Abstract
Objective
Obesity and estrogen are strong risk factors for endometrial cancer (EC). While diabetes also increases risk, little is known about related insulin resistance (IR). The purpose of this study was to determine the prevalence of IR in newly diagnosed EC patients.
Study Design
EC patients from a large, metropolitan county were prospectively enrolled from 2005–2008. Fasting serum was analyzed for glucose and insulin. IR was defined as a history of diabetes or a QUICKI [1/(log fasting insulin + log fasting glucose)] value of <0.357.
Results
Among 99 patients, diabetes was present in 30, and an abnormal QUICKI was found in 36 additional patients. Increased risk of IR was significantly associated with higher BMI (p<0.001), lower socioeconomic status (p=0.007), and nulliparity (p=0.029).
Conclusion
IR was highly prevalent in endometrial cancer patients, including non-obese women. Better characterization of metabolic risks in addition to obesity may provide avenues for targeted cancer prevention in the future.
Keywords: diabetes, endometrial cancer, insulin resistance, obesity
Introduction
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer overall in women in the United States, accounting for an estimated 43,470 new cancer diagnoses and 7,950 cancer-related deaths in 2010.1 Increased risk of endometrial cancer is attributed to reproductive conditions resulting in relative estrogen excess such as early menarche, late menopause, nulliparity, and chronic anovulation.2–6 Endometrial cancer is also strongly associated with obesity. While this association has been classically attributed to peripheral aromatization of androstenedione to estrone, some studies have suggested that this relationship is not entirely explained by unopposed estrogen.7, 8
Insulin resistance (IR) is a spectrum of disease that includes not only overt diabetes, but also prediabetes as well as varying degrees of preclinical insulinemia and glycemia. While diabetes has been shown to be a mild independent risk factor for endometrial cancer, little is known about the relationship between IR and endometrial cancer. IR is difficult to study retrospectively because it requires fasting blood samples, and consequently, few previous reports have investigated this relationship. However, an association between IR and endometrial cancer has been suggested by several retrospective case-control studies and a large prospective, nested case-control study employing a surrogate that does not require fasting blood, adiponectin.9–12 Additionally, preclinical studies have shown that insulin resistance accompanied by high circulating levels of insulin potentiates the effect of estrogen on endometrial proliferation.13 Thus, insulin resistance may further clarify the link between obesity and endometrial cancer. We hypothesized that the risk of endometrial cancer associated with diabetes alone results in an underestimation of the true relationship between IR and endometrial cancer. We performed a geographically-limited, case-case study to determine the prevalence of insulin resistance among women with endometrial cancer. In addition, we sought to describe the relationship of insulin resistance with other previously described risk factors for endometrial cancer.
Materials and Methods
A prospective, multi-institutional study evaluating insulin resistance and endometrial cancer was performed in a single metropolitan county in Texas, Harris County. Institutional Review Board approval was obtained, and patients with newly diagnosed endometrial cancer were recruited from 5 different hospitals and 1 private practice. Participating institutions included two private hospitals (St. Luke’s Episcopal Hospital and The Woman’s Hospital of Texas), one private practice group (Gynecologic Oncology of Houston), two public/county hospitals (Ben Taub General Hospital and Lyndon B. Johnson General Hospital), and one tertiary referral center (The University of Texas M.D. Anderson Cancer Center). English and Spanish speaking patients within 12 weeks of their primary diagnosis who were residents of Harris County, Texas were considered eligible.
Participants were required to complete a structured, self-administered questionnaire that addressed demographics, menstrual and reproductive history, family history, and personal medical history. Medical records were reviewed for tumor histology, stage and grade. Height and weight were measured and body mass index (BMI) was calculated by Quetelet Index as weight (kg)/ height (m)2.
