Abstract
Objective
Recent literature in ovarian cancer suggests differences in surgical outcomes depending on operative start time. We sought to examine the effects of operative start time on surgical outcomes for patients undergoing minimally invasive surgery for endometrial cancer.
Methods
A retrospective review was conducted of patients undergoing minimally invasive surgery for endometrial cancer at a single institution between 2000 and 2011. Surgical and oncologic outcomes were compared between patients with an operative start time before noon and those with a surgical start time after noon.
Results
A total of 380 patients were included in the study (245 with start times before noon and 135 with start times after noon). There was no difference in age (p=0.57), number of prior surgeries (p=0.28), medical comorbidities (p=0.19), or surgical complexity of the case (p=0.43). Patients with surgery starting before noon had lower median BMI than those beginning after noon, 31.2 vs. 35.3 respectively (p=0.01). No significant differences were observed for intraoperative complications (4.4% of patients after noon vs. 3.7% of patients before noon, p=0.79), estimated blood loss (median 100 cc vs. 100 cc, p=0.75), blood transfusion rates (7.4% vs. 8.2%, p=0.85), conversion to laparotomy (12.6% vs. 7.4%, p=0.10). There was no difference in operative times between the two groups (198 minutes vs. 216.5 minutes, p=0.10). There was no association between operative start time and postoperative non-infectious complications (11.9% vs. 11.0%, p=0.87), or postoperative infections (17.8% vs. 12.3%, p=0.78). Length of hospital stay was longer for surgeries starting after noon (median 2 days vs. 1 day, p=0.005). No differences were observed in rates of cancer recurrence (12.6% vs. 8.8%, p=0.39), recurrence-free survival (p=0.97), or overall survival (p=0.94).
Conclusion
Our results indicate equivalent surgical outcomes and no increased risk of postoperative complications regardless of operative start time in minimally invasive endometrial cancer staging, despite longer length of hospital stay for surgeries beginning after noon.
Introduction
In 1999, the Institute of Medicine released a Health Care Quality Initiative report showing that over a one year period, medical errors cost the health care system approximately $29 billion and resulted in as many as 98,000 patient deaths (1). The report identified surgical complications as the second most common cause of preventable morbidity and mortality. This national focus on patient safety has led the surgical community to closely examine current operating room practices and conduct evidence-based research as a means of implementing strategies to improve quality of care.
It is known that timing of a medical intervention can greatly affect health care outcomes. Over the decades, variations in outcomes have been described based on season of the year, day of the week and time of day (2–4). A study of pediatric emergency department admissions evaluated medication prescription errors over four divided time blocks in a day. The investigators found that there was an increased risk of medication prescribing errors between 4 AM and 8 AM when compared with 8 AM to 12 PM (5). The surgical community is well aware that similar problems may be encountered in the operating room. Recently, a Duke University group found a distinct association between operative start time and anesthetic adverse events, with a peak incidence of adverse events occurring in cases beginning in the afternoon (6). Specifically, cases that began between 3–6 pm had a higher rate of adverse events than cases that started between 6–9 am.
There are many potential factors that can contribute to these timing effects. Physician fatigue, resource availability and change-of-shift hand-offs are the most recognized and scrutinized performance factors for surgical subspecialties. The issue of physician fatigue and its relationship to human error recently earned the attention of the Accreditation Council for Graduate Medical Education and limitations on resident work-hours were established (7). Additionally, in a survey analysis of surgical errors at three teaching hospitals, 33% of incidents were at least partially attributed to fatigue or excess workload (8). Kelz et al. suggested that the change-of-shift for ancillary staff members may also play a role in adverse events related to timing of surgical procedures, in that medical errors can be attributed to lapses in communication during hand-offs (9).
In the field of gynecologic oncology, surgeons may perform two to three major operations in a day. With many surgical cases having an afternoon start time, issues of physician fatigue, multiple ancillary staff hand-offs and patient safety are of utmost importance. In an effort to identify a potentially controllable risk factor for adverse events we have performed a retrospective, single center analysis to examine the effects of operative start time on surgical and oncologic outcomes for patients undergoing laparoscopic or robotic surgery for endometrial cancer.
