Abstract
KABELIK, D., Weiss S. L. AND MOORE M. C. Steroid hormones alter neuroanatomy and aggression independently in the tree lizard. PHYSIOL BEHAV 00(0) 000-000, 0000. –Steroid hormones affect changes in both neuroanatomy and aggressive behavior in animals of various taxa. However, whether changes in neuroanatomy directly underlie changes in aggression is unknown. We investigate this relationship among steroid hormones, neuroanatomy, and aggression in a free-living vertebrate with a relatively simple nervous system, the tree lizard (Urosaurus ornatus). Weiss and Moore [1] manipulated testosterone and progesterone levels in adult male tree lizards and found that both hormones facilitated aggressive behavior toward a conspecific. In this study, we examined the brains of a subset of these animals to determine whether changes in limbic morphology were associated with hormone-induced changes in aggressive behavior. Specifically, we tested the hypothesis that testosterone and/or progesterone cause changes in neural morphology that are necessary for the expression of testosterone’s effects on aggressive behavior. We found that both hormones increased aggression; however, only testosterone induced changes in neuroanatomy. Testosterone increased the size of both the amygdala and nucleus sphericus. However, we could detect no individual correlations between neuroanatomy and aggression levels suggesting that the observed large-scale changes in neuroanatomy are not precisely reflective of changes in mechanisms underlying aggression.
Keywords: Testosterone, Progesterone, Aggression, Agonistic Behavior, Lizard, Reptile, Amygdala, Nucleus Sphericus
INTRODUCTION
Changes in steroid hormone levels are associated with changes in aggression in a variety of taxa [2–6]. Various neuroanatomical changes within brain regions known to regulate aggressive and reproductive behaviors are also regulated by testosterone or its metabolites [7–11]. However hormonal effects on the brain are diverse and adult hormone levels are known to modulate neuropeptide and neurotransmitter levels [12, 13], receptor abundance [14], enzymatic activity [15], neurogenesis and cell survival [16], neuronal structure [17], and gross neural morphology [18]. Yet, it is unclear which of these changes causally underlie the observed changes in aggressive behavior.
Gross neural morphology may be a measure of underlying changes that facilitate behavioral change. Recent studies in a variety of species have demonstrated that the volumes of certain brain nuclei can change with season or circulating steroid levels and are also associated with changes in behavior. Usually higher testosterone levels (or levels of androgenic and estrogenic metabolites) are associated with larger brain nuclei [7, 8]. These effects have been demonstrated most thoroughly in songbirds [19, 20], but exist also in mammals [11, 21] and reptiles [22, 23]. Larger brain regions have been associated with increased song production in songbirds [24] and reproductive behaviors in both mammals [25, 26] and birds [10, 27]. However, other studies show dissociations between brain structure and behavior [28, 29], and studies relating changes in neural structure to changes in aggressive behavior are limited.
In this study, we focused on steroid-mediated changes in the sizes of limbic brain nuclei in the tree lizard, Urosaurus ornatus, in relation to aggressive behavior. Using lizards as a model system provides benefits such as highly stereotyped and easily quantifiable aggressive displays [30]. In addition, limbic regions in reptiles comprise larger portions of their brains than mammals and birds [31]. Tree lizards are small, free-living iguanid lizards abundant in the deserts of southwestern North America. Males can be divided into morphological phenotypes (or morphs) that differ in dewlap (throat fan) coloration. Males with a bicolored orange-blue dewlap have been shown, under some circumstances, to be more aggressive and territorial than males with a unicolored orange dewlap [1, 32]. Ontogenetic testosterone and progesterone levels affect morph differentiation [33, 34], but morphs do not differ in adult hormone levels [32].
The work presented here is directly related to a pair of studies. First, Kabelik et al. [35] demonstrated seasonal changes in the volumes of limbic brain nuclei in tree lizards, and although testosterone levels were related to aggression levels, they were insufficient to explain all observed changes in neuroanatomy. Second, Weiss and Moore [1] conducted a hormone manipulation study in tree lizards in which they demonstrated that both increases in testosterone and progesterone levels induced increases in aggressive behavior. Progesterone’s role in the facilitation of aggression is much less established although it has been shown in other species to be involved in facilitating female aggression [36–38] and infanticidal male behavior [39]. Our interest in progesterone stems from its ability to affect tree lizard morph differentiation [33, 34] and thereby potentially also neural organization.
This present study uses the brains of male tree lizards from Weiss and Moore [1] to examine the hypothesis that testosterone and progesterone induce changes in morphology of limbic brain nuclei that underlie the accompanying increases in aggressive behavior.
METHODS
All procedures described herein were approved by the Arizona State University Institutional Animal Care and Use Committee, and were in accordance with NIH guidelines for the care and use of laboratory animals. Appropriate scientific collecting permits were obtained from the Arizona Game and Fish Department.
Subjects
The animals in this section are a subset of those whose behaviors were initially examined by Weiss and Moore [1]. Here, we present results from analyses of the brains of 39 of those animals. Unfortunately, due to equipment failure and overheating during tissue embedding, the remaining brains were damaged and not useable. We will briefly summarize the original study here, while detailing the methodology for our neural analyses.
Adult male tree lizards of both orange and orange-blue morphs were collected June – August 2000. Animals were housed in visual isolation in terraria (50×28×26) (l:w:h) divided in half by an opaque partition. Water and food (vitamin and calcium powdered crickets) were freely available.
Hormone manipulations
Surgeries were performed 07 July – 11 August, 2000. Males of each morph were randomly assigned to one of four treatment groups: blank (castrate with empty implant, n=8), sham (testes intact and no implant, n=12), progesterone (castrate with progesterone implant, n=12), and testosterone (castrate with testosterone implant, n=7). Bilateral castrations were performed according to Moore [3] and silastic implants (i.d. 1.47 mm, o.d. 1.96 mm, total length 1 cm, and steroid packing length of 2–3 mm) were then placed into the coelomic cavity of the animal.
Behavioral testing
The responses of focal males to orange-blue stimulus males were scored 22 days post-surgery. On the day before behavioral testing, each focal male and a size-matched stimulus male were placed into opposite ends of a partition-divided neutral test arena similar to their standard housing tanks. After a day of acclimation, the partition was removed and the lizards were allowed to interact for 15 min. We recorded the latency to display, the frequency of several displays (approaches, fullshows (push-up displays accompanied by lateral compression of the body and dewlap extension), chases, and bites), and maximal display intensity (low=no display, approach, or pushup without lateral compression of body; medium=fullshows and fullshow holds; high=chases; maximal=bites), as in Weiss and Moore [1].
Blood collection and hormone assays
Blood samples were taken from each focal male via the orbital sinus, 6–7 days following the behavioral test. The delay between testing and bleeding enabled hormone levels across groups to return to baseline levels. However, since hormone implants cause long-lasting increases in hormone levels, manipulated hormone levels at the time of bleeding should be similar to levels at the time of behavioral testing.
Blood samples were centrifuged within an hour of collection and the plasma fraction was frozen at −10°C until assayed. Plasma samples were assayed for progesterone and testosterone by radioimmunoassay following the methods of Moore [40]. Average testosterone and progesterone levels for the subset of animals examined in this study are presented in Table 1. Breeding season males normally have testosterone levels around a mean of 40–60 ng/ml [32], though individuals with levels equivalent to those in this experiment are sometimes seen. Therefore these testosterone values represent maximum physiological levels. However, progesterone treatment resulted in circulating progesterone levels about 100x greater than normally found in males (unpublished data), although much higher levels can be present in neonates [32]. Note that although sham animals were not castrated, steroid hormone levels were low in these animals. This may be the result of two factors. First, this experiment was conducted near the end of the breeding season (04 August – 08 September). Second, animals brought into the laboratory tend to have much lower testosterone levels than free-living individuals [41].
