Abstract
Objective
Treat to Target guidelines promote shared decision-making (SDM) in rheumatoid arthritis (RA). Also, due to high cost and potential toxicity of therapies, SDM is central to patient safety. Our objective was to examine patterns of perceived communication around decision-making in two cohorts of adults with RA.
Methods
Data were derived from patients enrolled in one of two longitudinal, observational cohorts (UCSF RA Cohort and RA Panel). Subjects completed a telephone interview in their preferred language that included a measure of patient-provider communication, including items about decision-making. Measures of trust in physician, education, and language proficiency were also asked. Logistic regression was performed to identify correlates of suboptimal SDM communication. Analyses were performed on each sample separately.
Results
Of 509 patients across two cohorts, 30% and 32% reported suboptimal SDM communication. Low trust in physician was independently associated with suboptimal SDM communication in both cohorts. Older age and limited English proficiency were independently associated with suboptimal SDM in the UCSF RA Cohort, as was limited health literacy in the RA Panel.
Conclusions
This study of over 500 adults with RA from two demographically distinct cohorts found that nearly one-third of subjects report suboptimal SDM communication with their clinicians, regardless of cohort. Lower trust in physician was independently associated with suboptimal SDM communication in both cohorts, as was limited English language proficiency and older age in the UCSF RA Cohort and limited health literacy in the Panel. These findings underscore the need to examine the impact of SDM on health outcomes in RA.
Key indexing terms: arthritis, rheumatoid, health communication, health literacy, trust
----“In a truly shared decision, physicians and patients mutually influence each other, each potentially ending up in a place different from where they began, with different understandings than either would have reached alone.”
–Hanson, Archives of Internal Medicine, July 14, 2008
Rheumatoid arthritis (RA) is the most common inflammatory arthritis, affecting up to 1% of the population. Due to the complexity, high cost, and potential toxicity of therapies for rheumatoid arthritis (RA), clear patient-clinician communication is central to safety and quality of care. Two of the six priorities outlined in the National Quality Strategy, a provision of the Affordable Care Act, are to ensure patient-centered care and promote effective communication (1). Implicit in this mandate to provide quality, person-centered care, is the need to involve patients in decision-making around all aspects of their care. Furthermore, recommendations from an international task force in rheumatology outline four overarching principles in the treatment of RA, the first of which states: “The treatment of rheumatoid arthritis must be based on a shared decision between patient and rheumatologist.”(2). Health authorities in several European nations have placed great emphasis patient engagement in decision making (3), however despite wide recognition of the benefits and ethical considerations in incorporating shared decision making and the use of decision aids in everyday practice a number of barriers to its uptake have been identified. Wennberg and colleagues outlined ways in which the U.S. government could improve care and reduce healthcare spending in their 2008 Dartmouth Atlas White Paper and included a focus on requiring informed patient choice and shared decision-making as ways to reduce unwanted or unnecessary procedures and treatments (4). While there is no consensus on a single definition or theoretical framework of what constitutes shared decision making, we define it here as a process whereby both patient and clinician take into account the best available evidence of risks and benefits across all available options as well as take into account patient values and preferences when making medical decisions (5). Evidence that greater levels of informed choice and patient involvement in decision making leads to increased knowledge of conditions and treatment, improved satisfaction with decisions, and greater adherence to medication (as seen in asthma) continues to build in other chronic conditions (6–8).
Several factors known to influence patient-provider communication and shared decision-making (SDM) in other conditions such as diabetes and coronary heart disease include trust in physician, race/ethnicity, education level, employment status, depression, and limited English proficiency (LEP) (9–15). While these factors have been associated with clinician-patient communication in other chronic diseases, they have not been examined in ethnically and linguistically diverse populations with RA, particularly among those patients at highest risk for poor outcomes and with barriers to communication, such as LEP and limited health literacy. Our study model (Figure 1) outlines our vision of the potential relationships between patient-level characteristics, trust in physician, shared decision-making and how these factors may be related to health outcomes in RA.
Figure 1.
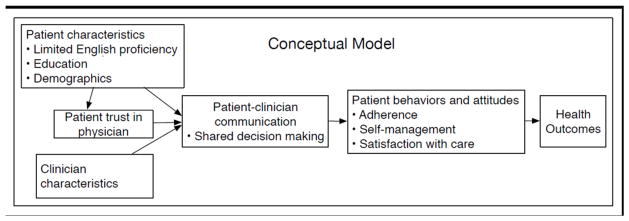
Conceptual model of the relationship among patient-level characteristics, trust, shared decision-making and health outcomes.
We conducted this study to examine patient perception of shared decision-making communication within two cohorts of adults with RA. In addition, we sought to better understand the influence of trust, language proficiency, and educational attainment on shared decision-making communication in RA.
Patients and Methods
Study design
This is a cross-sectional study of the results from a one-time telephone interview administered to two separate cohorts of adults with RA described below.
