Abstract
Background
The Safe Passage Study is a large, prospective, multidisciplinary study designed to (1) investigate the association between prenatal alcohol exposure, sudden infant death syndrome (SIDS), and stillbirth, and (2) determine the biological basis of the spectrum of phenotypic outcomes from exposure, as modified by environmental and genetic factors that increase the risk of stillbirth, SIDS, and in surviving children, fetal alcohol spectrum disorders.
Methods
The results provided are based on an interim assessment of 6004 women enrolled, out of the 12 000 projected, from the Northern Plains, US, and Cape Town, South Africa, areas known to be of high risk for maternal drinking during pregnancy. Research objectives, study design, and descriptive statistics, including consent, recruitment, and retention information, are provided.
Results
Overall visit compliance is 87%, and includes prenatal, delivery/newborn, and postnatal contacts through 1 year post-delivery. Pregnancy outcome ascertainment is 98% prior to medical chart review; less than 2% of women withdraw. Consent for the use of DNA and placental tissue exceed 94%, and consent to participate in the autopsy portion of the study is 71%.
Conclusions
The Safe Passage Study is the first multi-site study of SIDS and stillbirth to integrate prospectively collected exposure information with multidisciplinary biological information in the same maternal and fetal/ infant dyad using a common protocol. Essential components of the study design and its success are close ties to the community and rigorous systems and processes to ensure compliance with the study protocol and procedures.
Keywords: PASS, Safe Passage Study, fetal alcohol spectrum disorders, prenatal alcohol exposure, stillbirth, sudden infant death syndrome, study methodology
Prenatal alcohol exposure (PAE) is a major public health concern in the US; 52% of women report drinking alcohol during their childbearing years and 8% report drinking during pregnancy.1 Fetal alcohol spectrum disorders (FASD) is the umbrella term that encompasses a continuum of neurodevelopmental disabilities, craniofacial and somatic anomalies, and growth impairments attributed to the toxic effects of PAE, affecting 1% of individuals in the US.2 Diagnoses that fall under FASD include fetal alcohol syndrome (FAS), partial FAS, alcohol-related neurodevelopmental disorder, and alcohol-related birth defects.3 Emerging reports suggest an association between PAE and stillbirth and sudden infant death syndrome (SIDS), indicating that fetal and infant mortality, as well as neurodevelopmental morbidity, may be part of the spectrum of adverse outcomes.4,5
The global burden of stillbirth is large, with approximately 2.65 million third trimester stillbirths annually.6 In the US, stillbirth is defined as fetal death at or beyond 20 weeks gestation. SIDS is defined as the sudden death of an infant under 1 year of age that remains unexplained after a thorough case investigation, including the performance of a complete autopsy, an examination of the death scene, and a review of the infant’s clinical history.7 SIDS is the leading cause of postneonatal mortality in the US (overall infant mortality rate: 53.9 per 100 000 livebirths; postneonatal mortality rate: 49.1 per 100 000 livebirths).8
Virtually all existing evidence of PAE’s effect on early mortality is based on retrospective studies, and is often compromised by insufficient power and recall bias related to quantity, frequency, and timing of exposure.4,5,9 Recognising the need to elucidate the role of PAE in early mortality, as well as neurodevelopmental morbidity in survivors, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA) established a cooperative agreement in 2003 for the Prenatal Alcohol in SIDS and Stillbirth (PASS) Network. The National Institute on Deafness and Other Communication Disorders (NIDCD) joined the PASS Network in 2011. The objectives of the prospective Safe Passage Study conducted by the PASS Network are to (1) investigate the association between PAE, SIDS, and stillbirth, and (2) determine the biological basis of the spectrum of phenotypic outcomes from exposure, as modified by environmental and genetic factors that increase the risk of stillbirth, SIDS, and in surviving children, FASD. Women at high risk for maternal drinking during pregnancy are recruited in the Northern Plains (NP), US, and in Cape Town, South Africa (SA).9,10 The results provided are based on an interim assessment of 6004 women (half of the target) enrolled, and include an overview of the research objectives, study design, and descriptive statistics, including consent, recruitment, and retention information.
Methods
Overview of the PASS Network
The PASS Network is a collaborative effort among the NICHD, NIAAA, and NIDCD; two comprehensive clinical sites (CCS), one in the NP, US, and the other in Cape Town, SA; a developmental biology and pathology centre; a physiology assessment centre; and a centralised data coordinating and analysis centre. Internal network oversight is provided by the steering committee, and external oversight is provided by an independent advisory and safety monitoring board, as well as numerous institutional review boards (IRBs) and tribal communities.