Table 1.
Mean circulating steroid hormone levels (±1 SE) in male tree lizards, according to treatment group.
| Treatment Group |
||||
|---|---|---|---|---|
| Castrate | Sham | Progesterone | Testosterone | |
| Progesterone (ng/ml) | 0.78 ± 0.16 | 1.29 ± 0.22 | 388.66 ± 63.15 | 2.17 ± 0.50 |
| Testosterone (ng/ml) | 0.25 ± 0.13 | 4.07± 0.84 | 0.67 ± 0.13 | 185.22 ± 36.88 |
These values are from the subset of animals examined in the present study and thus differ slightly from those in Weiss and Moore [1].
Brain fixation and staining
Following bleeding, subjects were rapidly decapitated, incisions were made into the skull to allow for penetration of fixative, and the entire head was placed within 5% acrolein in 0.1 M sodium phosphate buffer for 120 min at room temperature. The brains were then dissected out and dehydrated via an alcohol dehydration series (15 min each at: 50%, 70%, 95%, 2×100% ethanol) followed by 1-h immersion in Hemo-De clearing solution, and finally placement in a 60 °C oven, first for 15 min within a 50:50 mixture of Hemo-De and paraffin, and then 15 min within 100% paraffin. The paraffin was allowed to cool and the paraffin blocks were trimmed. Sections (10 µm thick) were cut on a microtome. The sections were mounted onto gelatin-coated slides and dried on a slide-warmer. The mounted sections were then rehydrated, Nissl-stained for 5 min using 0.5% Cresyl Violet dye, dehydrated, and cover-slipped.
Image analyses
We captured digital brain images using a camera (Panasonic GP-US502) through a 4x objective on a light microscope (Olympus BX40), producing an image on screen similar to a 40x (ocular × objective) magnification as seen through the microscope. These images were imported into an image analysis program (Image-Pro Plus, version 4.0, Media Cybernetics, Silver Springs MD). We kept all microscope, camera, and computer settings constant across samples. We located brain nuclei according to neural landmarks, as no stereotaxic atlas exists for this species. However, our brain nuclei delineations were determined by reference to other lizard atlases [42–45]. Furthermore, our delineations agree with other published research involving lizard brain nuclei [e.g. 46, 47, 48]. Consistency in orientation, start and end points, and extent of nuclei were scrupulously ensured by comparison to landmarks. We obtained neural measurements by manually outlining Nissl-stained regions of dense cell clustering at every 100 µm. The image analysis program generated area measurements based on a conversion factor for the given magnification. Brain nuclei were measured on both hemispheres (as in [35]) and an average of all cross-sectional areas was determined. We conducted all measurements blind to treatment group.
Brain nuclei
We measured the average cross-sectional area of seven brain nuclei in this study (Fig. 1). For detailed descriptions of brain nucleus localization, see Kabelik et al. [35]. Six of these nuclei were chosen because of their previous implication in the control of aggression [49–52] and/or the presence of steroid receptors [46–48, 53]. The habenula (HAB) was chosen as a control nucleus because it is easily quantifiable and devoid of steroid receptors. We could not obtain accurate volumetric measurements of these brain nuclei because the start and end points were often difficult to determine without a sterotaxic atlas for this species, especially in these thin 10-um sections. Therefore, we only measured a set number of representative sections per brain nucleus that we were confidant were part of each nucleus. We then averaged these cross-sectional area measures to generate an average cross-sectional area measure for each brain nucleus.
Figure 1.
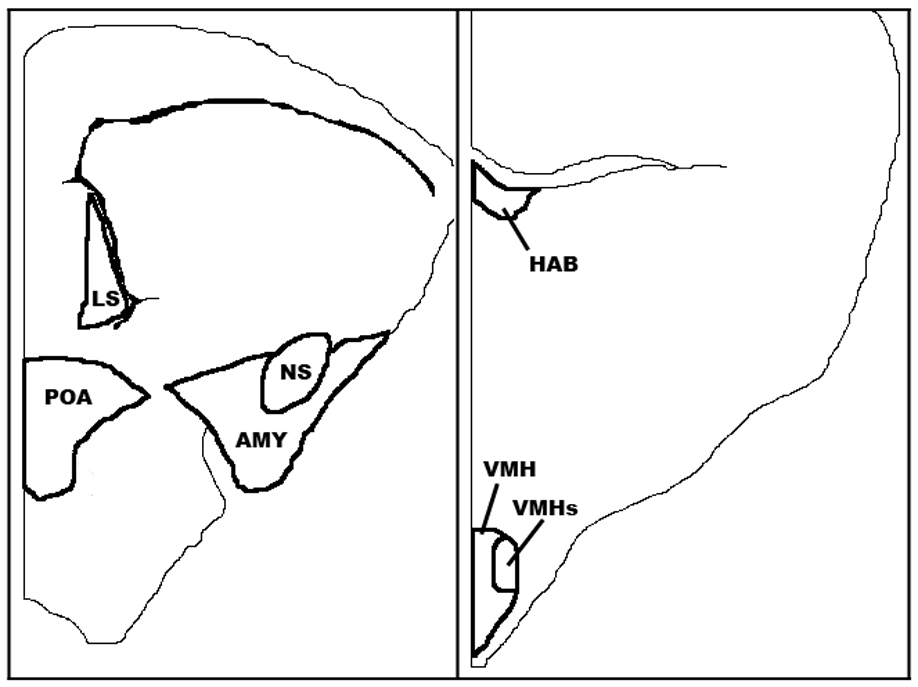
The brain nuclei quantified in this study: habenula (HAB), lateral septum (LS), preoptic area (POA), nucleus sphericus (NS), amygdala (AMY), ventromedial hypothalamic nucleus (VMH), and dorsolateral subdivision of ventromedial hypothalamic nucleus (VMHs), as traced from captured images. Modified from Kabelik et al. [35].
Habenula
The HAB was chosen as an easily quantifiable control region devoid of steroid receptors that we assumed would not be affected by changes in circulating steroid hormone levels. The tree lizard HAB, as in other species, is a very distinctive, darkly Nissl-staining region found adjacent to the third ventricle at the dorsal end of the diencephalon. We measured this nucleus across two brain sections.
Lateral Septum (LS)
Because the anterior end of the LS was difficult to consistently determine, we measured the lateral septum across four sections, near its posterior end, ending prior to the section on which it became longer along its medial-lateral axis than its dorsal-ventral axis.
Preoptic Area (POA)
We quantified the POA across three sections. At this point, the nucleus became less spherical and the dorsal end began to expand laterally. Although some lizard atlases treat this area as one continuous region [44, 45], our measures may have also included small portions of both the bed nucleus of the stria terminalis and suprachiasmatic nucleus, both of which are continuous with the POA in this and several other reptilian species [54]. Although these adjoining regions are small relative to the area of the POA [42], their inclusion may add some variation to our POA measures.
Nucleus Sphericus (NS)
The NS is a very distinctive spherical vomeronasal nucleus found in reptilian brains, associated with the amygdala and thought to be equivalent to the posteromedial cortical amygdala in mammals [55]. We quantified the NS across three sections where it was seen as a complete oval on a brain section (prior to its merging with the ventromedial wall of the dorsal ventricular ridge).
Amygdala (AMY)
Amygdaloid nuclei cannot be consistently distinguished from one another on Nissl-stained sections in this or related species [44], and so the entire amygdaloid region excluding the NS was measured as a whole and is referred to as AMY. This region is also known as the posterior dorsal ventricular ridge [56]. We measured the AMY across the same three brain sections as the NS.