Data sources
Subjects included in this study were participants in one of two longitudinal, observational RA cohorts: the RA Panel Study and the University of California, San Francisco (UCSF) RA Cohort. The RA Panel Study was initiated in 1982 by enrolling the universe of persons with RA being treated over a one-month period by a random sample of Northern California rheumatologists; subsequent enrollment occurred in 1989–1990, 1995–1996, 1999–2000, and 2003–2004, with an average of 85% of listed patients enrolled each time. RA Panel participants are interviewed by telephone annually in English. Follow-up rates in the RA Panel averaged 92% annually since the last enrollment wave. The data for this study consist of all interviews conducted in 2011, with a total of 275 participants from the RA Panel. The UCSF RA Cohort is a multi-site observational cohort whose enrollment began in October 2006. Subjects were consecutively enrolled from two outpatient clinics staffed by UCSF faculty and fellows, the Rheumatoid Arthritis Clinic at San Francisco General Hospital (SFGH) and the university-based UCSF Arthritis Center; approximately 90% of patients agreed to enroll in the cohort.. Data for the UCSF RA Cohort were obtained from patients and physicians at the time of each regular clinical visit and integrated with laboratory and radiology test results. The 234 patients from the UCSF RA Cohort included in this study were interviewed by telephone between 2007 and 2009 in their preferred language (English, Spanish, or Cantonese); they represented 85% of all active cohort members at that time. The research protocol for both cohorts was approved by the UCSF Committee on Human Research. All participants gave their informed consent to be part of the study. This study took place in the UCSF Collaborative Research Network.
Measures
Primary Outcome: Quality of Shared Decision-Making Communication
To measure the patient perception of communication around shared decision-making, we used a subscale from the Interpersonal Processes of Care (IPC) survey. The IPC is a valid and reliable measure (16) designed to measure specific components of doctor-patient communication in diverse populations (17); it was included in the telephone survey for both cohorts. Prior to administration of the IPC, all patients are told: “The next questions are about your experiences talking with your main rheumatology or arthritis doctor over the past twelve months….” If the patient reports seeing more than one physician, they are then prompted to answer about the doctor seen most often. The primary outcome for this study was the two-item validated decision-making subscale of the IPC that is calculated as the mean score for two items, “How often did you and your doctors work out a treatment plan together?” and, “If there were treatment choices, how often did doctors ask if you would like to help decide your treatment?” The five-item response ranged from 1 “never” to 5 “always. We created a summary score from the average of the two items. Mean scores <4 (corresponding to never/rarely/sometimes) were categorized as suboptimal communication, as has been done in prior studies (14). We also created an alternate measure of communication in decision making, in which anything besides the highest rating on both measures was considered suboptimal as has been done in other surveys of healthcare experiences (Consumer Assessments of Healthcare Providers and Systems or CAHPS) and considered the “top box” approach (18) to scoring which has also been recommended for the IPC (19).
Primary independent variables
Trust in Physician
Trust in physician has been shown to be associated with quality of communication (13, 14) as well as confidence in the decision to take a disease-modifying antirheumatic drug or DMARD in RA (20). We measured trust with the 11-item Trust in Physician scale (21) which has been validated in patients with RA(22), however, to our knowledge, it has not been validated in Spanish (David Thom, personal correspondence). Each question has a 5-point Likert scale response ranging from 1 “strongly disagree” to 5 “strongly agree.” Examples of questions include: “I trust my doctor so much I always try to follow his/her advice,” “I trust my doctor’s judgments about my medical care,” and “I trust my doctor to tell me if a mistake was made about my treatment.” Responses are summed and that value is transformed to a 0–100 scale. A score below the median (90.9) was considered to be suboptimal.
Education and income
Education level was ascertained during the telephone interview and dichotomized as less than high school (<HS) or high school graduate and beyond (HS/BA). Household income was also gathered in the telephone interview and included here as an alternate measure of socioeconomic status (SES), categorized as <$20,000, $20–80,000, and ≥$80,000.
Limited English language proficiency
In the UCSF RA Cohort interviews, English language proficiency was assessed using the U.S. Census question “How well do you speak English?” Those who reported “very well” or “well” were considered English proficient (EP) and those who reported “not well” or “not at all” were considered to have limited English-proficiency (LEP)(12, 23, 24). English language proficiency was not ascertained in the RA Panel, which was conducted exclusively in English.
Health literacy
Health literacy was measured in the RA Panel using the single item literacy screener, a self-report question developed by Morris and colleagues (25, 26) validated among diverse English and Spanish-speaking populations (27) and administered over the telephone in multiple prior studies (28–30).. This screener has also been used to measure health literacy in a large study of over 6,000 RA patients in the U.S. (31). The single-item question is “How often do you have someone like a family member, friend, hospital or clinic worker or caregiver help you read health plan materials, such as written information about your health or care you are offered?” Possible answers include: “always, often, sometimes, occasionally or never.” A response of “sometimes,” “often,” or “always” was considered to represent limited health literacy (26, 32).
Other variables
Patient age, gender, race/ethnicity, language, and date of diagnosis were obtained at time of enrollment into the cohorts. Patients provided a global assessment of their disease during the telephone interview, answering the following question, “Considering all the ways that your arthritis affects you, rate how well you are doing on a scale from 0 to 100, where 0 is very well and 100 is very poor” (33). The Patient Health Questionnaire-9 (PHQ-9)(34, 35) was collected in the UCSF RA Cohort and the Geriatric Depression Scale short form (GDS)(36, 37) was obtained in the RA Panel to measure depressive symptoms. The RA Panel has used the Geriatric Depression scale consistently since 1989; the UCSF RA Cohort has used the PHQ-9 since 2006 as it has been validated in multiple languages (35, 38–40) and used among patients with limited literacy (41) and English language proficiency (42). We used cutoffs for depressive symptoms in each scale that allowed for comparable thresholds.
