Abstract
Aging has independently been associated with regional brain atrophy, reduced non-rapid eye movement (NREM) slow-wave activity (SWA), and impaired long-term retention of episodic memories. However, that the interaction of these factors represents a neuropatholgical pathway associated with cognitive decline in later life remains unknown. Here, we show that age-related medial prefrontal cortex (mPFC) grey-matter atrophy is associated with reduced NREM SWA activity in older adults, the extent to which statistically mediates the impairment of overnight sleep-dependent memory retention. Moreover, this memory impairment was further associated with persistent hippocampal activation and reduced task-related hippocampal-prefrontal cortex connectivity, potentially representing impoverished hippocampal-neocortical memory transformation. Together, these data support a model in which age-related mPFC atrophy diminishes SWA, the functional consequence of which is impaired long-term memory. Such findings suggest that sleep disruption in the elderly, mediated by structural brain changes, represent a novel contributing factor to age-related cognitive decline in later life.
Introduction
A recognized and problematic feature of human aging is cognitive decline1, including impaired long-term retention of episodic memories2. These cognitive changes are paralleled by two prominent, albeit independently considered, signatures of aging. The first is structural brain atrophy, pronounced in midline frontal lobe regions3. The second is disrupted electroencephalographic (EEG) quality of non-rapid eye movement (NREM) slow wave sleep (SWS)4,5, evidenced in decreased slow wave activity (SWA). Despite these coinciding structural, physiological and cognitive changes in the elderly, the underlying nature of deficient NREM SWA in the elderly (i.e. whether age-related structural changes are associated with disrupted sleep physiology), and whether such structural and physiological changes are associated with age-related memory impairment, remain unknown. Here, we sought to determine whether these independently recognized features of aging are significantly interrelated or not, and if so, determine the precise nature in which their interaction predicts overnight memory retention.
Independent of aging, an emerging body of evidence in healthy young adults continues to support a role for NREM slow wave sleep physiology in the long-term consolidation of episodic memories6,7. Relative to equivalent time awake, NREM SWS beneficially limits the offline decay of episodic memory representations over time, resulting in superior retention8. Furthermore, electrical enhancement of SWA over prefrontal cortex (PFC) causally enhances retention of episodic memories9. Mechanistically, these findings have been considered within a postulated hippocampal-neocortical framework of memory consolidation10,11, whereby NREM SWS promotes the transformation of episodic representations from an initially hippocampal-dependent to an increasingly hippocampal-independent (and potentially more semanticized) state6,7,12. In doing so, older (more hippocampally-independent) memory representations11 are proposed to be less vulnerable to interference by ongoing encoding of new hippocampal-dependent episodic information7. Consistent with this framework, human neuroimaging findings suggest that NREM SWS may be associated with the degree of increasing hippocampal-independence during post-sleep memory retrieval8. Conversely, sleep deprivation after learning impairs long-term declarative memory retention, potentially resulting in a greater reliance of memory retrieval upon the hippocampus13. Such data suggest that one potential mechanism by which SWA promotes consolidation is the transformation of episodic memories from a labile, hippocampally-dependent state, to an increasingly hippocampal-independent state6,7,10,11.
These findings predict that the known age-related reduction of NREM SWA should result in impoverished hippocampal memory consolidation and hence next-day retention in the elderly. In support of these predictions, NREM SWS predicts memory retention across young and middle-aged adults, though, to date, examinations of this association have not controlled for age2. Furthermore, NREM SWA has been associated with the degree of overnight memory retention in healthy older adults and patients with amnestic mild cognitive impairment14. These findings support a role for NREM SWS in age-related memory decline, an effect potentially exacerbated in older individuals with memory disorders14. However, a preceding issue is the nature of age-related deficits in NREM SWS, and whether such a deficit is an inevitable consequence of the aging brain15. One candidate is structural brain atrophy in regions that promote NREM SWA. Specifically, the medial prefrontal cortex (mPFC) not only expresses some of the greatest grey matter reductions in older adults1,3, but is a region in young adults identified as the strongest EEG electrical current source generator of NREM slow waves16. Furthermore, NREM slow waves show a remarkable preponderance in origin and density over mPFC EEG derivations, as does the expression of associated NREM SWA17. These data further predict that the extent of age-related atrophy of mPFC should negatively impact the ability to generate NREM SWA in older adults.
Building on these findings, and given the role of prefrontal SWA in promoting episodic memory retention in young adults, it is possible that age-related reductions in SWA, mediated by reduced prefrontal grey matter atrophy, represents a novel neuropathological pathway associated with impaired long-term memory retention in older adults. Conversely, this collection of co-occurring changes in brain structure, sleep physiology, and episodic memory may, instead, be independent processes associated with aging that do not influence each other. Seeking to discriminate between these two possibilities, here we examine (1) whether age-related reductions in mPFC grey matter volume statistically mediate the effects of age on impaired NREM SWA, and (2) whether this age-related interaction between brain atrophy and NREM SWA consequently predicts age-related failure of overnight episodic memory retention, and with it, the persistent reliance (rather than increasing independence) of memory retrieval upon the hippocampus.
Results
In short (see Methods for details), a group of cognitively normal older adults and a group of healthy young adults performed an episodic associative (word-pair) memory task sensitive to sleep-dependent episodic memory retention6. All participants were initially trained to criterion on a set of word pairs in the evening, pre-sleep, followed by two separate recognition memory tests. The first (“short delay”) recognition memory test occurred 10 minutes after the initial study session, where a subset of the studied word pairs were tested. Following the short delay recognition test, participants were given an 8-hour sleep period in accordance with habitual sleep-wake habits, recorded using polysomnography with full-head EEG coverage. The next morning, participants performed the second (“long delay”) post-sleep recognition test, where the remaining subset of originally studied word pairs were tested. The long delay recognition testing was performed during an fMRI scan session to assess differences in retrieval activity, focused a priori on the hippocampus18. An additional structural MRI scan followed the fMRI session, allowing for assessment of grey matter differences between young and older adults. The measure of overnight memory retention was calculated by subtracting short delay recognition performance from long delay recognition performance2,13.
Age effects on sleep and grey matter volume
We first sought to characterize slow wave activity (SWA: 0.8–4.6Hz19) in older and young adults. As predicted, and similar to previous findings16,17, SWA was dominant over prefrontal EEG derivations, in both the young and older adult groups (Fig. 2A). Also consistent with prior reports4, older adults exhibited marked and significant reductions in SWA relative to young adults. This was true globally, averaged across the entire head (P<0.001) (Fig. 2B), and when focusing locally over prefrontal EEG derivations exhibiting highest SWA(P<0.001, Fig. 2B); the latter associated both with the dominant EEG source region of cortical slow waves16 and where experimental manipulations of SWA causally modulate episodic memory retention9. Similar age effects were detected for global (P<0.001) and prefrontal (P<0.001) absolute SWA (Fig. 2C–D). Sleep stage differences between groups are provided in Table S1, demonstrating expected reductions in NREM SWS amount in older adults4,5.
Figure 2.