Hypotheses
The primary hypothesis of the Safe Passage Study is that PAE increases the risk for SIDS and stillbirth. Multiple secondary hypotheses are based upon the premise that in addition to FASD, some stillbirths and SIDS cases may be part of a spectrum of disorders that result from PAE in combination with other maternal, environmental, and genetic factors that impinge directly on the central or autonomic nervous systems of the fetus, or secondarily upon the fetus via placental mechanisms. The secondary hypotheses relate to interactions among PAE and maternal, environmental, and genetic factors as modifiers of the function and structure of the placenta, development of the fetal and infant face and brain (three-dimensional ultrasound), autonomic function in the fetus and infant, cortical and brainstem activity in the infant [electroencephalography (EEG) and auditory function pathway assessments, respectively], and neurotransmitter and synaptic maturation in the cerebral cortex and brainstem of the fetus and infant (Figure 1).
Figure 1.
Model of adverse perinatal outcomes related to prenatal alcohol exposure and the role of environmental and genetic modifiers. ANS, autonomic nervous system; CNS, central nervous system.
Design
The planned enrolment period for the Safe Passage Study is 7.5 years, from August 2007 to January 2015, with subsequent follow-up for 1 year post-delivery. The main study protocol is implemented for 12 000 pregnant women enrolled in the study, while an embedded study protocol includes a randomly selected subset of 3750 women who were enrolled prior to 24 weeks gestation and consented to additional study assessments. The incorporation of the embedded study provides more in-depth information pertinent to specific secondary hypotheses, while minimising costs and resources.
Power calculations utilised published rates of SIDS, stillbirth, and PAE (see Table 1 and Supporting Information Appendix S1) and information from a retrospective study of antecedent risk factors for SIDS in the NP American Indians [Aberdeen Area Infant Mortality Study (AAIMS)] reporting a relative risk (RR) of 6.7 for SIDS among women with PAE in the first trimester as compared to unexposed women.9 Conservatively assuming that the risk of SIDS is tripled, 49% of women will be exposed to PAE during pregnancy, and 10% attrition, 11 622 women were required. The targeted sample size of 12 000 women enrolled (7000 from SA and 5000 in the NP) is expected to yield a sample of 98 stillbirths and 37 SIDS cases to ensure 80% power to detect an RR of 3 with a chi-square test for proportions with continuity correction and a two-sided level of significance of 5%. Based on the rates of stillbirth reported in Table 1 (8/1000 and 15/1000 in the NP and SA, respectively),14,15 a sample size of 12 000 will ensure >95% power to detect an RR of at least 2 comparing women with PAE with those without PAE. The study is not designed to investigate genetic and biological interactions or perform subgroup analyses (e.g. by site, cause of death). However, these relationships will be explored as appropriate.
Table 1.
Published sudden infant death syndrome (SIDS), stillbirth, and fetal alcohol syndrome (FAS) rates
Recruiting, screening, and enrolment
Each CCS is responsible for recruiting, enrolling, and following participants in accordance with the common study protocol and for disseminating information back to participating communities. The Northern Plains CCS is comprised of five clinical sites in North Dakota and South Dakota, including two sites on American Indian Reservations. The Stellenbosch CCS recruits from Bishop Lavis and Belhar residential areas within Cape Town, SA, and serves mainly the mixed ancestry population.19
Screening and enrolment occur at prenatal clinics affiliated with each CCS between 6 weeks gestation up to, but not including, delivery. During the recruitment or enrolment visit, participants provide informed consent that includes consent for specific study components/assessments (e.g. collection of placental tissue, use of specimens for future studies, contact for future studies) and authorisation for the collection of personally identifiable information. Women are provided these options so that they can decline aspects of the study that conflict with their personal needs or cultural beliefs. In the event of fetal or infant demise, a separate informed consent is required for collection of autopsy tissue, and access to the autopsy report and death scene investigation (infant demise only) for research. Of the participants who enrol prior to 24 weeks gestation, one in three are randomly selected and invited to participate in the embedded study. Educational materials on the potential effects of alcohol and tobacco exposure during pregnancy and safe sleeping practices for infants are provided to all participants.
Inclusion and exclusion criteria
A woman is eligible if all of the following criteria are met: (1) able to provide informed consent, (2) pregnant with one or two fetuses, (3) 16 years of age or older, (4) gestational age of at least 6 weeks, 0 days and not at the delivery admission, and (5) able to speak English or Afrikaans. A woman is excluded if any of the following criteria are met: (1) planned abortion, (2) planned relocation from catchment area prior to delivery, or (3) advice against participation by a health care provider (e.g. requires additional medical care).
Schedule of evaluations and events
Overview
The Safe Passage Study common protocol was developed and is maintained by the PASS Network steering committee. There are three clinical assessment periods: prenatal, delivery/newborn, and postnatal (Figure 2). Depending on the timing of enrolment, women have up to three prenatal visits: 20–24 weeks, 28–32 weeks (embedded study only), and 34+ weeks. Clinical coordinators at each CCS monitor labour and delivery admissions daily to determine if a study participant has delivered, and if so obtain pregnancy outcome information. If a liveborn infant is delivered preterm (prior to 37 weeks gestation), the newborn visit and all subsequent postnatal visits are adjusted for prematurity (39–41 weeks gestational age-adjusted interval). Postnatal visits occur at 1 month and 1 year, and at the 1 year visit, confirmation as to whether the infant is alive is made through personal contact and by review of both death records and medical charts. PASS personnel receive notifications of infant demises through well-established relationships with the forensic officers in the catchment areas.20 Upon notification of demise, an attempt is made to immediately consent women for the fetal or infant demise arm of the study. At any time during the study, the participant may withdraw.