Statistical analysis
We conducted all analyses on the two cohorts separately, examining the associations between the likelihood of reporting suboptimal communication in shared decision-making and the primary independent variables of education, health literacy or English language proficiency, and trust in physician. Because the dependent variable is dichotomous, we used binary logistic regression for all statistical modeling. In addition to the primary independent variables, we selected covariates on an a priori basis that are known or hypothesized to be associated with differences in patient-provider communication. These included patient age, gender, race/ethnicity, disease duration, and patient global disease assessment. Due to the relatively low number of RA Panel members who were ethnic minorities, whites were compared to nonwhites in that cohort.
We initially examined the relationship between each independent variable and the likelihood of suboptimal communication in shared decision-making in a series of bivariable logistic regression models. We next assessed the full model for multicollinearity. Finding no covariates with variance inflation factors (VIF) above 1.5 in either cohort, we determined that all variables could be appropriately included in the same multivariable models; there was likewise no evidence of excessive influence by any individual observations in the models. The models showed an adequate fit to the data based on both the classification table approach (>70% correctly classified in each model), and the Hosmer and Lemeshow goodness of fit test (43). Lastly, we assessed potential interactions among the primary independent variables: trust, education, and health literacy (in the RA Panel) or English language proficiency (in the UCSF RA Cohort). Finding no significant interactions in either model, we present only the main effects models.
We examined several alternate models to the ones presented here. These included replacing education with household income as an alternate measure of socioeconomic status, and adding a measure of depressive symptomatology, which has been associated with suboptimal communication in coronary heart disease and diabetes (13, 14), to the multivariable model for each cohort. We investigated the role of trust in physician as either an effect modifier or a mediator in the relationship of each of the main predictor variables to shared decision-making. As a sensitivity analysis, we used the more inclusive measure of suboptimal communication as the dependent variable in the logistic regression models.
All analyses were performed using STATA Version 12 (STATA Corp, College Station, TX).
Results
A total of 509 subjects were included in this study, 234 from the UCSF RA Cohort and 275 from the RA Panel. Patients from both cohorts were predominantly female (84% in the Cohort, 86% in the Panel), however, they were different in all other demographic characteristics (Table 1). The UCSF RA Cohort has great diversity by race/ethnicity and language with substantial numbers of ethnic minorities and non-English speakers (64% English, 22% Spanish and 14% Cantonese or Mandarin). The average age of the RA Panel patients was nearly ten years older, 83% were white and 95% had graduated high school. Limited health literacy was identified in 39 subjects (14%) in the RA Panel. RA Panel participants rated their disease as less active overall, with a mean rating of 24.2 vs. 41.9 for the UCSF RA Cohort patients, on a 0–100 scale. The two cohorts assessed depressive symptoms using two different measures and had differing degrees of depressive symptoms as well. In the UCSF RA Cohort, 25% of subjects had a PHQ-9 score ≥10, which has been shown to correspond to moderate depressive symptoms or greater. In the RA Panel, only 13% had a comparably high score in the GDS. Patients in both cohorts expressed a high degree of trust in their physicians, with half giving a score of over 90 on a 0–100 scale.
Table 1.
Characteristics of participants from two rheumatoid arthritis cohorts
| Characteristic | UCSF RA Cohort n=234 | RA Panel n=275 |
|---|---|---|
| n (%) or mean ± SD | ||
| Age, years ± SD | 55±14 | 64±11 |
| Female | 198 (84) | 236 (86) |
| Disease duration, years ± SD | 12±8 | 26±11 |
| Race | ||
| White | 84 (36) | 227 (83) |
| Latino | 76 (32) | 21 (8) |
| Asian/Pacific Islander | 46 (20) | 15 (5) |
| African American | 19 (8) | 4 (1) |
| Other | 9 (4) | 8 (3) |
| Language | ||
| English | 150 (64) | 275 (100) |
| Spanish | 52 (22) | 0 |
| Chinese | 32 (14) | 0 |
| Limited English language proficiency | 91 (39) | -- |
| Limited Health literacy | -- | 39 (14) |
| Less than high school education | 66 (28) | 14 (5) |
| Depressive Symptomology | ||
| PHQ-9, mean ± SD (range) | 6.5±5.9 (0–24) | -- |
| GDS, mean ± SD (range) | -- | 2.5±2.7 (0–13) |
| Proportion above cut-point for significant depressive symptoms1 | 25% | 13% |
| Patient global assessment, mean ± SD (range) | 41.9±30.2 (0–100) | 24.2±21.6 (0–97) |
| Trust in physician, (median, interquartile range) | 90.9 (85.5, 98.2) | 90.9 (81.8, 98.2) |
-- not measured in survey
For PHQ-9, score of ≥10 points; for GDS, score of ≥7 points.
Primary outcome
Despite the demographic differences, patients were nearly equally likely to report suboptimal shared decision-making communication (30% for the Cohort and 32% for the Panel, Table 2).
Table 2.
Proportion of study participants in two cohorts reporting suboptimal communication in shared-decision making
| Study | Sample size | Proportion reporting suboptimal communication in shared-decision making (95% CI) |
|---|---|---|
| UCSF RA Cohort | 234 | 30% (25–37%) |
| RA Panel | 275 | 32% (27–38%) |
Bivariate and multivariate results by sample
UCSF RA Cohort
In bivariate analyses, gender, age, and disease duration were not associated with suboptimal communication. Latino and Asian/Pacific Islander race/ethnicity, LEP, less than high school education, and low trust in physician were all associated with greater risk of reporting suboptimal SDM communication (Table 3). In the multivariate analyses, lower trust in physician remained strongly associated with suboptimal SDM communication with an adjusted odds ratio (AOR) of 2.11 (95% CI 1.10–4.07). In addition, an increase in age was a significant independent correlate of suboptimal SDM (AOR 1.28 per 10 years, 95% CI 1.01–1.62), as was LEP (AOR 5.11, 95% CI 1.56–16.7). Race/ethnicity and education were no longer significant in the multivariable model.