Normal weight was defined as a BMI < 25 kg/m2, overweight as a BMI 25–29.9 kg/m2, obese as a BMI 30–39.9 kg/m2, and morbidly obese as a BMI ≥ 40kg/m2.14 Socioeconomic status (SES) was classified by a combination of income and education levels. Low SES was defined as an annual household income <$20,000, or an income between $20-40,000 and education level less than high school graduate or equivalent, or an income between $40-60,000 and education level of 8th grade or less. Middle level SES was defined as an income between $20-40,000 and education level of at least high school graduate, or income between $40-60,000 and education level of at least some high school attendance, or income level between $60-80,000 and education level less than college graduate. High SES was defined as an income greater than $80,000 and any education level, or income between $60-80,000 and education of college or beyond. Infertility was defined as the inability to conceive after 1 year of trying or undergoing medical evaluation for difficulty conceiving. Chronic anovulation was characterized by self-reported irregular menses, decreased frequency of menses (>35 days or never regular), or assessment and/or treatment for irregular menstruation.
Fasting blood samples were collected after a minimum 8 hour fast from each participant. Serum insulin was determined by enzyme-immunoassay in triplicate (ALPCO Diagnostics, Salem, NH). Serum glucose levels were ascertained by The University of Texas M.D. Anderson Cancer Center CLIA-certified laboratory. A QUICKI was calculated by the formula [1/(log fasting insulin + log fasting glucose)].15, 16 Insulin resistance (IR) was defined as either (1) a history of type II diabetes mellitus (DM) or (2) a QUICKI value <0.357.17
Descriptive statistics were utilized to report demographic characteristics, anthropometrics, pathologic, and reproductive/menstrual variables. Fisher’s exact test was used to examine associations with insulin resistance. The Cochran-Armitage trend test was use to evaluate ordinal data. SAS version 9 (SAS Inc., Cary, NC) software was used for data analysis. Logistic regression was used to estimate the risk of IR associated with other factors among endometrial cancer patients. The initial model was created with all terms that were statistically significant in the univariate analysis with p < 0.10, and backward selection was employed to create the final model, with all terms in the final model statistically significant at p < 0.10. A p-value of <0.05 was used as an indicator of statistical significance in the final model.
Results
Ninety-nine patients with endometrial cancer were enrolled in the study and completed study requirements between November, 2005 and June, 2008. The mean age at diagnosis was 59.3 years old (range 26–87 years) (Table 1). The majority of patients were white, non-Hispanic (58.6%). Insulin resistance (IR) was identified in 66 of the 99 total study participants, of which 30 (45%) patients had a known, previous diagnosis of diabetes. However, in 36 (55%) of the patients with IR, the diagnosis was based on a low QUICKI (Figure 1). Age (p=0.028), recruiting site (p=0.004), socioeconomic status (p=0.002), and BMI (p<0.001) were significantly associated with IR in univariate analysis (Table 1). Among reproductive and menstrual factors, chronic anovulation (p=0.038) was the only factor more prevalent in IR patients by univariate analysis (Table 2). However, secondary to a p-value less than 0.10, nulliparity (p=0.063) was included in the regression analysis along with age, SES, BMI and chronic anolvulation to determine factors independently associated with risk of insulin resistance (Table 3). Although endometrial cancer patients enrolled from public hospitals were significantly more likely to be insulin resistant than other patients, this variable could not be modeled because 100% of women from the two public hospitals were found to have IR. Higher BMI, lower socioeconomic status, and nulliparity remained significant in the final reduced model.
Table 1.