Figure 2.
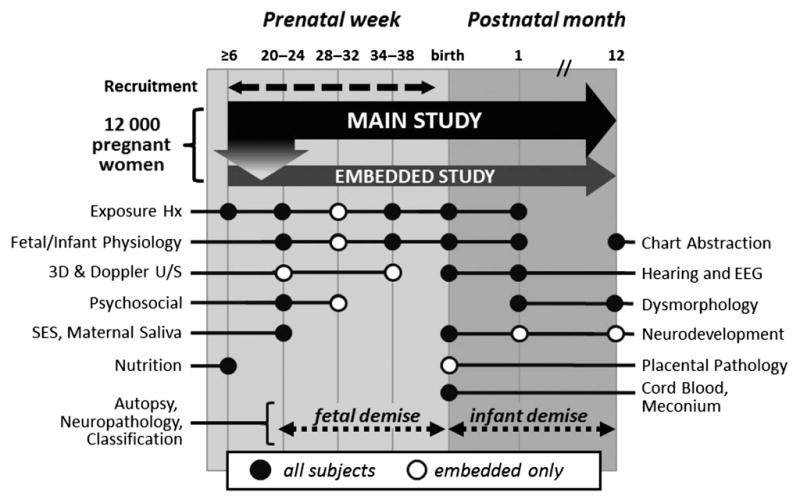
Schedule of evaluations and events. Hx, history; U/S, ultrasound.
Assessments (Figure 2)
Self-reported maternal characteristics (e.g. demographics, medical, and obstetric history), and dietary and psychosocial (i.e. depression, resilience, traumatic and threatening experiences, anxiety, and perceived stress) information, are collected at the earliest prenatal visit. Anthropometry, self-reported exposure (e.g. alcohol, tobacco, marijuana, methamphetamines), and fetal physiology [e.g. heart rate (HR), heart rate variability (HRV), movement, HR-movement coupling] are collected at each prenatal visit. Biometry and Doppler ultrasound velocimetry of uterine and fetal vessels are performed for embedded study participants. Postnatal newborn and/or 1 month visits include autonomic, cardiorespiratory, cortical activity, and auditory assessments, self-reported exposure, infant care practices, infant anthropometrics, facial dysmorphology photographs, and the Amiel-Tison Neurologic Assessment at term.21 At the 1 year visit, the Mullen Scales of Early Learning22 is administered to assess cognitive ability and motor development (embedded study), and infant anthropometrics and facial dysmorphology photographs are collected. Maternal (pregnancy through delivery) and infant (newborn to 1 year) charts are abstracted to obtain information regarding growth, physical exam/fetal structure, laboratory testing, medications and interventions, clinical events, co-morbidities, and diagnoses. Serious adverse events, unanticipated problems, and concomitant services are collected at each participant visit, contact, or event, and are reported according to regulatory guidelines.
Alcohol exposure assessment
Alcohol exposure information is captured using the validated Timeline Follow-Back (TLFB) method.23,24 At each prenatal visit, this information is collected for the last reported drinking day (and 30 days prior). Additionally, at the recruitment interview, peri-conception (2 weeks prior and 2 weeks following the last menstrual period) alcohol intake is collected. Detailed information is obtained to standardise and calculate the total grams of alcohol consumed on each drinking day or episode.25 Specifically, the type(s) of alcoholic beverage consumed, whether the drink was frozen or included ice, number and size of containers, number of persons sharing, and duration is collected for each drinking day. Total grams of alcohol consumed per drink is calculated and converted into standard drinks using the NIAAA definition of one standard drink equals 14 g of pure alcohol.25
Physiology assessments
The Physiology Assessment Center (PAC) leads the investigation into the relationship between potential adverse effects of PAE (alone or in combination with other exposures) and autonomic, respiratory, and brain function in the fetus and infant, as these effects may impair central homeostatic regulation and increase the risk for stillbirth and SIDS. Measures of fetal HR, HRV, movement, and HR-movement coupling are assessed at up to three fetal visits using ultrasound. Beat-to-beat variability in the fetus and mother are collected simultaneously using a transabdominal fetal electrocardiograph. Infant measures of cardiorespiratory function during sleep, as well as responses to head-up tilt challenge, are obtained at the newborn and 1 month visits. During the infant visits, cortical activity (EEG) and auditory function [auditory brainstem responses (ABR) and transient evoked otoacoustic emissions (TEOAE)] are assessed.