Table 3.
Odds ratios for suboptimal communication in shared decision-making among 234 UCSF RA Cohort participants, from unadjusted and adjusted logistic regression models
| Unadjusted odds ratio (95% CI) | Adjusted* odds ratio (95% CI) | |
|---|---|---|
| Female | 1.63 (0.70–3.79) | 1.57 (0.66–3.72) |
| Age, per 10 years | 1.15 (0.94–1.41) | 1.28 (1.01–1.62) |
| Disease duration, years | 0.98 (0.95–1.00) | 0.99 (0.95–1.02) |
| Patient global assessment (0–100) | 1.00 (0.99–1.01) | 0.99 (0.98–1.01) |
| Race/ethnicity | ||
| White | ref. | ref. |
| Latino | 2.72 (1.35–5.48) | 0.71 (0.18–2.79) |
| Asian/Pacific Islander | 2.77 (1.26–6.13) | 0.57 (0.16–2.08) |
| African American | 0.46 (0.10–2.20) | 0.47 (0.09–2.40) |
| Other | 1.13 (0.21–5.92) | 1.59 (0.29–8.69) |
| Less than high school education | 2.83 (1.56–5.16) | 1.36 (0.60–3.10) |
| Limited English language proficiency | 4.82 (2.66–8.78) | 5.11 (1.56–16.7) |
| Trust in physician, low | 2.70 (1.51–4.86) | 2.11 (1.10–4.07) |
Bold face type indicates p<0.05.
Model adjusted for all variables shown.
Hosmer-Lemeshow Goodness of Fit test: χ2(8)=9.8 p=0.28
RA Panel
In the bivariate analyses, the Panel showed similar results with respect to low trust in physician (odds ratio 5.22, 95% CI 2.93–9.31), but lower education, race/ethnicity, age, gender and disease duration were not associated with suboptimal SDM communication. However, worse patient global rating of disease was associated with suboptimal SDM communication (Table 4). In multivariate analysis, low trust in physician remained significant (AOR 5.57, 95% CI 3.05–10.15) as did limited health literacy (AOR 2.80, 95% CI 1.25–6.28).
Table 4.
Odds ratios for suboptimal communication in shared decision-making among 275 RA Panel participants, from unadjusted and adjusted logistic regression models
| Unadjusted odds ratio (95% CI) | Adjusted* odds ratio (95% CI) | |
|---|---|---|
| Female | 0.73 (0.36–1.47) | 0.77 (0.36–1.68) |
| Age, per 10 years | 1.24 (0.99–1.55) | 1.14 (0.87–1.50) |
| Disease duration, years | 1.01 (0.99–1.03) | 1.01 (0.98–1.03) |
| Patient global assessment (0–100) | 1.02 (1.00–1.03) | 1.01 (0.99–1.02) |
| Nonwhite race/ethnicity | 1.18 (0.61–2.27) | 1.02 (0.50–2.07) |
| Less than high school education | 1.61 (0.54–4.78) | 1.28 (0.44–3.66) |
| Limited health literacy | 2.88 (1.44–5.74) | 2.80 (1.25–6.28) |
| Trust in physician, low | 5.22 (2.93–9.31) | 5.57 (3.05–10.15) |
Bold face type indicates p<0.05.
Model adjusted for all variables shown.
Hosmer-Lemeshow Goodness of Fit test: χ2(8)=4.7 p=0.79
We conducted several sensitivity analyses as part of the study. Given the use of a “top box” method of scoring healthcare experience surveys as discussed above, we created a more stringent definition of optimal communication, only including participants who responded ‘always’ to both decision-making items. Under this definition, more than 50% of either cohort report sub-optimal communication around decision-making. Nevertheless, the results for the main analysis are essentially unchanged, with strong associations with limited English proficiency and suboptimal trust in physician in the UCSF RA Cohort and with health literacy and suboptimal trust in the RA Panel. When we estimated the models using annual household income as an alternate measure of socioeconomic status, the findings were similarly unchanged. Depressive symptoms have been shown to be associated with suboptimal patient-clinician communication in other chronic diseases such as diabetes and coronary heart disease. Measures of depressive symptoms were available on a subset of patients in both cohorts. We re-calculated the multivariate models for both cohorts separately and included the measures of depressive symptoms. Depressive symptoms were not associated with suboptimal communication in either multivariate model, nor did the inclusion of depressive symptoms change the main results. Because we anticipated that trust in physician could modify the association of the primary independent variables with shared decision-making communication, we added interaction terms to the models for both cohorts, but found no significant effect modification. Similarly, trust could play a mediating role in the pathway between these variables and shared decision-making. However, there was no evidence of mediation in either cohort.
Discussion
This study of two demographically distinct cohorts including a total of over 500 adults with RA found that nearly one-third of subjects reported suboptimal shared-decision making communication with their clinicians, regardless of the study sample. To our knowledge, this is the first study of perceptions of shared decision-making communication in RA that has included a diverse, multilingual population.