Characteristics of endometrial cancer patients by insulin resistance status
| TOTAL (N=99) |
Insulin Resistant (N=66) |
Non- Insulin Resistant (N=33) |
p-valuea | |
|---|---|---|---|---|
| Age (years) | Mean (SD) | Mean (SD) | Mean (SD) | 0.028 |
| 59.3 (11.8) | 57.4 (12.3) | 62.9 (10.0) | ||
| N (%) | N (%) | N (%) | 0.281 | |
| <50 | 18 (18.2) | 15 (22.7) | 3 (9.1) | |
| 50–59 | 32 (32.3) | 22 (33.3) | 10 (30.3) | |
| 60–69 | 31 (31.3) | 19 (28.8) | 12 (36.4) | |
| ≥70 | 18 (18.2) | 10 (15.2) | 8 (24.2) | |
| Race/Ethnicity | N (%) | N (%) | N (%) | 0.178 |
| White, Non-Hispanic | 58 (58.6) | 38 (57.6) | 20 (60.6) | |
| White, Hispanic | 15 (15.2) | 12 (18.2) | 3 (9.1) | |
| Black | 11 (11.1) | 8 (12.1) | 3 (9.1) | |
| Asian | 7 (7.1) | 2 (3.0) | 5 (15.2) | |
| Other | 2 (2.0) | 1 (1.5) | 1 (3.0) | |
| Not reported | 6 (6.1) | 5 (7.6) | 1 (3.0) | |
| Recruiting Site | N (%) | N (%) | N (%) | 0.004 |
| Private Hospitals | 48 (48.5) | 32 (48.5) | 16 (48.5) | |
| Public Hospitals | 14 (14.1) | 14 (21.2) | 0 (0.0) | |
| Tertiary Referral Center | 37 (37.4) | 20 (30.3) | 17 (51.5) | |
| SES | N (%) | N (%) | N (%) | 0.002b |
| Low | 22 (22.2) | 19 (28.8) | 3 (9.1) | |
| Middle | 36 (36.4) | 25 (37.9) | 11 (33.3) | |
| High | 29 (29.3) | 13 (19.7) | 16 (48.5) | |
| Not reported | 12 (12.1) | 9 (13.6) | 3 (9.1) | |
| BMI (kg/m2) |
Median (Range) |
Median (Range) |
Median (Range) |
<0.001 |
| 32.9 (19.5–71.1) |
37.6 (22.6–71.1) |
27.3 (19.5–47.5) |
||
| N (%) | N (%) | N (%) | <0.001 | |
| Normal Weight (<25) | 17 (17.2) | 4 (6.1) | 13 (39.4) | |
| Overweight (25–29.9) | 23 (23.2) | 13 (19.7) | 10 (30.3) | |
| Obese (30–39.9) | 28 (28.3) | 23 (34.8) | 5 (15.2) | |
| Morbidly Obese (≥40) | 28 (28.3) | 24 (36.4) | 4 (12.1) | |
| Not reported | 3 (3.0) | 2 (3.0) | 1 (3.0) |
Univariate analysis; Missing values not included in statistical testing
Cochrane-Armitage trend test reported
Figure 1. CONSORT diagram of endometrial cancer patients by insulin resistance.
A majority of patients had some form of insulin resistance, and over half of these women had been previously undiagnosed. Severity of insulin resistance in these undiagnosed women ranged from abnormal QUICKI values to overt diabetes.
Table 2.
Reproductive and menstrual characteristics by insulin resistance status
| TOTAL (N=99) |
Insulin Resistant (N=66) |
Non- Insulin Resistant (N=33) |
p-valuea | |
|---|---|---|---|---|
|
Parity (Number of pregnancies) |
Median (Range) |
Median (Range) |
Median (Range) |
0.051 |
| 2 (0–11) |
2 (0–11) |
2.5 (0–10) |
||
| N (%) | N (%) | N (%) | 0.063 | |
| Nulliparous | 19 (19.2) | 16 (24.2) | 3 (9.1) | |
| Parous | 74 (74.7) | 45 (68.2) | 29 (87.9) | |
| Not reported | 6 (6.1) | 5 (7.6) | 1 (3.0) | |
| Fertility | N (%) | N (%) | N (%) | 0.400 |
| Fertile | 77 (77.8) | 52 (78.8) | 25 (75.8) | |
| Infertile | 16 (16.2) | 9 (13.6) | 7 (21.2) | |
| Not reported | 6 (6.0) | 5 (7.6) | 1 (3.0) | |
| Chronic anovulation | N (%) | N (%) | N (%) | 0.038 |
| Ovulatory | 62 (62.6) | 35 (53.0) | 26 (78.8) | |
| Anovulatory | 31 (31.3) | 25 (37.9) | 6 (18.2) | |
| Not reported | 6 (6.1) | 6 (9.1) | 1 (3.0) | |
| Age at menarche (years) |
Median (Range) |
Median (Range) |
Median (Range) |
0.291 |
| 13 (9–16) |
12 (9–16) |
13 (9–16) |
||
| N (%) | N (%) | N (%) | 0.228 | |
| < 12 years old | 25 (25.2) | 19 (28.8) | 6 (18.2) | |
| ≥ 12 years old | 68 (68.7) | 42 (63.6) | 26 (78.8) | |
| Not reported | 6 (6.1) | 5 (7.6) | 1 (3.0) | |
| Age at menopause (years) |
Median (Range) |
Median (Range) |
Median (Range) |
0.273 |
| 51 (26–67) |
50 (26–67) |
52 (43–61) |
||
| N (%) | N (%) | N (%) | 0.767 | |
| ≥ 55 years old | 15 (15.2) | 11 (16.7) | 4 (12.1) | |
| < 55 years old | 76 (76.8) | 51 (77.3) | 25 (75.8) | |
| Not reported | 8 (8.0) | 4 (6.1) | 4 (12.1) |
Univariate analysis; Missing values not included in statistical testing
Table 3.