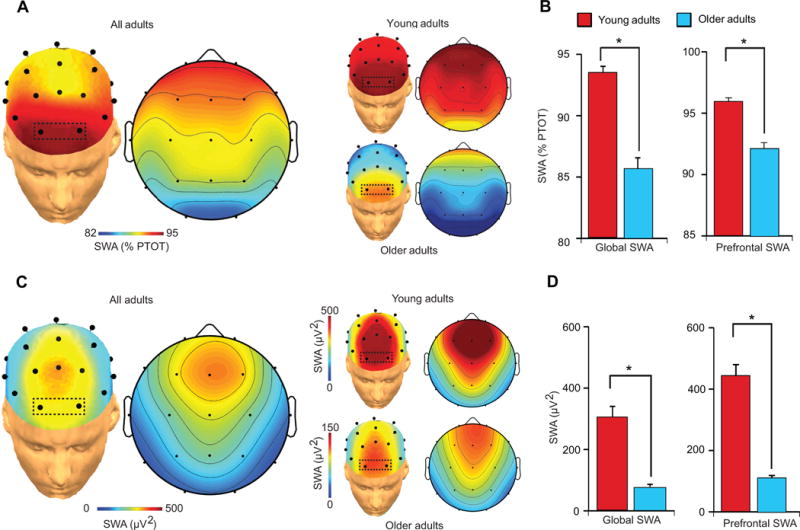
EEG topographic plots of relative (a) slow wave activity (SWA: 0.8Hz–4.6Hz) during slow wave sleep in Young and Older adults, and the relative (b) SWA difference between Young and Older adults both averaged across all electrode sites for a metric of global SWA (left) and averaged across prefrontal electrode sites (right), outlined in the box in (a: Fp1 and Fp2) exhibiting peak relative SWA in both groups. (c) EEG topographic plots of absolute slow wave activity (SWA: 0.8Hz–4.6Hz) during slow wave sleep in all adults collapsed and Young (top plot) and Older (bottom plot) adults separately, and (d) the SWA difference between Young and Older adults both averaged across all electrode sites for a metric of global SWA (left) and averaged across prefrontal electrode sites (right), outlined in the box in (a: Fp1 and Fp2) exhibiting prefrontal derivations where absolute SWA was averaged and then compared between groups. Error bars indicate s.e.m.
*denotes significance at P<0.001.
%PTOT denotes percentage of total spectral power (0.4–50Hz)
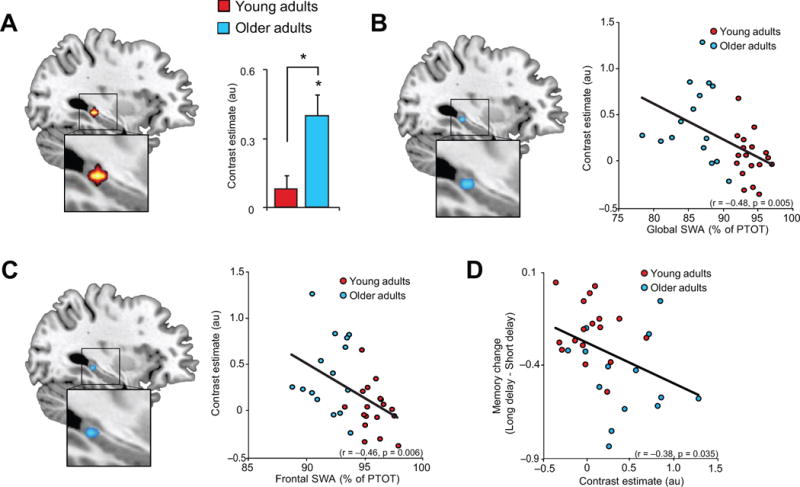
Given the described homology between prefrontal dominance in both slow wave generation and age-related brain atrophy, we next examined group differences in grey matter volume, focusing a priori on mPFC. Consistent with previous findings1,3, the peak significant difference in grey matter volume between young and older adults, when examining across the whole brain, was detected in medial prefrontal cortex (mPFC) (Fig. 3A). Significant reductions in grey matter volume in older relative to young adults were additionally detected in bilateral insula and posterior cingulate cortex (Fig. S2); notable as these regions have both also been associated with slow wave source generation, albeit to a lesser degree than the mPFC16.
Figure 3.
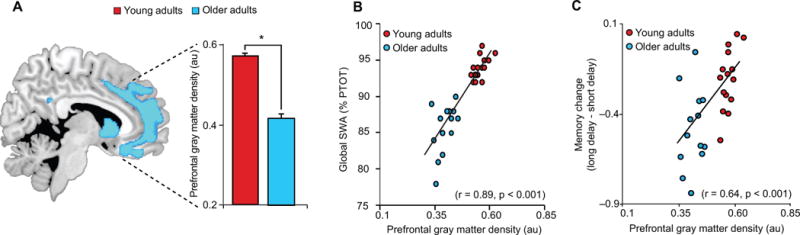
(a) Age effects (Older<Young adults) in grey matter volume, with mean medial prefrontal grey matter volume in young (red) and older (blue) adults plotted to the right. Regression between mean prefrontal grey matter volume and global slow wave activity (SWA) (b), defined as the average relative SWA across all electrode sites, and (c) associative episodic memory change. Activations are displayed and considered significant at the voxel level of P<0.05 family-wise error (FWE) corrected for multiple comparisons across the whole brain volume. Cool colors represent the extent of reduced grey matter volume in Older relative to Young adults. Error bars indicate s.e.m.
*denotes significance at P<0.05 FWE whole brain corrected.
au denotes arbitrary units.
%PTOT denotes percentage of total spectral power (0.4–50Hz)
Since age was associated both with reduced SWA and prefrontal grey matter atrophy, we next sought to determine whether age effects upon SWA were statistically mediated by changes in mPFC grey matter volume, examined using mediation analyses. Older age was associated with decreasing global SWA (r=−0.86, P<0.001) and decreasing mPFC grey matter volume (r=−0.94, P<0.001). However, age no longer significantly predicted the extent of global SWA when mPFC grey matter volume was included in the statistical model (P=0.009, Age P=0.446), reflected in a significant mediation effect (Sobel test20: P=0.005). This association between mPFC grey matter volume and global SWA (r=0.89, P<0.001; Fig. 2B) was present in young (r=0.60, P=0.01) and older (r=0.52, P=0.05) adults separately. Therefore, age was not independently associated with reduced mPFC grey matter and reduced global SWA. Instead, the SWA decrease with age was significantly mediated by the reduction in mPFC grey matter. To examine whether the mediating role of grey matter in age-related changes in SWA was specific to mPFC, mediation analyses were repeated in other regions associated with age-related memory decline: the precuneus, hippocampus. and temporal lobe1,21. Older age was associated with decreasing precuneus (r=−0.74, P<0.001), hippocampus (r=−0.42, P=0.014), and temporal lobe (r=−0.83, P<0.001) grey matter volume, also reflected in age group effects (precuneus: p<0.001; hippocampus: p=0.003; temporal lobe: p<0.001). However, precuneus (P=0.157, Age P<0.001), hippocampus (P=0.586, Age P<0.001), and temporal lobe (P=0.679, Age P<0.001) grey matter volume did not predict SWA when age was included in the statistical model, suggesting that grey matter atrophy in these regions did not significantly mediate the effects of age on SWA (all Sobel test20: P>0.13), nor the effects of SWA on long-term memory retention (see below; all P<0.001 for SWA in models including age and grey matter). Therefore, age-related changes in SWA were statistically mediated by regionally-specific changes in grey matter volume which include the mPFC.
Age effects on memory
Next, we examined whether the overnight change in episodic memory retention was impaired in older adults relative to young adults (Fig. 4A, Table 1). A two-way, repeated measures ANOVA revealed significant main effects of group (young/older; P<0.001), testing session (short delay [10 min; pre-sleep]/long delay [10 hr; post-sleep]; P<0.001). Most relevant, there was also a group×testing session interaction effect (P=0.001). Specifically, the decline from pre- to post-sleep episodic memory, expressed as the memory savings difference between short (10 min; pre-sleep) and long (10 hr; post-sleep) delay recognition performance, was significantly impaired (i.e. greater) in older relative to young adults (P=0.001, Fig. 4B, Table 1). These findings indicate that (1) memory performance in older adults was worse relative to young adults, (2) both groups demonstrated a deterioration in retention of memory overnight, relative to short delay (10 min) recognition testing, and (3) older adults exhibited a significant impairment in sleep-dependent memory retention compared to young adults.