Ventromedial hypothalamic nucleus (VMH)
We measured the ventromedial hypothalamic nucleus (VMH) across three sections where it was distinctly visible. The anterior region of the VMH was identified as a small oval clustering of cells on the ventral end of the diencephalon, immediately posterior to the disappearance of the optic chiasm. The VMH ended when the clustering of cells that defined its borders disappeared along the edge of the third ventricle.
VMH dorsolateral subdivision (VMHs)
A region of the dorsolateral VMH that is particularly dense in cells and steroid receptors [46, 48, 53] was also quantified separately and termed the VMH dorsolateral subdivision. This nucleus appears to be homologous with the lateral or ventrolateral subdivision of the VMH in mammals and birds [57–59], an area that shows activity during social stress [59, 60]. This small nucleus was measured across a single section where it was clearly distinguishable.
Whole Brain Size
To verify consistency of brain size across treatment groups, we estimated average cross-sectional brain area by averaging area measures of the entire brain section at the location of three landmarks throughout the tree lizard forebrain: 1. the anterior portion of the nucleus septalis impar [43], 2. the anterior portion of the habenula, 3. the midpoint of the posterior commisure.
Data analyses
Morph type and hormone treatment versus aggression frequency and latency analyses were conducted by Analysis of Variance (ANOVA), with LSMeans Student’s t post hoc analyses. Aggression frequency and latency data were log transformed to meet assumptions of normality, although figures display untransformed data. Morph type and hormone treatment versus aggression intensity analyses were conducted by ordinal logistic analysis. Brain nucleus comparisons were conducted using Multivariate Analysis of Variance (MANOVA) with hormone treatment or morph type as factors. If Wilks’ Lambda tests showed a significant multivariate effect, individual one-way ANOVAs were performed for each dependent variable (brain region). Post hoc analyses were conducted with the LSMeans Student’s t post hoc comparisons tests with α<0.05. Correlations between hormones and brain nuclei measures, as well as between brain nuclei and measures of aggressive behavior were conducted using Spearman’s rho, as were follow-up correlations of testosterone or progesterone versus aggression frequency. Animals with ambiguous dewlap coloration were excluded from morph comparisons, reducing the sample size of those analyses by five. Due to tissue damage, the sample size for image analyses of the SPH, AMY, VMH, and VMHs were reduced by one progesterone-treated orange-blue male, and analyses of the VMH and VMHs were reduced by one further sham-treated orange male.
RESULTS
Hormone Treatment and Aggression
Although Weiss and Moore [1] demonstrated an effect of testosterone and progesterone on aggression, and no morph differences in behavior, we wanted to verify those results in the subset of males examined in this study. We likewise found no differences between morphs types in aggression frequency (t=0.32, n=34, p=0.75), latency to display (t=1.69, n=34, p=0.10), or intensity (χ2=0.00, n=34, p=1.00). In addition, we found no effect of morph type on measures of average cross-sectional brain nucleus areas (overall MANOVA: F(7,24)=0.64, p=0.72; all one-way ANOVAs for individual brain areas: p>0.05). We thus pooled morph type in all subsequent analyses.
We found that testosterone treatment increased the frequency of fullshow displays to a conspecific in relation to blank-treated or sham males, while progesterone-treated males showed a greater aggression frequency than blank-treated males (F(3,35)=5.49, p=0.003, Fig. 2, top). An ordinal logistic model also showed a marginal effect of hormone treatment on behavioral intensity (χ2=7.51, n=39, p=0.057, Fig. 2, middle), which is in line with the data in Weiss and Moore [1]. An analysis of latency to display was nonsignificant due to high variance (F(3,35)=2.17, p=0.11, Fig. 2, bottom, figure depicts untransformed data), though the trend was also consistent with Weiss and Moore [1].
Figure 2.
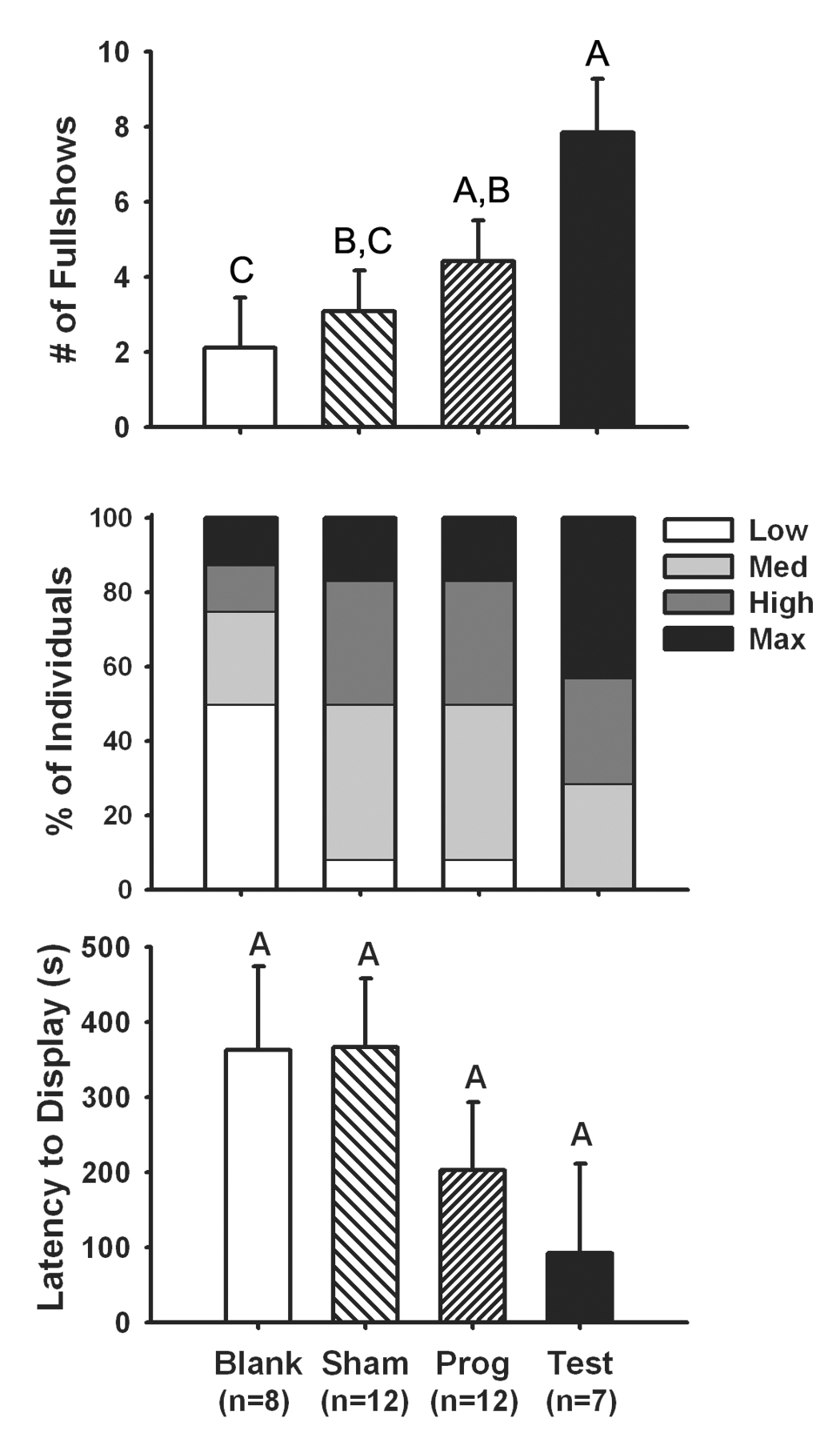
(Top) Testosterone treatment increased the frequency of fullshow displays by male tree lizards to male conspecifics relative to late-season sham surgery males, or castrated males treated with a blank implant. Progesterone treatment increased aggression frequency relative to castrated males treated with a blank implant. (Middle) A marginal positive effect of testosterone treatment on maximal aggression intensity was also observed. (Bottom) A non-significant trend showing a negative effect of testosterone treatment on latency to attack was consistent with data in Weiss and Moore [1]. Bars represent 1 S.E. Common letters above bars designate homogenous groups at α=0.05. See text for statistics.