Logistic regression model to predict insulin resistance among endometrial cancer patients
| Full Model | Reduced Model | |||||
|---|---|---|---|---|---|---|
| OR | P-Value | 95% CI | OR | P-Value | 95% CI | |
| Age | 0.99 | 0.700 | 0.93 – 1.05 | |||
| BMI | 1.16 | 0.001 | 1.06 – 1.26 | 1.15 | < 0.001 | 1.06 – 1.24 |
| SES | 0.25 | 0.006 | 0.09 – 0.67 | 0.31 | 0.007 | 0.13 – 0.73 |
| Parity | 0.13 | 0.022 | 0.02 – 0.74 | 0.16 | 0.029 | 0.03 – 0.83 |
| Menstrual Pattern | 0.41 | 0.271 | 0.08 – 2.02 | |||
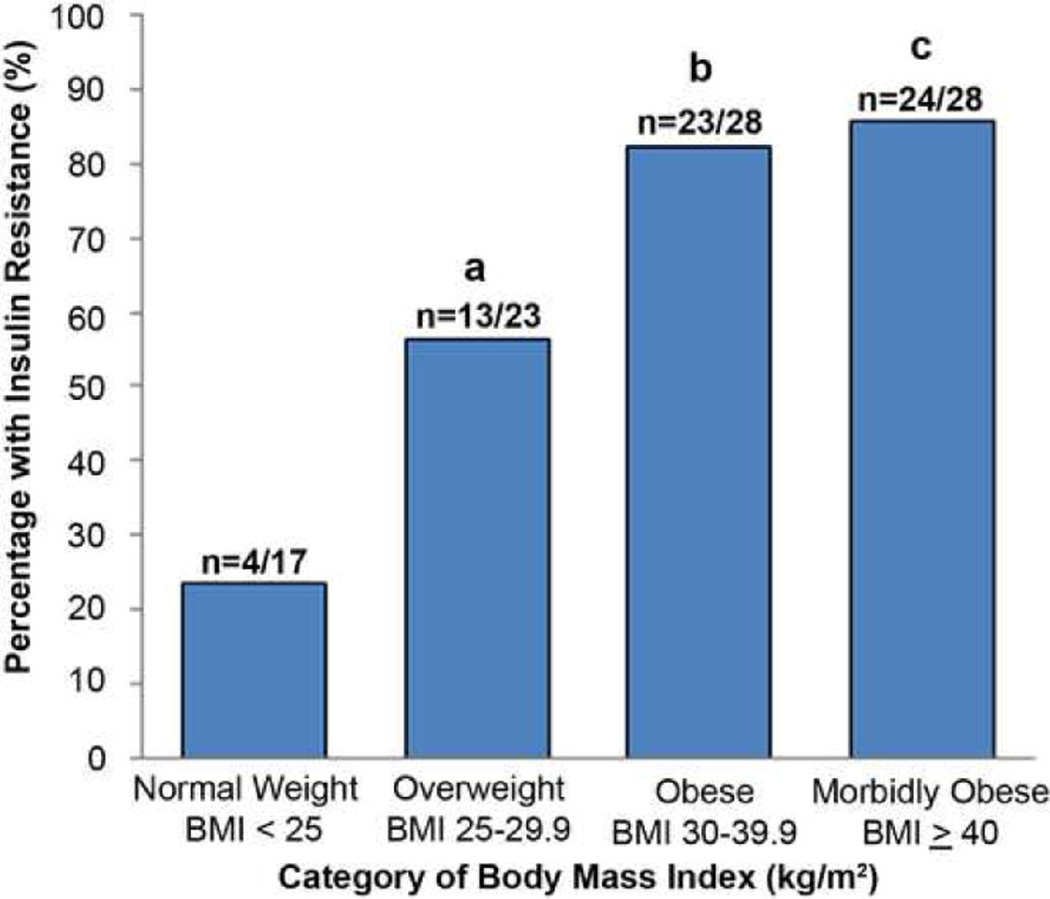
As expected, IR was common among obese patients; among the 56 women with a BMI ≥ 30kg/m2, 47 (84%) had IR. IR was also present in a high proportion, 17 of 40 (43%), of non-obese (BMI< 30 kg/m2) women. Specifically, 13 of the 23 (57%) overweight women and 4 of 17 (24%) of normal weight women were insulin resistant. The proportion of women with IR who were overweight was significantly higher than that among normal weight women (p=0.04). The difference was of borderline significance when comparing overweight to obese women (p=0.05) but was not different when comparing obese to morbidly obese women (p=0.72).
Overall, the majority of endometrial cancer patients in this study had tumors with favorable characteristics (Table 4). Seventy of 96 (73%) had stage I disease, 79 of 87 (91%) were grade 1 or 2, and 74 of 94 (79%) were of endometrioid histology. There was no difference in tumor characteristics by IR status.
Table 4.
Tumor characteristics by insulin resistance status
| TOTAL (N=99) |
Insulin Resistant (N=66) |
Non- Insulin Resistant (N=33) |
p-valuea | |
|---|---|---|---|---|
| Stageb | N (%) | N (%) | N (%) | 0.259 |
| I | 70 (70.7) | 46 (69.7) | 24 (72.7) | |
| II | 9 (9.1) | 7 (10.7) | 2 (6.1) | |
| III | 10 (10.1) | 8 (12.1) | 2 (6.1) | |
| IV | 6 (6.1) | 2 (3.0) | 4 (12.1) | |
