. J Neural Eng. 2008 Mar 15;5(1):75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blakely T, Miller KJ, Zanos SP, Rao RPN, Ojemann JG. Robust, long-term control of an electrocorticographic brain-computer interface with fixed parameters. Neurosurg Focus. 2009 Jul 1;27(1):E13. doi: 10.3171/2009.4.FOCUS0977. [DOI] [PubMed] [Google Scholar]
- 31.Moritz CT, Fetz EE. Volitional control of single cortical neurons in a brain–machine interface. J Neural Eng [Internet] 2011;8 doi: 10.1088/1741-2560/8/2/025017. Available from: stacks.iop.org/JNE/8/025017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birbaumer N. Breaking the silence: Brain–computer interfaces (BCI) for communication and motor control. Psychophysiology. 2006;43(6):517–32. doi: 10.1111/j.1469-8986.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- 33.Cromer JA, Roy JE, Miller EK. Representation of Multiple, Independent Categories in the Primate Prefrontal Cortex. Neuron. 2010 Jun 10;66(5):796–807. doi: 10.1016/j.neuron.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rigotti M, Barak O, Warden MR, Wang X-J, Daw ND, Miller EK, Fusi S. The importance of mixed selectivity in complex cognitive tasks. Nature. 2013 May 30;497(7451):585–90. doi: 10.1038/nature12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron. 2006 Sep 21;51(6):871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 36.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007 Oct 1;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Widge AS, Moritz CT, Matsuoka Y. Direct Neural Control of Anatomically Correct Robotic Hands. In: Tan DS, Nijholt A, editors. Brain-Computer Interfaces [Internet] Springer; London: 2010. [2013 Jun 4]. pp. 105–19. Available from: http://link.springer.com/chapter/10.1007/978-1-84996-272-8_7. [Google Scholar]
- 38.Widge AS, Moritz CT. Pre-frontal control of closed-loop limbic neurostimulation by rodents using a brain-computer interface. J Neural Eng. 2014 doi: 10.1088/1741-2560/11/2/024001. accepted; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gage GJ, Ludwig KA, Otto KJ, Ionides EL, Kipke DR. Naïve coadaptive cortical control. J Neural Eng. 2005;2(2):52. doi: 10.1088/1741-2560/2/2/006. [DOI] [PubMed] [Google Scholar]
- 40.Marzullo TC, Miller CR, Kipke DR. Suitability of the cingulate cortex for neural control. IEEE Trans Neural Syst Rehabil Eng. 2006 Dec;14(4):401–409. doi: 10.1109/TNSRE.2006.886730. [DOI] [PubMed] [Google Scholar]
- 41.Foa EB, Liebowitz MR, Kozak MJ, Davies S, Campeas R, Franklin ME, Huppert JD, Kjernisted K, Rowan V, Schmidt AB, Simpson HB, Tu X. Randomized, Placebo-Controlled Trial of Exposure and Ritual Prevention, Clomipramine, and Their Combination in the Treatment of Obsessive-Compulsive Disorder. Am J Psychiatry. 2005 Jan 1;162(1):151–61. doi: 10.1176/appi.ajp.162.1.151. [DOI] [PubMed] [Google Scholar]
- 42.Ellard KK, Fairholme CP, Boisseau CL, Farchione TJ, Barlow DH. Unified Protocol for the Transdiagnostic Treatment of Emotional Disorders: Protocol Development and Initial Outcome Data. Cogn Behav Pract. 2010 Feb;17(1):88–101. doi: 10.1016/j.cbpra.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev. 2010 Aug;30(6):635–41. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;44:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- 45.Nishimura Y, Perlmutter SI, Eaton RW, Fetz EE. Spike-Timing-Dependent Plasticity in Primate Corticospinal Connections Induced during Free Behavior. Neuron. 2013 Dec;80(5):1301–9. doi: 10.1016/j.neuron.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olds J. Self-stimulation of the brain: its use to study local effects of hunger, sex, and drugs. Science. 1958;127(3294):315–24. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- 47.Talwar SK, Xu S, Hawley ES, Weiss SA, Moxon KA, Chapin JK. Behavioural neuroscience: rat navigation guided by remote control. Nature. 2002;417(6884):37–8. doi: 10.1038/417037a. [DOI] [PubMed] [Google Scholar]