Figure 4.
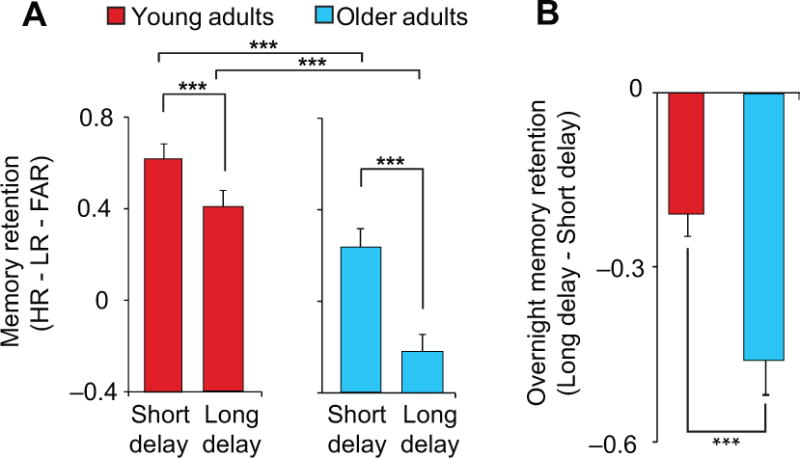
(a) Recognition performance (HR–LR–FAR: [hit rate to originally studied word pairs – false alarm rate to originally studied word pairs – false alarm rate to new, unstudied words]) in Young (red) and Older (blue) adults both at pre (short delay) and post-sleep (long delay) testing, and (b) the change in recognition performance (long delay – short delay), reflecting a measure of associative episodic memory change. Age differences in recognition performance were driven by changes in hit rate (P<0.001) and not Lure rate (P=0.16) or False alarm rate (P=0.17), suggesting that age differences in recognition performance were driven by changes in memory and not response bias. Error bars indicate s.e.m.
*denotes significance at P<0.05, **P<0.01, ***P<0.001.
Table 1.
Demographic and Neuropsychological Measures (mean±s.d.)
| Variable | Young (n = 18) | Older (n = 15) |
|---|---|---|
| Age (yr) | 20.4±2.1 | 72.1±6.6*** |
| Gender | 10 Female | 12 Female |
| Education (yr) | 14.3±1.8 | 17.8±1.6*** |
| MMSE | 29.6±0.8 | 29.3±0.8 |
| Mean bed time | 0:28±0:48 | 22:28±1:09*** |
| Mean wake time | 8:34±0:48 | 6:55±1:03** |
| Mean prestudy time in bed (hr) | 8.10±0.61 | 8.46±0.81 |
| Mean prestudy sleep time (hr) | 7.79±0.63 | 6.91±1.07* |
| Mean prestudy sleep latency (min) | 14.8±9.1 | 46.2±56.1 |
| Mean prestudy sleep efficiency (%) | 96.3±2.2 | 82.6±13.2** |
| Scan time relative to pre-study wake time | 1:21±1:16 | 1:33±0:57 |
| Short delay recognition (HR-LR-FAR) | 0.62±0.07 | 0.24±0.08*** |
| Long delay recognition (HR-LR-FAR) | 0.41±0.07 | −0.22±0.07*** |
| Memory Change (long-short delay) | −0.21±0.04 | −0.46±0.06*** |
| Neuropsychological Measures | ||
| CVLT (long delay, # free recalled) | 11.5±3.2 | |
| WMS (visual reproduction %) | 83.3±12.2 | |
| Trailmaking B (seconds) | 69.8±42.4 | |
| Stroop (# correct in 60 seconds) | 54.3±13.0 |
denotes P<0.05
P<0.01
P<0.001
That differences in memory change were not driven by differences at encoding was explored by two additional analyses. First, recognition performance at the short delay (10 min) did not predict the amount of overnight memory change across all subjects combined or within subgroups (all r2<0.02, P≥0.35). Second, and to further ensure that differences at memory encoding between young and older adults did not drive differences following sleep, a subset analysis was performed matching the highest older adult memory performers at the short delay (n=8; 0.42±0.05) with the lowest young adult memory performers at the short delay (n=8; 0.40±0.08), scores that were not different between these subgroups (P=0.855). The highest performing older adults at the short delay test, similar to the overall older adult cohort, exhibited a significantly greater decrease in memory between the short and long delay tests than the lowest performing young adults (−0.54±0.06 versus −0.21±0.05, respectively, P<0.001). To further ensure differences in memory change were not driven by encoding differences, participants were trained to criterion. Specifically, participants were tested until they correctly identified all paired associates. To examine differences in criterion accuracy, defined as the number correct divided by the total number attempted, across age or experimental groups, a two way ANOVA with age (Young/Older) and experimental (Sleep/Wake) group as between subject factors was performed. No significant effects were detected. Taken together, these findings suggest that overnight differences in the memory retention between the young and older adult groups are not likely to be accounted for by differences in memory at the initial short delay test.
To determine whether diminished memory retention in older relative to young adults was unique to offline periods of sleep (rather than simply time), two separate groups of young and older adult participants performed the same word-pair memory task but spanning a delay period of wakefulness during the day (see Table S1). A three-way repeated measures ANOVA was performed with test-session (short delay/long delay) as a within subject factor, and age group (young/older) and experimental group (sleep/wake) as between subject factors. Significant main effects of test-session (P<0.001) and age group (P<0.001) were detected, suggesting that recognition performance was lower at long (10 hr) than at short (10 min) delay testing, and worse in older relative to young adults. Importantly, however, significant age group × testing session (P<0.001) and experimental group × test-session (P=0.005) interaction effects were detected. Older adults expressed significantly worse offline retention relative to young adults across periods including sleep (−0.46±0.06 in older versus −0.21±0.04 in young adults, P=0.001) and periods including only wake (−0.62±0.09 in older versus −0.40±0.04 in young adults, P=0.031). However, and consistent with prior reports6,9,13, only young adults demonstrated superior offline memory retention following sleep compared to wake (P=0.003), while older adults showed no such sleep relative to wake memory retention benefit (P=0.161).
Sleep associations with memory
Having established disruptions in prefrontal grey matter volume, SWA, and episodic memory change in older adults, we next tested the hypothesis that the extent of this SWA disruption significantly and statistically mediated the effects of age and reductions in mPFC grey matter on successful overnight episodic memory change. Both global (r=0.81, P<0.001; for young adults only r=0.77, P=0.001; for older adults only r=0.71, P=0.006) and local relative SWA over prefrontal EEG derivations (r=0.81, P<0.001; for young adults only r=0.80, P<0.001; for older adults only r=0.69, P=0.009) were positively and significantly associated with the success of overnight memory change across all participants combined (Fig. 5A). Additionally, this significant positive relationship was identified in young and older adult groups separately, with peak correlations over frontal electrodes (Fig. 5B), significant when corrected for multiple comparisons22. These findings establish that SWA, especially over prefrontal cortex, was associated with the pre- to post-sleep change in episodic memory retention, such that deficits in the degree of overnight memory change were proportional to the extent of SWA impairment.
Figure 5.