| Early stage | ||||
| Early stage (Iab) | 59 (59.6) | 36 (54.6) | 23 (69.7) | 0.186 |
| ≥Ib | 36 (36.4) | 27 (40.9) | 9 (27.3) | |
| Not reported | 4 (4.0) | 3 (45.5) | 1 (3.0) | |
| Grade | N (%) | N (%) | N (%) | 0.131 |
| 1 | 23 (23.2) | 13 (19.7) | 10 (30.3) | |
| 2 | 56 (56.6) | 37 (56.1) | 19 (57.6) | |
| 3 | 8 (8.1) | 7 (10.6) | 1 (3.0) | |
| Not reported | 12 (12.1) | 9 (13.6) | 3 (9.1) | |
| Histology | N (%) | N (%) | N (%) | >0.999 |
| Endometrioid | 74 (74.7) | 50 (75.8) | 24 (72.7) | |
| Non-endometrioid | 20 (20.2) | 14 (21.2) | 6 (18.2) | |
| Not reported | 5 (5.1) | 2 (3.0) | 3 (9.1) |
Univariate analysis
Revised International Federation of Gynecology & Obstetrics (FIGO) Staging, 2009
Although there was not a significant difference in IR status by race/ethnicity, several other observations were made by univariate analysis between endometrial cancer patients in this study with different backgrounds, specifically between women who identified themselves as white and Hispanic compared to all other non-Hispanic ethnicities (Figure 2). Hispanic women were younger than non-Hispanic women (p=0.005). Three of the 15 (20%) white, Hispanic women were less than 40 years old at the time of diagnosis compared to 3 of the 78 (4%) women other races and ethnicities (p=0.051). A greater proportion of Hispanic women (8 of 15, 53%) were morbidly obese than other women (18 of 75, 24%, p=0.031), and BMI was higher among Hispanic women (p=0.005). Seven of the 14 (50%) Hispanic women were of low SES, compared to 15 of 73 (21%) non-Hispanic women (p= 0.039). SES was also significantly lower in Hispanic women by trend analysis (p=0.019). Lastly, a high proportion of Hispanic women were found to have IR (12 of 15, 80%), although this was not significantly different when compared to non-Hispanic women (49 of 78, 63%, p=0.247).