Materials and Methods
Approval to conduct this study was obtained from The University of Texas MD Anderson Cancer Center Institutional Review Board. All data were de-identified before transfer to the research analysis team. Patients who underwent minimally invasive surgery for endometrial cancer at The University of Texas MD Anderson Cancer Center were identified from surgical databases between January 2000 and January 2011. From 2000 to 2007 all minimally invasive procedures were laparoscopy. Since 2007 there is an even distribution between laparoscopy and robotic surgery for minimally invasive cases. Patient age, BMI, prior surgical history and medical comorbidities were retrospectively collected by electronic medical record review. Additionally, operative start times, total operative times, estimated blood loss, transfusion requirements, surgical complications, conversion to laparotomy rates and age, grade, histology and stage of tumor were retrospectively collected by electronic medical record review.
Operative start time was categorized as either beginning before or after 12 noon. We elected the time of noon since in our institution the time of start of shift changes for the morning crew and thus transfer to alternate crews is 12–2 pm. During this time there is a transition from specialty specific experienced crews to covering teams that could theoretically impact efficiency and performance. Additionally, noon is generally the time when surgeons transition from first major case to second major case and thus allows for comparisons of fatigue, focus, mental concentration in morning hours vs. afternoon hours. Case allocation to morning versus afternoon was based solely on the order of scheduling.
Variables of interest from surgical cases beginning before or after noon were compared. All cases were performed Monday through Friday. For the purpose of data analysis, patient medical comorbidities and surgical complications were compared as “none” versus “any”. Additionally, postoperative complications were categorized as those occurring within the first 30 days after surgery and those occurring greater than 30 days from surgery. Surgical procedures were divided into 3 groups (low, intermediate, and high) based on degree of complexity. Low-complexity procedures included diagnostic and second-look laparoscopy. Intermediate-complexity procedures included unilateral or bilateral salpingo-oophorectomy, unilateral or bilateral ovarian cystectomy, lysis of adhesions, and hysterectomy with or without salpingo-oophorectomy. High-complexity procedures included radical hysterectomy, pelvic and/or para-aortic lymphadenectomy, splenectomy, and small bowel or colonic resection. These criteria are as defined by the MD Anderson Department of Gynecologic Oncology Innovative Surgical Research & Education Group (10).
Fisher’s exact test was used to compare categorical variables. For continuous variables, two-sample t-test was used to compare means; unless the means and medians were significantly different, suggesting skewed or non-normal distributions, in which case the Wilcoxon test was used to compare medians. Though the number of lymph nodes is displayed as a frequency distribution, the Wilcoxon test was used to compare the two groups of patients with respect to the median number of lymph nodes. Statistical significance was set at a p-value < 0.05.
Results
Patient and Tumor Characteristics
Three-hundred and eighty met inclusion criteria for this study. Complete description of patient and tumor characteristics are listed in Table 1. The cohort of patients had a median age of 60.0 years, ranging from 19 to 92 years of age (SD 12.6). Patient BMI ranged from 17.5 to 72.3 with a median BMI of 32.2 (SD 9.9) and patients who underwent surgery before noon had a lower BMI compared to those who underwent surgery after noon (31.2 vs. 35.3, p = 0.01). The majority of cases were Stage I (N=336, 88.4%), followed by stage II (N=7, 1.8%), stage III (N=29, 7.6%), and stage IV (N=8, 2.1%). Overall, there was no difference in surgical stage (p=0.477), medical comorbidities (p = 0.191) or prior surgical history (p = 0.283) between the two groups. The most common histologic subtype of endometrial cancer was endometrioid (73.4%).
Table 1.