Developmental biology and pathology assessments
The Developmental Biology and Pathology Center (DBPC) leads the investigation into the potential adverse effects of PAE (alone or in combination with other exposures) upon the placenta and developing fetal and infant brain. The main objectives of the neuropathological studies are to determine the relationship between PAE and the (1) neurotransmitter development of brainstem sites that control homeostatic function, relative to SIDS and stillbirth, and (2) neurotransmitter development and synaptogenesis in areas of the cerebral cortex related to abnormal cognitive function in individuals with FASD. Genetic research includes investigating the effect of PAE and modifications to genetic mechanisms that impact phenotypic outcomes (i.e. stillbirth, SIDS, or FASD). For each demise, all relevant clinical, autopsy, and other pertinent (death scene investigation, placental pathology) information is reviewed by the pathology subcommittee led by the DBPC, and comprised of experts in paediatrics, obstetrics, dysmorphology, genetics, paediatric/placental pathology, paediatric neuropathology, and forensic pathology. The demises are classified according to previously published systems, as well as a stillbirth classification system developed for the study (see Supporting Information Appendix S1).7,26–30 Demise adjudication requires 100% consensus while blinded to prenatal exposures. Specimens collected for the Safe Passage Study include maternal and infant saliva or umbilical cord blood for DNA analysis (all participants), meconium (all), placental samples (embedded study), and brain samples (autopsied stillbirths and infant demises). Specimens are shipped for long-term storage to Fisher Bioservices in Rockville, MD.
Biostatistics, regulatory compliance, quality assurance, and data management
The Data Coordinating and Analysis Center (DCAC) is responsible for the study design and data analysis, biostatistical issues, and the development of systems and processes to provide centralised data management and ensure compliance with good clinical practices31 and regulatory guidelines. The DCAC oversees (1) the development and maintenance of the common protocol, ancillary studies, consent forms, manual of operations, and regulatory binder, and ensures that yearly IRB protocol and consent form approvals are completed and documented at each site/centre; (2) the development and maintenance of all case report forms (CRFs) and electronic web-based tracking, data entry, and reporting systems; and (3) quality assurance monitoring of all protocols and study procedures, as well as monitoring of statistical assumptions utilised to size the study.
Statistical methods
The results presented are based on an interim (halfway through enrolment target, n = 6004) assessment and are descriptive in nature, and as agreed by the steering committee do not include information regarding exposure or outcome rates related to the primary hypothesis. Reporting results on exposure or primary outcome information prior to study completion may bias or impact recruitment and retention of participants, cause therapeutic drift (i.e. alter medical provider practices at a given site/centre), alter the focus of information collected by study staff, or alter participant report. Analyses were performed using sas/stat® software, Version 9.3, Copyright © 2011 (Cary, NC, US).
Results
Screening, consent, and enrolment
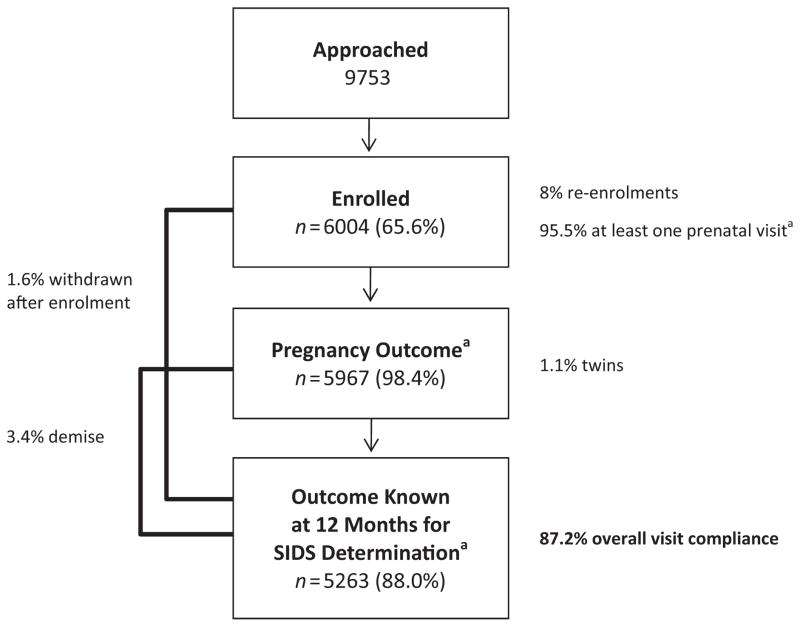
As of 7 July 2011, 9753 women were approached and 6004 were enrolled (Figure 3); these women and their infants were followed for one age-adjusted year post-delivery (through 5 April 2013). The enrolled women represent three diverse populations: Caucasians (25%), American Indians (17%), and women of mixed ancestry (57%) [other/not specified (1%)]. Of the 26% of women who refused to participate, most indicated a lack of interest (65%) or time (25%).
Figure 3.
Consolidated standards of reporting trials chart. aOf those eligible for the contact.