Figure 2. Characteristics of Hispanic endometrial cancer patients.
In univariate analysis, Hispanic women were found to be younger, more obese, and of lower socioeconomic status (SES) than women of other races. Eighty percent of all Hispanic women were insulin resistant.
Comment
While previous studies have demonstrated an association between diabetes and endometrial cancer, this study demonstrates that the insulin-resistant state is also highly prevalent among women with endometrial cancer. In our study, 66% of women were insulin resistant at the time of diagnosis. More than half of these insulin resistant women were non-diabetic and had not been previously diagnosed. Prospective investigations measuring fasting indices of insulin resistance are required to capture the full impact of insulin resistance on endometrial cancer. Past studies without fasting serum samples have been limited by their retrospective nature and inability to examine the association of non-diabetic insulin resistance and endometrial cancer.
Compared to previous studies, we found a higher prevalence of both overt diabetes and non-diabetic IR among endometrial cancer patients. One of the largest case-control studies of endometrial cancer by Brinton et al. found that 58 of the 405 (14%) cases enrolled between 1987 and 1990 had a self-reported diagnosis of diabetes, a proportion lower than in our study participants (36%) who were diagnosed more recently, between 2005 and 2008.2 This difference may be related to an increasing prevalence of obesity in recent years. In a Russian study by Berstein et al. published in 2004 in which diabetics were excluded, authors found a 36% prevalence of IR in endometrial cancer patients using the HOMA index, while 52% of the non-diabetic women in our study were insulin-resistant.18 Ethnic differences between their Russian patients and our patients from metropolitan Texas may account for this difference in prevalence of IR.
In general, insulin resistance is a metabolic state in which target tissues become less responsive to circulating insulin, resulting in elevations in blood glucose and circulating insulin. Insulin has been shown to have a mitogenic effect on a number of different cancer cell lines, including endometrial cancer22. Preclinical studies conducted by our group suggest that hyperinsulinemia and insulin resistance associated with obesity may contribute to endometrial proliferation.13 In addition, we have found that correction of hyperinsulinemia with insulin-sensitizing agents reverses the proliferative drive (unpublished data). Given the high prevalence of insulin resistance in our population of patients and potential contribution of IR to the carcinogenesis of endometrial cancer, insulin-sensitizing agents may have a role in treatment and prevention of this disease.
As expected, higher BMI was associated with increasing risk of IR among our endometrial cancer patients. However, we did find that insulin resistance was present in a moderate proportion of overweight and normal weight women. This finding emphasizes the importance of including both obese and non-obese women in future studies of insulin resistance. Although additional prospective studies will be needed, insulin resistance in non-obese women may represent a risk factor for the development of endometrial cancer.
In the general population, lower socioeconomic status is associated with increased frequency of obesity and related comborbidities, including diabetes. In our study of endometrial cancer patients, lower SES was associated with an increased risk of insulin resistance, independent of other risk factors such as BMI. Although there is no standard definition, most investigators use some combination of income and education level to stratify SES. We chose to use a combination of income level and education level to define SES. Income level stratifications were based upon mean income levels and poverty income levels for Harris County, Texas. Although, this measure has not been validated, the correlation between income and education in our study was r= 0.60 (P<0.001). Additionally, there was a high correlation between our definition of low SES and treatment at a public hospital (p<0.001). These patients may represent an underserved population that would benefit from targeted education and screening for both IR and possibly endometrial cancer.