Patient demographics and Tumor Characteristics
| Surgical Start Time | |||||||
|---|---|---|---|---|---|---|---|
| Before 12p | After 12p | Total | P-value | ||||
| N | % | N | % | N | % | ||
| Stage | 0.477 | ||||||
| IA | 185 | 75.5 | 105 | 77.8 | 290 | 76.3 | |
| IB | 30 | 12.2 | 16 | 11.9 | 46 | 12.1 | |
| II | 6 | 2.5 | 1 | 0.7 | 7 | 1.8 | |
| IIIA | 3 | 1.2 | 4 | 3.0 | 7 | 1.8 | |
| IIIC1 | 9 | 3.7 | 1 | 0.7 | 10 | 2.6 | |
| IIIC2 | 7 | 2.9 | 5 | 3.7 | 12 | 3.2 | |
| IVA | 1 | 0.4 | 0 | 0.0 | 1 | 0.3 | |
| IVB | 4 | 1.6 | 3 | 2.2 | 7 | 1.8 | |
| Tumor Histology | 0.390 | ||||||
| Grade | 0.163 | ||||||
| 1 | 56 | 22.9 | 25 | 18.5 | 81 | 21.3 | |
| 2 | 125 | 51.0 | 66 | 48.9 | 191 | 50.3 | |
| 3 | 39 | 15.9 | 32 | 23.7 | 71 | 18.7 | |
| Unknown | 25 | 10.2 | 12 | 8.9 | 37 | 9.7 | |
| Age | 0.567 | ||||||
| N | 245 | 135 | 380 | ||||
| Median | 60.0 | 60.0 | 59.5 | ||||
| Minimum | 24.0 | 19.0 | 19.0 | ||||
| Maximum | 92.0 | 83.0 | 92.0 | ||||
| BMI | 0.010 | ||||||
| N | 245 | 135 | 380 | ||||
| Median | 60.0 | 60.0 | 59.5 | ||||
| Minimum | 24.0 | 19.0 | 19.0 | ||||
| Maximum | 92.0 | 83.0 | 92.0 | ||||
| Prior Surgical History | 0.283 | ||||||
| Medical Comorbidities | 0.191 | ||||||
Intraoperative Complications, Surgical Staging
Overall, intraoperative complications were uncommon; 96.3% of cases beginning before noon and 95.6% of cases beginning after noon (p=0.785) were without incident. Complications that did occur included vascular (2.2% after noon vs. 1.6% before noon), bowel (0.7% after noon vs. 0.4% before noon), urinary (0.7% after noon vs. 0.0% before noon), or uterine perforation (0.0% after noon vs. 0.4% before noon), and these complications rates did not differ significantly between the two groups (p=0.785). Overall 47.4% of cases had lymphadenectomy performed, and there was no significant difference between the group undergoing surgery before noon (49.8%) and after noon (43.0%) (p=0.238), nor did it differ by mode of minimally invasive surgery, laparoscopic (p= 0.434) or robotic (p=0.612). Furthermore, the number of lymph nodes obtained from both the pelvic (median before noon=12, after noon=11, p=0.656) and para-aortic (median before noon=6, after noon=4, p=0.327) regions did not differ between the two groups.
Operative Time, Estimated Blood Loss, Conversion Rates, Complexity
There was no significant difference in median operative time between cases that began before noon than those that began after noon, median 216.5 minutes compared to 198.0 minutes (p = 0.102). The median estimated blood loss for surgeries beginning before noon and after noon was 100 cc (p = 0.751). In addition, blood product transfusion rates did not differ significantly between the two groups. 8.2% of cases beginning before noon required a blood transfusion, compared to 7.4% of cases beginning after noon (p = 0.845). Conversion rates from minimally invasive to laparotomy also did not differ, with a conversion rate of 7.4% for cases beginning before noon and 12.6% for those beginning after (p = 0.098). The most common reasons for conversion to laparotomy were difficulty achieving optimal visualization (25.7%) and adhesions (20.0%). Overall the surgical complexity of cases did not differ significantly between the two groups (p=0.434).
Hospital Length of Stay, Postoperative Complications, Survival
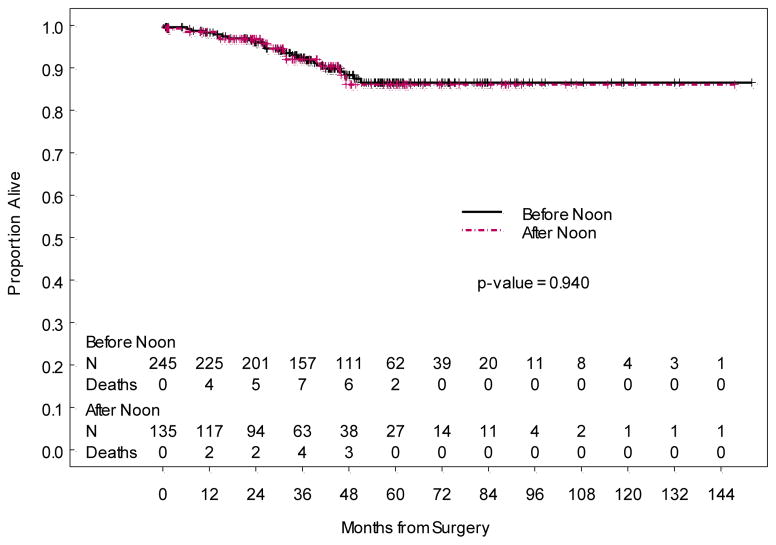
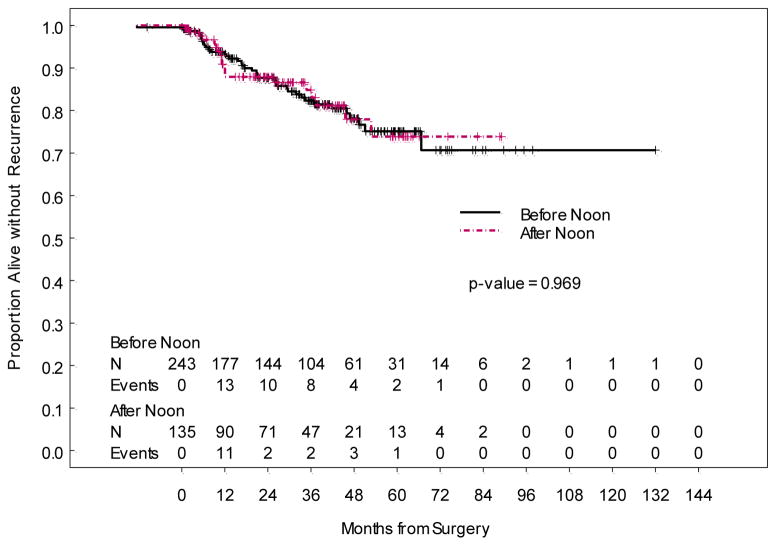
The median length of hospital stay was significantly different for cases beginning before noon (1 day, range 0 to 19 days) compared to those beginning after noon (2 days, range 0 to 8 days) (p = 0.005). Three of 245 (1.2%) patients with operative start time in the morning were outpatients, while 1 of 135 (0.7%) patients with operative start time in the afternoon was an outpatient. Most common complications occurring within 30 days postoperatively were urinary tract infection, fever and wound cellulitis. Those occurring greater than 30 days postoperatively were most commonly lymphocysts and renal/urinary complications. Infectious complication rates less than 30 days postoperatively (p = 0.775), non-infectious complication rates less than 30 days postoperatively (p=0.866), and non-infectious complication rates greater than 30 days postoperatively (p = 0.851), did not differ significantly between surgeries that began before noon and those that began after noon. Finally, overall survival (p=0.940) and disease-free survival (p=0.969) also did not differ between the two groups.