Of the 6004 women enrolled in the study, 99% agreed to participate in all study components/ evaluations (Figure 2), with the exception of collection of placental tissue (94% agreed), use of specimens for future studies (96% agreed), and contact for future studies (96% agreed). Consent rates to be contacted for future studies were mixed ancestry (98%), Caucasian (92%), and American Indian (94%). Consent rates for collection of placental tissue were mixed ancestry (90%), Caucasian (100%), and American Indian (96%). Of the parents who experienced a fetal or infant demise, 71% consented to participate in the autopsy portion of the study, and of those, 92% provided consent to brain tissue donation and 84% to specimen use in future studies.
Participant characterisation, follow-up information, and compliance rates
The 6004 women enrolled represent 6066 infants/ fetuses (1.1% were twin pregnancies) and 95 (1.6%) women withdrew consent (Figure 3). Women were 25.9 ± 5.8 (mean ± standard deviation) years of age at the time of enrolment. High school completion rates were Caucasian (96%), American Indian (57%), and mixed ancestry (23%). The overall visit compliance was 87.2% for all visits, including prenatal, delivery/ newborn, and postnatal contacts (Table 2). Over 95% of women completed at least one prenatal study visit in addition to the recruitment interview. Pregnancy outcome was obtained in 98% of women prior to review of medical records. Women delivered at 39.0 ± 2.1 weeks gestation; 12% delivered preterm. The newborn or 1 month assessment was completed for 96% of women, and the 1 year outcome has been ascertained on 88%; the latter has increased to over 90% in the past year (data not shown). Approximately 3% of the participants experienced a fetal or infant demise. Visit compliance for American Indian women (72%) was lower on every scheduled assessment compared with Caucasian and mixed ancestry women (89% and 91%, respectively) (Table 2). Over 48 000 fetal (21 505) and infant (27 268) physiology files, 145 911 CRFs, 2359 ultrasounds, 20 183 specimens, and 46 286 infant photographs have been collected and are currently being analysed.
Table 2.
Visit compliance by race
| American Indian n = 1042 |
Mixed ancestry n = 3410 |
Caucasian n = 1493 |
Other n = 47 |
Total n = 5992 |
||
|---|---|---|---|---|---|---|
| Overall participant compliance | Mean (SD) median | 69.1 (29.3) 80.0 | 90.7 (17.1) 100.0 | 87.6 (21.0) 100.0 | 74.8 (29.7) 84.5 | 86.1 (22.2) 100.0 |
| Overall visit compliance | # compliant/# eligible (%) | 4248/5909 (71.9%) | 17 799/19 552 (91.0%) | 8125/9087 (89.4%) | 209/275 (76.0%) | 30 381/34 823 (87.2%) |
| 20–24 weeks | # compliant/# eligible (%) | 644/848 (75.9%) | 2031/2173 (93.5%) | 1303/1442 (90.4%) | 33/38 (86.8%) | 4011/4501 (89.1%) |
| 28–32 weeksa | # compliant/# eligible (%) | 307/380 (80.8%) | 924/951 (97.2%) | 540/578 (93.4%) | 13/17 (76.5%) | 1784/1926 (92.6%) |
| 34+ weeks | # compliant/# eligible (%) | 714/893 (80.0%) | 3023/3151 (95.9%) | 1267/1352 (93.7%) | 39/44 (88.6%) | 5043/5440 (92.7%) |
| Delivery | # compliant/# eligible (%) | 809/967 (83.7%) | 3180/3340 (95.2%) | 1387/1442 (96.2%) | 35/45 (77.8%) | 5411/5794 (93.4%) |
| Newborn | # compliant/# eligible (%) | 686/931 (73.7%) | 2667/3324 (80.2%) | 1288/1412 (91.2%) | 28/43 (65.1%) | 4669/5710 (81.8%) |
| 1 month | # compliant/# eligible (%) | 527/949 (55.5%) | 3003/3320 (90.5%) | 1202/1433 (83.9%) | 34/44 (77.3%) | 4766/5746 (82.9%) |
| 12 months | # compliant/# eligible (%) | 561/941 (59.6%) | 2971/3293 (90.2%) | 1138/1428 (79.7%) | 27/44 (61.4%) | 4697/5706 (82.3%) |
| Participants with at least one prenatal visit | # compliant/# eligible (%) | 868/998 (87.0%) | 3232/3317 (97.4%) | 1431/1478 (96.8%) | 43/46 (93.5%) | 5574/5839 (95.5%) |
| Participants with pregnancy outcome ascertainedb | # compliant/# eligible (%) | 995/1042 (95.5%) | 3403/3410 (99.8%) | 1450/1493 (97.1%) | 47/47 (100.0%) | 5895/5992 (98.4%) |
| Outcome known at 12 months for SIDS determination | # compliant/# eligible (%) | 937/1037 (90.4%) | 2965/3408 (87.0%) | 1324/1492 (88.7%) | 37/47 (78.7%) | 5263/5984 (88.0%) |
| Outcome known at approximately 12 months | # compliant/# eligible (%) | 937/1037 (90.4%) | 3156/3408 (92.6%) | 1324/1492 (88.7%) | 38/47 (80.9%) | 5455/5984 (91.2%) |
The number displayed in each column header represents the total number of women enrolled with a recruitment visit. The compliance computations account for whether the participant was due for the study visit.