Strikingly, low trust in physician was independently associated with suboptimal SDM communication in both cohorts. In addition, limited English proficiency was associated with greater likelihood of reporting suboptimal communication, after controlling for age, gender, race/ethnicity, disease duration, and trust in physician in the more language and racially diverse UCSF RA Cohort. Limited health literacy was also an independent correlate of suboptimal communication in the largely white, more educated, older subjects in the RA Panel. While older age and suboptimal trust in physician have been associated with poorer communication in prior studies of patients with RA(20, 44), limited English proficiency and limited health literacy have not been studied as a correlate of shared decision-making in RA.
The emphasis on shared decision-making as an integral part of delivering high quality, patient-centered care is reflected in national and international directives such as the Institute of Medicine’s Crossing the Quality Chasm, which concerns all medical conditions, and the 2010 Treat to Target guidelines for RA, which concerns this one. Despite the goal of an informed, activated patient truly sharing a decision with the clinician, multiple barriers to this ideal exist (3). Specific barriers include health beliefs, and educational, cultural, or literacy backgrounds that pose challenges to understanding or applying existing evidence to certain decisions, such as whether or not to begin a biologic or triple therapy with synthetic DMARDs when faced with moderate to high disease activity. Such barriers can then result in disenfranchisement or further alienate patients from fully engaging in healthcare decisions (45).
As evidenced by this current study, patients with limited health literacy and limited English proficiency had much greater risk of suboptimal SDM communication. It is these very populations who are also at increased risk of poorer health outcomes in RA, even if they have access to the most current state of the art treatments (46). In a cross-sectional study of over 1,000 community-based RA patients (6.5% non-White and 8.8% with limited health literacy), Martin and colleagues found that health literacy was independently associated with risk perception and willingness to take a DMARD but that depression was not (47). This study also found that risk perception mediates the effect of health literacy on willingness to take a DMARD. This finding underscores the importance of creating interventions to ensure that more vulnerable groups are truly informed of risks and benefits of treatments and are engaged in making treatment decisions with their clinicians. In other chronic diseases, suboptimal patient-clinician communication has been associated with lower medication adherence to cardiometabolic medications in heart disease, and to hypoglycemic medications in diabetes (11), which suggests a possible role for communication in addressing health disparities in general (48). While associations with poorer adherence or health outcomes have not yet been demonstrated in RA, it is a reasonable next step in acquiring a better understanding of whether a link exists between patient-provider communication and health disparities.
Prior studies have reported on decision making in RA, but have largely examined the concept in majority populations with higher levels of education. Kjeken et al studied over 1,000 Norwegian RA patients and found that younger age, high levels of formal education, high levels of satisfaction with care and received information were all associated with greater patient involvement in medical decisions (44). In a study of 628 U.S. adults with RA who were 90% white and over half of whom had some college education or more, Martin et al identified trust as an independent correlate of confidence in a medical decision (20). While our study confirms the findings of prior work, it adds substantially to the current literature in that it included populations most vulnerable to poor outcomes and suboptimal communication. While it is perhaps not surprising that those with limited health literacy and limited English proficiency reported suboptimal communication around shared decision-making, the size of the effect of these barriers was notable, at minimum in the case of health literacy, tripling the odds of reporting suboptimal communication. These findings emphasize and underscore the need to identify these characteristics of our patients, and promote and support patient-centered care in these groups.
Our study has several limitations. The cross-sectional design does not allow inferences regarding causation, particularly with regard to the relationship between trust and shared decision-making, even though our theoretical model proposes a causal pathway. Patient reports of communication may be subject to recall bias (11), and such bias may be greater among those with low trust or poor understanding of the health care encounter. The measure of decision making communication used was one of self-report and not one of direct observation, however the IPC subscale of decision making has been developed and validated in diverse populations and administered to similar patient populations to those in this study (14, 16, 17, 49). Our measure of health literacy in the RA Panel is self-reported and does not capture all domains of this complex concept, nor does it measure numeracy; use of the single item screener that focuses on reading ability may lead to measurement bias when compared to a longer instrument of health literacy. We did not have a measure of health literacy in the UCSF RA Cohort, which one might assume would have an even greater number of subjects with limited literacy. We combined race/ethnicity into a single category for matters of simplicity and power, however we acknowledge that an analysis examining the association of ethnicity (Latino v. non-Latino) with communication around decision making may be of interest in future studies to evaluate whether English-speaking Hispanics experiences differs from English-speaking non-Hispanics.
What can be done to improve the quality of communication and to promote shared decision-making in rheumatology care? It has been reported that physicians engage in less actual shared decision-making than they perceive (50). Clinicians often report they do not have time for engaging in shared decision-making and believe that patients want to be told what to do or have their doctors decide what is best (51). However, despite reservations on the part of clinicians, there is evidence to support the practice of patient-centered care and shared decision-making as well as the use of decision aids to improve communication and enhance health outcomes. A 2011 Cochrane review of 86 decision aids reported that these tools appear to have a positive effect on patient-clinician communication, increase patient involvement, and improve knowledge and realistic perception of outcomes (52). The effects on adherence, cost-effectiveness, and use with lower literacy populations were deemed to require further evaluation. To date, several investigators have published studies on the development and testing of decision support tools designed for patients with RA (53–55), but only one addressed the needs of patients with low literacy (54) and none addressed the barriers of limited English proficiency. Given the findings of this study and what is known about disparities in RA, we could begin by enhancing the provision of literacy-appropriate educational resources that describe the condition, therapies, risks/benefits and potential harms to all patients with RA; training clinicians who care for RA patients in communication skills and techniques of delivering patient-centered care (56); increasing awareness of shared decision-making and the use of decision support tools; and promoting the development and implementation of low literacy decision aids to facilitate shared decision-making around complex therapies. In addition to targeting clinician and patient-level enhancements in communication, Brach and colleagues in a 2012 paper published by the Institute of Medicine outline ten attributes of a “health literate organization.” The authors underscore that the provision of truly health literate care to patients is “a necessary prerequisite to assuring patient safety, promoting adherence, enhancing self-efficacy, and improving patient outcomes” (57).