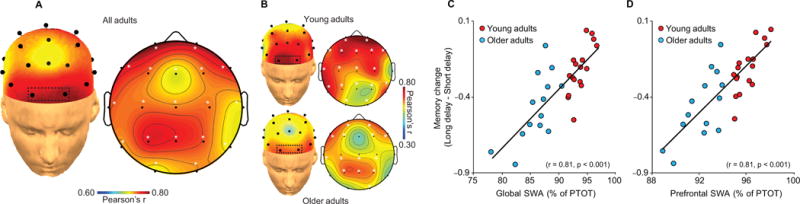
Topographic plots of the association between relative slow wave activity (SWA: 0.8Hz–4.6Hz) during slow wave sleep and associative episodic memory change in (a) all participants collapsed and (b) in Young (top plots) and Older (bottom plots) adults, with corresponding regressions for Young (red) and Older (blue) adults plotted for global relative SWA (c), defined as the average relative SWA across all electrode sites, and prefrontal SWA (d), defined as the average at prefrontal electrodes outlined in the box in (a & b: Fp1 and Fp2) exhibiting peak relative SWA in both groups. Associations were specific to SWA, as measures of subjective sleepiness and alertness, objective alertness, circadian preference, neurocognitive status, fast spindle density during stage 2 sleep (Fig. S1), total sleep time, wake after sleep onset time, and sleep efficiency did not correlate with episodic memory change in either young or older adults separately (Table S3). Similar to relative slow wave activity, both global absolute SWA (r=0.57, P=0.001) and prefrontal absolute SWA (Fp1 & Fp2 mean; r=0.60, P<0.001) predicted episodic memory change across all participants. While not significant in all cases, a similar association was detected within each group separately for both global (young adults: r=0.49 P=0.045, older adults: r=0.36 P=0.21) and prefrontal (young adults: r=0.56 P=0.019, older adults: r=0.24 P=0.41) absolute SWA.
*denotes significance at P<0.05 corrected22.
%PTOT denotes percentage of total spectral power (0.4–50Hz)
Building on these associations, and to formally examine the inter-relationships between age, mPFC grey matter, SWA and overnight memory change, mediation analyses were performed. Age and mPFC grey matter were significantly associated with SWA, both globally, averaged across all EEG derivations (age r=−0.86, P<0.001; mPFC grey matter r=0.89, P<0.001) and locally, over prefrontal EEG derivations (r=−0.83, P<0.001; mPFC grey matter r=0.86, P<0.001). Furthermore, age and mPFC grey matter were also significantly associated with deficits in episodic memory retention (age r=−0.61, P=0.001; mPFC grey matter r=0.64, P=0.001). However, neither age nor mPFC grey matter significantly predicted the extent of overnight episodic memory retention when SWA was included in the statistical model. This was the case for global SWA (SWA P<0.001, Age P=0.178; SWA P<0.001, mPFC grey matter P=0.186) as well as prefrontal SWA (SWA P<0.001, Age P=0.249; SWA P<0.001, mPFC grey matter P=0.357), reflected in significant mediation effects for both measures (Sobel test20: all P<0.001). These findings indicate that while both age and prefrontal grey matter changes were independently associated with episodic memory change, such effects were significantly mediated by SWA when this variable was added to the same statistical model. Such data support the hypothesis that offline episodic memory consolidation deteriorates with age, in part, due to mPFC grey matter atrophy that reduces NREM SWA (Fig. 6). It should be noted that these data alone do not establish causality. Furthermore, they do not prove that the disruption in NREM SWA directly causes impaired memory retention in older adults, as there may be unmeasured factors beyond the collection of co-factors controlled for in the current study that could account for the statistical associations between each of these variables. However, our findings nevertheless make clear that these three a priori targets (mPFC brain atrophy, NREM SWA, and delayed memory retention) are not independent of each other, and that other potentially unaccounted for factors would themselves be associated with these specific sleep and atrophy changes in predicting memory.
Figure 6.
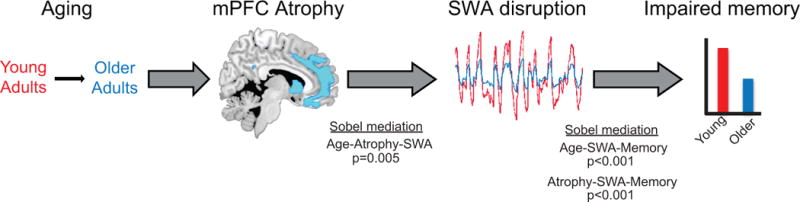
Model schematic of mediation findings. Aging is associated with grey matter atrophy, which mediates the degree of slow wave activity disruption, with slow wave activity, in turn, mediating the degree of impaired memory retention.
Influence of co-factors on memory
To determine whether age and sleep effects on memory were explained by the influence of circadian rhythms, alertness, neurocognitive status, or hippocampal volume, follow-up analyses were performed. First, recognition performance at short delay did not differ significantly between evening (sleep) and morning (wake) groups in young adults (0.62±0.07 versus 0.62±0.08, P=0.942) or older adults (0.24±0.08 versus 0.40±0.08, P=0.175). Second, a measure of circadian preference58 did not predict memory change scores in young or older adults (all r2<0.063, P>0.35). Third, testing time relative to habitual wake time did not differ between groups (P=0.798), or predict memory change in either group (all r2<0.11, P>0.245). Fourth, subjective sleepiness and alertness did not differ by age group (all P>0.25)54,59 or explain memory change in young or older (r2<0.16, P>0.16) adults. Fifth, reaction times during short and long delay recognition testing, and the change in reaction times, did not predict memory at short delay, long delay, or memory change in young or older (all r2<0.10, P>0.15) adults. Sixth, episodic memory change demonstrated no significant association with any of the neurocognitive metrics of assessment described in Table 1 (all P>0.45). Finally, hippocampal volume also did not predict memory change (P=0.454). These data suggest that age and sleep effects on memory were not parsimoniously explained by the influence of circadian rhythms, alertness, neurocognitive status, or hippocampal volume. However, these data also do not suggest that these factors cannot influence memory.
Furthermore, no association was detected between fast spindle density (13.5–15Hz, calculated as described previously60) during stage 2 NREM sleep and episodic memory change when examined across all participants or within each group separately. Stage 2 sigma power (12–15Hz) also did not predict memory change at any one of the 19 electrode derivations (all r2<0.04, P>0.30). Moreover, no sleep stage metric predicted episodic memory change in either young or older adults (all r2<0.12, P>0.16, Table S3). When examined across participants, TST (r=0.40, P=0.027), SE (r=0.40, P=0.028), and percent stage 1 sleep (r=−0.36, P=0.045) did significantly predict memory change. However, when age was included in the model, none of these variables significantly predicted memory (for TST P=0.79, for SE P=0.98, and for %S1 P=0.94). Further, when SWA and age were included in the model with these other variables (total sleep time, sleep efficiency, and percent stage 1 sleep time), neither age nor these other variables remained significant, while SWA (both global and prefrontal), remained a significant predictor of memory change. Taken together, these data support the conclusion that slow wave activity during slow wave sleep specifically predicts episodic memory change in young and older adults.