Although a combined race and ethnicity variable was not significantly associated with increased insulin resistance, we did note a high prevalence (80%) of insulin resistance among women of Hispanic ethnicity who had endometrial cancer. The Hispanic women were significantly younger, more obese, and of lower SES than women of other ethnicities. According to Texas Cancer Registry data, 23% of endometrial cancer patients in the state are Hispanic, slightly higher than the 17% in our study population.19 Comparatively, nationwide, 9% of incident cases of endometrial cancer diagnosed between 2002–2006 were among Hispanic women.20 Given the high prevalence of IR, and well as the young age of onset of endometrial cancer among the Texas Hispanic women in our study, targeted education and prevention measures should be considered in this population.
Classic models of endometrial cancer pathogenesis suggest that obesity and related estrogen excess are associated with low grade, early stage disease of well-differentiated endometrioid histology.21 However, in the current study, we did not see an association between insulin resistance and these tumor characteristics. Although negative findings such as this could be affected by small sample size, we feel that this finding is intriguing and should be further investigated in larger studies that have been adequately powered to determine the true relationship between IR and tumor histology. It may be that IR contributes to all grades of endometrial cancer. Biological plausibility supports this theory; insulin is a growth factor and in vitro studies demonstrate a mitogenic effect of insulin on endometrial cancer cell lines.22
This case-case study assessed the prevalence of IR as well as risk factors associated with increased risk of IR among endometrial cancer patients. Although a mean number of 278 new cases of endometrial cancer were diagnosed in Harris County per year during the study time period, we were able to enroll 99 patients who completed study requirements. Although this is a fraction of all cases diagnosed in Harris County over the study period, the demographic distribution of the patients approximates that reported for the same time period. For 2005–2007, the Texas Cancer Registry reports that 55.8% of patients in Harris County with newly diagnosed endometrial cancer were white non-Hispanic, 19.7% were white Hispanic, and 17.3% were black. We feel that this is an accurate representation of the population. However, exploratory analyses of certain subgroups of patients including non-obese women, young women and women of certain racial and ethnic minorities lacked sufficient numbers to evaluate associations with sufficient statistical power. Furthermore, because of the descriptive nature of this paper, we did not adjust for multiple comparisons.
In summary, we report a high prevalence of insulin resistance, both non-diabetic and diabetic, which has relevance for etiologic investigation, chemoprevention, and treatment of endometrial cancer. Larger case-control studies will be necessary to determine the changing demographics of endometrial cancer in this country and more specifically, the contribution of the full spectrum of IR to this disease.
Figure 3. Characteristics of Hispanic endometrial cancer patients.
Acknowledgments
Funding: This study has been supported in part by The University of Texas M. D. Anderson Cancer Center Uterine Cancer Specialized Program of Research Excellence (SPORE) P50 CCA098258. Additionally, this research is supported in part by the National Institutes of Health through M. D. Anderson's Cancer Center Support Grant CA016672. Additionally, this research was supported in part by the National Institute of General Medical Sciences U54 CA96300 05 S1(PP-15) grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- The 40th Annual Meeting on Women’s Cancer, Society of Gynecologic Oncologists, San Antonio, Texas, February 5th, 2009. Poster presentation.