Future research must investigate patient-clinician communication as a potential contributor to health disparities, particularly in the rheumatic diseases. If we as a subspecialty are to have any impact on the persistent health disparities documented among our patients, we need to closely examine all facets of care. If patients lack trust or feel their doctors do not respect them or involve them in making decisions about their health, patients may in turn be less likely to adhere to therapy and be at higher risk of poorer outcomes. A disenfranchised patient then enters a vicious cycle of disempowerment and disenchantment with medical care. It is the responsibility of the medical community to confront deficiencies and variation in care resulting from the health care system, even those occurring in the interaction in the exam room.
Implications
This study highlights that nearly one-third of adults with RA report suboptimal SDM communication with their clinician. Patients with limited health literacy, limited English proficiency, and lower trust in physician had significantly greater odds of suboptimal shared decision-making communication. These findings underscore the need for more research into the association of SDM and health outcomes and potentially, the development of more literacy- and language-appropriate interventions.
Acknowledgments
Drs. Barton, Imboden, Katz, and Yelin’s work was supported by funding the Rosalind Russell Medical Research Center for Arthritis, University of California, San Francisco. Drs. Barton, Imboden, and Yelin received funding from the Agency for Healthcare Research and Quality (R18 HS19209). Drs. Barton, Yelin, and Katz, and Ms. Trupin’s work was also supported by NIAMS (grant P60 AR053308, Multidisciplinary Clinical Research Center). Dr. Schillinger’s work was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131.
Footnotes
This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Services HaH, editor. National Strategy for Quality Improvement in Health Care. Bethesda: 2011. [Google Scholar]
- 2.Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Annals of the rheumatic diseases. 2010;69(4):631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legare F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health affairs. 2013;32(2):276–84. doi: 10.1377/hlthaff.2012.1078. [DOI] [PubMed] [Google Scholar]
- 4.Wennberg JEBS, Fisher ES, Skinner JS, Weinstein JN. Improving Quality and Curbing Health Care Spending: Opportunities for the Congress and the Obama Administration. Dartmouth, NH: The Dartmouth Institute for Health Policy & Clinical Practice; 2008. [PubMed] [Google Scholar]
- 5.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient education and counseling. 2006;60(3):301–12. doi: 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Arora NK, Ayanian JZ, Guadagnoli E. Examining the relationship of patients’ attitudes and beliefs with their self-reported level of participation in medical decision-making. Medical care. 2005;43(9):865–72. doi: 10.1097/01.mlr.0000173560.18607.67. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. American journal of respiratory and critical care medicine. 2010;181(6):566–77. doi: 10.1164/rccm.200906-0907OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes-Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane database of systematic reviews. 2011;(10):CD001431. doi: 10.1002/14651858.CD001431.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Peek ME, Gorawara-Bhat R, Quinn MT, Odoms-Young A, Wilson SC, Chin MH. Patient Trust in Physicians and Shared Decision-Making Among African-Americans With Diabetes. Health communication. 2012 doi: 10.1080/10410236.2012.710873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peek ME, Wilson SC, Gorawara-Bhat R, Odoms-Young A, Quinn MT, Chin MH. Barriers and facilitators to shared decision-making among African-Americans with diabetes. Journal of general internal medicine. 2009;24(10):1135–9. doi: 10.1007/s11606-009-1047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratanawongsa N, Karter AJ, Parker MM, Lyles CR, Heisler M, Moffet HH, et al. Communication and medication refill adherence: the Diabetes Study of Northern California. JAMA internal medicine. 2013;173(3):210–8. doi: 10.1001/jamainternmed.2013.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez A, Schillinger D, Warton EM, Adler N, Moffet HH, Schenker Y, et al. Language barriers, physician-patient language concordance, and glycemic control among insured Latinos with diabetes: the Diabetes Study of Northern California (DISTANCE) Journal of general internal medicine. 2011;26(2):170–6. doi: 10.1007/s11606-010-1507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swenson SL, Rose M, Vittinghoff E, Stewart A, Schillinger D. The influence of depressive symptoms on clinician-patient communication among patients with type 2 diabetes. Medical care. 2008;46(3):257–65. doi: 10.1097/MLR.0b013e31816080e9. [DOI] [PubMed] [Google Scholar]
- 14.Schenker Y, Stewart A, Na B, Whooley MA. Depressive symptoms and perceived doctor-patient communication in the Heart and Soul study. Journal of general internal medicine. 2009;24(5):550–6. doi: 10.1007/s11606-009-0937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legare F, O’Connor AM, Graham ID, Wells GA, Tremblay S. Impact of the Ottawa Decision Support Framework on the agreement and the difference between patients’ and physicians’ decisional conflict. Medical decision making: an international journal of the Society for Medical Decision Making. 2006;26(4):373–90. doi: 10.1177/0272989X06290492. [DOI] [PubMed] [Google Scholar]