Hippocampal association with memory and SWA
We finally tested the hypothesis that this age-related compromise in overnight memory retention, statistically mediated by deficient SWA, was associated with persistent (i.e. greater) hippocampal activation6–8,10,13, and diminished hippocampal-neocortical functional connectivity13 during memory retrieval. Consistent with this prediction, age effects were detected in the hippocampus, such that older adults exhibited greater (rather than progressively less) post-sleep hippocampal-dependent retrieval activation (Fig. 7A). Additionally, older adults also demonstrated significantly impaired retrieval-related hippocampal-prefrontal functional connectivity relative to young adults (Fig. S3). Further consistent with this sleep-dependent hippocampal memory transformation model7,8,10,11,13, both global SWA (Fig. 7B) and prefrontal SWA (Fig. 7C) negatively correlated with hippocampal activation during successful retrieval, such that greater SWA was associated with significantly less hippocampal-dependent activity. Importantly, hippocampal voxels that correlated negatively with SWA also correlated negatively with the offline measure of episodic memory change (Fig. 7D), indicating that activity within this region of the hippocampus that was not simply sensitive to retrieval, but sensitive to the change in overnight memory retention itself. Finally, and also congruent with these above associations and model predictions, retrieval-related hippocampal-prefrontal functional connectivity was also positively associated with SWA and episodic memory change (Fig. S3).
Figure 7.
(a) Age effects (Older>Young adults) in left hippocampal activation greater during successful associative episodic retrieval than correct rejection of novel words (Hits-Correct Rejections; 6mm-sphere ROI: [x=−33, y=−32, z=−7])18. No age differences in activation were detected outside the Hippocampus when employing FWE correction across the whole brain. Regressions between global slow wave activity (SWA) (b), defined as the average relative SWA across all electrode sites, and prefrontal SWA (c), defined as the average at prefrontal electrodes exhibiting peak relative SWA in both groups and left hippocampal activation (Hits-Correct Rejections; 6mm-sphere ROI: [x=−33, y=−32, z=−7])18. Regression between associative episodic memory change (d) and left hippocampal activation (Hits-Correct Rejections) at peak of both SWA correlations. Activations are displayed and considered significant at the voxel level of P<0.05 family-wise error (FWE) corrected for multiple comparisons within the a priori hippocampal regions of interest18. Hot colors represent the extent of increased activation in Older relative to Young adults, and cold colors represent the extent of the negative correlation between hippocampal activation and SWA. While the 6mm-sphere ROIs used to correct for multiple comparisons did extend outside the hippocampus, no effects were detected or presented outside the hippocampus in the current report. Error bars indicate s.e.m.
*denotes significance at P<0.05 FWE corrected.
au denotes arbitrary units.
%PTOT denotes percentage of total spectral power (0.4–50Hz)
Discussion
Together, our results support a framework in which deficits in age-related prefrontal grey matter predict the extent of disrupted NREM SWA, the degree to which represents a contributing factor (alongside other established pathways1,3,21,23) to impaired long-term memory in older adults. These findings establish that the degree of medial prefrontal grey matter atrophy is significantly associated with the extent of impoverished SWA in older adults. Moreover, the extent of impaired SWA in turn is associated with the degree of impaired long-term episodic memory retention, and furthermore, when included in the same statistical model, significantly accounts for the effects of age and medial prefrontal grey matter atrophy on such memory failure. Finally, our findings demonstrate that the extent of age-related impairment in overnight memory retention is additionally associated with the persistence (rather than progressive independence) of hippocampal dependence at later post-sleep retrieval, together with reduced hippocampal-mPFC functional connectivity during memory retrieval, indicative of impoverished overnight memory transformation in aging.
These data suggest that age-related differences in memory retention reflect an impaired sleep-dependent memory consolidation mechanism in older adults, as previously proposed2. While other factors such as circadian rhythms and alertness can also influence memory change across time, these factors did not appear to strongly account for the memory changes observed in the current experiment. Further, young adults exhibited a significant sleep-dependent stabilization of memory—stabilization, here, in the context of superior memory retention over time asleep relative to time awake——while older adults did not. Additionally, the degree of this overnight memory retention benefit was significantly predicted by the amount of intervening NREM SWA. Moreover, that NREM SWA negatively predicted hippocampal activation and positively predicted hippocampal-neocortical functional connectivity during retrieval conforms to model predictions of systems-level hippocampal-neocortical memory consolidation11.
Our findings build on and importantly extend previous evidence demonstrating that middle-age and older adults experience impaired long-term episodic memory across offline time periods that include sleep2, associated with reductions in time spent in NREM slow wave sleep2. However, the underlying neuropathological factors predicting these canonical reductions in NREM slow wave sleep and associated SWA has remained unknown. Furthermore, whether these reductions in SWA account for age and brain atrophy effects on episodic memory instead of age and brain atrophy independently impairing both sleep and memory has similarly remained unclear. Finally, the neural correlates of sleep-dependent memory impairment in aging have remained unexplored.
Founded on these outstanding issues, the current study indicates that impaired sleep-dependent memory consolidation potentially contributes to diminished long-term memory retention in old age, associated with canonical reductions in SWA in the elderly. Depending on the task and the nature of the memory assessment, some reports show no effect of age on long-term memory retention24, while others, particularly those that examine memory retention after delays long enough to include periods of sleep, show pronounced age effects1,25. Congruent with this literature, the current findings demonstrate that sleep offers a significantly greater overnight memory retention benefit in young adults relative to older adults. Importantly, this is not to suggest sleep does not benefit memory retention in older adults; an interpretation further supported by the fact that SWA still significantly predicted memory retention in older adults. Instead, this benefit was diminished in older adults, the degree to which was proportional to the extent of impoverished SWA.
In light of our current findings, we offer a hypothesized neuropathological model whereby age-related prefrontal atrophy may partially explain the prominent and well documented reductions in NREM SWA in aging4,5. Specifically, age-sensitive changes in grey matter atrophy within the mPFC consequently mediates the canonical age-related decline in SWA. Our data do not, by themselves, prove that prefrontal atrophy causes SWA decline in aging. However, this remains a parsimonious interpretation considering previous evidence in young adults demonstrating that EEG current source generators of NREM slow waves are maximally localized to remarkably similar mPFC regions16 to those identified in the current report showing maximal grey matter atrophy in older adults (and significant mediation of age-related changes in SWA), together with evidence linking NREM SWA changes with prefrontal brain structure development across ontogeny26.
In addition, our results and proposed framework offer a potential mechanistic basis supporting an association between sleep disruption and age-related cognitive decline2. However, our findings suggest that it is not age, per se, that independently governs these sleep and memory impediments2. Instead, the influence of age and concomitant prefrontal atrophy on impoverished overnight memory retention is statistically mediated by the degree of deficient SWA. Therefore, age alone does not independently reduce prefrontal grey matter and SWA and also diminish memory retention. Rather, our data suggest these three canonical signatures of the aging brain are significantly inter-related, with the deficiency in SWA mediating the degree of compromised offline episodic memory retention in the aging, atrophied brain. Given these results, it appears tenable that the reductions in prefrontal SWA in older adults, associated with medial prefrontal lobe atrophy, compromise the offline transformation and consolidation of episodic memories. Consistent with this functional relationship, the degree of prefrontal SWA impairment and overnight memory change was associated with persistent next-day hippocampal retrieval activation, as well as reduced hippocampal-mPFC functional connectivity. Such findings conform to predictions made by the model of sleep-dependent hippocampal memory transformation, when SWA is reduced6,7,10, in a similar manner to experimental manipulations of SWA in young adults9.
Building on these current findings, it will be important to determine whether such sleep-related changes represent an early predisposing factor to, or accelerant of, cognitive decline in the elderly23, and further, what role, if any, similar NREM SWA disruption plays in degenerative diseases with comorbid sleep abnormalities14,15. They further relate to the emerging proposal that factors such as sleep are important features determining healthy aging, beyond age per se15. At a translational level, and in light of this literature, our findings endorse the possibility that improvements of SWA in older adults, through physiological, behavioral or pharmacological means9,27,28, may represent a novel treatment target for minimizing the cognitive decline associated with deficient long-term memory retention in later life.