Hormone Treatment and Brain Morphometrics
As expected, there were no differences among treatment groups in snout-vent length (F(3,35)=0.01, p=1.0), body mass (F(3,35)=0.12, p=0.95), or our average cross-sectional brain area (F(3,35)=2.617, p=0.067). Because average cross-sectional brain area was near significance, we also conducted analyses with this as a covariate and obtained similar results.
We detected a significant overall effect of hormone treatment on brain nucleus sizes (F(21,78)=2.16, p=0.008; Fig. 3). One-way ANOVAs showed that this relationship was significant for the NS (F(3,34)=2.97, p=0.046) and AMY (F(3,34)=3.43, p=0.028), but not any other brain nuclei (p>0.05 for all). Posthoc analyses revealed that the NS and AMY are larger in testosterone-treated animals than in any other group.
Figure 3.
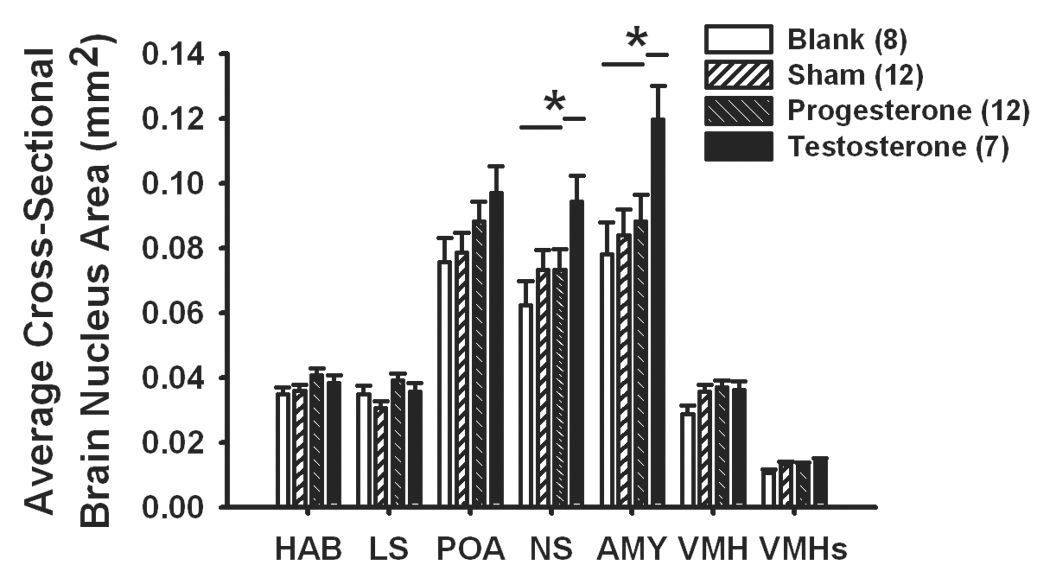
Testosterone treatment increased average cross-sectional areas of the nucleus sphericus (NS) and amygdala (AMY) within male tree lizards, relative to late-season sham surgery males, or castrated males treated with a blank implant or a progesterone-filled implant. HAB=habenula, LS=lateral septum, POA=preoptic area, VMH=ventromedial hypothalamus, VMHs=dorsolateral subdivision of the ventromedial hypothalamus. Bars represent 1 S.E. Sample sizes per group are listed within parentheses within the legend. * denotes a difference between groups at p<0.05 and lines over bars denote homogenous groups. Morph types did not significantly differ in the volume of any brain region. See text for statistics.
Because hormone levels differed substantially between treatment groups, we also examined within-treatment correlations between hormones and brain nucleus measures (Fig. 4). We only examined sham or hormone-manipulated groups of animals with non-minimal levels of testosterone (sham and testosterone treatment) or progesterone (sham and progesterone treatment). We found that testosterone level was significantly correlated with the sizes of both the NS (rs=0.56, n=19, p=0.012) and AMY (rs=0.62, n=19, p=0.005). Sample sizes within individual treatment groups are small, but Pearson's correlations trends are similar to the combined data, with only testosterone treatment by AMY size being significant (NS: sham, r=0.42, n=12, p=0.17; testosterone, r=0.56, n=7, p=0.19; AMY: sham, r=0.40, n=12, p=0.20; testosterone, r=0.77, n=7, p=0.044). Progesterone level was not related to either the size of the NS (rs=0.17, n=23, p=0.44) or AMY (rs=−0.018, n=23, p=0.94), and within-group analyses were all nonsignificant (p>0.05 for all). Within the sham treatment group, some animals likely experienced seasonal declines in hormone levels more recently than others, thus creating the observed variation in brain measurements.
Figure 4.
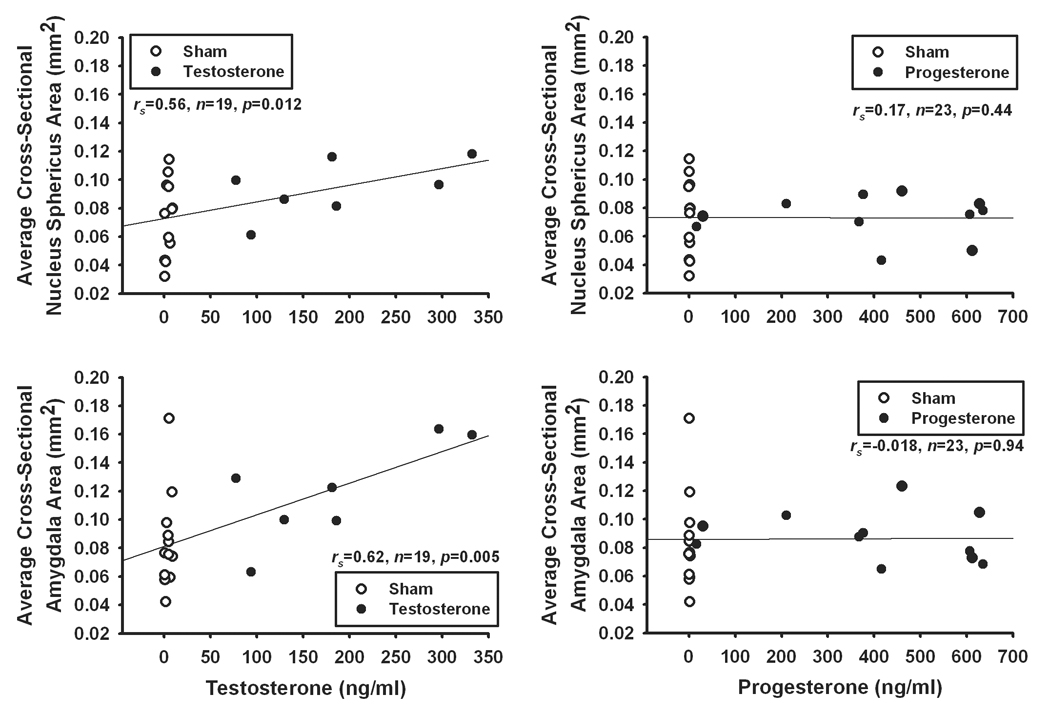
Scatterplots of Spearman’s correlations between testosterone or progesterone level and brain nucleus sizes in sham surgery and hormone-treated male tree lizards. Testosterone level was found to correlate with the average cross-sectional area of both the NS (top left) and AMY(bottom left). Contrarily, progesterone level was not related to the size of either the NS (top right) or AMY (bottom right).