Applies to the embedded study only.
In the case of twins, a participant will have two deliveries. Of the outcomes ascertained, there were 5956 total deliveries.
Comments
The Safe Passage Study is the first multi-site study of SIDS and stillbirth to integrate prospectively collected adverse exposure information with multidisciplinary biological information in the same maternal and fetal/infant dyad using a common protocol. The collection of physiological assessments of fetuses and infants who may succumb to stillbirth or SIDS is unique, may identify abnormal physiological profiles of risk for early death, and will produce the largest database of prospective serial HR, HRV, respiratory patterns, tilt responses, EEGs, ABRs, and TEOAEs available to date. These rich physiological data, linked with prospectively collected serial exposure information, will provide critically needed information about the effect of PAE upon a spectrum of neural processes. The neuropathological studies are also unique in that they represent the first ever analysis of prospectively collected exposure information linked with cellular, neurochemical, and molecular parameters in the human brain in autopsied fetuses and infants. The linkage of neuropathological studies with prospectively collected physiological information has the potential to increase our understanding of the pathological mechanism of SIDS, which has remained elusive for decades. A potential significant contribution of the Safe Passage Study is the development of algorithms for the early identification of fetuses and infants potentially at risk for adverse outcomes, such as stillbirth, SIDS, and FASD. The risk profile will be based upon the analysis and integration of physiological profiles in the fetus/infant and placental, genetic, and maternal factors. The PASS Network will comply with the data sharing policy of the National Institutes of Health (NIH). Availability of tribal data will be dependent upon the requirements of the tribal review boards.
The reliability and validity of maternal self-reported exposure and biomarkers to detect exposure during pregnancy vary among study designs. As compared with biomarkers, the granular detail with respect to timing, frequency, and amount of exposure is more easily obtained through self-report.32 However, there are inherent issues with self-report, such as recall bias,33 fear of social stigma, punishment, guilt, and other factors.32,34 Further, data collected during pregnancy as compared with post pregnancy have demonstrated issues with test–retest reliability.35 In the Safe Passage Study, women are eligible to enrol as early as 6 weeks gestation; however, it has been shown that women who are heavily exposed may not participate, may enrol later in pregnancy, or may be less compliant with visits.36 Despite these potential limitations, the TLFB method has been widely accepted as a measure of self-reported exposure. Our data indicate that women are drinking various and sometimes hazardous amounts throughout pregnancy, and preliminary analyses demonstrate excellent performance characteristics between self-report and meconium biomarkers (PASS data, publications pending).
Over the course of the Safe Passage Study, the enrolment, follow-up, and retention rates, as well as exposure and outcome rates, have been extensively monitored and are consistent with the estimates used to size/power the study (Table 1). Given that obtaining consent for research [in particular, the use of autopsy (brain) and DNA samples] can be problematic in socio-economically disadvantaged and minority populations,37,38 the rates in the Safe Passage Study are exceptional and attest to early involvement and ongoing commitment of the communities to the mission of the Safe Passage Study (Supporting Information Appendix S1). Based upon the experience of the clinical sites in the Safe Passage Study in obtaining autopsy tissues for research, we have reported suggested guidelines for obtaining such consent in socioeconomically disadvantaged populations that we consider of potential value to investigators involved in similar types of research (Odendaal et al. 2011).38
Stillbirth, SIDS, and FASD disproportionately afflict socio-economically disadvantaged populations and minority populations.9,10,12,14–18 However, it is likely that these findings will be universally applicable due to potential associations found between common cellular and molecular mechanisms of PAE upon the developing fetus and placenta. These findings may translate to preventive and intervention strategies in communities most burdened by these disorders and to the population at large.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the cooperation of the study participants, PASS investigators, and members of the NICHD advisory safety monitoring board: Elizabeth Thom, PhD (Chair); Reverend Phillip Cato, PhD; James W. Collins, Jr, MD, MPH; Terry Dwyer, MD, MPH; George Macones, MD; Philip A. May, PhD; Jeff Murray, MD; Richard M. Pauli, MD, PhD; Raymond W. Redline, MD; and Michael Varner, MD. The PASS Research Network is supported by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute on Deafness and Other Communication Disorders through the Cooperative Agreement Mechanism (U01 HD055154, U01 HD045935, U01 HD055155, U01 HD045991, and U01 AA016501). The following institutions and researchers comprise the PASS Network (additional network members other than authors listed):