Methods
Thirty-six healthy adult participants completed the study (Young adults n=18, 10 females, mean±s.d., 20.4±2.1 years and Older adults n=18, 14 females, mean±s.d., 72.4±6.1 years). The study was approved by the local human studies committee, with all participants providing written informed consent. Exclusion criteria included presence of neurologic, psychiatric or sleep disorders, current use of antidepressant or hypnotic medications, or being left handed. Two older participants were excluded due to the presence of sleep apnea, while another was excluded due to technical difficulties during data collection. Young and older adults were free of depressive symptoms29,30, and performed similarly on the mini mental state exam31 (P=0.43). Further, and in addition to neuroradiological assessments and medical interviews (cf.21; obtained within 0–6 months of study entry), participants performed within two standard deviations of their age-matched control group on tests of 1) episodic memory32,33 and 2) frontal function34,35 (Table 1). Prior to study entry, older adults underwent sleep disorders screening with a polysomnography (PSG) recording night (described below) reviewed by a board certified sleep medicine specialist (author B.L.). Participants were excluded if they displayed evidence of a parasomnia or an Apnea/Hypopnea Index ≥1536.
All participants abstained from caffeine, alcohol, and daytime naps for the 48 hr before and during the entire course of the study. Participants kept normal, habitual sleep-wake rhythms and averaged 7–9 hr of reported time in bed per night prior to study participation, verified by sleep logs (Table 1).
General Experimental Design
Participants entered the lab in the evening and trained to criterion on a sleep-dependent episodic memory task (describe below), followed by a short delay (10 min) recognition test. Participants were then given an 8 hour sleep opportunity, measured with PSG, starting at their habitual bed time (Table 2) to minimize the influence of age-related circadian differences37. Approximately two hours post-awakening, participants performed an event-related functional MRI (fMRI) scanning session while performing a long delay (10 hr) recognition test.
Episodic Memory Task
The word-pairs task had an intentional encoding phase immediately followed by a criterion phase (see Fig. 1 & Supplemental Information), which was then followed by a short delay recognition test (10 min; 30 studied trials and 15 foil trials) and a long delay recognition test (10 hr, occurring 2 hours post-awakening within the MRI scanner; 90 studied trials and 45 foil trials) testing.
Figure 1.
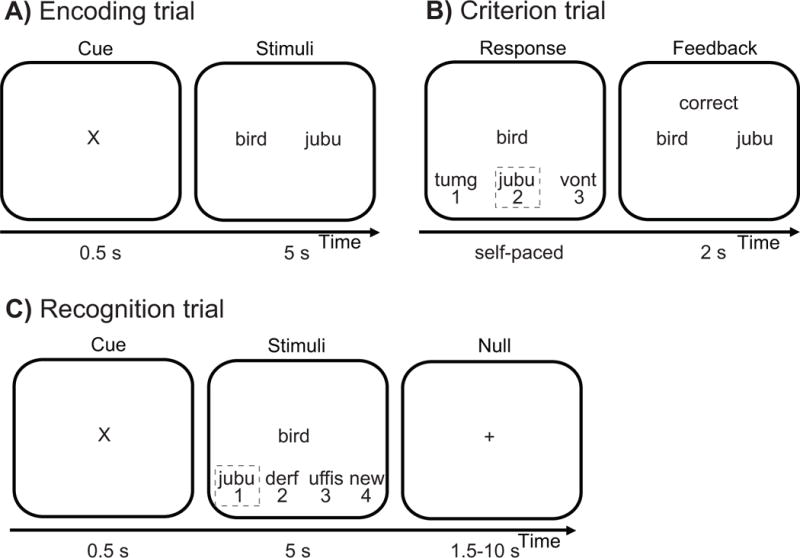
The sleep-dependent episodic word-pair task6,9 utilized word-nonsense word pairs to 1) maximize the novel episodic and associated hippocampal-dependent demands of the task38, and 2) minimize the semantic, and thus hippocampal-independent, demands of the task11,12,38. Words were 3–8 letters in length and drawn from a normative set of English words39. Nonsense words were 6–14 letters in length, derived from groups of common phonemes38. The word pair task began with an encoding phase composed of 120 word-nonsense word trials. (a) During each encoding trial, a word-nonsense word pair was shown for 5 s. Criterion training occurred immediately after encoding. (b) During each self-paced criterion trial, a previously studied probe word was presented with its original nonsense word associate from encoding (outlined in the grey box) and two new nonsense words not previously shown. Upon responding, the participant was given feedback for 1 s, with incorrect responses resulting in trial repetition at random intervals. (c) During recognition trials, either a previously studied probe word or a new (foil) probe word was shown for 5 s with four response options presented below. When a previously studied probe word was presented, the following response options were presented below: (1) the nonsense word originally paired with that probe word at encoding (‘Hit’), (2) a previously studied nonsense word, but one presented with a different previously studied probe word (‘Lure’), (3) a new nonsense word never seen during encoding, and (4) an option designating the shown probe word as “new”. New nonsense words were only presented once during the entire experiment, while previously studied nonsense words were presented twice during recognition testing, always in the same session, as a lure on one trial and the correct paired associate on another. When a foil probe word was presented, the four responses options consisted of three new nonsense words never presented during learning (which if chosen, would designate a ‘False alarm’), and an option designating this foil probe word as “new” (which if chosen, would designate a ‘Correct rejection’). Forty-five null events, consisting of a fixation display (1.5 s – 10 s), were interspersed throughout long delay recognition testing during fMRI acquisition, jittering trial onsets.
Associative recognition memory was calculated by subtracting both the ‘False alarm rate’ (FAR; proportion of foil words endorsed as “previously studied”) and the ‘Lure rate’ (LR; proportion of previously studied words recognized but erroneously paired with the lure and hence incorrect nonsense word associate) from the ‘Hit rate’ (HR; proportion of previously studied probe words accurately paired with the correct nonsense word associate)38. Episodic memory change was subsequently calculated as the difference in short and long delay recognition memory performance [long delay – short delay]40. Two participants, one young and one older adult, were excluded from analysis as outliers (memory performance more than 1.5 standard deviations below the mean).
MRI scanning
Scanning was performed on a Siemens Trio 3 Tesla scanner equipped with a 32-channel head coil. Functional scans were acquired using a susceptibility-weighted, single-shot echo-planar imaging (EPI) method to image the regional distribution of the blood oxygenation level-dependent signal (TR/TE, 2000/23 ms; flip angle, 90°; FOV, 224 mm; matrix, 64×64; 37 3mm slices). Three functional runs were acquired (159 volumes, 5.3 minutes). Following functional scanning, two high-resolution T1-weighted anatomical image was acquired using a 3D MPRAGE protocol with the following parameters: repetition time (TR), 1900 ms; echo time (TE), 2.52 ms; flip angle, 9°; field of view (FOV), 256 mm; matrix, 256 × 256; slice thickness, 1.0 mm; and 176 slices.
fMRI analysis
fMRI data were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm/software/) beginning with standardized preprocessing (realignment, slice timing correction, and coregistration), and with normalization accomplished using a mixed young-old template generated from 400 individual T1-weighted anatomical images41, consistent with recommendations for use in studies comparing young and older adults41,42.