For comparison purposes, we also conducted similar analyses between hormone level and aggression frequency (Fig. 5). Testosterone level was correlated with aggression frequency (rs=0.54, n=19, p=0.016), and progesterone exhibited a marginally-significant trend in the same direction (rs=0.39, n=24, p=0.059). However, as in the hormone to brain comparisons, no within group analyses were significant (p>0.05 for all).
Figure 5.
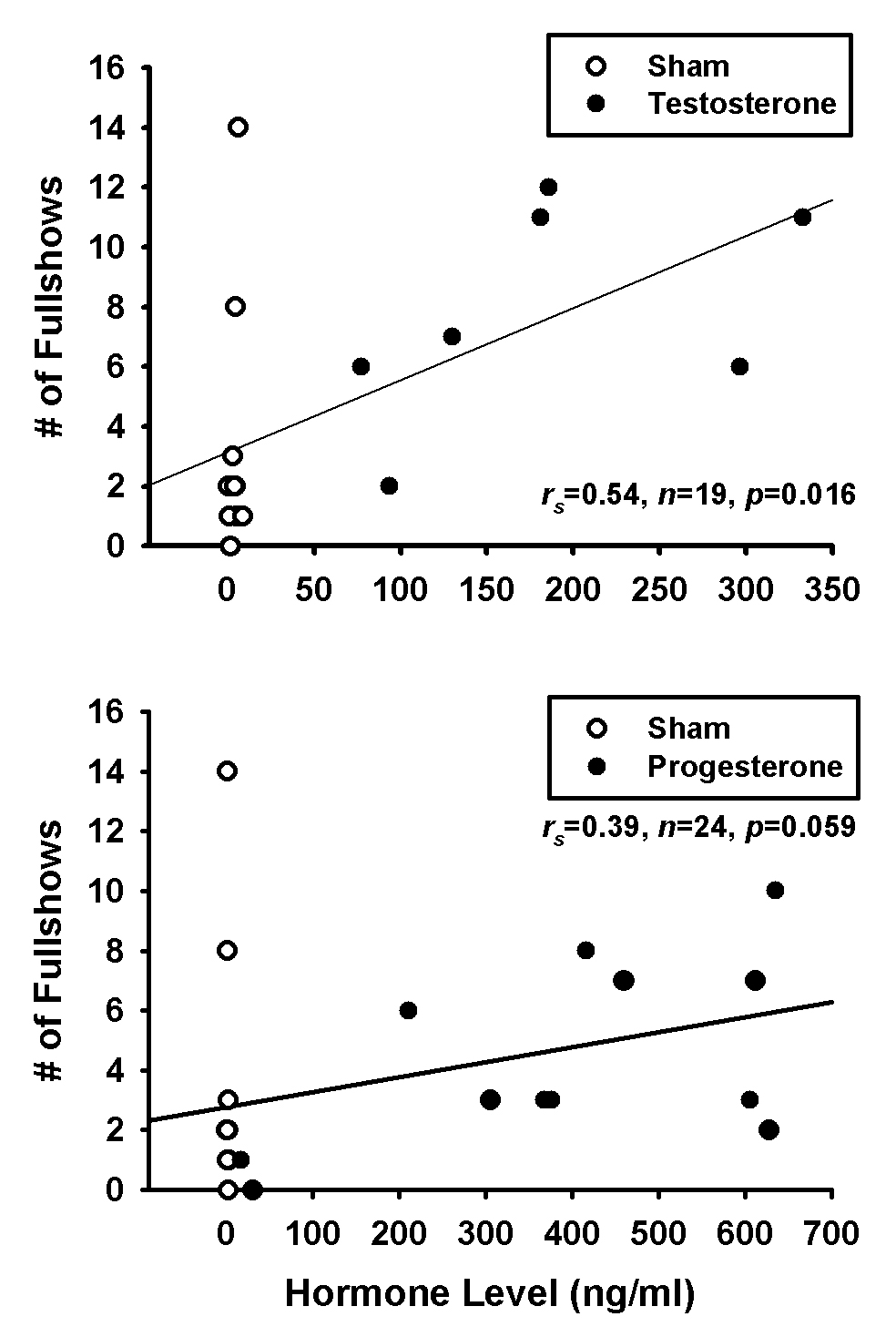
Scatterplots of Spearman's correlations between (Top) testosterone or (Bottom) progesterone level and frequency of fullshow displays.
Brain nuclei and aggression
For these analyses, we again only examined groups of animals with non-minimal levels of testosterone (sham and testosterone treatment). We found no significant correlations between either AMY or NS size and the number of bites at an intruder, the number of fullshow displays to an intruder, or the latency to performing the first aggressive display (Table 2). Weiss and Moore [1] had found all three of these behaviors to be altered by testosterone treatment. To confirm this lack of a correlation between brain nuclei and behavior, we repeated the correlations, this time using animals across all treatment groups. Even though testosterone treatment increased both aggression and the size of brain nuclei, when examining animals across all treatment groups, we found no individual-level correlations between neuroanatomy and behavior (Table 2).
Table 2.
Spearman’s correlations of nucleus sphericus or amygdala size in male tree lizards with frequency of bites, frequency of fullshows, or latency to display (either within sham and testosterone (T) treatment groups, or across all males regardless of treatment group).
| Nucleus Sphericus | Amygdala | |||||||
|---|---|---|---|---|---|---|---|---|
| sham and T (n=19) | all males (n=38) | sham and T (n=19) | all males (n=38) | |||||
| rs | p | rs | p | rs | p | rs | p | |
| # bites | 0.31 | 0.19 | 0.25 | 0.13 | 0.25 | 0.30 | 0.23 | 0.16 |
| # fullshows | 0.30 | 0.21 | 0.24 | 0.15 | 0.32 | 0.18 | 0.26 | 0.11 |
| Latency | −0.27 | 0.27 | −0.13 | 0.42 | −0.30 | 0.20 | −0.12 | 0.49 |
DISCUSSION
This study demonstrated a positive relationship between the steroid hormone testosterone and the sizes of limbic brain nuclei, specifically the AMY and NS. Although both testosterone and progesterone have previously been shown to increase aggression in male tree lizards [1, 35], this study demonstrates that only testosterone treatment and not progesterone treatment concurrently alters the size of limbic brain nuclei. This finding suggests that progesterone alters aggression independently of morphological size changes of the examined limbic brain nuclei. Although we found a positive effect of testosterone treatment on AMY and NS size, as well as on aggression levels, the size of these brain regions were not directly correlated with measures of aggressive behavior to a conspecific. This finding, taken together with other published literature demonstrating dissociations between limbic nucleus structure and function, suggests that the effects of testosterone on aggression also need not involve the large-scale changes in neural structure observed in the limbic system following increases in testosterone level.
Hormones and brain nuclei
Reptiles, with limbic regions comprising a larger portion of their brains than those of mammals and birds, present the prospect of a simplified neural circuit regulating aggression, while still sharing many neural characteristics with other vertebrates. In the present study, subjects treated with testosterone for four weeks had larger AMY and NS sizes than did blank, sham, or progesterone treated individuals. These results support a role for testosterone in effecting changes in neuroanatomy [7, 11, 24]. The precise changes underlying these gross morphological changes (e.g., larger soma sizes, more extensive dendritic connectivity, a greater number of cells) are not clear and require further investigation, but our results suggest the AMY and NS as targets of such further research.
As in most other vertebrate species, the tree lizard limbic system possesses numerous steroid receptors, including androgen and estrogen receptors in the diencephalon and amygdala region of the telencephalon, and progesterone receptors within the diencephalon (unpublished results). These findings are corroborated by other researchers who have demonstrated the presence of androgen and estrogen receptors within the reptilian limbic system [46, 48, 53, 61], as well as presence of the enzyme aromatase [62] in these regions.