Discussion
When our cohort was divided into two start time subgroups, comprised of cases beginning before noon and after noon, our analysis revealed variable incidence of surgical and oncologic outcomes between the two start times. Our results indicate equivalent surgical outcomes and no increased risk of postoperative complications regardless of operative start time in minimally invasive endometrial cancer staging, despite longer length of hospital stay for surgeries beginning after noon. No statistically significant differences were observed for intraoperative complications, median estimated blood loss, blood transfusion rates, conversion rates to laparotomy, percent of patients who underwent lymphadenectomy, or number of nodes obtained during lymphadenectomy. There was no association between operative start time and postoperative complications. However, median length of hospital stay was shown to be longer for cases beginning after noon, compared to those beginning before.
In an effort to reduce wait times associated with gynecologic cancer surgery surgeon caseloads have increased, leading to more surgeries being performed in the afternoon. There is support in the literature that patient outcomes are adversely affected by time-of-day and fatigue in operator-dependent cognitive and technical tasks (11). Specifically laparoscopic procedures, cardiac interventions, and endotracheal intubations have been shown to have inferior outcomes when performed later in the day (6,12,13). These findings potentially have implications for physician productivity, procedural outcomes, and more importantly, patient safety. To our knowledge prior to this study there have been no investigations on the influence of operative start time on surgical and oncologic outcomes in patients undergoing minimally invasive surgery for gynecologic cancer. In contrast to other studies in the literature the findings of this study show no clinically significant differences when outcomes were compared by operative start time of cases beginning before and after noon. These results lend support to the continued use of current surgical practices in the field of gynecologic oncology, however larger studies are needed to validate these findings.
Our study is limited by potential misclassification bias and generalizability. First, while every effort was made to ensure the accuracy of the data set, we acknowledge that retrospective data collection is subject to errors including but not limited to data entry errors, inaccuracies or omissions in the original records and inconsistencies in the interpretation of primary data by different reviewers. Next, the time stratification scheme chosen here was one of many possible categorizations, and it is conceivable that other categorizations may have identified differences among start times not observed among the strata we ultimately chose. Similarly, because of the small number of absolute complications, it is possible that different groupings of individual complications may have identified differences or trends we did not observe. The single center nature of this analysis potentially limits its generalizability. Although of interest, details such as the experience level and case volume of the attending surgeon, and the makeup of the care team were not included in this analysis. It is difficult to account for ‘surgeon expertise’ given that this is a continuous variable. In other words, it is a variable that is constant only at the beginning of the study and impacted by many other factors through the course of study, such as surgical volume and case complexity. Given that we are in a fellowship program, all cases involved the combined effort of a fellow and faculty staff. Therefore, it is challenging to account for direct contribution solely from the fellow or staff. Finally, 88% of our cohort was stage I and overall survival rates for this patient population are excellent. It is possible that these variables could impact the outcomes observed.