- 48.Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007 Nov;2(11):2987–95. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 49.Flint RD, Lindberg EW, Jordan LR, Miller LE, Slutzky MW. Accurate decoding of reaching movements from field potentials in the absence of spikes. J Neural Eng. 2012 Aug 1;9(4):046006. doi: 10.1088/1741-2560/9/4/046006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flint RD, Wright ZA, Scheid MR, Slutzky MW. Long term, stable brain machine interface performance using local field potentials and multiunit spikes. J Neural Eng. 2013 Oct 1;10(5):056005. doi: 10.1088/1741-2560/10/5/056005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curran EA, Stokes MJ. Learning to control brain activity: A review of the production and control of EEG components for driving brain–computer interface (BCI) systems. Brain Cogn. 2003 Apr;51(3):326–36. doi: 10.1016/s0278-2626(03)00036-8. [DOI] [PubMed] [Google Scholar]
- 52.Price DD. Psychological and Neural Mechanisms of the Affective Dimension of Pain. Science. 2000 Jun 9;288(5472):1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 53.Felton EA, Wilson JA, Williams JC, Garell PC. Electrocorticographically controlled brain-computer interfaces using motor and sensory imagery in patients with temporary subdural electrode implants: report of four cases. J Neurosurg. 2007;106(3):495–500. doi: 10.3171/jns.2007.106.3.495. [DOI] [PubMed] [Google Scholar]
- 54.Carmena JM, Lebedev MA, Crist RE, O’Doherty JE, Santucci DM, Dimitrov DF, Patil PG, Henriquez CS, Nicolelis MAL. Learning to Control a Brain-Machine Interface for Reaching and Grasping by Primates. PLoS Biol. 2003;1(2):193–208. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanchez JC, Carmena JM, Lebedev MA, Nicolelis MAL, Harris JG, Principe JC. Ascertaining the Importance of Neurons to Develop Better Brain-Machine Interfaces. IEEE Trans Biomed Eng. 2004 Jun;51(6):943–53. doi: 10.1109/TBME.2004.827061. [DOI] [PubMed] [Google Scholar]
- 56.Ifft PJ, Shokur S, Li Z, Lebedev MA, Nicolelis MAL. A Brain-Machine Interface Enables Bimanual Arm Movements in Monkeys. Sci Transl Med. 2013 Nov 6;5(210):210ra154–210ra154. doi: 10.1126/scitranslmed.3006159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mikhail M, El–Ayat K, Coan JA, Allen JJB. Using minimal number of electrodes for emotion detection using brain signals produced from a new elicitation technique. Int J Auton Adapt Commun Syst. 2013 Jan 1;6(1):80–97. [Google Scholar]
- 58.Allison BZ, Leeb R, Brunner C, Müller-Putz GR, Bauernfeind G, Kelly JW, Neuper C. Toward smarter BCIs: extending BCIs through hybridization and intelligent control. J Neural Eng. 2012 Feb 1;9(1):013001. doi: 10.1088/1741-2560/9/1/013001. [DOI] [PubMed] [Google Scholar]
- 59.Pfurtscheller G, Allison BZ, Brunner C, Bauernfeind G, Solis-Escalante T, Scherer R, Zander TO, Mueller-Putz G, Neuper C, Birbaumer N. The Hybrid BCI. [2013 Oct 30];Front Neurosci [Internet] 2010 Apr 21;4 doi: 10.3389/fnpro.2010.00003. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2891647/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S-P, Simeral JD, Hochberg LR, Donoghue JP, Friehs GM, Black MJ. Point-and-Click Cursor Control With an Intracortical Neural Interface System by Humans With Tetraplegia. IEEE Trans Neural Syst Rehabil Eng. 2011 Apr;19(2):193–203. doi: 10.1109/TNSRE.2011.2107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lim S-Y, O’Sullivan SS, Kotschet K, Gallagher DA, Lacey C, Lawrence AD, Lees AJ, O’Sullivan DJ, Peppard RF, Rodrigues JP, Schrag A, Silberstein P, Tisch S, Evans AH. Dopamine dysregulation syndrome, impulse control disorders and punding after deep brain stimulation surgery for Parkinson’s disease. J Clin Neurosci. 2009 Sep;16(9):1148–52. doi: 10.1016/j.jocn.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Widge AS, Agarwal P, Giroux M, Farris S, Kimmel RJ, Hebb AO. Psychosis from subthalamic nucleus deep brain stimulator lesion effect. [2013 Dec 7];Surg Neurol Int [Internet] 2013 Jan 18;4 doi: 10.4103/2152-7806.106265. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3589868/ [DOI] [PMC free article] [PubMed] [Google Scholar]