Following preprocessing, retrieval trials were sorted into “Hits” (correct word-nonsense word recognition), “Lures” (selection of the incorrect, previously studied, nonsense word), “Misses” (incorrect selection of never studied nonsense word or endorsement of word as “new”), Correct Rejections (novel words correctly endorsed as “new”), False Alarms (novel words incorrectly endorsed as “studied”), and Omissions (trials with no subject response)43,44, with each trial modeled using a canonical hemodynamic response function. To generate a validated contrast for retrieval-related activity, Hit events were contrasted with Correct Rejection events [Hits – Correct Rejections]43,44. Activation maps at the subject level were thentaken to a second-level random effects analysis to examine group effects. Activations were assessed at the voxel level of p<0.05 family wise error (FWE)45, corrected for multiple comparisons within an a priori hippocampal region of interest (ROI; 6 mm sphere [x=−33, y=−32, z=−7]18).
To determine whether hippocampal involvement during delayed recognition was inversely associated with the degree of preceding SWA8,13, regression models relating global and prefrontal slow wave activity (SWA) to hippocampal activation [Hits – Correct Rejections] were examined.
Finally, to examine the role of SWA on hippocampal-neocortical connectivity expressly during the act of memory retrieval, psychophysiologic interaction (PPI) analysis was employed46,47 using SPM8 (Wellcome Department of Imaging Neuroscience), with an a priori focus on coupling with ventromedial prefrontal cortex (vmPFC)6–8,10,11,13,48. Specifically, within each individual, psychophysiologic interaction effects were calculated using a hippocampal seed region defined by the peak hippocampal activation SWA correlations (6mm-sphere ROI: [x=−34, y=−35, z=−4]), detected in the above described hippocampal ROI18. General linear models were constructed for each subject and included (i) a regressor for the deconvolved blood oxygen level dependent (BOLD) signal from the hippocampal seed region, (ii) a regressor accounting for the cognitive context of successful memory retrieval [Hits relative to Correct Rejections], (iii) the psychophysiologic interaction term of the first and second regressors, and (iv) movement regressors to account for noise relating to head motion. The contrast images for the psychophysiologic interaction term were then forwarded to the second level and regressed against global SWA. In order to ensure effects were also related to group differences, effects were inclusively masked (p<0.05). Connectivity values were assessed at the voxel level of p<0.05 family wise error (FWE) corrected for multiple comparisons, targeting the vmPFC a priori (8mm-sphere ROI: [x=−2, y=32, z=−10])8. The cluster average of significant voxels was extracted using Marsbar49 and regressed against memory change to determine whether the interaction between the hippocampus and vmPFC, in the context of memory retrieval, was associated with the metric of successful memory retention.
Structural MRI analysis
To measure grey matter volume, optimized voxel-based morphometry (VBM) was performed using SPM8 (Wellcome Department of Imaging Neuroscience) with the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html) and the Diffeomorphic Anatomical Registration through Exponentiated Lie algebra (DARTEL) toolbox in order to improve registration of older brains to the normalized MNI template50,51. To enhance signal to noise ratio, two T1-weighted MPRAGE images were first coregistered and averaged. Averaged images were then segmented applying the Markov random field approach52, and then registered, normalized, and modulated using DARTEL. Grey matter and white matter segmentations were inputted into DARTEL and utilized to create a study specific template, which was then used to normalize individual brains into MNI space. Modulated grey matter maps were then smoothed using an 8 mm Gaussian kernel. Grey matter maps were then forwarded into a general linear model. Measures of total intracranial volume (TIV) for each participant were estimated from the sum of grey matter, white matter, and CSF segmentation, and then included as a nuisance regressor. Due to data collection error, anatomical scans from two younger participants and one older participant were excluded from analysis, leaving 14 older and 16 younger adults for all related analyses. An independent samples t-test was used to determine the effects of age on grey matter volume, assessed and displayed at the voxel level of p<0.05 family wise error (FWE) corrected for multiple comparisons across the whole brain. Mean gray matter density within medial prefrontal cortex, defined using Brodmann’s definition53, exhibiting significant age effects was extracted using the Marsbar toolbox49 and used in the below described mediation analyses.
Measures of subjective and objective alertness
Subjective measures of sleepiness and alertness were measured using a validated visual analog scale54 collected every two hours throughout the study while subjects were awake. Subjective ratings were compared from before to after sleep to assess the change in subjective sleepiness and alertness. The after sleep assessment occurred approximately 2 hr post awakening and immediately before scanning, to best estimate subjective sleepiness and alertness immediately preceding delayed recognition in the scanner. Overall reaction time during immediate and delayed recognition, and the change in reaction time from immediate to delayed [delayed – immediate] testing were used to determine if objective alertness predicted memory change in young and older adults.
Sleep monitoring and EEG analysis
Polysomnography (PSG) sleep monitoring on the experimental night was recorded using a Grass Technologies Comet XL system (Astro-Med, inc., West Warwick, RI), including 19-channel electroencephalography (EEG) placed using the standardized 10–20 system, electrooculography (EOG) recorded at the right and left outer canthi (right superior; left inferior), and electromyography (EMG). Reference electrodes were recorded at both the left and right mastoid (A1, A2). Data were digitized at 400Hz, and stored unfiltered (recovered frequency range of 0.1–100 Hz), except for a 60-Hz notch filter. Sleep was scored using standard criteria55.
Sleep monitoring on the screening night was recorded using a Grass Technologies AURA PSG Ambulatory system (Astro-Med, inc., West Warwick, RI), similar to as described above save for the following exceptions: 1) EEG was recorded at 9 derivations (F3, FZ, F4, C3, CZ, C4, P3, P4, OZ), 2) data were digitized at 200Hz. During full PSG screening nights, nasal/oral airflow, abdominal and chest belts, and pulse oximetry were additionally monitored to screen for the presence of sleep apnea.
EEG data from the experimental night were imported into EEGLAB (http://sccn.ucsd.edu/eeglab/) and epoched into 5 s bins. Epochs containing artifacts were rejected, and the remaining epochs were filtered between 0.4–50Hz. A fast Fourier transform (FFT) was then applied to the filtered EEG signal at 5-second intervals with 50% overlap and employing hanning windowing. Analyses in the current report focused, a priori, on slow wave activity (SWA), defined as absolute and relative spectral power between 0.8–4.6Hz during slow wave sleep19. Spectral power during slow wave sleep was chosen, because staging requires absolute amplitude above a standard threshold55 requiring, by definition, slow wave detection. Relative spectral power was chosen, because it accounts for individual differences in overall absolute spectral power potentially due to differences in brain to scalp distance, skull thickness, impedance, head size, and the potential effects of different sleep recording systems56, standardizing spectral power across subjects.
Statistical Analysis
A two-way repeated measures ANOVA was used to compare episodic memory retention between young and older adults, with group (young/older) as a between subjects factor and testing session (immediate/delayed) as a within-subjects factor. To assess whether this effect was specific to sleep, a three-way repeated measures ANOVA was utilized with group (young/older) and condition (sleep/wake) as between subjects factors and testing session (short delay–10 min/long delay–10 hr) as a within-subjects factor. Group differences in sleep variables were assessed using independent, two-sample t-tests. Associations between prefrontal atrophy, sleep measures, and episodic memory change were assessed using Pearson’s correlations. SWA and episodic memory change correlations were examined and considered significant if they were significant at P<0.05 FDR corrected across the electrode array22.
To test the hypothesis that the effects of age on prefrontal atrophy mediated the effects of age on relative slow wave activity (SWA) and the effects of age on relative SWA mediated the effects of age and prefrontal atrophy on episodic memory change, mediation analyses were performed using established methods20,21,57. In short, these analyses determine whether the influence of independent variable X on dependent variable Y is accounted for by mediator M, i.e. is the value of the direct path coefficient between X and Y reduced by the inclusion of M? Reduction to 0 is interpreted as mediation, partial reduction is interpreted as partial mediation, and non-significant reduction is interpreted as no evidence for mediation. Effects were formally tested using the Sobel test of mediation20,57. Analyses were completed using SPSS version 18.0 (SPSS, Inc., Chicago, IL).