Because of the presence of both androgen and estrogen receptors within tree lizard limbic systems, it is possible that testosterone's effects on the AMY and NS can be via either androgenic or estrogenic pathways. Conversely, the lack of progesterone receptors in these nuclei [53] is consistent with our failure to detect an effect of this hormone on the size of the AMY and NS.
In our parallel field study [35], we found changes in the size of the POA, both with season and with male reproductive state (reflective of long-term steroid hormone levels), suggesting an effect of testosterone on this region. In the current study, we only observed a nonsignificant trend, with testosterone treated animals seeming to possess a slightly larger POA than animals in the other treatment groups. It is possible that low power in the current study prevented us from detecting a difference in this brain nucleus. Unlike the AMY and NS, the POA in reptiles contains not only androgen and estrogen, but also progesterone receptors [unpublished research; 48, 53]. Despite this fact, no effect of progesterone on POA size was observed, further suggesting that progesterone does not alter brain nucleus sizes as does testosterone.
The seasonal changes in AMY, NS, and POA volumes observed by Kabelik et al. [35] were however only partly explained by male reproductive state, suggesting the additional involvement of seasonally varying factors other than steroid levels. Females in that study also exhibited seasonal variation in brain nuclei volumes, though these were completely independent of steroid hormone levels. Other studies have also demonstrated partial to complete independence of neural plasticity and circulating testosterone levels [24, 29, 63, 64]. Therefore, firm conclusions regarding the involvement of steroid hormones in seasonal neural variation are sometimes difficult to draw. The present study demonstrates that testosterone treatment indeed causes increases of male limbic brain nuclei and therefore suggests that this hormone plays at least a partial role in regulating seasonal neural plasticity.
Aggression
Testosterone, and to a lesser degree progesterone, were both found to increase the expression of aggressive behavior in the study by Weiss and Moore [1]. We examined brains from a portion of these animals for the current study, and the behavioral data for these individuals are displayed in Figure 2. Although the sample sizes in this study are smaller than in Weiss and Moore [1], the behavioral trend of this subgroup are the same, with testosterone and progesterone treatment both inducing significant increases in aggressive display to a conspecific, though treatment with testosterone showed more robust effects. It is also important to point out that whereas the testosterone treatment resulted in high but physiological circulating levels of this hormone, progesterone treatment resulted in supraphysiological hormone levels. Therefore, it is unclear whether the effects of progesterone on aggression were via traditional progesterone pathways, or by interacting with other signaling systems.
AMY and NS size differed among hormone treatment groups similarly to how aggression differed among these groups. Both aggression scores and brain nucleus sizes were greatest in the testosterone treatment group and least in the blank-implant group, seemingly supporting the hypothesis that increases in the size of steroid sensitive limbic brain nuclei underlie increases in aggression. However, these could also be coincidental changes, both driven by hormone level but independent of one another. Therefore, to examine the relationship between brain nucleus sizes and aggression in more detail, we calculated correlations between these variables (Table 2). None of these analyses detected a direct relationship between structure size and behavior on an individual level, even when examining animals across all treatment groups. Such an analysis should be biased toward finding a correlation since low-testosterone animals have both lower aggression and smaller AMY and NS sizes than high-testosterone animals. However, the brain nucleus sizes also seemed affected by other unknown factors and thus no clear correlations between structure size and function was present.
We also conducted similar analyses in a parallel field study [35]. Whereas in the present study we used a neutral arena paradigm to maximize aggression, the field study employed a resident-intruder paradigm. However, as in this study, no correlations were detected between brain nucleus sizes and levels of aggressive behavior. Together, these results suggest that the effects of testosterone on brain nuclei are at least somewhat independent of testosterone’s affects on aggressive behavior. It is still possible that some changes in neural morphology are necessary to achieve a change in aggression level, but these changes may be of a much more limited magnitude than those examined in these studies.
In order to allow hormone levels to return to baseline, animals in the present study were not sacrificed until a week following behavioral trials. It is thus possible that the aggressive encounter itself may have had some influence on neural morphology, perhaps even synergistically with the increased hormone levels. However, because we found no direct correlations between behavior and brain morphology, it seems that any neural changes resulted primarily from changes in testosterone level. It is also possible that other factors induced changes in neural structure during the week between behavioral testing and tissue harvesting. However, we obtained parallel results from our seasonal field study [35], during which animals were bled and sacrificed immediately following a 3-min behavioral trial. We did not find any associations between brain nucleus sizes and behavior in that study either, suggesting that the delay between behavioral and neural sampling was not the reason for the lack of associations.
Similar dissociations between testosterone’s effects on brain morphology and on reproductive behavior were also observed in whiptail lizards [23] and anoles [65]. On the other hand, correlations between brain nucleus volumes and behavior are often present in the songbird literature [24], though dissociations between song and song nucleus volumes have also been detected [28]. Correlations between limbic brain nuclei and reproductive behaviors have also been observed in rodents [25, 26] and birds [10, 27]. Why structure-function relationships between brain nucleus sizes and reproductive/aggressive behaviors are observed under some but not other conditions is unclear and requires further investigation.
The regulation of aggression by testosterone is complex and within a species, regulation in one season may differ from regulation in another season [66]. Regulation may also be species specific and depend upon other hormone-related factors such as the presence or absence of parental care. Although previous research involving tree lizards had suggested that testosterone must simply be present in sufficient concentration to surpass an activational threshold for the full expression of aggressive behaviors [67], aggression in this species may be even more directly tied to immediate testosterone levels. In our field study [35], we found that immediate measures of testosterone level following a three-minute field behavioral encounter were more closely related to aggressive display than long-term seasonal differences in aggression. This suggests that very rapid changes in neurochemistry may be involved in testosterone's regulation of aggression, and such changes in neurochemistry must also be considered in addition to any changes in neural structure.
Male morphs
In previous studies, male tree lizard morphs differed in aggression level [32]. However, under the environmental conditions of recent experiments, no such differences have been observed [1, 35], suggesting that differences between the morphs may be more facultative than previously believed. Likewise, we observed no neural differences between morphs in this study, or in our recent field study [35]. Morphs do not differ in adult hormone level and their behaviors seem to be organized during development [32]. Hence, it is unclear whether the lack of an observed morph difference in behavior, especially in our sham surgery group, was due to late-season gonadal recrudescence and laboratory housing, thus leading to lower than normal testosterone levels in both morphs, or a natural change in this population minimizing behavioral and potentially also neural differences between morphs [1].
Conclusion
Past studies in various species have demonstrated that steroid hormone levels alter both neural morphology and aggressive behavior. A field study that we conducted in tree lizards showed seasonal plasticity in limbic brain nucleus volumes, but did not provide conclusive evidence for the involvement of steroid hormones in this process. The current study demonstrated that testosterone, but not progesterone, can alter the size of the AMY and NS. Therefore, testosterone level is also likely one of the factors involved in mediating seasonal volumetric plasticity in the brains of male tree lizards. We also conducted correlations between AMY and NS size and levels of displayed aggression to a conspecific. Even though testosterone-treated animals had the largest AMY and NS sizes, and also the greatest aggression levels, we were unable to detect individual correlations between these variables. These results confirmed those of our field study and suggest that testosterone has at least partly independent effects on brain nucleus morphology and aggressive behavior, though it alters these variables simultaneously.