While our analysis revealed an overall variable incidence of surgical and oncologic outcomes among the start time strata, cases beginning after noon did have a statistically significant longer length of hospital stay. Reasons for a longer hospital stay might be attributed to several factors that are difficult to capture from a retrospective analysis. Such reasons might include the fact that patients did not meet criteria for discharge, patient preference, surgeon preference, and/or issues of transportation. A multicenter analysis or national database analysis would enable the evaluation of a larger patient population that would improve the statistical power of the study. This evaluation of our single center cohort underscores the need for continued assessment and for efforts to identify and implement practices that ensure the safest possible operation and any time of the day.
Figure 1.
Overall Survival
Figure 2.
Recurrence-Free Survival
Table 2.
Operative complications and surgical staging
| Intraoperative Complications | 0.785 | ||||||
| None | 236 | 96.3 | 129 | 95.6 | 365 | 96.1 | |
| Vascular Injury | 4 | 1.6 | 3 | 2.2 | 7 | 1.8 | |
| Bowel | 1 | 0.4 | 1 | 0.7 | 2 | 0.5 | |
| Ureteral/Bladder | 0 | 0.0 | 1 | 0.7 | 1 | 0.3 | |
| Uterine Perforation | 1 | 0.4 | 0 | 0.0 | 1 | 0.3 | |
| Other | 4 | 1.6 | 3 | 2.2 | 7 | 1.8 | |
| Lymphadenectomy | 0.238 | ||||||
| Yes | 122 | 49.8 | 58 | 43.0 | 180 | 47.4 | |
| No | 123 | 50.2 | 77 | 57.0 | 200 | 52.6 | |
Table 3.
Operative time, conversion rates, blood loss and transfusion rates
| Surgical Start Time | |||||||
|---|---|---|---|---|---|---|---|
| Before 12p | After 12p | Total | |||||
| N | % | N | % | N | % | P-value | |
| Operative Time (minutes) | 0.102 | ||||||
| N | 134 | 244 | 378 | ||||
| Mean | 213.4 | 231.1 | 224.9 | ||||
| Minimum | 73.0 | 81.0 | 73.0 | ||||
| Maximum | 393.0 | 633.0 | 633.0 | ||||
| Surgical Complexity | 0.434 | ||||||
| Conversion to Open | 0.098 | ||||||
| No | 118 | 87.4 | 227 | 92.7 | 345 | 90.8 | |
| Yes | 17 | 12.6 | 18 | 7.4 | 35 | 9.2 | |
| Reason | 0.196 | ||||||
| Adhesions | 2 | 11.8 | 5 | 27.8 | 7 | 20.0 | |
| Unable to Keep Trendelenberg | 3 | 17.7 | 1 | 5.6 | 4 | 11.4 | |
| Difficulty to Achieve Visualization | 3 | 17.7 | 6 | 33.3 | 9 | 25.7 | |
| Vascular Injury | 1 | 5.9 | 3 | 16.7 | 4 | 11.4 | |
| Enterotomy | 1 | 5.9 | 1 | 5.6 | 2 | 5.7 | |
| Obesity | 1 | 5.9 | 2 | 11.1 | 3 | 8.6 | |
| Large and Bulky Lymph Nodes | 1 | 5.9 | 0 | 0.0 | 1 | 2.9 | |
| Uterus Removal | 4 | 23.5 | 0 | 0.0 | 4 | 11.4 | |
| Estimated Blood Loss (cc) | 0.751 | ||||||
| N | 135 | 245 | |||||
| Mean | 142.5 | 161.5 | 154.8 | ||||
| Minimum | 10 | 10 | 10 | ||||
| Maximum | 1000 | 35000 | 3500 | ||||
| Blood Transfusion | 0.845 | ||||||
| No | 125 | 92.6 | 224 | 91.4 | 349 | 91.8 | |
| Yes | 10 | 7.4 | 20 | 8.2 | 30 | 7.9 | |
Table 4.