- 16.Stewart AL, Napoles-Springer A, Perez-Stable EJ. Interpersonal processes of care in diverse populations. The Milbank quarterly. 1999;77(3):305–39. 274. doi: 10.1111/1468-0009.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart AL, Napoles-Springer AM, Gregorich SE, Santoyo-Olsson J. Interpersonal processes of care survey: patient-reported measures for diverse groups. Health services research. 2007;42(3 Pt 1):1235–56. doi: 10.1111/j.1475-6773.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tom JO, Mangione-Smith R, Solomon C, Grossman DC. Integrated personal health record use: association with parent-reported care experiences. Pediatrics. 2012;130(1):e183–90. doi: 10.1542/peds.2011-1786. [DOI] [PubMed] [Google Scholar]
- 19.Haggerty JL, Beaulieu C, Lawson B, Santor DA, Fournier M, Burge F. What Patients Tell Us about Primary Healthcare Evaluation Instruments: Response Formats, Bad Questions and Missing Pieces. Healthcare policy = Politiques de sante. 2011;7(Spec Issue):66–78. [PMC free article] [PubMed] [Google Scholar]
- 20.Martin RW, Head AJ, Rene J, Swartz TJ, Fiechtner JJ, McIntosh BA, et al. Patient decision-making related to antirheumatic drugs in rheumatoid arthritis: the importance of patient trust of physician. The Journal of rheumatology. 2008;35(4):618–24. [PubMed] [Google Scholar]
- 21.Thom DH, Ribisl KM, Stewart AL, Luke DA. Further validation and reliability testing of the Trust in Physician Scale. The Stanford Trust Study Physicians. Medical care. 1999;37(5):510–7. doi: 10.1097/00005650-199905000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Freburger JK, Callahan LF, Currey SS, Anderson LA. Use of the Trust in Physician Scale in patients with rheumatic disease: psychometric properties and correlates of trust in the rheumatologist. Arthritis and rheumatism. 2003;49(1):51–8. doi: 10.1002/art.10925. [DOI] [PubMed] [Google Scholar]
- 23.Karliner LS, Napoles-Springer AM, Schillinger D, Bibbins-Domingo K, Perez-Stable EJ. Identification of limited English proficient patients in clinical care. Journal of general internal medicine. 2008;23(10):1555–60. doi: 10.1007/s11606-008-0693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenker Y, Karter AJ, Schillinger D, Warton EM, Adler NE, Moffet HH, et al. The impact of limited English proficiency and physician language concordance on reports of clinical interactions among patients with diabetes: the DISTANCE study. Patient education and counseling. 2010;81(2):222–8. doi: 10.1016/j.pec.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Family medicine. 2004;36(8):588–94. [PubMed] [Google Scholar]
- 26.Morris NS, MacLean CD, Chew LD, Littenberg B. The Single Item Literacy Screener: evaluation of a brief instrument to identify limited reading ability. BMC family practice. 2006;7:21. doi: 10.1186/1471-2296-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarkar U, Schillinger D, Lopez A, Sudore R. Validation of self-reported health literacy questions among diverse English and Spanish-speaking populations. Journal of general internal medicine. 2011;26(3):265–71. doi: 10.1007/s11606-010-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. Journal of general internal medicine. 2008;23(5):561–6. doi: 10.1007/s11606-008-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller MJ, Allison JJ, Schmitt MR, Ray MN, Funkhouser EM, Cobaugh DJ, et al. Using single-item health literacy screening questions to identify patients who read written nonsteroidal anti-inflammatory medicine information provided at pharmacies. Journal of health communication. 2010;15(4):413–27. doi: 10.1080/10810731003753091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD. Brief report: screening items to identify patients with limited health literacy skills. Journal of general internal medicine. 2006;21(8):874–7. doi: 10.1111/j.1525-1497.2006.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caplan L, Wolfe F, Michaud K, Quinzanos I, Hirsh JM. Health literacy is strongly associated with functional status among rheumatoid arthritis patients: A cross-sectional study. Arthritis care & research. 2013 doi: 10.1002/acr.22165. [DOI] [PubMed] [Google Scholar]
- 32.Al Sayah F, Williams B, Johnson JA. Measuring health literacy in individuals with diabetes: a systematic review and evaluation of available measures. Health education & behavior: the official publication of the Society for Public Health Education. 2013;40(1):42–55. doi: 10.1177/1090198111436341. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity: Patient (PtGA) and Provider (PrGA) Global Assessment of Disease Activity, Disease Activity Score (DAS) and Disease Activity Score with 28-Joint Counts (DAS28), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), Patient Activity Score (PAS) and Patient Activity Score-II (PASII), Routine Assessment of Patient Index Data (RAPID), Rheumatoid Arthritis Disease Activity Index (RADAI) and Rheumatoid Arthritis Disease Activity Index-5 (RADAI-5), Chronic Arthritis Systemic Index (CASI), Patient-Based Disease Activity Score With ESR (PDAS1) and Patient-Based Disease Activity Score without ESR (PDAS2), and Mean Overall Index for Rheumatoid Arthritis (MOI-RA) Arthritis care & research. 2011;63 (Suppl 11):S14–36. doi: 10.1002/acr.20621. [DOI] [PubMed] [Google Scholar]
- 34.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, 3rd, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA: the journal of the American Medical Association. 1994;272(22):1749–56. [PubMed] [Google Scholar]