Acknowledgements
We would like to thank DF Denardo, P Deviche, M Orchinik, RL Rutowski, and two anonymous reviewers for comments on the manuscript and also T Crombie and TA Sultani for help in the laboratory. This research was funded by NIMH grants (5R01MH048564-10) to MCM and (MH12112) to SLW, as well as NSF DDIG (0408009) to DK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiss SL, Moore MC. Activation of aggressive behavior by progesterone and testosterone in male tree lizards, Urosaurus ornatus. Gen Comp Endocrinol. 2004;136(2):282–288. doi: 10.1016/j.ygcen.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Beeman EM. The effect of male hormone on aggressive behaviour in mice. Physiol Zool. 1947;20:373–405. doi: 10.1086/physzool.20.4.30151969. [DOI] [PubMed] [Google Scholar]
- 3.Moore MC. Castration affects territorial and sexual behavior of free-living male lizards, Sceloporus jarrovi. Anim Behav. 1987;35:1193–1199. [Google Scholar]
- 4.Schwabl H, Kriner E. Territorial aggression and song of male European robins (Erithacus rubecula) in autumn and spring: effects of antiandrogen treatment. Horm Behav. 1991;25(2):180–194. doi: 10.1016/0018-506x(91)90049-n. [DOI] [PubMed] [Google Scholar]
- 5.Cardwell JR, Liley NR. Androgen control of social status in males of a wild population of stoplight parrotfish, Sparisoma viride (Scaridae) Horm Behav. 1991;25(1):1–18. doi: 10.1016/0018-506x(91)90035-g. [DOI] [PubMed] [Google Scholar]
- 6.Ros AF, Bruintjes R, Santos RS, Canario AV, Oliveira RF. The role of androgens in the trade-off between territorial and parental behavior in the Azorean rock-pool blenny, Parablennius parvicornis. Horm Behav. 2004;46(4):491–497. doi: 10.1016/j.yhbeh.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23(6):251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- 8.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: Principles and mechanisms. Front Neuroendocrinol. 1998;19(4):323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 9.Charlier TD, Ball GF, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J Neurosci. 2005;25(4):906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38(4):250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- 11.Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96(13):7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brot MD, De Vries GJ, Dorsa DM. Local implants of testosterone metabolites regulate vasopressin mRNA in sexually dimorphic nuclei of the rat brain. Peptides. 1993;14(5):933–940. doi: 10.1016/0196-9781(93)90069-s. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy MM, Pfaus JG. Steroid modulation of neurotransmitter function to alter female reproductive behavior. Trends Endocrin Met. 1996;7(9):327–333. doi: 10.1016/s1043-2760(96)00157-9. [DOI] [PubMed] [Google Scholar]
- 14.McIntyre KL, Porter DM, Henderson LP. Anabolic androgenic steroids induce age-, sex-, and dose-dependent changes in GABA(A) receptor subunit mRNAs in the mouse forebrain. Neuropharmacology. 2002;43(4):634–645. doi: 10.1016/s0028-3908(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 15.Fusani L, Hutchison JB, Gahr M. Testosterone regulates the activity and expression of aromatase in the canary neostriaturn. J Neurobiol. 2001;49(1):1–8. doi: 10.1002/neu.1061. [DOI] [PubMed] [Google Scholar]
- 16.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64(1):34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- 18.Tramontin AD, Wingfield JC, Brenowitz EA. Androgens and estrogens induce seasonal-like growth of song nuclei in the adult songbird brain. J Neurobiol. 2003;57(2):130–140. doi: 10.1002/neu.10263. [DOI] [PubMed] [Google Scholar]
- 19.Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214(4527):1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 20.Smith GT, Brenowitz EA, Wingfield JC. Roles of photoperiod and testosterone in seasonal plasticity of the avian song control system. J Neurobiol. 1997;32(4):426–442. doi: 10.1002/(sici)1097-4695(199704)32:4<426::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 21.Commins D, Yahr P. Adult testosterone levels influence the morphology of a sexually dimorphic area in the Mongolian gerbil brain. J Comp Neurol. 1984;224(1):132–140. doi: 10.1002/cne.902240112. [DOI] [PubMed] [Google Scholar]
- 22.Wade J, Crews D. The relationship between reproductive state and "sexually" dimorphic brain areas in sexually reproducing and parthenogenetic whiptail lizards. J Comp Neurol. 1991;309(4):507–514. doi: 10.1002/cne.903090407. [DOI] [PubMed] [Google Scholar]
- 23.Wade J, Huang JM, Crews D. Hormonal control of sex differences in the brain, behavior and accessory sex structures of whiptail lizards (Cnemidophorus species) J Neuroendocrinol. 1993;5(1):81–93. doi: 10.1111/j.1365-2826.1993.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 24.Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front Neuroendocrinol. 2002;23(2):137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- 25.Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43(2):336–346. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 26.Cooke BM, Chowanadisai W, Breedlove SM. Post-weaning social isolation of male rats reduces the volume of the medial amygdala and leads to deficits in adult sexual behavior. Behav Brain Res. 2000;117(1–2):107–113. doi: 10.1016/s0166-4328(00)00301-6. [DOI] [PubMed] [Google Scholar]
- 27.Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17(1):51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- 28.Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria) Horm Behav. 2005;47(4):467–476. doi: 10.1016/j.yhbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Leitner S, Voigt C, Garcia-Segura LM, Van't Hof T, Gahr M. Seasonal activation and inactivation of song motor memories in wild canaries is not reflected in neuroanatomical changes of forebrain song areas. Horm Behav. 2001;40(2):160–168. doi: 10.1006/hbeh.2001.1700. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter CC. Ritualistic social behaviors in lizards. In: Greenberg N, MacLean PD, editors. Behavior and Neurology of Lizards. Rockville, MD: NIMH; 1978. pp. 253–267. [Google Scholar]
- 31.MacLean PD. Why brain research on lizards? In: Greenberg N, MacLean PD, editors. Behavior and Neurology of Lizards. Rockville, MD: NIMH; 1978. pp. 1–10. [Google Scholar]
- 32.Moore MC, Hews DK, Knapp R. Hormonal control and evolution of alternative male phenotypes: Generalizations of models for sexual differentiation. Am Zool. 1998;38(1):133–151. [Google Scholar]
- 33.Hews DK, Knapp R, Moore MC. Early exposure to androgens affects adult expression of alternative male types in tree lizards. Horm Behav. 1994;28(1):96–115. doi: 10.1006/hbeh.1994.1008. [DOI] [PubMed] [Google Scholar]
- 34.Hews DK, Moore MC. A critical period for the organization of alternative male phenotypes of tree lizards by exogenous testosterone? Physiol Behav. 1996;60(2):425–429. doi: 10.1016/s0031-9384(96)80014-x. [DOI] [PubMed] [Google Scholar]
- 35.Kabelik D, Weiss SL, Moore MC. Steroid hormone mediation of limbic brain plasticity and aggression in free-living tree lizards, Urosaurus ornatus. Horm Behav. 2006;49(5):587–597. doi: 10.1016/j.yhbeh.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Gleason PE, Michael SD, Christian JJ. Effects of gonadal steroids on agonistic behavior of female Peromyscus leucopus. Horm Behav. 1979;12(1):30–39. doi: 10.1016/0018-506x(79)90024-2. [DOI] [PubMed] [Google Scholar]