Supplementary Material
Acknowledgments
We thank Maggie Belshe, Meghna Bhatter, Michelle Binod, Sam Bowditch, Catherine Dang, Amynta Hayenga, April Horn, Emily Hur, Candace Markeley, Elizabeth Mormino, Molly Nicholas, Lily Zhang, and Alyssa Zhu for their assistance; Anthony Mander for his aid in task design; and Michael Rubens and Adam Gazzaley for use of their aging template brain. This work was supported by awards R01-AG031164(MPW), R01-AG034570(WJ) and F32-AG039170(BAM), from the National Institutes of Health.
Footnotes
Author contributions: B.A.M. designed the study, conducted the experiments, analyzed the data, and wrote the manuscript. V.R. aided in data analysis and manuscript preparation. B.L. aided in study screening procedures and manuscript preparation, J.M.S. provided data analytic tools, aided in data analysis, and manuscript preparation. J.R.L. aided in conducting the experiment and manuscript preparation. S.A.I aided in study design and manuscript preparation. W.J, provided the elderly subject pool and data analytic tools, aided in study design and manuscript preparation. M.P.W. designed the study, aided data analysis, and wrote the manuscript.
References
- 1.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Backhaus J, et al. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem. 2007;14:336–341. doi: 10.1101/lm.470507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowell ER, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 4.Dijk DJ, Beersma DG, van den Hoofdakker RH. All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiology of aging. 1989;10:677–682. doi: 10.1016/0197-4580(89)90004-3. [DOI] [PubMed] [Google Scholar]
- 5.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. Jama. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 6.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 7.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 8.Takashima A, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756–761. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 10.Buzsaki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 11.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 12.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 13.Gais S, et al. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci U S A. 2007;104:18778–18783. doi: 10.1073/pnas.0705454104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westerberg CE, et al. Concurrent Impairments in Sleep and Memory in Amnestic Mild Cognitive Impairment. J Int Neuropsychol Soc. 2012:1–11. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitiello MV. Recent Advances in Understanding Sleep and Sleep Disturbances in Older Adults: Growing Older Does Not Mean Sleeping Poorly. Current Directions in Psychological Science. 2009;18:316–320. [Google Scholar]
- 16.Murphy M, et al. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marzano C, Ferrara M, Curcio G, De Gennaro L. The effects of sleep deprivation in humans: topographical electroencephalogram changes in non-rapid eye movement (NREM) sleep versus REM sleep. J Sleep Res. 2010;19:260–268. doi: 10.1111/j.1365-2869.2009.00776.x. [DOI] [PubMed] [Google Scholar]
- 18.Nadel L, Campbell J, Ryan L. Autobiographical memory retrieval and hippocampal activation as a function of repetition and the passage of time. Neural Plast. 2007;2007:90472. doi: 10.1155/2007/90472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dijk DJ, Hayes B, Czeisler CA. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993;626:190–199. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 20.MacKinnon DP, Warsi G, Dwyer JH. A simulation study of mediated effect measures. Multivariate Behavioral Research. 1995;30:41–62. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mormino EC, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate – a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- 23.Kang JE, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science (New York, NY. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albert MS. The ageing brain: normal and abnormal memory. Philos Trans R Soc Lond B Biol Sci. 1997;352:1703–1709. doi: 10.1098/rstb.1997.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis HP, et al. Acquisition, recall, and forgetting of verbal information in long-term memory by young, middle-aged, and elderly individuals. Cortex. 2003;39:1063–1091. doi: 10.1016/s0010-9452(08)70878-5. [DOI] [PubMed] [Google Scholar]
- 26.Buchmann A, et al. EEG sleep slow-wave activity as a mirror of cortical maturation. Cereb Cortex. 2011;21:607–615. doi: 10.1093/cercor/bhq129. [DOI] [PubMed] [Google Scholar]
- 27.Naylor E, et al. Daily social and physical activity increases slow-wave sleep and daytime neuropsychological performance in the elderly. Sleep. 2000;23:87–95. [PubMed] [Google Scholar]
- 28.Van Cauter E, et al. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young Men. J Clin Invest. 1997;100:745–753. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rush AJ, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 30.Yesavage JA, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 31.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 32.Delis D, Kramer J, Kaplan E, Ober B. California verbal learning test. The Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- 33.Wechsler D. Wechsler memory scale-revised. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 34.Reitan RM. Validity of the trail-making test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 35.Zec RF. The stroop color-word test: A paradigm for procedural learning. Arch Clin Neuropsychol. 1986;1:274–275. [Google Scholar]
- 36.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 37.Munch M, Silva EJ, Ronda JM, Czeisler CA, Duffy JF. EEG sleep spectra in older adults across all circadian phases during NREM sleep. Sleep. 2010;33:389–401. doi: 10.1093/sleep/33.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otten LJ, Sveen J, Quayle AH. Distinct patterns of neural activity during memory formation of nonwords versus words. J Cogn Neurosci. 2007;19:1776–1789. doi: 10.1162/jocn.2007.19.11.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida free association, rhyme, and word fragment norms. Behav Res Methods Instrum Comput. 2004;36:402–407. doi: 10.3758/bf03195588. [DOI] [PubMed] [Google Scholar]
- 40.Cohen DA, Robertson EM. Preventing interference between different memory tasks. Nat Neurosci. 2011;14:953–955. doi: 10.1038/nn.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bollinger J, Rubens MT, Masangkay E, Kalkstein J, Gazzaley A. An expectation-based memory deficit in aging. Neuropsychologia. 2011;49:1466–1475. doi: 10.1016/j.neuropsychologia.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckner RL, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- 44.Spaniol J, et al. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 45.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 47.Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 48.Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav Brain Res. 2011;218:325–334. doi: 10.1016/j.bbr.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 49.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract]. NeuroImage; 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan: 2002. [Google Scholar]
- 50.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 51.Mak HK, et al. Efficacy of voxel-based morphometry with DARTEL and standard registration as imaging biomarkers in Alzheimer’s disease patients and cognitively normal older adults at 3.0 Tesla MR imaging. J Alzheimers Dis. 2011;23:655–664. doi: 10.3233/JAD-2010-101659. [DOI] [PubMed] [Google Scholar]
- 52.Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging. 1997;16:176–186. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- 53.Brodmann K. Brodmann’s Localisation in the cerebral cortex : the principles of comparative localisation in the cerebral cortex based on cytoarchitectonics. Springer; New York: 1909. [Google Scholar]
- 54.Monk TH. A Visual Analogue Scale technique to measure global vigor and affect. Psychiatry Res. 1989;27:89–99. doi: 10.1016/0165-1781(89)90013-9. [DOI] [PubMed] [Google Scholar]
- 55.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques an scoring system of sleep stages in human subjects. UCLA, Brain Information Services; Los Angeles; 1968. [Google Scholar]
- 56.Gasser T, Bacher P, Steinberg H. Test-retest reliability of spectral parameters of the EEG. Electroencephalogr Clin Neurophysiol. 1985;60:312–319. doi: 10.1016/0013-4694(85)90005-7. [DOI] [PubMed] [Google Scholar]
- 57.Mander BA, et al. EEG measures index neural and cognitive recovery from sleep deprivation. J Neurosci. 2010;30:2686–2693. doi: 10.1523/JNEUROSCI.4010-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 59.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 60.Mander BA, Santhanam S, Saletin JM, Walker MP. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21:R183–184. doi: 10.1016/j.cub.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