- 35.Margaretten M, Yelin E, Imboden J, Graf J, Barton J, Katz P, et al. Predictors of depression in a multiethnic cohort of patients with rheumatoid arthritis. Arthritis and rheumatism. 2009;61(11):1586–91. doi: 10.1002/art.24822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris A, Yelin EH, Panopalis P, Julian L, Katz PP. Long-term patterns of depression and associations with health and function in a panel study of rheumatoid arthritis. Journal of health psychology. 2011;16(4):667–77. doi: 10.1177/1359105310386635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katz PP, Yelin EH. The development of depressive symptoms among women with rheumatoid arthritis. The role of function. Arthritis and rheumatism. 1995;38(1):49–56. doi: 10.1002/art.1780380108. [DOI] [PubMed] [Google Scholar]
- 38.Huang FY, Chung H, Kroenke K, Delucchi KL, Spitzer RL. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. Journal of general internal medicine. 2006;21(6):547–52. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wulsin L, Somoza E, Heck J. The Feasibility of Using the Spanish PHQ-9 to Screen for Depression in Primary Care in Honduras. Primary care companion to the Journal of clinical psychiatry. 2002;4(5):191–5. doi: 10.4088/pcc.v04n0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Margaretten M, Barton J, Julian L, Katz P, Trupin L, Tonner C, et al. Socioeconomic determinants of disability and depression in patients with rheumatoid arthritis. Arthritis care & research. 2011;63(2):240–6. doi: 10.1002/acr.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss BD, Francis L, Senf JH, Heist K, Hargraves R. Literacy education as treatment for depression in patients with limited literacy and depression: a randomized controlled trial. Journal of general internal medicine. 2006;21(8):823–8. doi: 10.1111/j.1525-1497.2006.00531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez K, Watt TT. Collaborative care for the treatment of depression in primary care with a low-income, spanish-speaking population: outcomes from a community-based program evaluation. The primary care companion to CNS disorders. 2012;14(6) doi: 10.4088/PCC.12m01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.SHDWJaL . Applied Logistic Regression. New York: John Wiley & Sons; 2000. [Google Scholar]
- 44.Kjeken I, Dagfinrud H, Mowinckel P, Uhlig T, Kvien TK, Finset A. Rheumatology care: Involvement in medical decisions, received information, satisfaction with care, and unmet health care needs in patients with rheumatoid arthritis and ankylosing spondylitis. Arthritis and rheumatism. 2006;55(3):394–401. doi: 10.1002/art.21985. [DOI] [PubMed] [Google Scholar]
- 45.Smith QW, Street RL, Jr, Volk RJ, Fordis M. Differing levels of clinical evidence: exploring communication challenges in shared decision making. Medical care research and review: MCRR. 2013;70(1 Suppl):3S–13S. doi: 10.1177/1077558712468491. [DOI] [PubMed] [Google Scholar]
- 46.Barton JL, Trupin L, Schillinger D, Gansky SA, Tonner C, Margaretten M, et al. Racial and ethnic disparities in disease activity and function among persons with rheumatoid arthritis from university-affiliated clinics. Arthritis care & research. 2011;63(9):1238–46. doi: 10.1002/acr.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin RW, McCallops K, Head AJ, Eggebeen AT, Birmingham JD, Tellinghuisen DJ. Influence of patient characteristics on perceived risks and willingness to take a proposed anti-rheumatic drug. BMC medical informatics and decision making. 2013;13:89. doi: 10.1186/1472-6947-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Julian LJ, Yelin E, Yazdany J, Panopalis P, Trupin L, Criswell LA, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis and rheumatism. 2009;61(2):240–6. doi: 10.1002/art.24236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Napoles AM, Gregorich SE, Santoyo-Olsson J, O’Brien H, Stewart AL. Interpersonal processes of care and patient satisfaction: do associations differ by race, ethnicity, and language? Health services research. 2009;44(4):1326–44. doi: 10.1111/j.1475-6773.2009.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heisler M, Tierney E, Ackermann RT, Tseng C, Narayan KM, Crosson J, et al. Physicians’ participatory decision-making and quality of diabetes care processes and outcomes: results from the triad study. Chronic illness. 2009;5(3):165–76. doi: 10.1177/1742395309339258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanson JL. Shared decision making: have we missed the obvious? Archives of internal medicine. 2008;168(13):1368–70. doi: 10.1001/archinte.168.13.1368. [DOI] [PubMed] [Google Scholar]
- 52.O’Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane database of systematic reviews. 2009;(3):CD001431. doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Fraenkel L, Peters E, Charpentier P, Olsen B, Errante L, Schoen RT, et al. Decision tool to improve the quality of care in rheumatoid arthritis. Arthritis care & research. 2012;64(7):977–85. doi: 10.1002/acr.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li LCAP, Townsend AF, Lacaille D, Yousefi C, Turnau S, et al. A web-based methotrexate decision aid for patients with rheumatoid arthritis. Arthritis Rheum. 2011;63(10):S652. [Google Scholar]
- 55.Martin RW, Brower ME, Geralds A, Gallagher PJ, Tellinghuisen DJ. An experimental evaluation of patient decision aid design to communicate the effects of medications on the rate of progression of structural joint damage in rheumatoid arthritis. Patient education and counseling. 2012;86(3):329–34. doi: 10.1016/j.pec.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Levinson W, Lesser CS, Epstein RM. Developing physician communication skills for patient-centered care. Health affairs. 2010;29(7):1310–8. doi: 10.1377/hlthaff.2009.0450. [DOI] [PubMed] [Google Scholar]
- 57.Brach C, Dreyer BP, Schillinger D. Physicians’ Roles in Creating Health Literate Organizations: A Call to Action. Journal of general internal medicine. 2013 doi: 10.1007/s11606-013-2619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