- The American Society of Clinical Oncology, Annual Meeting, June 6th, 2010. Discussion poster presentation.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010 doi: 10.3322/caac.20073. Epub. [DOI] [PubMed] [Google Scholar]
- 2.Brinton LA, Berman ML, Mortel R, et al. Reproductive, menstrual, and medical risk factors for endometrial cancer: results from a case-control study. Am J Obstet Gynecol. 1992;167:1317–1325. doi: 10.1016/s0002-9378(11)91709-8. [DOI] [PubMed] [Google Scholar]
- 3.Elwood JM, Cole P, Rothman KJ, Kaplan SD. Epidemiology of endometrial cancer. J Natl Cancer Inst. 1977;59:1055–1060. doi: 10.1093/jnci/59.4.1055. [DOI] [PubMed] [Google Scholar]
- 4.Goodman MT, Hankin JH, Wilkens LR, et al. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Res. 1997;57:5077–5085. [PubMed] [Google Scholar]
- 5.Henderson BE, Casagrande JT, Pike MC, Mack T, Rosario I, Duke A. The epidemiology of endometrial cancer in young women. Br J Cancer. 1983;47:749–756. doi: 10.1038/bjc.1983.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Vecchia C, Franceschi S, Decarli A, Gallus G, Tognoni G. Risk factors for endometrial cancer at different ages. J Natl Cancer Inst. 1984;73:667–671. [PubMed] [Google Scholar]
- 7.MacDonald PC, Edman CD, Hemsell DL, Porter JC, Siiteri PK. Effect of obesity on conversion of plasma androstenedione to estrone in postmenopausal women with and without endometrial cancer. Am J Obstet Gynecol. 1978;130:448–455. doi: 10.1016/0002-9378(78)90287-9. [DOI] [PubMed] [Google Scholar]
- 8.Potischman N, Hoover RN, Brinton LA, et al. Case-control study of endogenous steroid hormones and endometrial cancer. J Natl Cancer Inst. 1996;88:1127–1135. doi: 10.1093/jnci/88.16.1127. [DOI] [PubMed] [Google Scholar]
- 9.Cust AE, Kaaks R, Friedenreich C, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92:255–263. doi: 10.1210/jc.2006-1371. [DOI] [PubMed] [Google Scholar]
- 10.Dal Maso L, Augustin LS, Karalis A, et al. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab. 2004;89:1160–1163. doi: 10.1210/jc.2003-031716. [DOI] [PubMed] [Google Scholar]
- 11.Petridou E, Mantzoros C, Dessypris N, et al. Plasma adiponectin concentrations in relation to endometrial cancer: a case-control study in Greece. J Clin Endocrinol Metab. 2003;88:993–997. doi: 10.1210/jc.2002-021209. [DOI] [PubMed] [Google Scholar]
- 12.Soliman PT, Wu D, Tortolero-Luna G, et al. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106:2376–2381. doi: 10.1002/cncr.21866. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Shen Q, Celestino J, et al. Enhanced estrogen-induced proliferation in obese rat endometrium. Am J Obstet Gynecol. 2009;200:186 e1–188 e1. doi: 10.1016/j.ajog.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Practical guide, identification, evaluation, and treatment of overweight and obesity in adults: National Institute of Health/ National Heart, Lung, and Blood Institute. 2000;2009 [Google Scholar]
- 15.Chen H, Sullivan G, Quon MJ. Assessing the predictive accuracy of QUICKI as a surrogate index for insulin sensitivity using a calibration model. Diabetes. 2005;54:1914–1925. doi: 10.2337/diabetes.54.7.1914. [DOI] [PubMed] [Google Scholar]
- 16.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 17.Hrebicek J, Janout V, Malincikova J, Horakova D, Cizek L. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metab. 2002;87:144–147. doi: 10.1210/jcem.87.1.8292. [DOI] [PubMed] [Google Scholar]
- 18.Berstein LM, Kvatchevskaya JO, Poroshina TE, et al. Insulin resistance, its consequences for the clinical course of the disease, and possibilities of correction in endometrial cancer. J Cancer Res Clin Oncol. 2004;130:687–693. doi: 10.1007/s00432-004-0587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Texas Cancer Registry: Statistical Data: Texas Department of State Health Services. 2009 [Google Scholar]
- 20.SEER Web Site. 2010 [Google Scholar]
- 21.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 22.Nagamani M, Stuart CA, Dunhardt PA, Doherty MG. Specific binding sites for insulin and insulin-like growth factor I in human endometrial cancer. Am J Obstet Gynecol. 1991;165:1865–1871. doi: 10.1016/0002-9378(91)90047-u. [DOI] [PubMed] [Google Scholar]