- 37.Kapusta J. Gonadal hormones and intrasexual aggressive behavior in female bank voles (Clethrionomys glareolus) Aggressive Behav. 1998;24(1):63–70. [Google Scholar]
- 38.Meisel RL, Sterner MR. Progesterone inhibition of sexual behavior is accompanied by an activation of aggression in female Syrian hamsters. Physiol Behav. 1990;47(3):415–417. doi: 10.1016/0031-9384(90)90102-a. [DOI] [PubMed] [Google Scholar]
- 39.Schneider JS, Stone MK, Wynne-Edwards KE, Horton TH, Lydon J, O'Malley B, Levine JE. Progesterone receptors mediate male aggression toward infants. Proc Natl Acad Sci U S A. 2003;100(5):2951–2956. doi: 10.1073/pnas.0130100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore MC. Elevated testosterone levels during nonbreeding-season territoriality in a fall-breeding lizard, Sceloporus jarrovi. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1986;158(2):159–163. doi: 10.1007/BF01338559. [DOI] [PubMed] [Google Scholar]
- 41.Moore MC, Thompson CW, Marler CA. Reciprocal changes in corticosterone and testosterone levels following acute and chronic handling stress in the tree lizard, Urosaurus ornatus. Gen Comp Endocrinol. 1991;81(2):217–226. doi: 10.1016/0016-6480(91)90006-r. [DOI] [PubMed] [Google Scholar]
- 42.Greenberg N. A forebrain atlas and stereotaxic technique for the lizard, Anolis carolinensis. J Morphol. 1982;174(2):217–236. doi: 10.1002/jmor.1051740210. [DOI] [PubMed] [Google Scholar]
- 43.Smeets WJ, Hoogland PV, Lohman AH. A forebrain atlas of the lizard Gekko gecko. J Comp Neurol. 1986;254(1):1–19. doi: 10.1002/cne.902540102. [DOI] [PubMed] [Google Scholar]
- 44.Northcutt RG. Arthitectonic studies of the telencephalon of Iguana iguana. J Comp Neurol. 1967;130(2):109–148. doi: 10.1002/cne.901300203. [DOI] [PubMed] [Google Scholar]
- 45.Butler AB, Northcutt RG. Architectonic studies of the diencephalon of Iguana iguana (Linnaeus) J Comp Neurol. 1973;149(4):439–462. doi: 10.1002/cne.901490404. [DOI] [PubMed] [Google Scholar]
- 46.Rosen G, O'Bryant E, Matthews J, Zacharewski T, Wade J. Distribution of androgen receptor mRNA expression and immunoreactivity in the brain of the green anole lizard. J Neuroendocrinol. 2002;14(1):19–28. doi: 10.1046/j.0007-1331.2001.00735.x. [DOI] [PubMed] [Google Scholar]
- 47.Moga MM, Geib BM, Zhou D, Prins GS. Androgen receptor-immunoreactivity in the forebrain of the Eastern Fence lizard (Sceloporus undulatus) Brain Res. 2000;879(1–2):174–182. doi: 10.1016/s0006-8993(00)02771-2. [DOI] [PubMed] [Google Scholar]
- 48.Morrell JI, Crews D, Ballin A, Morgentaler A, Pfaff DW. 3H-estradiol, 3H-testosterone and 3H-dihydrotestosterone localization in the brain of the lizard Anolis carolinensis: an autoradiographic study. J Comp Neurol. 1979;188(2):201–223. doi: 10.1002/cne.901880202. [DOI] [PubMed] [Google Scholar]
- 49.Sugerman RA, Demski LS. Agonistic behavior elicited by electrical stimulation of the brain in western collared lizards, Crotaphytus collaris. Brain Behav Evol. 1978;15(5–6):446–469. doi: 10.1159/000123793. [DOI] [PubMed] [Google Scholar]
- 50.Nelson RJ, Chiavegatto S. Molecular basis of aggression. Trends Neurosci. 2001;24(12):713–719. doi: 10.1016/s0166-2236(00)01996-2. [DOI] [PubMed] [Google Scholar]
- 51.Tarr RS. Role of the amygdala in the intraspecies aggressive behavior of the iguanid lizard, Sceloporus occidentalis. Physiol Behav. 1977;18(6):1153–1158. doi: 10.1016/0031-9384(77)90022-1. [DOI] [PubMed] [Google Scholar]
- 52.Greenberg N, Scott M, Crews D. Role of the amygdala in the reproductive and aggressive behavior of the lizard, Anolis carolinensis. Physiol Behav. 1984;32(1):147–151. doi: 10.1016/0031-9384(84)90088-x. [DOI] [PubMed] [Google Scholar]
- 53.Young LJ, Lopreato GF, Horan K, Crews D. Cloning and in situ hybridization analysis of estrogen receptor, progesterone receptor, and androgen receptor expression in the brain of whiptail lizards (Cnemidophorus uniparens and C. inornatus) J Comp Neurol. 1994;347(2):288–300. doi: 10.1002/cne.903470210. [DOI] [PubMed] [Google Scholar]
- 54.Crosby EC, Showers MJC. Comparative anatomy of the preoptic and hypothalamic areas. In: Haymaker W, Anderson E, Nauta WJH, editors. The Hypothalamus. Springfield, Ill.: Thomas; 1969. pp. 61–135. [Google Scholar]
- 55.Lanuza E, Halpern M. Afferent and efferent connections of the nucleus sphericus in the snake Thamnophis sirtalis: convergence of olfactory and vomeronasal information in the lateral cortex and the amygdala. J Comp Neurol. 1997;385(4):627–640. doi: 10.1002/(sici)1096-9861(19970908)385:4<627::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 56.Greenberg N, MacLean PD, Ferguson JL. Role of the paleostriatum in species-typical display behavior of the lizard (Anolis carolinensis) Brain Res. 1979;172(2):229–241. doi: 10.1016/0006-8993(79)90535-3. [DOI] [PubMed] [Google Scholar]
- 57.Lynch CS, Story AJ. Dihydrotestosterone and estrogen regulation of rat brain androgen-receptor immunoreactivity. Physiol Behav. 2000;69(4–5):445–453. doi: 10.1016/s0031-9384(99)00257-7. [DOI] [PubMed] [Google Scholar]
- 58.Wood RI, Newman SW. Androgen receptor immunoreactivity in the male and female Syrian hamster brain. J Neurobiol. 1999;39(3):359–370. doi: 10.1002/(sici)1097-4695(19990605)39:3<359::aid-neu3>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 59.Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46(4):371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66(3):721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- 61.Rhen T, Crews D. Distribution of androgen and estrogen receptor mRNA in the brain and reproductive tissues of the leopard gecko, Eublepharis macularius. J Comp Neurol. 2001;437(4):385–397. doi: 10.1002/cne.1290. [DOI] [PubMed] [Google Scholar]
- 62.Krohmer RW, Bieganski GJ, Baleckaitis DD, Harada N, Balthazart J. Distribution of aromatase immunoreactivity in the forebrain of red-sided garter snakes at the beginning of the winter dormancy. J Chem Neuroanat. 2002;23(1):59–71. doi: 10.1016/s0891-0618(01)00145-4. [DOI] [PubMed] [Google Scholar]
- 63.Deviche P, Gulledge CC. Vocal control region sizes of an adult female songbird change seasonally in the absence of detectable circulating testosterone concentrations. J Neurobiol. 2000;42(2):202–211. doi: 10.1002/(sici)1097-4695(20000205)42:2<202::aid-neu4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 64.Tramontin AD, Perfito N, Wingfield JC, Brenowitz EA. Seasonal growth of song control nuclei precedes seasonal reproductive development in wild adult song sparrows. Gen Comp Endocrinol. 2001;122(1):1–9. doi: 10.1006/gcen.2000.7597. [DOI] [PubMed] [Google Scholar]
- 65.O'Bryant EL, Wade J. Seasonal and sexual dimorphisms in the green anole forebrain. Horm Behav. 2002;41(4):384–395. doi: 10.1006/hbeh.2002.1778. [DOI] [PubMed] [Google Scholar]
- 66.Wingfield JC. Control of territorial aggression in a changing environment. Psychoneuroendocrinology. 1994;19(5–7):709–721. doi: 10.1016/0306-4530(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 67.Thompson CW, Moore MC. Behavioral and hormonal correlates of alternative reproductive strategies in a polygynous lizard: tests of the relative plasticity and challenge hypotheses. Horm Behav. 1992;26(4):568–585. doi: 10.1016/0018-506x(92)90023-o. [DOI] [PubMed] [Google Scholar]