DCAC: Biostatistics: Tara Tripp, MA, Fay Robinson, MPH; Project Management/Regulatory Affairs: Julie M. Petersen, BA, Rebecca A. Young, MPH; Data Management/Information Technology: Travis Baker, BS, Derek Petersen, BS, Gregory Toland, MS
DBPC: Assistant Director: Robin L. Haynes, PhD; Co-investigators: David S. Paterson, PhD, Kevin G. Broadbelt, PhD, Kyriacos Markianos, PhD, Ingrid A. Holm, MD, Theonia Boyd, MD, Drucilla Roberts, MD, Richard G. Goldstein, MD, Hanno Stein, PhD; Technicians: Claire Maggiotto, BS, Catherine Hassett, BS
CCS NP: Co-investigators: Jyoti Angal, MPH, Donald Habbe, MD, H. Eugene Hoyme, MD, William Massello III, MD, Bradley Randall, MD, Mary Ann Sens, MD, PhD, Catherine Stoos, MD, Peter Van Eerden, MD; Project Management: Whitney Adler, BA, Elizabeth Berg, RN, Jessica Gromer, RN, Bethany Norton, MA, Liz Swenson, RN, Deb Tobacco, MA
CCS SA: Co-investigator and Project Manager: Coen Groenewald, MBChB, MMed, FCOG environmental, M Comm; Project Management: Erna Carstens, RN, Jean Coldray, Nat Dipl, Mandy Potter, RN, Lucy Brink, MSc, Rosemary Meyer, BTech, Carlie du Plessis, RN, Elaine Geldenhuys, Nat Dipl
PAC: Project Management: J. David Nugent, BA, Carmen Condon, BA; Data Analysis: Margaret C. Shair, BA, Tracy Thai, BA
-
NIH: Project Scientist: Chuan-Ming Li, MD, PhD (NIDCD); Program Officers: Bill Dunty, PhD (NIAAA), Tonse Raju, MD, DCH (NICHD), Gordon B. Hughes, MD (NIDCD)
Further, the following individuals made significant contributions to the research and warrant recognition:
DCAC: Idania Ramirez, MPH, Jamie Collins, MA, Laura Spurchise, MPH
DBPC: Richard A. Belliveau, BA, Kristin McMillan, BA, Megan Minter, MS
PAC: Johnston T. Grier, BA, Emilia F. Vignola, BA, Joseph J. Violaris, BA
Footnotes
The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service (IHS) or the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), or the National Institute on Deafness and Other Communication Disorders (NIDCD).
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- 1.Centers for Disease Control and Prevention. Alcohol use and binge drinking among women of childbearing age –United States, 2006–2010. MMWR Morbidity and Mortality Weekly Report. 2012;61:534–538. [PubMed] [Google Scholar]
- 2.Warren KR, Hewitt BG. Fetal alcohol spectrum disorders: when science, medicine, public policy, and laws collide. Developmental Disabilities Research Reviews. 2009;15:170–175. doi: 10.1002/ddrr.71. [DOI] [PubMed] [Google Scholar]
- 3.Hoyme HE, May PA, Kalberg WO, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake during pregnancy and the risk of stillbirth and death in the first year of life. American Journal of Epidemiology. 2002;155:305–312. doi: 10.1093/aje/155.4.305. [DOI] [PubMed] [Google Scholar]
- 5.Strandberg-Larsen K, Gronboek M, Andersen AM, Andersen PK, Olsen J. Alcohol drinking pattern during pregnancy and risk of infant mortality. Epidemiology (Cambridge, Mass) 2009;20:884–891. doi: 10.1097/EDE.0b013e3181bbd46c. [DOI] [PubMed] [Google Scholar]
- 6.Bhutta ZA, Yakoob MY, Lawn JE, et al. Stillbirths: what difference can we make and at what cost? Lancet. 2011;377:1523–1538. doi: 10.1016/S0140-6736(10)62269-6. [DOI] [PubMed] [Google Scholar]
- 7.Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatric Pathology/affiliated with the International Paediatric Pathology Association. 1991;11:677–684. doi: 10.3109/15513819109065465. [DOI] [PubMed] [Google Scholar]
- 8.Heron M. Deaths: leading causes for 2009. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2012;61:1–96. [PubMed] [Google Scholar]
- 9.Iyasu S, Randall LL, Welty TK, et al. Risk factors for sudden infant death syndrome among northern plains Indians. JAMA. 2002;288:2717–2723. doi: 10.1001/jama.288.21.2717. [DOI] [PubMed] [Google Scholar]
- 10.May PA, Brooke L, Gossage JP, et al. Epidemiology of fetal alcohol syndrome in a South African community in the Western Cape Province. American Journal of Public Health. 2000;90:1905–1912. doi: 10.2105/ajph.90.12.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2006 period linked birth/infant death data set. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2010;58:1–32. [PubMed] [Google Scholar]
- 12.Molteno CD, Kibel MA. Postneonatal mortality in the Matroosberg Divisional Council area of the Cape Western Health Region. South African Medical Journal. 1989;75:575–578. [PubMed] [Google Scholar]
- 13.MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2009;57:1–19. [PubMed] [Google Scholar]
- 14.North Dakota Department of Health, Division of Vital Records. Fetal deaths by race (2000–2004) 2005. [Google Scholar]
- 15.Talip Q, Theron G, Steyn W, Hall D. Total perinatally related losses at Tygerberg Hospital – a comparison between 1986, 1993 and 2006. South African Medical Journal. 2010;100:250–253. doi: 10.7196/samj.3812. [DOI] [PubMed] [Google Scholar]
- 16.May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Research & Health. 2001;25:159–167. [PMC free article] [PubMed] [Google Scholar]
- 17.Duimstra C, Johnson D, Kutsch C, et al. A fetal alcohol syndrome surveillance pilot project in American Indian communities in the Northern Plains. Public Health Reports. 1993;108:225–229. [PMC free article] [PubMed] [Google Scholar]
- 18.Poitra BA, Marion S, Dionne M, et al. A school-based screening program for fetal alcohol syndrome. Neurotoxicology and Teratology. 2003;25:725–729. doi: 10.1016/j.ntt.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 19.City of Cape Town: Census 2011. [last accessed July 2014];Trends and Change – 10 Years: Census 2001 – Census 2011. 2012 http://www.capetown.gov.za/en/stats/Documents/2011%20Census/2011_Census_Cape_Town_Profile_Change_from_2001-2011.pdf.