Length of hospital stay, post-operative complications
| Surgical Start Time | |||||||
|---|---|---|---|---|---|---|---|
| After 12p | Before 12p | Total | |||||
| N | % | N | % | N | % | P-value | |
| Hospital Length of Stay (days) | 0.005 | ||||||
| N | 131 | 240 | |||||
| Minimum | 0 | 0 | |||||
| Median | 2 | 1 | |||||
| Maximum | 8 | 19 | |||||
| Post-Op Infections <30 Days | 0.775 | ||||||
| Post-Op Non-Infectious Complications <30 Days | 0.866 | ||||||
Highlights.
There were no differences in intraoperative outcomes in patients undergoing surgery before or after noon.
Rate of staging or lymph node count did not differ among patients undergoing surgery before or after noon.
Length of stay was longer in patients undergoing surgery after noon
Acknowledgments
Supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kohn LT, Corrigan J, Donaldson MS. To err is human: Building a safer health system. Vol. 200. Washington, DC: National Academy Press; [PubMed] [Google Scholar]
- 2.Spencer FA, Goldberg RJ, Becker RC, Gore JM. Seasonally distribution of acute myocardial infarction in the second national registry of myocardial infarction. J Am Coll Cardiol. 1998;31:1226–1233. doi: 10.1016/s0735-1097(98)00098-9. [DOI] [PubMed] [Google Scholar]
- 3.Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends compared with weekdays. N Eng J Med. 2001;345:663–668. doi: 10.1056/NEJMsa003376. [DOI] [PubMed] [Google Scholar]
- 4.Magid DJ, Wang Y, Herrin J, et al. Relationship between time of day, day of week, timeliness of reperfusion, and in-hospital mortality for patients with acute st-segment elevation myocardial infarction. JAMA. 2005;294:803–812. doi: 10.1001/jama.294.7.803. [DOI] [PubMed] [Google Scholar]
- 5.Kozer E, Scolnik D, Macpherson A, Keays T, Shi K, Luk T, et al. Variables associated with medication errors in Pediatric Medicine. Pediatrics. 2002;110:737–742. doi: 10.1542/peds.110.4.737. [DOI] [PubMed] [Google Scholar]
- 6.Wright MC, Phillips-Bute B, Mark JB, Stafford-Smith M, Grichnik KP, Andregg BC, et al. Time of day effects on the incidence of anesthetic adverse events. Qual Saf Health Care. 2006;15:815–820. doi: 10.1136/qshc.2005.017566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goitein L, Shanafelt TD, Wipf JE, et al. The effects of work-hour limitations on resident well-being, patient care, and education in an internal medicine residency program. Arch Intern Med. 2005;165:2601–2606. doi: 10.1001/archinte.165.22.2601. [DOI] [PubMed] [Google Scholar]
- 8.Gawande AA, Zinner MJ, Studdert DM, Brennan TA. Analysis of errors reported by surgeons at three teaching hospitals. Surgery. 2003;133:614–623. doi: 10.1067/msy.2003.169. [DOI] [PubMed] [Google Scholar]
- 9.Kelz R, Freeman K, Hosokawa P, Asch D, Sptiz F, Moskowitz M, et al. Time of day is associated with postoperative morbidity. Ann Surg. 2008;247:544–552. doi: 10.1097/SLA.0b013e31815d7434. [DOI] [PubMed] [Google Scholar]
- 10.Zand B, Frumovitz M, Jofre MF, Nick AM, Dos Reis R, et al. Risk factors for prolonged hospitalization after gynecologic laparoscopic surgery. Gynecol Oncol. 2012;126(3):428–431. doi: 10.1016/j.ygyno.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peskun C, Walmsley D, Waddell J, Schemitsch E. Effect of surgeon fatigue on hip and knee arthroplasty. Can J Surg. 2012;55(2):81–86. doi: 10.1503/cjs.032910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taffinder NJ, McManus IC, Gul Y, Russell RC, Darzi A. Effect of sleep deprivation on surgeons’ dexterity on laparoscopy simulator. Lancet. 1998;352:1191. doi: 10.1016/s0140-6736(98)00034-8. [DOI] [PubMed] [Google Scholar]
- 13.Kuon E, Dahm JB, Schmitt M, Glaser C, Gefeller O, et al. Time of day influences patient radiation exposure from percutaneous cardiac intervention. Br J Radiol. 2003;76:189–191. doi: 10.1259/bjr/14780035. [DOI] [PubMed] [Google Scholar]