- 20.Dempers JJ, Folkerth RD, Kinney HC, et al. The Institution of a Standardized SIDS Investigation Protocol in South Africa: Feasibility Study of 11 Cases in the Western Cape. International SIDS Meeting; Portsmouth, England. 2008. [Google Scholar]
- 21.Amiel-Tison C. Update of the Amiel-Tison neurologic assessment for the term neonate or at 40 weeks corrected age. Pediatric Neurology. 2002;27:196–212. doi: 10.1016/s0887-8994(02)00436-8. [DOI] [PubMed] [Google Scholar]
- 22.Mullen EM. Mullen Scales of Early Learning, Manual. Circle Pines, MN: American Guidance Service, Inc; 1995. AGS edn. [Google Scholar]
- 23.Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- 24.Sobell LC, Sobell MB. Timeline Followback: A Technique for Assessing Self Reported Ethanol Consumption. Vol. 17. Totowa, NJ: Humana Press; 1992. [Google Scholar]
- 25.Brick J. Standardization of alcohol calculations in research. Alcoholism, Clinical and Experimental Research. 2006;30:1276–1287. doi: 10.1111/j.1530-0277.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 26.Dudley DJ, Goldenberg R, Conway D, et al. A new system for determining the causes of stillbirth. Obstetrics and Gynecology. 2010;116 (Pt 1):254–260. doi: 10.1097/AOG.0b013e3181e7d975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardosi J, Kady SM, McGeown P, Francis A, Tonks A. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ (Clinical Research Ed) 2005;331:1113–1117. doi: 10.1136/bmj.38629.587639.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyd T, Wright C, Colby K, et al. O139 PASS stillbirth classification: incorporating mechanism, etiology and recurrence. Prenatal alcohol stillbirth and SIDS (PASS) network, NIAAA/NICHD. International Journal of Gynecology & Obstetrics. 2009;S132:133. [Google Scholar]
- 29.Krous HF, Beckwith JB, Byard RW, et al. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114:234–238. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 30.Randall BB, Wadee SA, Sens MA, et al. A practical classification schema incorporating consideration of possible asphyxia in cases of sudden unexpected infant death. Forensic Science, Medicine, and Pathology. 2009;5:254–260. doi: 10.1007/s12024-009-9083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. [last accessed July 2014];1996 International Conference on Harmonisation E-6(R1), Guidelines for Good Clinical Practice. 1996 http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6_R1/Step4/E6_R1__Guideline.pdf.
- 32.Babor TF, Steinberg K, Anton R, Del Boca F. Talk is cheap: measuring drinking outcomes in clinical trials. Journal of Studies on Alcohol. 2000;61:55–63. doi: 10.15288/jsa.2000.61.55. [DOI] [PubMed] [Google Scholar]
- 33.Northcote J, Livingston M. Accuracy of self-reported drinking: observational verification of ‘last occasion’ drink estimates of young adults. Alcohol and Alcoholism. 2011;46:709–713. doi: 10.1093/alcalc/agr138. [DOI] [PubMed] [Google Scholar]
- 34.Bearer CF, Stoler JM, Cook JD, Carpenter SJ. Biomarkers of alcohol use in pregnancy. Alcohol Research & Health. 2004;28:38–43. [PMC free article] [PubMed] [Google Scholar]
- 35.Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109:815–825. doi: 10.1542/peds.109.5.815. [DOI] [PubMed] [Google Scholar]
- 36.Cunradi CB, Moore R, Killoran M, Ames G. Survey nonresponse bias among young adults: the role of alcohol, tobacco, and drugs. Substance Use & Misuse. 2005;40:171–185. doi: 10.1081/ja-200048447. [DOI] [PubMed] [Google Scholar]
- 37.Odendaal HJ, Elliott A, Kinney HC, et al. Consent for autopsy research for unexpected death in early life. Obstetrics and Gynecology. 2011;117:167–171. doi: 10.1097/AOG.0b013e318200cb17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annual Review of Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.